Abstract
We previously reported that angiotensin II type 1 receptor (AT1R) blockade attenuates renal inflammation/fibrogenesis in immune-mediated glomerulonephritis via angiotensin II type 2 receptor (AT2R). In the present study, further in vivo experiments revealed that AT2R was expressed in tubular epithelial cells of nephritic kidneys in mice, and feedback activation of the renin-angiotensin system during AT1R blockade significantly reduced p-ERK, but not intranuclear nuclear factor-κB, levels via AT2R. This led to reduction in mRNA levels of the proinflammatory mediator monocyte chemoattractant protein-1 and overall interstitial inflammation and subsequent fibrogenesis. Specific blockade of ERK expression in tubular epithelium by anti-sense oligodeoxynucleotides also attenuated interstitial inflammation, mimicking the anti-inflammatory action of AT2R in nephritic kidneys. Alternatively, we succeeded in confirming such an AT2R function by demonstrating that AT1R blockade did not confer renoprotection in nephritic, AT2R gene-deficient mice. Additional in vitro experiments revealed that AT2R activation did not affect nuclear factor-κB activation by tumor necrosis factor-α in cultured tubular epithelial cells, although it inhibited ERK phosphorylation, which reduced monocyte chemoattractant protein-1 mRNA levels. These results suggest that feedback activation of AT2Rs in tubular epithelium of nephritic kidneys plays an important role in attenuating interstitial inflammation.
The potent vasoactive peptide angiotensin II (Ang II) binds two main subtypes of receptors, ie, type 1 and 2 receptors (AT1R and AT2R) that belong to the superfamily of G protein-coupled receptors but that display primarily opposite biological and physiological effects.1 Animals with anti-glomerular basement membrane (anti-GBM) nephritis, protein-overload nephropathy, subtotal nephrectomy, unilateral ureteral obstruction, or diabetic nephropathy that were treated with AT1R blockers, as well as AT1AR gene-deficient mice with such kidney diseases, exhibited renoprotection.2–7 Although the anti-inflammatory and anti-fibrotic effects of AT1R blockade have been reported in the cardiovascular system,8,9 similar effects have also been found in the liver.10 These findings suggest that AT1R mediates not only hemodynamic but also proinflammatory/fibrogenic functions. The effects of AT1R blockers, however, cannot be attributed solely to the action of AT1R. In most instances, feedback activation of the renin-angiotensin system may result in increased stimulation of AT2Rs that are freed from AT1R blockers.2
In contrast to AT1R, the pathophysiological role of AT2R remains controversial and has not yet been fully elucidated.11 AT2R activates unconventional signaling pathways that generally do not involve coupling to classical regulatory G proteins. In many cell types, AT2R is capable of activating protein tyrosine phosphatases (PTPs) such as MAP kinase phosphatase-1 (MKP-1), src homology 2 domain-containing PTP (SHP-1), and serine/threonine phosphatase PP2A.11 Activation of PTPs blocks the ERK pathway, which is activated by AT1R and other growth factors.12,13 In addition, although albumin was found to activate the ERK pathway and AP-1 through transactivation of epidermal growth factor receptor tyrosine kinase in tubular epithelial cells,14 AT2R was reported to transinactivate epidermal growth factor, basic fibroblast growth factor and insulin receptor tyrosine kinases by targeting autophosphorylation of the receptor and inhibiting ERK activation.11
Although tissue remodeling is markedly influenced by AT2R expression and signaling, it is still unclear whether stimulation of AT2R aggravates or attenuates renal interstitial inflammation and fibrogenesis.2,15–20 Recently, we demonstrated that feedback activation of AT2R in response to AT1R blockade had anti-inflammatory and anti-fibrotic effects on advanced immune-mediated glomerulonephritis in SJL mice.2 In that study, AT2R blockade alone did not influence the native course of interstitial inflammation in the day 56 anti-GBM nephritic kidney via AT1R, possibly because of a lack of feedback-mediated increase in Ang II synthesis. In contrast, AT1R blockade alone significantly attenuated such interstitial inflammation, ie, MCP-1 expression and immune cell infiltration, and fibrogenesis, ie, α1(I) procollagen (α1COLI) expression and extracellular matrix deposition, and it caused a feedback-mediated increase in Ang II synthesis. The renoprotective effects by an AT1R blocker were clearly abolished by simultaneous co-treatment with an AT2R blocker, suggesting an important anti-inflammatory/anti-fibrotic function of feedback activation of AT2R in the nephritic kidney.2 This study also suggested that pharmacologically unmodified, native renin-angiotensin system had no significant effects via AT1R as well as AT2R on the course of immune-mediated glomerulonephritis. Therefore, in the present study, we investigated how AT2R mediates renoprotection through feedback activation of the renin-angiotensin system during AT1R blockade in immune-mediated glomerulonephritis with severe inflammation.
Materials and Methods
Anti-GBM Nephritis in Mice
The preparation and characterization of the murine anti-GBM nephritis model used in this study were performed as previously described.2 Animal care and treatment were provided in conformity with the institutional guidelines.
In Vivo Experiment 1
Four groups of male, 5- to 6-week-old SJL mice were used in this experiment. Anti-GBM antiserum was injected on days 1 to 3. A group of nephritic mice (n = 10) were treated with the AT1R blocker CGP-48933 (kindly provided by Novartis Pharma AG, Basel, Switzerland) at a dose of 10 mg/kg intraperitoneally (i.p.) injected once a day from day 28 to day 30 (CGP group). Another group of nephritic mice (n = 10) were treated with both CGP-48933 and the AT2R blocker PD-123319 (Sigma, St. Louis, MO) at a dose of 20 mg/kg i.p., injected twice a day from day 28 to day 30 (CGP/PD group). Nephritic mice in the positive control (PC) group (n = 10) and nephritis-free mice in the negative control (NC) group (n = 10) received daily intraperitoneal injections of 4 mg/kg hydralazine (Sigma) to normalize blood pressure to the same levels that were seen in the CGP and CGP/PD groups2 and saline, respectively. Treatment with this dose of hydralazine did not significantly influence the disease activity of anti-GBM nephritis in mice.3
In Vivo Experiment 2
As an independent, long-term experiment, a group of nephritic mice (n = 10) were injected with ERK anti-sense oligodeoxynucleotides (ODNs) at a concentration of 1.0 mg/kg every 2 days from day 28 to day 56 (ASd56 group) to suppress tubular ERK expression and the ERK pathway and to determine the roles of the ERK pathway in the tubular epithelium in the nephritic kidney. Another group of nephritic mice (n = 10) was treated with mutated anti-sense ODN (mASd56 group). The ERK anti-sense ODN sequence was 5′-GCCGCCGCCGCCGCCAT-3′, and the mutated anti-sense ODN sequence was 5′-GCAGTCACTGACTCAAT-3′ (mutated sequence in bold); these sequences were chosen as previously described.21 We and others reported that when a given anti-sense ODN was injected intravenously into rodents, it was absorbed into the renal tubular epithelium where it was retained for nearly 48 hours and during which time it could block transcription of a target gene.22,23 Nephritic mice in the PC (PCd56) group (n = 10) and nephritis-free mice in the NC (NCd56) group (n = 8) received saline every 2 days from day 28 to the day 56. Two mice from each group were sacrificed 72 hours later (day 31) (ASd31, mASd31, PCd31, and NCd31 groups, respectively) to assess the effects of these injections on renal ERK protein expression.
In Vivo Experiment 3
Male 5- to 6-week-old AT2R gene-deficient mice (Agtr2−)24 and wild-type mice (Agtr2+) with SJL background were used in this experiment. Because the AT2R gene is located on the X chromosome, heterozygous females were mated with Agtr2+ males to obtain Agtr2− and Agtr2+ males. Animal genotyping was performed as previously described.24 Anti-GBM antiserum was injected on days 1 to 3. Each group of nephritic Agtr2− and Agtr2+ mice (n = 10) was treated with CGP-48933 at a dose of 10 mg/kg i.p. injected once a day from day 28 to day 56 (Agtr2−CGP and Agtr2+CGP groups). Each PC group of nephritic Agtr2− and Agtr2+ mice (n = 10) received daily intraperitoneal injections of 4 mg/kg hydralazine from day 28 to day 56 (Agtr2−PC and Agtr2+PC groups). Each NC group of nephritis-free Agtr2− and Agtr2+ mice (n = 10) received saline from day 28 to day 56 (Agtr2−NC and Agtr2+NC groups). Three mice from each group were sacrificed on day 31 to assess ERK phosphorylation in the kidneys. Twenty-four hour urine samples were collected on day 56, and urinary protein concentration was determined by the Bradford method.
At sacrifice on day 31 (in vivo experiment 1) and on day 56 (in vivo experiments 2 and 3), one of the kidneys of each mouse was harvested for protein/RNA extraction. A small piece of the contralateral kidney was directly snap-frozen in OCT compound without fixation for laser-captured microdissection while the remaining tissue was fixed in 4% paraformaldehyde in phosphate-buffered saline overnight and processed into paraffin blocks for histopathological analysis.
Cell Culture
To determine the effects of Ang II on AT2R in cultured murine proximal tubular epithelial cells (mProx24),25 those cells were stably transfected with a retrovirus construct (pLXSN-AT2,26 generously provided by Dr. H. Sasamura at Keio University School of Medicine, Tokyo, Japan) to generate a subline that constitutively expressed AT2R (mProx.at2r). Native mProx24 cells were used as the control. AT2R protein expression and binding in mProx.at2r cells were assessed by Western blotting as described below and by whole cell receptor-binding assay as previously described,26 respectively. mProx24 cells and mProx.at2r cells were maintained in growth medium (10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin in Dulbecco’s modified Eagle’s medium). Cells were seeded in six-well plates (1 × 105 cells/well) and cultured overnight in growth medium, which was then replaced with K-1 medium (50:50 Ham’s F-12/Dulbecco’s modified Eagle’s medium with 5 μg/ml transferrin, 5 μg/ml insulin, and 5 × 10−8 mol/L hydrocortisone). After 72 hours, recombinant murine tumor necrosis factor-α (rmTNF-α) (R&D Systems, Minneapolis, MN) was added to the medium at a final concentration of 10 ng/ml with or without co-treatment with Ang II (10−5 mol/L) (Sigma) plus CGP-48933 (10−4 mol/L) or co-treatment with both compounds plus PD123319 (10−4 mol/L). In an additional experiment, the MEK inhibitor U0126 (1.0 μmol/L) (Sigma) was used to specifically inhibit the ERK pathway. Cells in the six-well plates were harvested for Western blotting and electrophoretic mobility shift assay after another 30 minutes of incubation, or after 3 hours for the RNase protection assay, as described below.
RNase Protection Assay
Total RNA was extracted from the homogenates of kidney tissues and cultured cells with TRIzol (Gibco BRL, Grand Island, NY) according to the manufacturer’s instructions. The RNase protection assay was performed as previously described2 using cRNA probes generated from cDNA fragments encoding MCP-1, transforming growth factor-β1 (TGF-β1), α1COLI, the EIIIA isoform of fibronectin (FN-EIIIA), and GAPDH. To quantify the level of each corresponding mRNA, the autoradiograph bands were analyzed by computerized densitometry using Mac SCOPE software (version 2.5; Mitani Corp., Fukui, Japan). Data are presented as the ratio of specific mRNA to GAPDH mRNA to equalize the quantity of RNA in each sample.
Western Blot
Using protein samples from kidney tissues and cultured cells, Western blotting was performed as described previously.27 The following primary antibodies were used in this study: rabbit polyclonal anti-ERK2 (sc-154; Santa Cruz Biotechnology, Santa Cruz, CA), anti-phosphorylated ERK1/2 (p-ERK1/2) (no. 442705; Calbiochem, San Diego, CA), anti-SHP-1 (sc-287; Santa Cruz Biotechnology), anti-AT2R antibody28 (generously provided by Prof. Robert M. Carey, University of Virginia Health System, Charlottesville, VA), mouse monoclonal anti-p38 (no. 05-454; Upstate, Lake Placid, NY), anti-phosphorylated p38 (p-p38) (M8177; Sigma), and anti-actin (sc-8432; Santa Cruz Biotechnology). The immunoreactive proteins were detected by enhanced chemiluminescence and captured on X-ray film. Biotinylated molecular weight standards were run with each blot.
Morphological Examination and Immunostaining
Sections (4 μm) cut from paraffin blocks were processed for hematoxylin and eosin (H&E) staining and Masson’s trichrome staining. Glomerulosclerosis or interstitial inflammation and fibrosis were quantitatively determined with Mac SCOPE in 30 glomeruli and in 10 high-power (×200) cortical fields in tissue sections, respectively. The glomerulosclerosis and interstitial fibrosis indices were expressed as the mean percentage area in blue per one glomerulus and per one cortical field in Masson’s trichrome-stained sections, respectively.22 To calculate the interstitial inflammation index, the mean percentage area in brown per one cortical field in the sections treated immunohistochemically with anti-CD45 antibody was used.23 The indirect immunoperoxidase method was used as described previously.27 Rabbit anti-AT2R, anti-ERK2, and anti-p-ERK1/2, and rat anti-CD45 (no. 553076; BD PharMingen, San Jose, CA) were applied as primary antibodies. The following immunoreactions were performed using biotin-conjugated anti-rabbit IgG and anti-rat IgG as the secondary antibodies and the catalyzed signal amplification system peroxidase (K1500; DAKO, Carpinteria, CA) according to the manufacturer’s instructions. Negative control sections were treated as described above, but the primary antibody was omitted.
Laser-Captured Microdissection
Under RNase-free conditions, frozen tissue blocks were sectioned at 6 μm in a cryostat, mounted on clean glass slides covered with thermoplastic film, and left to dry for 30 minutes at room temperature. Sections were subsequently dipped into 70% ethanol to dissolve the OCT, counterstained with Harris modified hematoxylin (Sigma), rinsed in distilled H2O for 30 seconds, dehydrated in graded ethanol solutions (2 times at 70% for 1 minute each, two times at 95% for 1 minute each, three times at 100% for 1 minute each), and cleared in xylene (two times for 1 minute each). After being air-dried for 30 minutes, a robot-microbeam system (PALM; Mikrolaser Technology AG, Bernried, Germany) was used to perform the microdissection. After selecting areas of interest such as the glomeruli, cortical proximal tubules, and papillary collecting ducts, boundary fields were photolysed by the microbeam, and the selected areas were launched from the slide into a collecting cap using the pin-pointed microbeam. This process was repeated until 200 glomeruli, 100 proximal tubules, or 100 collecting ducts were collected.
Real-Time Quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from microdissected tissues using an RNeasy mini kit (Qiagen, Basel, Switzerland). All RNA samples were treated with the RNase-free DNase set (Qiagen), followed by a clean-up with an RNeasy mini kit. A real-time quantitative one-step RT-PCR assay was performed to quantify AT2R, MCP-1, TGF-β1, and GAPDH mRNAs using QuantiTect SYBR Green RT-PCR (Qiagen) and the ABI Prism 7700 sequence detection system (Applied Biosystems, Tokyo, Japan). The primers used for real-time RT-PCR were as follows: AT2R primer, forward 5′-CTTCAGTTTTGCTGCCACCA-3′, reverse 5′-TGTGTGAGCAATTAAAGGCGG-3′; MCP-1 primer, forward 5′-CTCTCTTCCTCCACCACCAT-3′, reverse 5′-ACTGCATCTGGCTGAGCCA-3′; TGF-β1 primer, forward 5′-CAGTGGCTGAACCAAGGAGAC-3′, reverse 5′-ATCCCGTTGATTTCCACGTG-3′; and GAPDH primer, forward 5′-TGCAGTGGCAAAGTGGAGATT-3′, reverse 5′-TTGAATTTGCCGTGAGTGGA-3′. All of these oligonucleotides were designed using Primer Express software (Perkin Elmer, Foster City, CA), and preliminary RT-PCR procedures using these primer sets successfully yielded appropriately sized single products.
Electrophoretic Mobility Shift Assay
Nuclear proteins from cultured cells were prepared using NE-PER nuclear extraction reagent (Pierce Chemical Co., Rockford, IL), and those from renal tissues were prepared using a procedure described by von Harsdorf and colleagues.29 Protein concentration was determined by the Bradford method, and aliquots were frozen at −80°C until use. Eight μg of nuclear protein was incubated with 100 pg of 32P-labeled probes containing the nuclear factor (NF)-κB consensus site or the AP-1 consensus site (5′-AGTTGAGGGGACTTTCCCAGGC-3′ and 5′-GTAAAGCATGAGTCAGACACCTC-3′, respectively) in buffer containing 10 mmol/L HEPES, pH 7.8, 2 mmol/L MgCl2, 50 mmol/L KCl, 1 mmol/L dithiothreitol, 0.1 mmol/L ethylenediaminetetraacetic acid, and 20% glycerol in the presence of poly(dI-dC) (Pharmacia Biotech, Piscataway, NJ) for 20 minutes at room temperature. The mixture was loaded on a 4% polyacrylamide 0.5× TBE gel. The gels were dried under vacuum, after which standard autoradiography was performed. To quantify the intranuclear translocation of binding proteins, the autoradiograph bands were analyzed by a computerized densitometry using Mac SCOPE software.
Statistical Analysis
Values are presented as the means ± SE. Statistical differences between groups were evaluated using a Bonferroni/Dunnett’s test and considered significant at P < 0.05.
Results
Effects of 72-Hour Activation of AT2R Under AT1R Blockade (in Vivo Experiment 1)
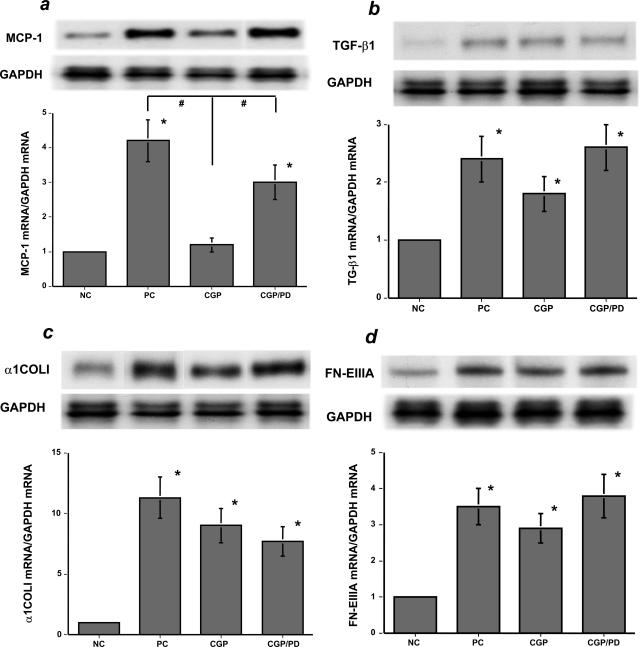
Among the up-regulated, representative proinflammatory/fibrogenic genes tested in this study, (eg, MCP-1, TGF-β1, α1COLI, FN-EIIIA) (Figure 1, a–d), feedback activation of AT2R significantly lowered MCP-1 mRNA levels in the nephritic kidney during AT1R blockade (Figure 1a). We focused on MCP-1 in the tubular epithelium because of its pivotal proinflammatory role in anti-GBM nephritis as shown previously.23 There were no significant differences in renal histopathology and urinary protein excretion among those nephritic groups on day 31 (data not shown).
Figure 1.
Seventy-two hours of AT2R-mediated effects on the day 31 nephritic kidneys (in vivo experiment 1). a: MCP-1 mRNA levels were significantly increased in the day 31 nephritic kidneys in the untreated PC group and the group co-treated with AT1R and AT2R blockers (CGP/PD) but were significantly suppressed in the group treated with an AT1R blocker (CGP). In contrast, the mRNA levels of TGF-β1 (b), α1COLI (c), and FN-EIIIA (d) were not significantly affected by 72 hours of activation of AT2R in the CGP group. NC, untreated, negative control mice without nephritis (versus NC, *P < 0.05; versus CGP, #P < 0.05). Each blot is representative of three independent experiments, and the quantitative densitometric data were obtained from these three blots.
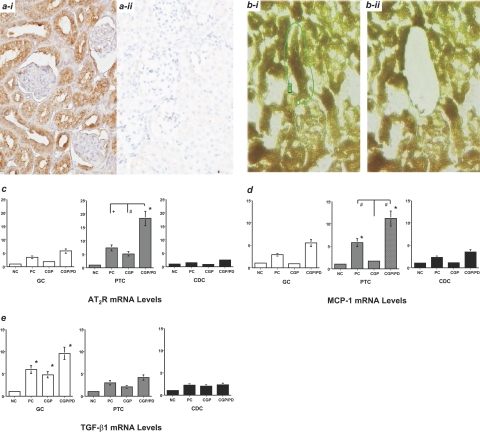
AT2R was immunohistochemically identified on tubular epithelial cells (Figure 2a) in addition to a few foci of densely infiltrating mononuclear cells (data not shown) in the day 28 nephritic kidney. AT2R mRNA levels were enhanced in proximal tubular cells (PTC) compared with glomerular cells and collecting duct cells in the nephritic kidneys (Figure 2c). Similarly, MCP-1 mRNA levels were enhanced in PTC on day 31 in the PC group (Figure 2d) but were significantly suppressed by AT1R blockade in the CGP group and further increased by the dual-blockade in the CGP/PD group (Figure 2d). These data suggest that Ang II likely reduces MCP-1 mRNA levels in PTC in the nephritic kidney via feedback activation of AT2R during AT1R blockade. In contrast, enhanced TGF-β1 mRNA levels in glomerular cells relative to PTC were seen in the day 31 nephritic kidney in the PC group, and such enhancement was not suppressed by AT1R blockade in the CGP group (Figure 2e).
Figure 2.
Seventy-two hours of AT2R-mediated effects on microdissected tissues from the day 31 nephritic kidneys (in vivo experiment 1). ai: AT2R expression in tubular epithelial cells in the day 28 nephritic kidney. aii: The NC for ai (diaminobenzidine) (DAB). bi: Before harvesting, a given proximal tubule that was directly connected to a glomerulus was targeted and marked. bii: A targeted tubule was harvested by LCM (hematoxylin). c: AT2R mRNA levels. There was an increase in PTCs relative to glomerular cells and collecting duct cells in the untreated PC group, which was further enhanced in the group co-treated with AT1R and AT2R blockers (CGP/PD). d: MCP-1 mRNA levels were significantly increased in PTC in the PC and CGP/PD groups but were suppressed in the group treated with an AT1R blocker (CGP). e: TGF-β1 mRNA levels were significantly increased in glomerular cells in the day 31 nephritic kidneys in the PC, CGP, and CGP/PD groups (versus NC, *P < 0.05; versus CGP, #P < 0.05; versus PC, +P < 0.05). These data were obtained from three independent experiments. Original magnifications: ×200 (a, b).
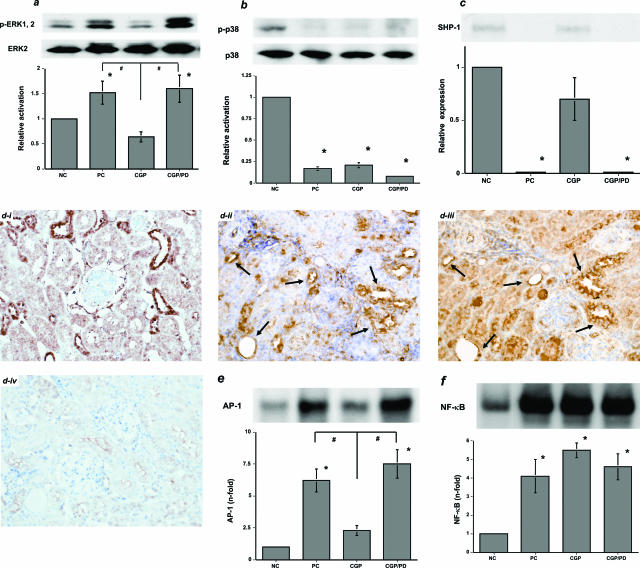
Effects of 72-Hour Activation of AT2R on MAP Kinase Pathways and NF-κB (in Vivo Experiment 1)
In contrast to the AT1AR-mediated activation of tyrosine kinases (such as MAP kinases), Ang II, via the AT2R, likely activates tyrosine phosphatases and serine/threonine phosphatases. In general, phosphatases inactivate tyrosine kinases and suppress the cell growth and extracellular matrix synthesis that is stimulated by various growth factors, including Ang II. To elucidate the role of MAP kinase pathways, Western blotting was performed using protein samples from the day 31 nephritic kidneys. Activation of AT2R under AT1R blockade significantly reduced the phosphorylated ERK1/2/ERK2 ratio in the CGP group compared with the PC and CGP/PD groups, both of which showed significant activation of the ERK pathway (Figure 3a). In contrast, although phosphorylation of JNK was also increased in the CGP group (data not shown), phosphorylation of p38 was suppressed in the PC, CGP, and CGP/PD groups (Figure 3b). The protein levels of SHP-1 were significantly decreased in the PC and CGP/PD groups, whereas those in the CGP group were increased to the levels seen in the NC group (Figure 3c). Although ERK2 was expressed in nearly all tubulointerstitial components on day 28 (Figure 3di), activation of ERK (p-ERK1/2) was restricted to tubular epithelial cells at random (Figure 3dii). Analysis of serial kidney sections from the PC group revealed that most of the tubular epithelial cells that were positive for p-ERK1/2 were also positive for AT2R (Figure 3d, ii and iii). However, in the kidneys of animals in the CGP group, p-ERK1/2 was nearly undetectable by immunohistochemistry (data not shown). Levels of AP-1-binding proteins in the nuclear proteins extracted from the nephritic kidneys were reduced by activation of AT2R, presumably because MAP kinase pathways induce their transport into the nucleus (Figure 3e). On the other hand, levels of NF-κB were unaffected by AT1R blockade (Figure 3f).
Figure 3.
AT2R-mediated effects on MAP kinase pathways and intranuclear translocation of AP-1-binding proteins and NF-κB in the day 31 nephritic kidneys (in vivo experiment 1). a: The ERK pathway was significantly activated in the untreated, PC group and the group co-treated with AT1R and AT2R blockers (CGP/PD). In contrast to the CGP/PD group, the ERK pathway was inactivated in the group treated with an AT1R blocker (CGP). b: Phosphorylated p38 was not detected in the day 31 nephritic kidneys. c: The protein levels of SHP-1 were significantly decreased in the day 31 nephritic kidneys in the PC and CGP/PD groups, whereas those in the CGP group were detected. di: ERK2 was found in almost all cells in the day 31 nephritic kidney in the PC group. dii: p-ERK1/2 was found in tubular epithelial cells in a random manner. diii: AT2R was found in serially sectioned tubular epithelial cells, some of which were also positive for p-ERK1/2 (arrows). div: The NC for di to diii (DAB). e: AP-1-binding proteins in nuclear proteins extracted from the day 31 nephritic kidneys. Intranuclear translocation of AP-1-binding proteins was significantly increased in the PC group. AT1R blockade alone significantly suppressed it in the CGP group, but simultaneous AT2R blockade abolished this suppression in the CGP/PD group. f: NF-κB in nuclear proteins extracted from the day 31 nephritic kidneys. The intranuclear translocation of NF-κB was significantly increased in the PC, CGP, and CGP/PD groups (versus NC, *P < 0.05; versus CGP, #P < 0.05). Each blot in a–c, e, and f is representative of three independent experiments; the quantitative densitometric data were obtained from these three blots. Original magnifications, ×200.
The ERK Pathway in Tubular Epithelium Mediates Inflammation (in Vivo Experiment 2)
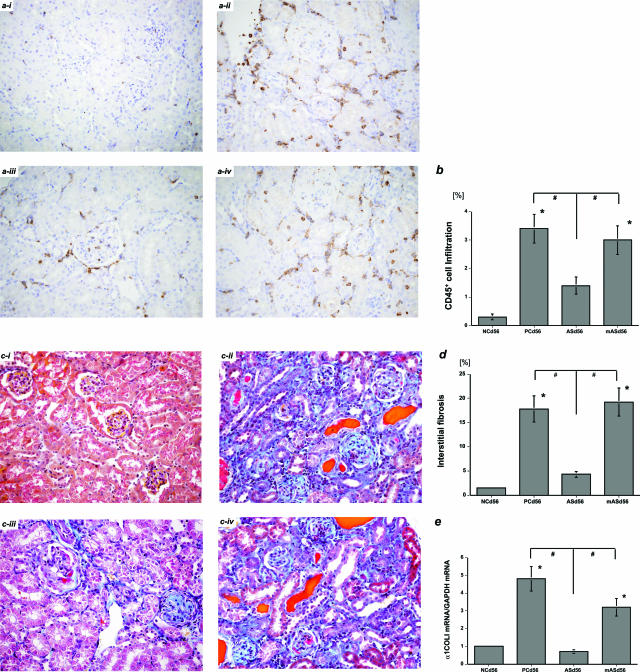
Although ERK activation was not affected by treatment with ERK mutated anti-sense ODN (mASd31 group), protein levels of p-ERK1/2 were significantly reduced in parallel with those of ERK2 after treatment with ERK anti-sense ODN (ASd31 group) (Figure 4a). It was unlikely attributable to nonspecific effects by ODN because the actin protein levels were not affected (Figure 4a). Levels of AP-1-binding proteins in nuclear proteins extracted from nephritic kidneys were significantly reduced by ERK anti-sense ODN treatment in the ASd31 group (Figure 4b). In contrast, it did not affect levels of NF-κB in the ASd31 group (Figure 4c). Levels of p-ERK1/2 and ERK2 were significantly reduced in tubular epithelial cells in the ASd31 group (p-ERK1/2; Figure 4di, ERK2; data not shown) as determined by immunohistochemistry. MCP-1 mRNA levels were also reduced in the ASd31 group compared with those in the PCd31 and mASd31 groups, although TGF-β1 mRNA levels were not affected (Figure 4, e and f). Long-term treatment with ERK anti-sense ODN from day 28 to day 56 significantly attenuated renal interstitial CD45+ immune cell infiltration and subsequent fibrogenesis in the ASd56 group compared with the PCd56 and mASd56 groups (Figure 5), possibly because of anti-inflammatory effects exerted by the continuous suppression of p-ERK1/2 levels in tubular epithelial cells. These results were mostly identical to the anti-inflammatory actions of AT2R in the day 56 nephritic kidneys after long-term treatment with an AT1R blocker from day 28 to day 56.2
Figure 4.
Single treatment with ERK anti-sense ODN reduces p-ERK1/2 levels in tubular epithelial cells in the day 31 nephritic kidneys (in vivo experiment 2). a: ERK anti-sense ODN treatment for 72 hours significantly reduced p-ERK1/2 levels in parallel with those of ERK2 in the day 31 nephritic kidneys in the ASd31 group. ERK mutated anti-sense ODN treatment had no effects in the mASd31 group. NCd31, untreated, NC mice without nephritis; PCd31, untreated PC mice with nephritis. b: Intranuclear translocation of AP-1-binding proteins was significantly increased in the PCd31 group. ERK anti-sense ODN treatment significantly suppressed it in the ASd31 group, but the mutated anti-sense ODN had no effects in the mASd31 group. c: The intranuclear translocation of NF-κB was significantly increased in the PCd31, ASd31, and mASd31 groups. di: p-ERK1/2 was exclusively found in tubular epithelial cells (arrows) in the mASd31 group. dii: Treatment with ERK anti-sense ODN significantly reduced p-ERK1/2 in tubular epithelial cells in the ASd31 group (DAB). e: MCP-1 mRNA levels were significantly attenuated by treatment with ERK anti-sense ODN in the ASd31 group relative to the PCd31 and mASd31 groups. f: TGF-β1 mRNA levels were not affected by 72 hours of treatment with ERK anti-sense ODN (versus NCd31, *P < 0.05; versus ASd31, #P < 0.05). The data in a–c, and in e and f were obtained from two and three independent experiments, respectively. Original magnifications, ×200.
Figure 5.
Long-term, multiple treatments with ERK anti-sense ODN attenuate interstitial immune cell infiltration and fibrogenesis in the day 56 nephritic kidneys (in vivo experiment 2). CD45+ immune cell infiltration in the day 56 nephritic kidney-negative (ai) and -positive (aii) controls (NCd56 and PCd56 groups, respectively). Interstitial inflammation in the day 56 nephritic kidneys was significantly reduced by long-term treatment with ERK anti-sense ODN from day 28 to day 56 (ASd56 group, aiii) as compared with the PCd56 group (aii) and the group treated with mutated anti-sense ODN (mASd56, aiv) (DAB). b: The percentage of interstitial area occupied by infiltrating CD45+ immune cells in each group was quantitated as described in Materials and Methods. Expansion of interstitial fibrosis in the NCd56 (ci) and PCd56 (cii) groups. Interstitial fibrosis in the day 56 nephritic kidneys was significantly reduced in the ASd56 group (ciii) as compared with the PCd56 (cii) and mASd56 (civ) groups (Masson’s trichrome). d: The percentage of interstitial area occupied by extracellular matrix in each group was quantitated as described in Materials and Methods. e: α1COLI mRNA levels were significantly reduced in the day 56 nephritic kidneys in the ASd56 group (versus NCd56, *P < 0.05; versus ASd56, #P < 0.05). The data in e were obtained from three independent experiments. Original magnifications, ×200.
AT1R Blockade Does Not Have Renoprotective Effects on Immune-Mediated Glomerulonephritis in the Agtr2− Mice (in Vivo Experiment 3)
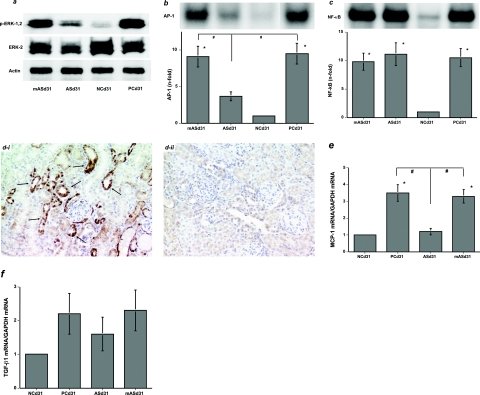
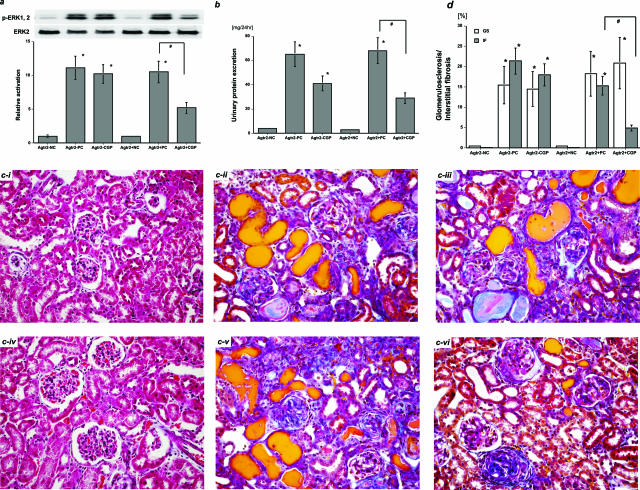
To confirm the role of feedback activation of AT2R in renoprotection against immune-mediated glomerulonephritis during AT1R blockade, Agtr2− mice with anti-GBM nephritis were treated with the AT1R blocker, CGP-48933 from day 29 to day 56. The ERK pathway was significantly activated in the day 31 nephritic kidneys in the Agtr2+PC group, and this was not substantially influenced by AT2R deficiency in the Agtr2−PC group (Figure 6a). Although AT1R blockade significantly suppressed ERK activation in the day 31 nephritic kidneys in the Agtr2+CGP group, it did not affect it in the Agtr2−CGP group (Figure 6a). On day 56, urinary protein excretion was significantly increased in the Agtr2+PC and Agtr2−PC groups regardless of AT2R-deficiency (Figure 6b). AT1R blockade lowered urinary protein excretion in the Agtr2+CGP and Agtr2−CGP groups (Figure 6b). In addition, significant glomerulosclerosis and interstitial fibrosis were found at the same degrees in the Agtr2+PC and Agtr2−PC groups regardless of AT2R deficiency [Figure 6, c (ii and v) and d]. AT1R blockade did not significantly affect glomerulosclerosis in the Agtr2+CGP and Agtr2−CGP groups [Figure 6, c (iii and vi) and d]. In contrast, whereas AT1R blockade significantly attenuated interstitial fibrosis in the day 56 nephritic kidneys in the Agtr2+CGP group [Figure 6, c (vi) and d], it did not affect it in the Agtr2−CGP group [Figure 6, c (iii) and d].
Figure 6.
AT1R blockade did not have renoprotective effects on immune-mediated glomerulonephritis in the AT2R gene-deficient mice. a: The ERK pathway was significantly activated in the day 31 nephritic kidneys in the Agtr2+PC and Agtr2−PC groups. Although AT1R blockade significantly suppressed ERK activation in the Agtr2+CGP group, it did not affect it in the Agtr2−CGP group. b: Urinary protein excretion was significantly increased in the Agtr2+PC and Agtr2−PC groups by day 56. AT1R blockade significantly lowered urinary protein excretion in the Agtr2+CGP group, and it also reduced proteinuria in the Agtr2−CGP group. ci: The day 56 kidney tissues in the Agtr2−NC group. cii: The Agtr2−PC group. ciii: The Agtr2−CGP group. civ: The Agtr2+NC group. cv: The Agtr2+PC group. cvi: The Agtr2+CGP group (Masson’s trichrome). d: Glomerulosclerosis (GS) and interstitial fibrosis (IF) in the day 56 nephritic kidneys from each group were quantitated as described in Materials and Methods (versus NC, *P < 0.05; versus PC, #P < 0.05). The data in a were obtained from three independent experiments. Original magnifications, ×200.
Activation of AT2R in Tubular Epithelial Cells Reduces MCP-1 mRNA Levels via Inactivation of the ERK Pathway
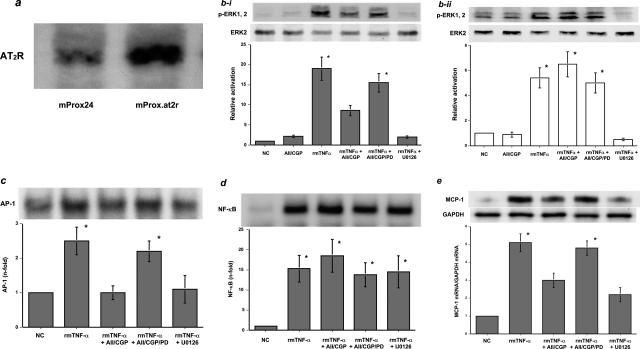
To determine whether AT2R activation reduces MCP-1 mRNA levels in proximal tubular epithelial cells, cultured proximal tubular epithelial cells that constitutively expressed AT2R were generated and tested. Thus, cultured murine proximal tubular epithelial cells, mProx24 cells, were stably transfected with pLXSN-AT2, a subline (mProx.at2r) was established, and expression of AT2R was confirmed (Figure 7a). A whole cell receptor-binding assay revealed that AT2R was present in mProx.at2r cells at a concentration of 220 ± 35 fmol/mg protein. To mimic the natural environment of proximal tubules in the nephritic kidney, mProx.at2r cells were stimulated with rmTNF-α for 30 minutes; TNF-α has been reported to play a pivotal role in the anti-GBM nephritic kidney.30 Such treatment significantly increased p-ERK1/2 levels in mProx.at2r as well as in mProx24 cells (Figure 7b, i and ii). However, simultaneous AT2R activation by Ang II under AT1R blockade significantly suppressed ERK phosphorylation in the mProx24.at2r cells (Figure 7bi) but had no significant effects on the control, mProx24 cells (Figure 7bii). Consistent with these findings, AP-1-binding proteins were found to be shuttled into the nucleus by rmTNF-α treatment, an effect that was significantly blocked by AT2R activation and by the MEK inhibitor U0126 (Figure 7c). In contrast, rmTNF-α treatment facilitated the intranuclear translocation of NF-κB, which was not affected by AT2R activation under AT1R blockade or by U0126 (Figure 7d). Despite intranuclear translocation of NF-κB, AT2R activation significantly reduced MCP-1 mRNA levels in the mProx.at2r cells in response to rmTNF-α treatment for 3 hours; this effect may have been attributable to the inactivation of the ERK pathway (Figure 7e).
Figure 7.
a: Increased AT2R protein expression in cultured murine proximal tubular epithelial cells stably transfected with an AT2R expression vector (mProx.at2r cells), compared with native mProx24 cells. bi: ERK activation in the mProx.at2r cells by rmTNF-α treatment. rmTNF-α treatment led to significant phosphorylation of ERK in the mProx.at2r cells (rmTNF-α). However, AT2R activation by Ang II in the presence of CGP-48933 inhibited ERK phosphorylation (rmTNF-α + AII/CGP). This effect was attenuated by co-treatment with PD123319 (rmTNF-α + AII/CGP/PD). Treatment with a MEK inhibitor, U0126, also blocked ERK activation (rmTNF-α + U0126). NC, untreated negative control mProx.at2r cells; AII/CGP, mProx.at2r cells treated with Ang II in the presence of the AT1R blocker. bii: In contrast, AT2R activation did not significantly affect ERK activation in the control mProx24 cells (rmTNF-α + AII/CGP). c: rmTNF-α treatment significantly increased the shuttling of AP-1-binding proteins into the nucleus of mProx.at2r cells (rmTNF-α), whereas Ang II treatment under AT1R blockade and U0126 treatment significantly blocked this effect (rmTNF-α + AII/CGP and rmTNF-α + U0126). The effect by AT1R blockade was abolished by co-treatment with the AT2R blocker (rmTNF-α + AII/CGP/PD). d: rmTNF-α treatment significantly enhanced the shuttling of NF-κB into the nucleus of mProx.at2r cells (rmTNF-α). Neither AT2R activation nor U0126 treatment affected the enhancement (rmTNF-α + AII/CGP and rmTNF-α + U0126). e: rmTNF-α treatment significantly increased MCP-1 mRNA levels in the mProx.at2r cells (rmTNF-α). Both Ang II treatment under AT1R blockade and U0126 treatment significantly suppressed rmTNF-α-mediated increases in MCP-1 mRNA levels in the mProx.at2r cells (rmTNF-α + AII/CGP and rmTNF-α + U0126). The effect of AT1R blockade was abolished by co-treatment with the AT2R blocker (rmTNF-α + AII/CGP/PD) (versus NC, *P < 0.05). Each blot is representative of three independent experiments, and the quantitative densitometric data were obtained from these three blots.
Discussion
In this study, we found an important anti-inflammatory function for the feedback activation of AT2R on the tubular epithelium in anti-GBM nephritic kidneys during AT1R blockade. In addition, a cDNA microarray analysis has revealed that reduced levels of most mRNAs in the nephritic kidney by treatment with an AT1R blocker were AT2R-dependent because co-treatment with an AT2R blocker nearly abolished such reduction (manuscript in preparation), which suggests that few genes are up-regulated in an AT1R-dependent manner in the immune-mediated nephritic kidney. Feedback activation of AT2R significantly decreased mRNA levels of proinflammatory mediators such as MCP-1, but not fibrogenic molecules such as TGF-β1 and α1COLI, in the middle stage of nephritis, which likely reduced the expansion of interstitial inflammation and the subsequent development of fibrogenesis. The possible involvement of ERK pathways in progressive renal diseases has been the focus of a number of studies in recent years.31–33 The ERK pathway was reported to play an essential role in mesangial proliferation in anti-GBM and anti-Thy1 nephritis in rats.31,32 In contrast to the ERK pathway, the p38 pathway was found not to be activated in anti-GBM nephritis at ∼day 31 in this study, consistent with the notion that the p38 pathway was transiently involved in the very early phase of anti-GBM nephritis.34 Furthermore, p-ERK was also found to be distributed in the tubular epithelium, in a pattern similar to that seen for AT2R in the anti-GBM nephritic kidney. AT2R induction in tubular epithelial cells has been reported to occur in most renal injury models.20,28,35 AT2R induction during inflammation is believed to occur as a result of the activity of potential cis-elements such as the CCAAT/enhancer-binding protein (C/EBP) sites, the interferon regulatory factor-I site, and the nuclear factor of activated T cells site in the promoter region of the AT2R gene that respond to proinflammatory mediators.11 In addition, our finding that mRNA levels of AT2R in PTCs were further increased by the dual-blockade in the CGP/PD group compared with the PC group in Figure 2c suggests that a native AT2R function down-regulates its own expression. ERK activation in tubular epithelial cells is likely induced by proinflammatory factors and growth factors derived from adjacent tissues.36 MCP-1 was induced in the tubular epithelium in anti-GBM nephritis in the same context, and we previously demonstrated that such MCP-1 was predominantly responsible for interstitial inflammation and subsequent fibrogenesis.23 The ERK pathway was also reported to be involved in the albumin-induced activation of AP-1 and NF-κB and in the induction of MCP-1 expression in cultured tubular epithelial cells.14,25 Recently, inflammation mediated by ERK pathway in the lung has been found, and Lee and colleagues37 reported that interleukin-13 induced a number of chemokines and proteases in the lung via ERK activation and caused lung injury and remodeling.
Our data suggest that Ang II inactivates the ERK pathway via AT2Rs and subsequently decreases MCP-1 mRNA levels in cultured tubular epithelial cells (mProx.at2r), even under conditions of sustained NF-κB activation. Anti-growth effects of AT2R are known to be associated with the activation of PTPs, such as MKP-1, SHP-1, and PP2A, which inhibit ERK in neuronal cells, adrenal cells, R3T3 fibroblasts, vascular smooth muscle cells, and cardiac myocytes.11,12,26 The same mechanisms probably occur in nephritic kidneys, especially in the tubular epithelial cells. Although the overall PTP activities in nephritic kidneys were not measured in this study, we detected SHP-1 protein expression in the day 31 nephritic kidneys during AT1R blockade, suggesting that feedback activation of AT2R might activate SHP-1 to inhibit the ERK pathway.38 A recent study also revealed a functional role for ERK as a temporal regulator of NF-κB activation.39,40 In cultured vascular smooth muscle cells, sustained activation of ERK was required for interleukin-1β to persistently activate NF-κB. Inhibition of ERK attenuated I-κBβ degradation and inactivated NF-κB, and thereby reduced the expression of some NF-κB-dependent genes such as inducible nitric oxide synthase and cyclooxygenase-2.39 In addition, NF-κB and AP-1 were shown to be involved in ERK-mediated control of matrix metalloproteinase-9 expression in vascular smooth muscle cells in response to TNF-α.40 Prolonged TNF-α expression and NF-κB activation are hallmark features of the anti-GBM nephritic kidney.30 NF-κB is activated in the kidney not only by proinflammatory cytokines such as TNF-α but also by Ang II itself.18 However, the contribution of Ang II seemed negligible in the advanced anti-GBM nephritic kidneys, because AT1R blockade alone or in combination with AT2R blockade had no effect on NF-κB activation (Figure 3f). Although the interrelationship between the ERK pathway and NF-κB in tubular epithelial cells remains to be clarified, we suggest that the expression of some NF-κB-dependent genes, ie, MCP-1, may be independently co-regulated by the ERK pathway.
To eliminate the possibility of incomplete blockade of AT2R in our study, experiments were performed in Agtr2− mice. Although fine descriptions concerning baseline kidney phenotype of Agtr2− mice have never been reported, we did not find apparent differences in renal function and life span between Agtr2− and Agtr2+ mice.9,24 The nephritic process was found to be similar in the Agtr2−PC and Agtr2+PC groups through day 56. We previously reported that AT2R was induced in activated splenic lymphocytes and attenuated their immune responses.41 This may influence the inflammatory system in Agtr2− mice. In fact, cuff placement on the femoral artery in Agtr2+ mice increased the expression of TNF-α and MCP-1 and infiltration of CD45+ immune cells, causing mild inflammation in the arteries, and further enhanced them in Agtr2− mice.9 However, based on our finding, AT2R unlikely had a significant renoprotective function at least in such severely nephritic kidneys in mice with pharmacologically unmodified, native renin-angiotensin system. These mice were treated with CGP-48933 from day 28 when glomerular inflammation developed in this study. It attenuated interstitial fibrosis in the day 56 nephritic kidneys in Agtr2+ mice, but not in Agtr2− mice (Figure 6d), despite the reduction in urinary protein excretion in both groups which was possibly because of hemodynamic effects by AT1R blockade (Figure 6b). In contrast, AT1R blockade had no protective effects on glomerular lesions in the day 56 nephritic kidneys in Agtr2− and Agtr2+ mice (Figure 6d). This was likely because by day 28, inflammation in the glomeruli had progressed and become unresponsive to AT1R blockade. These results are consistent with the pharmacological results of the present study and to those from the previous pharmacological experiments,2 and strongly supported our notion that the anti-inflammatory effects of AT1R blockade on the tubulointerstitium are dependent on the feedback activation of AT2R in tubular epithelial cells. In contrast, infused Ang II often acts as a proinflammatory factor not only via AT1R but also AT2R in the kidney and the heart.42–45 Studies in Ang II-infused rats by Navar’s group46 demonstrated that the uptake of circulating Ang II by an AT1R-dependent mechanism significantly augments intrarenal Ang II levels. This likely contributes to the induction of AT2R and to the pathological activation of NF-κB in mesangial cells, tubular epithelial cells, monocytes, and vascular smooth muscle cells.35,44,46,47 We believe that an increase in intrarenal Ang II levels caused by systemic infusion in these studies affected AT2R functions differently from the feedback increases in Ang II synthesis during AT1R blockade in this study.
In conclusion, AT1R blockade had an anti-inflammatory effect on tubular epithelial cells in advanced, immune-mediated glomerulonephritis, which was mediated through the feedback activation of AT2R. We also found that such treatment reduced the mRNA levels of proinflammatory molecules such as MCP-1 as a result of the inactivation of the ERK pathway, despite the concomitant activation of NF-κB.
Acknowledgments
We thank T. Senbonmatsu (Department of Pharmacology, Saitama Medical School, Saitama) for his critical review of the manuscript, and M. Otobe for her technical assistance.
Footnotes
Address reprint requests to Hiromichi Suzuki, M.D., Ph.D., Professor of Medicine, Department of Nephrology, Saitama Medical School, 38 Morohongo, Moroyama-cho, Irumagun, Saitama 350-0495, Japan. E-mail: iromichi@saitama-med.ac.jp.
Supported in part by a grant-in-aid for scientific research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
A portion of this work was presented at the 35th Annual Meeting of the American Society of Nephrology in San Diego, CA, in 2003.
References
- Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- Okada H, Watanabe Y, Inoue T, Kobayashi T, Kikuta T, Kanno Y, Ban S, Suzuki H. Angiotensin II type 1 receptor blockade attenuates renal fibrogenesis in an immune-mediated nephritic kidney through counter-activation of angiotensin II type 2 receptor. Biochem Biophys Res Commun. 2004;314:403–408. doi: 10.1016/j.bbrc.2003.12.105. [DOI] [PubMed] [Google Scholar]
- Hisada Y, Sugaya T, Yamanouchi M, Uchida H, Fujimura H, Sakurai H, Fukamizu A, Murakami K. Angiotensin II plays a pathogenic role in immune-mediated renal injury in mice. J Clin Invest. 1999;103:627–635. doi: 10.1172/JCI2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Lopez-Franco O, Gomez-Garre D, Tejera N, Gomez-Guerrero C, Sugaya T, Bernal R, Blanco J, Ortega L, Egido J. Renal tubulointerstitial damage caused by persistent proteinuria is attenuated in AT1-deficient mice. Am J Pathol. 2001;159:1895–1904. doi: 10.1016/S0002-9440(10)63036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafayette R, Mayer G, Park SK, Meyer TW. Angiotensin II receptor blockade limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1992;90:766–771. doi: 10.1172/JCI115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Kashihara N, Yamasaki Y, Maruyama K, Okamoto K, Maeshima Y, Sugiyama H, Sugaya T, Murakami K, Makino H. Renal interstitial fibrosis is reduced in angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol. 2001;12:317–325. doi: 10.1681/ASN.V122317. [DOI] [PubMed] [Google Scholar]
- Allen TJ, Cao Z, Youssef S, Hulthen UL, Cooper ME. Role of angiotensin II and bradykinin in experimental diabetic nephropathy: functional and structural studies. Diabetes. 1997;46:1612–1618. doi: 10.2337/diacare.46.10.1612. [DOI] [PubMed] [Google Scholar]
- Yu CM, Tipoe GL, Wing-Hon Lai K, Lau CP. Effects of combination of angiotensin-converting enzyme inhibitor and angiotensin receptor antagonist on inflammatory cellular infiltration and myocardial interstitial fibrosis after acute myocardial infarction. J Am Coll Cardiol. 2001;38:1207–1215. doi: 10.1016/s0735-1097(01)01518-2. [DOI] [PubMed] [Google Scholar]
- Wu L, Iwai M, Nakagami H, Li Z, Chen R, Suzuki J, Akishita M, de Gasparo M, Horiuchi M. Roles of angiotensin II type 2 receptor stimulation associated with selective angiotensin II type 1 receptor blockade with valsartan in the improvement of inflammation-induced vascular injury. Circulation. 2001;104:2716–2721. doi: 10.1161/hc4601.099404. [DOI] [PubMed] [Google Scholar]
- Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Fukui H. Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology. 2001;34:745–750. doi: 10.1053/jhep.2001.28231. [DOI] [PubMed] [Google Scholar]
- Stoll M, Unger T. Angiotensin and its AT2 receptor: new insights into an old system. Regul Pept. 2001;99:175–182. doi: 10.1016/s0167-0115(01)00246-4. [DOI] [PubMed] [Google Scholar]
- Lehtonen JY, Daviet L, Nahmias C, Horiuchi M, Dzau VJ. Analysis of functional domains of angiotensin II type 2 receptor involved in apoptosis. Mol Endocrinol. 2004;13:1051–1060. doi: 10.1210/mend.13.7.0303. [DOI] [PubMed] [Google Scholar]
- Moore SA, Huang N, Hinthong O, Andres RD, Grammatopoulos TN, Weyhenmeyer JA. Human angiotensin II type-2 receptor inhibition of insulin-mediated ERK-2 activity via a G-protein coupled signaling pathway. Mol Brain Res. 2004;124:62–69. doi: 10.1016/j.molbrainres.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Reich H, Tritchler D, Herzenberg AM, Kassiri Z, Zhou X, Gao W, Scholey J. Albumin activates ERK via EGF receptor in human renal epithelial cells. J Am Soc Nephrol. 2005;16:1266–1278. doi: 10.1681/ASN.2004030222. [DOI] [PubMed] [Google Scholar]
- Ma J, Nishimura H, Fogo A, Kon V, Inagami T, Ichikawa I. Accelerated fibrosis and collagen deposition develop in the real interstitium of angiotensin type 2 receptor null mutant mice during ureteral obstruction. Kidney Int. 1998;53:937–944. doi: 10.1111/j.1523-1755.1998.00893.x. [DOI] [PubMed] [Google Scholar]
- Morrissey JJ, Klahr S. Effect of AT2 receptor blockade on the pathogenesis of renal fibrosis. Am J Physiol. 1999;276:F39–F45. doi: 10.1152/ajprenal.1999.276.1.F39. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Maeshima Y, Satoh M, Odawara M, Sugiyama H, Kashihara N, Matsubara H, Yamasaki Y, Makino H. Overexpression of angiotensin type 2 receptor ameliorates glomerular injury in a mouse remnant kidney model. Am J Physiol. 2004;286:F516–F525. doi: 10.1152/ajprenal.00294.2003. [DOI] [PubMed] [Google Scholar]
- Esteban V, Lorenzo O, Ruperez M, Suzuki Y, Mezzano S, Blanco J, Kretzler M, Sugaya T, Egido J, Ruiz-Ortega M. Angiotensin II, via AT1 and AT2 receptors and NF-κB pathway, regulates the inflammatory response in unilateral ureteral obstruction. J Am Soc Nephrol. 2004;15:1514–1529. doi: 10.1097/01.asn.0000130564.75008.f5. [DOI] [PubMed] [Google Scholar]
- Cao Z, Bonnet F, Candido R, Nesteroff SP, Burns WC, Kawachi H, Shimizu F, Carey RM, de Gasparo M, Cooper ME. Angiotensin type 2 receptor antagonism confers renal protection in a rat model of progressive renal injury. J Am Soc Nephrol. 2002;13:1773–1787. doi: 10.1097/01.asn.0000019409.17099.33. [DOI] [PubMed] [Google Scholar]
- Tejera N, Gomez-Garre D, Lazaro A, Gallego-Delgado J, Alonso C, Blanco J, Ortiz A, Egido J. Persistent proteinuria up-regulates angiotensin II type 2 receptor and induces apoptosis in proximal tubular cells. Am J Pathol. 2004;164:1817–1826. doi: 10.1016/S0002-9440(10)63740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santibanez JF, Guerrero J, Quintanilla M, Fabra A, Martinez J. Transforming growth factor-β1 modulates matrix metalloproteinase-9 production through the Ras/MAPK signaling pathway in transformed keratinocytes. Biochem Biophys Res Commun. 2002;296:267–273. doi: 10.1016/s0006-291x(02)00864-1. [DOI] [PubMed] [Google Scholar]
- Okada H, Kikuta T, Kobayashi T, Inoue T, Kanno Y, Takigawa M, Sugaya T, Kopp JB, Suzuki H. Connective tissue growth factor expressed in tubular epithelium plays a pivotal role in renal fibrogenesis. J Am Soc Nephrol. 2005;16:133–143. doi: 10.1681/ASN.2004040339. [DOI] [PubMed] [Google Scholar]
- Okada H, Moriwaki K, Kalluri R, Imai H, Ban S, Takahama M, Suzuki H. Inhibition of monocyte chemoattractant protein-1 expression in tubular epithelium attenuates tubulointerstitial alteration in rat Goodpasture syndrome. Kidney Int. 2000;57:927–936. doi: 10.1046/j.1523-1755.2000.00909.x. [DOI] [PubMed] [Google Scholar]
- Akishita M, Iwai M, Wu L, Zhang L, Ouchi Y, Dzau VJ, Horiuchi M. Inhibitory effect of angiotensin II type 2 receptor on coronary arterial remodeling after aortic banding in mice. Circulation. 2000;102:1684–1689. doi: 10.1161/01.cir.102.14.1684. [DOI] [PubMed] [Google Scholar]
- Takaya K, Koya D, Isono M, Sugimoto T, Sugaya T, Kashiwagi A, Haneda M. Involvement of ERK pathway in albumin-induced MCP-1 expression in mouse proximal tubular cells. Am J Physiol. 2003;284:F1037–F1045. doi: 10.1152/ajprenal.00230.2002. [DOI] [PubMed] [Google Scholar]
- Mifune M, Sasamura H, Shimizu-Hirota R, Miyazaki H, Saruta T. Angiotensin II type 2 receptors stimulate collagen synthesis in cultured vascular smooth muscle cells. Hypertension. 2000;36:845–850. doi: 10.1161/01.hyp.36.5.845. [DOI] [PubMed] [Google Scholar]
- Okada H, Watanabe Y, Kikuta T, Kobayashi T, Kanno Y, Sugaya T, Suzuki H. Bradykinin decreases plasminogen activator inhibitor-1 expression and facilitates matrix degradation in the renal tubulointerstitium under angiotensin-converting enzyme blockade. J Am Soc Nephrol. 2004;15:2402–2413. doi: 10.1097/01.ASN.0000136132.20189.95. [DOI] [PubMed] [Google Scholar]
- Okada H, Inoue T, Kanno Y, Kobayashi T, Watanabe Y, Kopp JB, Carey RM, Suzuki H. Interstitial fibroblast-like cells express renin-angiotensin system components in a fibrosing murine kidney. Am J Pathol. 2002;160:765–772. doi: 10.1016/S0002-9440(10)64898-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Harsdorf R, Edwards JG, Shen YT, Kudej RK, Dietz R, Leinwand LA, Nadal-Ginard B, Vatner SF. Identification of a cis-acting regulatory element conferring inducibility of the atrial natriuretic factor gene in acute pressure overload. J Clin Invest. 1997;100:1294–1304. doi: 10.1172/JCI119643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Ha IS, Hwang CI, Lee YJ, Kim J, Yang SH, Kim YS, Cao YA, Choi S, Park WY. Gene expression profiling of anti-GBM glomerulonephritis model: the role of NF-κB in immune complex kidney disease. Kidney Int. 2004;66:1826–1837. doi: 10.1111/j.1523-1755.2004.00956.x. [DOI] [PubMed] [Google Scholar]
- Bokemeyer D, Guglielmi KE, McGinty A, Sorokin A, Lianos EA, Dunn MJ. Activation of extracellular signal-regulated kinase in proliferative glomerulonephritis in rats. J Clin Invest. 1997;100:582–588. doi: 10.1172/JCI119568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokemeyer D, Panek D, Kramer HJ, Lindemann M, Kitahara M, Boor P, Kerjaschki D, Trzaskos JM, Floege J, Ostendorf T. In vivo identification of the mitogen-activated protein kinase cascade as a central pathway in experimental mesangioproliferative glomerulonephritis. J Am Soc Nephrol. 2002;13:1473–1480. doi: 10.1097/01.asn.0000017576.50319.ac. [DOI] [PubMed] [Google Scholar]
- Masaki T, Stambe C, Hill PA, Dowling J, Atkins RC, Nikolic-Paterson DJ. Activation of the extracellular-signal regulated protein kinase pathway in human glomerulopathies. J Am Soc Nephrol. 2004;15:1835–1843. doi: 10.1097/01.asn.0000130623.66271.67. [DOI] [PubMed] [Google Scholar]
- Stambe C, Atkins RC, Tesch GH, Kapoun AM, Hill PA, Schreiner GF, Nikolic-Paterson DJ. Blockade of p38a MAPK ameliorates acute inflammatory renal injury in rat anti-GBM glomerulonephritis. J Am Soc Nephrol. 2003;14:338–351. doi: 10.1097/01.asn.0000048715.12315.fd. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Esteban V, Suzuki Y, Ruperez M, Mezzano S, Ardiles L, Justo P, Ortiz A, Egido J. Renal expression of angiotensin type 2 receptors during kidney damage. Kidney Int. 2003;64(Suppl 86):S21–S26. doi: 10.1046/j.1523-1755.64.s86.5.x. [DOI] [PubMed] [Google Scholar]
- Tian W, Zhang Z, Cohen DM. MAPK signaling and the kidney. Am J Physiol. 2000;279:F593–F604. doi: 10.1152/ajprenal.2000.279.4.F593. [DOI] [PubMed] [Google Scholar]
- Lee PJ, Zhang X, Shan P, Ma B, Lee CG, Homer RJ, Zhu Z, Rincon M, Mossman BT, Elias JA. ERK1/2 mitogen-activated protein kinase selectively mediates IL-13-induced lung inflammation and remodeling in vivo. J Clin Invest. 2006;116:163–173. doi: 10.1172/JCI25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki Y, Matsubara H, Nozawa Y, Mori Y, Masaki H, Kosaki A, Tsutsumi Y, Uchiyama Y, Fujiyama S, Nose A, Iba O, Tateishi E, Hasegawa T, Horiuchi M, Nahmias C, Iwasaka T. Angiotensin II type 2 receptor inhibits epidermal growth factor receptor transactivation by increasing association of SHP-1 tyrosine phosphatase. Hypertension. 2001;38:367–372. doi: 10.1161/01.hyp.38.3.367. [DOI] [PubMed] [Google Scholar]
- Jiang B, Xu S, Hou X, Pimentel DR, Brecher P, Cohen RA. Temporal control of NF-κB activation by ERK differentially regulates interleukin-1β-induced gene expression. J Biol Chem. 2004;279:1323–1329. doi: 10.1074/jbc.M307521200. [DOI] [PubMed] [Google Scholar]
- Moon SK, Cha BY, Kim CH. ERK1/2 mediates TNF-α-induced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-κB and AP-1: involvement of the ras dependent pathway. J Cell Physiol. 2004;198:417–427. doi: 10.1002/jcp.10435. [DOI] [PubMed] [Google Scholar]
- Okada H, Watanabe Y, Kobayashi T, Kikuta T, Kanno Y, Suzuki H. Angiotensin II type 1 and type 2 receptors reciprocally modulate pro-inflammatory/pro-fibrotic reactions in activated splenic lymphocytes. Am J Nephrol. 2004;24:322–329. doi: 10.1159/000078496. [DOI] [PubMed] [Google Scholar]
- Wolf G, Ziyadeh FN, Thaiss F, Tomaszewski J, Caron RJ, Wenzel U, Zahner G, Helmchen U, Stahl RAK. Angiotensin II stimulates expression of the chemokine RANTES in rat glomerular endothelial cells. J Clin Invest. 1997;100:1047–1058. doi: 10.1172/JCI119615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Lorenzo O, Ruperez M, Blanco J, Egido J. Systemic infusion of angiotensin II into normal rats activates nuclear factor-κB and AP-1 in the kidney. Am J Pathol. 2001;158:1743–1756. doi: 10.1016/s0002-9440(10)64130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban V, Ruperez M, Vita JR, Lopez ES, Mezzano S, Plaza JJ, Egido J, Ruiz-Ortega M. Effects of simultaneous blockade of AT1 and AT2 receptors on the NFκB pathway and renal inflammatory response. Kidney Int. 2003;64(Suppl 86):S33–S38. doi: 10.1046/j.1523-1755.64.s86.7.x. [DOI] [PubMed] [Google Scholar]
- Ichihara S, Senbonmatsu T, Price E, Ichiki T, Gaffney A, Inagami T. Angiotensin II type 2 receptor is essential for left ventricular hypertrophy and cardiac fibrosis in chronic angiotensin II-induced hypertension. Circulation. 2001;104:346–351. doi: 10.1161/01.cir.104.3.346. [DOI] [PubMed] [Google Scholar]
- Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Lorenzo O, Ruperez M, Koenig S, Wittig B, Egido J. Angiotensin II activates nuclear transcription factor κB through AT1 and AT2 in vascular smooth muscle cells. Circ Res. 2000;86:1266–1272. doi: 10.1161/01.res.86.12.1266. [DOI] [PubMed] [Google Scholar]