Abstract
Vitiligo presents with depigmented cutaneous lesions following localized melanocyte death. Multiple factors contribute to cell death, including genetically determined susceptibility to trauma, and environmental factors, such as exposure to 4-tert-butylphenol (4-TBP). We demonstrate that 4-TBP induces oxidative stress that is more readily overcome by melanocytes from normally pigmented individuals than from two individuals with vitiligo. The antioxidant catalase selectively and significantly reduced death of melanocytes derived from two individuals with vitiligo, indicating a role for oxidative stress in vitiligo pathogenesis. In normal melanocytes, oxidative stress results in reduced expression of microphthalmia-associated transcription factor (MITF). Melanocyte-stimulating hormone-induced expression of MITF protein caused increased sensitivity to 4-TBP, whereas sensitivity of melanomas correlated with MITF expression. MITF stimulates melanin synthesis by up-regulating expression of melanogenic enzymes such as tyrosinase-related protein-1 (Tyrp1). Although melanin content per se did not affect sensitivity to 4-TBP, expression of Tyrp1 significantly increased sensitivity. Melanocytes and melanomas that express functional Tyrp1 were significantly more sensitive to 4-TBP than Tyrp1-null cells. Thus, normal melanocytes respond to 4-TBP by reducing expression of MITF and Tyrp1. We hypothesize that melanocytes in vitiligo demonstrate reduced ability to withstand oxidative stress due, partly, to a disruption in MITF regulation of Tyrp1.
Vitiligo is an acquired condition characterized by depigmented, cutaneous lesions that result from the death of pigment-producing cells, melanocytes, in delimited areas of the skin.1 It affects about 1% of the world’s population2 and has significant impact on both the physical and mental health of patients. Following melanocyte loss, the skin is deprived of pigment protection, leaving it more susceptible to solar damage, and occasionally, compromised immunity may result.3 Multiple factors contribute to susceptibility for vitiligo.4 Genetic predetermination is evident by the higher than expected number of patients who have affected primary family members,5 whereas anecdotal evidence demonstrates a correlation between environmental factors and onset. In contact/occupational vitiligo, the environmental trigger is exposure to specific chemotoxins, which include phenol and catechol derivatives6,7,8 such as 4-tert-butylphenol (4-TBP).9 A number of these chemicals induce cellular oxidative stress.
Phenol and catechol derivatives such as 4-TBP are thought to have increased toxicity in melanocytes due to their structural similarity to the melanin precursor tyrosine. They act as substrates for tyrosinase, the enzyme that initiates melanin biosynthesis, producing toxic intermediates.2,10 Tyrosinase mediates the conversion of both 4-TBP and 4-tert-catechol to quinones that react with glutathione or cysteine.11,12 Thus, 4-TBP, a competitive inhibitor of tyrosinase,13 is metabolized in melanocytes and may generate reactive oxygen species capable of damaging these cells and inducing apoptosis. Ironically, expression levels of tyrosinase do not correlate with sensitivity to 4-TBP-induced apoptosis,14 suggesting an alternative enzymatic mediator may be more critical.
We have shown previously that normal melanocytes cultured in the presence of 4-TBP undergo dose-dependent apoptosis.14 The percentage of cells that die following exposure to 4-TBP are unaffected by 12-O-tetradecanoylphorbol-13-acetate but can be reduced significantly by excluding α-melanocyte-stimulating hormone (MSH)15 from the culture medium. MSH, a potent stimulator of melanogenesis, is a ligand for the melanocortin 1 receptor (MC1R). Binding of MSH to MC1R activates adenylate cyclase.16 The subsequent increase in cyclic AMP leads to expression of the melanocyte-specific isoform of the microphthalmia-associated transcription factor (MITF),17 which in turn trans-activates expression of downstream genes, including tyrosinase and tyrosinase-related protein 1 (Tyrp1),18 ultimately resulting in increased melanin production. MITF is therefore considered the “master regulator of the melanocyte,”17 controlling expression of the major melanocyte-specific proteins required for melanin synthesis. MITF is most active in its phosphorylated state but is rapidly degraded following ubiquitination.19
Although MITF promotes survival of melanocytes and melanoma cells in some instances, it can also act as a proapoptotic signal. MITF has recently been shown to be a target of caspases, and in melanoma cells, the products of caspase-mediated MITF digestion induce apoptosis.20 Furthermore, expression of MITF results in a reduced response to oxidative stress induced by hydrogen peroxide,21 suggesting a role for this transcription factor in melanocyte survival and response to oxidative stress. Since melanocytes are preferentially targeted in vitiligo, we considered MITF an ideal candidate for investigation of melanocyte death induced by 4-TBP.
In this study, we explore the hypothesis that normal melanocytes are capable of combating 4-TBP-induced oxidative stress, whereas melanocytes in contact vitiligo fail to do so. Indicators of sustained oxidative stress are present in both serum and skin of individuals with vitiligo,22–24 suggesting a reduced capacity to maintain intracellular redox balance. Melanocytes in vitiligo, subjected to additional oxidative stress, such as the influx of a chemotoxin, may undergo apoptosis more readily than normal melanocytes. Because normal melanocytes can be sensitized to 4-TBP by MSH, we have investigated whether regulation of melanogenesis by MITF and expression of melanocyte-specific genes modulate sensitivity of the melanocytes to 4-TBP. Characterization of these pathways is crucial for establishing the underlying etiology of vitiligo.
Materials and Methods
Cell Culture
Primary cultures of human melanocytes were established following trypsin digestion of skin as previously described.25 Neonatal foreskins removed during routine circumcision or adult skin removed during breast reduction surgery was obtained with University of Cincinnati Institutional review board approval. A 4-mm punch biopsy was obtained from an individual with contact vitiligo and an unaffected individual (matched to the vitiligo patient for age, skin type, and sex) who had been enrolled in a protocol approved by the Institutional review board and who had signed for informed consent. The individual with vitiligo was a factory worker, who acquired vitiligo following contact with a bearing lubricant containing a phenol derivative (ie, benzenamine, N-phenyl). The individual initially developed vitiligo on both hands and generalized vitiligo developed within 18 months.
Basal medium consisted of MCDB153 supplemented with 4% fetal bovine serum, 5 μg/ml insulin, 1 μg/ml vitamin E, 0.6 ng/ml basic fibroblast growth factor, and 1% penicillin-streptomycin (10,000 units/ml and 10,000 g/ml, respectively; Gibco, BRL, Grand Island, NY). For complete melanocyte growth medium, the basal medium was further supplemented with 13 μg/ml bovine pituitary extract (Upstate Biotechnology, Lake Placid, NY) and 8 nmol/L 12-O-tetradecanoylphorbol-13-acetate. Unless stated otherwise, all reagents were purchased from Sigma (St. Louis, MO). The cultures were maintained in a humidified incubator with 5% CO2 at 37°C.
Melanocytes were maintained in complete culture medium. Before testing for the effects of media supplements, subcultures of each line were switched to basal medium for five days to allow any residual MSH to be expended. Basal medium was then supplemented with 10 nmol/L MSH, 1 μmol/L forskolin, or 0.1 nmol/L ET-1 and cells cultured for a further 5 days.
Immortalized human melanocyte cell lines PIG1 (normal matched control) and PIG3V (vitiligo cell line) (a gift from Dr. I.C. Le Poole, Loyola University, Chicago, IL) were cultured as previously described.26
Neonatal melanocytes shown to carry homozygous loss-of-function mutations at the MC1R locus (a gift from Dr. Zalfa Abdel-Malek, University of Cincinnati, OH) were maintained in complete medium. Line M1 was shown to be a compound heterozygote for the R160W/D29H mutations, and M2 was a compound heterozygote for the R160W/R151C mutations27 (personal communication with Dr. Abdel-Malek).
Cutaneous fibroblast cultures were established from the dermis of neonatal foreskins as previously described.14 Fibroblasts were cultured in DMEM medium (Sigma) supplemented with 8% fetal bovine serum, 1 mmol/L glutamine, 0.5 mmol/L sodium pyruvate, and 1% penicillin-streptomycin (10,000 units/ml and 10,000 mg/ml, respectively), (Gibco, Carlsbad, CA). Melanoma lines IIB-MEL-J28, WM 129, 130, 134 (a gift from M. Herlyn, Wistar Institute, Philadelphia, PA) and SK-MEL188 (a gift from R. Srinivasan, Sloan-Kettering Institute for Cancer Research, New York, NY) were also maintained in the supplemented DMEM medium.
To alter melanin content of SK-MEL188 melanomas, cells were cultured in RPMI-based medium. These cultures were then supplemented with increasing concentrations of tyrosine (0, 69, 138, 207, and 276 μmol/L, respectively) such that five individual cultures with varying melanin content resulted. Cells were then cultured for 3 days under identical conditions in medium containing 4% serum content to reduce proliferation and dilution of melanin following removal of the excess tyrosine. After 3 days, cells were plated and cell viability determined as described below.
Administration of 4-TBP
4-TBP was dissolved in 70% ethanol and added to the culture medium to bring the concentration to the final molarity indicated (0 to 1000 μmol/L 4-TBP for viability assays). Control melanocytes were treated with the vehicle, 70% ethanol, such that the final concentration of alcohol (maximum of 0.5% final concentration of alcohol) was equal in cells with and without 4-TBP.
Detection of Reactive Oxygen Species
Primary melanocytes from a single donor were plated in six-well dishes and allowed to attach for 48 hours. Melanocytes were dosed with a final concentration of 250 μmol/L 4-TBP. Cells were labeled at 0 (no 4-TBP added), 30, and 60 minutes using the Image-iT LIVE Green Reactive Oxygen Species Detection Kit (Molecular Probes, Eugene, OR). A nonfluorescent dye, H2DCFDA, is added to the culture medium and enters the cell, where it is deacetylated by esterases. On contact with reactive oxygen species (ROS), the fluorescein is oxidized and emits a green fluorescence. Cells were viewed and imaged using both phase-contrast and fluorescence microscopy.
Measurement of Cell Viability
Cells were seeded in triplicate in 96-well plates (6000 melanocytes per well and 4000 fibroblasts or melanoma cells per well) and allowed to attach for 24 hours. Cells were then treated with either 4-TBP or vehicle (70% ethanol) for 72 hours. After treatment, viability was determined using the CellTiter kit (Promega, Madison, WI).
Western Blotting
Fifteen micrograms of total protein was loaded on a 12% sodium dodecyl sulfate-polyacrylamide gel for detection of tyrosinase and Tyrp1. Forty micrograms of proteins were used for analysis of MITF. After transfer to a membrane, the following antibodies were used to detect the proteins of interest: polyclonal anti-human tyrosinase antibody Pep7h (1:7000) (a gift from Drs. R. King and W. Oetting, Minnesota, MN), polyclonal anti-TYRP1 antibody Pep 1 (1:2000) (a gift from Dr. V. Hearing, National Institutes of Health, Bethesda, MD), Mel5 (1:1500) monoclonal anti-TYRP1 antibody (Signet, Dedham, MA), and C5 anti-MITF (1:100) (a gift from Dr. D. Fisher, Boston, MA), for both MITF and phospho-MITF. A horseradish peroxidase conjugated antibody against actin was used as a loading control (1:500) (sc-1615; Santa Cruz Biotechnology, Santa Cruz, CA). Immunoreactivity was determined using a chemiluminescence reaction (Amersham, Arlington Heights, IL), followed by densitometric analysis. Relative protein expression was calculated as a ratio of band density at a given time point and band density at 0 hours.
Real-Time PCR
RNA was extracted using the RNeasy kit (Qiagen). Following quantitation, 2 μg of RNA was used to generate cDNA using random primers and Superscript III reverse transcriptase (Invitrogen). The cDNA was diluted 10-fold, and 1 μl used for the real-time reaction. Real-time PCR was performed using the TaqMan assay kit (Applied Biosystems) in an Applied Biosystems 7300 Real Time PCR System. The TaqMan PCR reagents and commercial assay-on-demand primers and probes for amplification of MITF (assay ID Hs00165156_m1, 5-carboxyfluorescein dye) and glyceraldehyde-3-phosphate dehydrogenase (catalog number 4326317E) were purchased from Applied Biosystems. PCR was performed in individual wells. Expression was normalized to that of glyceraldehyde-3-phosphate dehydrogenase and shown relative to expression of MITF in untreated melanocytes (0 hours) using the standard curve method for relative quantitation (SDS software; Applied Biosystems) (ABI Prism 7700User Bulletin 2).
Melanin Assay
Total melanin content was determined as previously described.14 Cells were pelleted following trypsinization and lysed (50 mmol/L Tris, pH 7.54, 2 mmol/L ethylenediamine tetraacetic acid, pH 7.87, 150 mmol/L sodium chloride, and 1% Triton X-100) in the presence of protease inhibitors (Complete; Roche, Indianapolis, IN). Cell extracts were spun at 14,000 rpm for 5 minutes at 4°C. The supernatant was removed and protein content determined (Pierce, Rockford, IL). The pellet was washed twice in ethanol/ether (1:1) and dissolved in 2 N sodium hydroxide, 20% DMSO at 60°C. A 200-μl aliquot was measured for absorbance at 490 nm in a plate reader (BioRad, Hercules, CA).
Vector Construction
A previously described expression plasmid containing tyrosinase was used for transfection.14 An expression plasmid for Tyrp1 was generated using an EcoRI fragment containing full-length cDNA excised from a previously constructed plasmid.29 The fragment was cloned into the EcoRI site of pcDNA 3.1(+) (Invitrogen). The orientation of the insert was determined by restriction enzyme analysis and confirmed by DNA sequencing.
The wild-type Tyrp1 plasmid was used for site-directed mutagenesis to generate a plasmid (Tyrp1-b110) encoding a sequence equivalent to the mouse brown mutation. Amino acid 86 of the mouse sequence is mutated from cysteine to tyrosine (G>A) in mice with the brown phenotype.30 Alignment of the mouse and human Tyrp1 sequences indicated that amino acid 110 of the human sequence is homologous to mouse residue 86. The GeneEditor kit (Promega) was used according to manufacturer’s instructions to generate the mutant plasmid. Mutagenesis was confirmed by sequencing and by loss of an HpyC4 III (NEB, Beverly, MA) restriction site.
Transfection
Human melanoma cells (IIB-MEL-J) were stably transfected using Effectene transfection reagent (Qiagen). Stable clones were selected using 1.5 mg/ml G418 (Invitrogen) 72 hours after transfection. Colonies appeared 10 to 14 days after selection. Transfection was confirmed by Western blot analysis.
Statistical Analysis
Student’s t-test was used to evaluate the significance of difference in viability. The Pearson coefficient was used to determine whether there was statistically significant correlation between trends.
Results
Oxidative Stress Induced by Exposure to 4-TBP Leads to Increased Melanocyte Death in Vitiligo
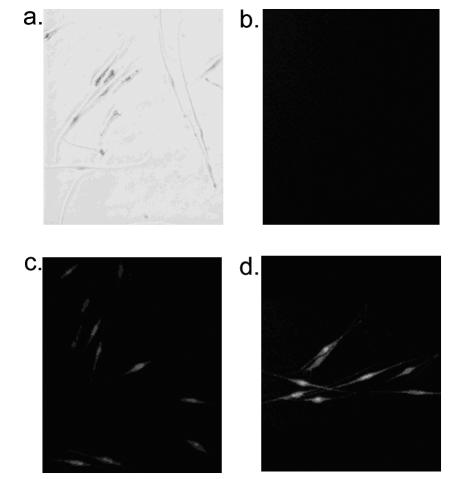
Exposure to 4-TBP was found to result in oxidative stress in melanocytes (Figure 1). Normal human melanocytes were dosed with 4-TBP, and cells were assessed for ROS at 0 (a, b), 30 (c), and 60 minutes (d). Fluorescence, indicative of the presence of ROS, was increased in melanocytes subjected to 4-TBP for 30 minutes as compared with untreated controls, whereas melanocytes incubated for 60 minutes displayed the highest level of ROS.
Figure 1.
4-TBP induces oxidative stress in melanocytes. Normal human melanocytes (a, b) were dosed with 250 μmol/L 4-TBP for (c) 30 minutes and (d) 60 minutes. Cells were labeled with a nonfluorescent dye that is oxidized on contact with ROS and emits a green fluorescence. a and b represent phase-contrast and fluorescence images, respectively, of the same cells. ROS concentrations, indicated by fluorescence, increased with 4-TBP incubation times.
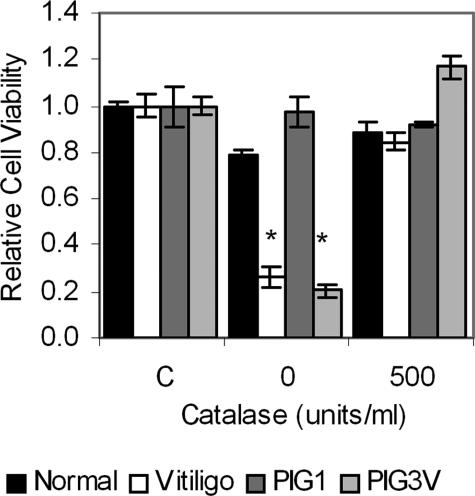
To test our hypothesis that melanocytes from individuals with vitiligo are genetically prone to death induced by oxidative stress and therefore more sensitive to 4-TBP, we challenged melanocytes derived from pigmented regions of skin of two vitiligo patients, one with contact and a second with idiopathic vitiligo. We found that indeed, melanocytes from both individuals were significantly (P < 0.01) more sensitive to 4-TBP than melanocytes from normally pigmented controls matched for age, skin type, and sex (Figure 2). The reduction in viability of vitiligo melanocytes could be reversed slightly by 100 units/ml catalase (26% viability versus 39% in contact vitiligo melanocytes), and cells were rendered equally sensitive to 4-TBP as normal melanocytes by 500 units/ml catalase. Two additional neonatal melanocyte lines were assessed to determine whether catalase reduced the toxicity of 4-TBP. There was no significant increase in viability in the presence of catalase when normal melanocytes were treated with 400 μmol/L 4-TBP.
Figure 2.
Melanocytes from two individuals with vitiligo are more sensitive to 4-TBP-induced oxidative stress than normal melanocytes. Melanocytes were derived from either vitiligo patients or normally pigmented control individuals, matched for age, sex, and skin type. PIG cells were immortalized by infection with the HPV16 E6/E7 genes.26 Cells were treated with 500 μmol/L 4-TBP in the absence or presence of 500 units/ml catalase for 72 hours, after which viability was determined. Control cells (C) were treated with vehicle alone. Bars indicate SD of triplicates within the experiment. Melanocytes from the individuals with vitiligo were significantly more sensitive to 4-TBP (* indicates P < 0.01), except in the presence of catalase. The experiment was repeated three times; a representative experiment is shown.
Oxidative Stress Induced Down-Regulation of the Microphthalmia Associated Transcription Factor
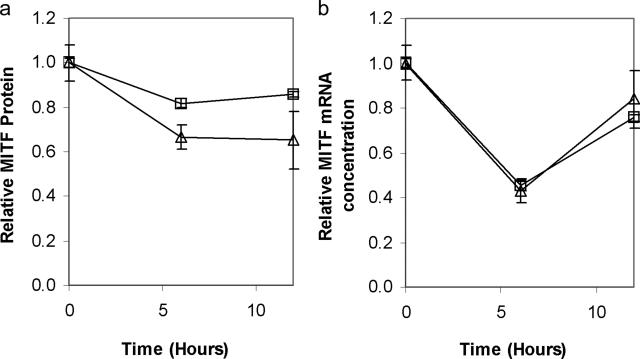
Oxidative stress induced by hydrogen peroxide has been shown to result in reduced expression of MITF in melanoma cells.21 We therefore determined the effect of 4-TBP on expression of MITF in normal human melanocytes (Figure 3). Both MITF protein and RNA were reduced 6 hours after exposure to 4-TBP.
Figure 3.
4-TBP exposure results in reduced expression of both MITF RNA and protein in normal melanocytes. Cells from two separate normal human melanocyte lines were treated with 200 μmol/L 4-TBP. Samples were harvested over a 12-hour period. a: Cell lysates were prepared and normalized for protein content, then subjected to Western blot analysis followed by densitometry using an antibody against MITF. Total (both un- and phosphorylated) MITF protein content decreased within a 6-hour period. b: Cells from each of the two lines were also harvested for RNA extraction. Real-time PCR was performed in triplicate. MITF RNA concentrations were normalized to glyceraldehyde-3-phosphate dehydrogenase expression and are shown relative to MITF at 0 hours. Error bars show SD of the triplicates. Levels of MITF RNA were reduced to below 50% 6 hours after treatment with 4-TBP.
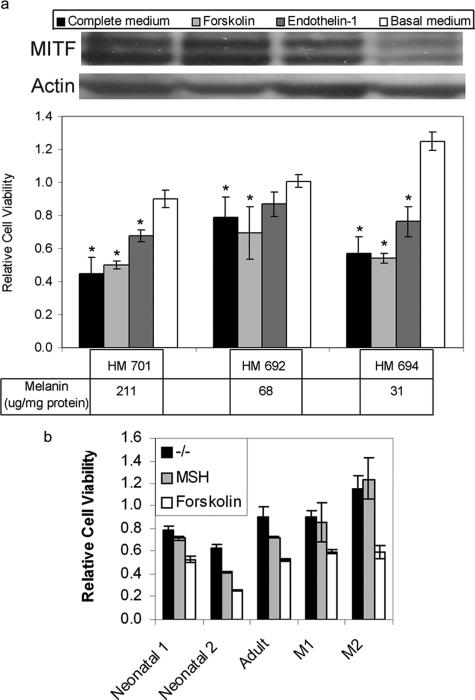
We have shown previously that the addition of MSH, which increases expression of MITF, increases the cytotoxic effect of 4-TBP.15 To confirm that increased MITF expression caused an increase in 4-TBP toxicity, we determined the effect of a second cytokine, endothelin-1 (ET-1), which in synergy with MSH and basic fibroblast growth factor stimulates an increase in MITF expression and phosphorylation.31 Forskolin, which like MSH triggers an increase in cyclic AMP leading to expression of MITF,32 was used as a control. Melanocytes cultured in the presence of ET-1 were significantly more sensitive to 4-TBP (P < 0.05) than cells cultured in the absence of ET-1; similarly, cells grown in the presence of either bovine pituitary extract, which contains MSH,25 or forskolin were also more sensitive to 4-TBP than cells grown in the absence of these factors (Figure 4a). To confirm that sensitivity to 4-TBP is not limited to neonatal, foreskin-derived melanocytes, we determined the effect of 4-TBP and MSH on adult melanocytes derived from breast skin tissue and found no difference in response (Figure 4b). We also confirmed the sensitizing effect of MSH on melanocytes by testing two melanocyte lines that have been shown to carry loss-of-function mutations at the MC1R locus.27 Whereas MSH had no effect on the sensitivity of these mutant melanocytes to 4-TBP-induced toxicity, treatment with forskolin, which acts independently of the MSH receptor, increased sensitivity to 4-TBP (Figure 4b).
Figure 4.
Melanocyte sensitivity to 4-TBP is increased in the presence of factors that promote MITF expression. a: Melanocyte cultures, established from three normally pigmented individuals (HM), varied 6.8-fold in melanin content (ie, between 31 and 211 μg/mg protein). Cells were transferred to basal culture medium for 5 days and then to either complete medium or basal medium supplemented with either 0.1 nmol/L ET-1 or 1 μmol/L forskolin. After 5 days, cells were either harvested for protein extraction or dosed with 300 μmol/L 4-TBP. Cell lysates were prepared and normalized for protein content, then subjected to Western blot analysis to detect MITF and actin. MITF expression was increased in cell cultured in forskolin, ET-1, and complete medium. Viability was determined after 72 hours for cells dosed with 4-TBP. Viability is shown relative to viability of melanocytes, cultured in the appropriate medium, and treated with vehicle alone. The toxicity of 4-TBP did not correlate with pigment status but was significantly increased (*P < 0.05) in the presence of forskolin, ET-1, and complete medium (which contains bovine pituitary extract as a source of MSH). b: To confirm that melanocytes derived from adult breast skin are similarly sensitized to 4-TBP by MSH, whereas melanocytes that lack functional receptors for MSH do not, we determined the sensitivity of five melanocyte lines: one adult, breast-derived; and four neonatal foreskin-derived—two normally pigmented and two with loss-of-function MC1R mutations. All normal lines were from type IV skin (darkly pigmented). Melanocyte viability was determined in the absence and presence of either 1 μmol/L forskolin or 10 nmol/L MSH as described above. As with neonatal melanocytes, adult melanocytes were more sensitive to 4-TBP in the presence of MSH than in basal media and most sensitive in the presence of forskolin. Melanocytes that lack functional receptors for MSH showed equal sensitivity to 4-TBP in the absence and presence of MSH but were sensitized by forskolin. Bars indicate SD of triplicates within a representative experiment.
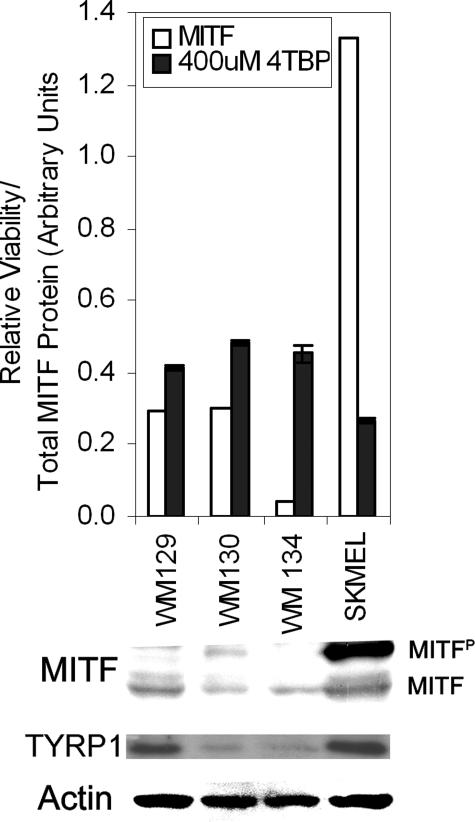
Thus, factors that promote MITF expression increase sensitivity to 4-TBP. To test this hypothesis further, we determined whether 4-TBP toxicity correlated with expression of MITF in melanoma cells. We tested four melanoma lines that have varying levels of MITF expression for sensitivity to 4-TBP. The line that expressed the most MITF was also the most sensitive to 4-TBP (Figure 5). The Pearson co-efficient of correlation was used to determine whether increase in sensitivity (proportion of nonviable cells) correlated with MITF expression (band density following Western blot analysis). There was a statistically significant correlation between melanocyte death and total MITF (both un- and phosphorylated, r = 0.8088, P < 0.05).
Figure 5.
Melanoma sensitivity to 4-TBP correlates with expression of MITF. Melanoma cells were either harvested for protein extraction or plated to test sensitivity to 4-TBP. Cells were attached for 48 hours and then dosed with 400 μmol/L 4-TBP for 72 hours. The proportion of viable cells was determined and is shown relative to cells treated with vehicle alone. Densitometry was performed following Western blot analysis for MITF, Tyrp1 (Pep1), and actin, total MITF protein is expressed as the sum of band densities for un- and phosphorylated MITF normalized to actin. SK-MEL188 that expressed the most MITF, and in the potent phosphorylated form, as well as Tyrp1, was significantly more sensitive than each of the other lines, which express less MITF (P < 0.05). Error bars indicate SD of triplicates within a representative experiment.
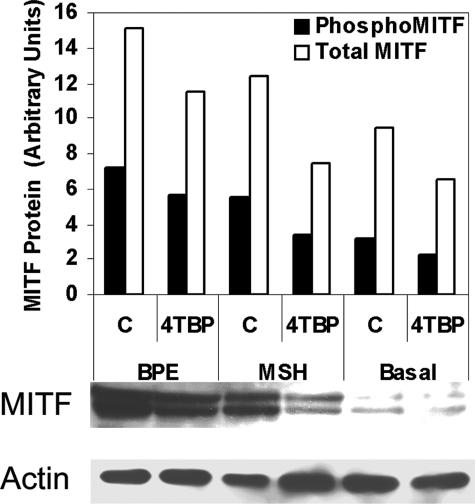
We therefore determined the effect of MSH on MITF expression in melanocytes subjected to 4-TBP (Figure 6). MITF expression was reduced following exposure to 4-TBP, even in the presence of MSH. Expression was lowest in melanocytes cultured in the absence of MSH and the presence of 4-TBP.
Figure 6.
MITF expression is reduced by 4-TBP in the presence of MSH. Cells from a normal human melanocyte line were cultured in basal medium supplemented with either 13 μg/ml bovine pituitary extract (BPE) or 10 nmol/L MSH. Cells were then treated with either vehicle (C) or 200 μmol/L 4-TBP. Samples were harvested 6 hours after treatment, normalized for protein content and subjected to Western blot analysis followed by densitometry. Density values for phospho-MITF and total MITF (both un- and phosphorylated) protein are expressed relative to the density of the actin band. MITF content decreased in cells treated with 4-TBP compared with their respective controls. Cells cultured in the absence of MSH (Basal media) demonstrate a dramatic reduction in MITF protein, which is further diminished following exposure to 4-TBP.
Melanin Content Does Not Affect the Sensitivity of Melanoma Cells to 4-TBP
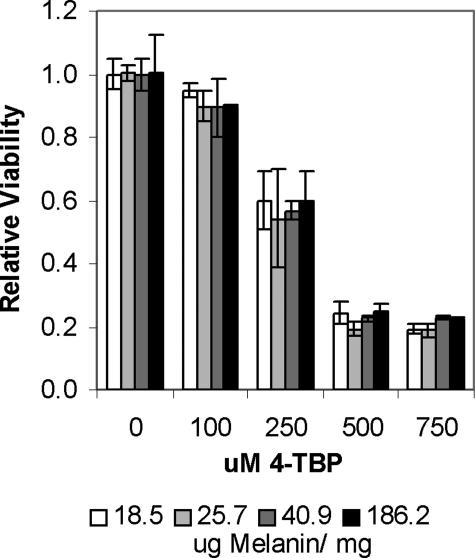
We investigated some of the changes that MITF instigates in melanocytes to determine their effect on sensitivity to 4-TBP. The overall effect of MITF expression is an increase in melanin synthesis. We therefore investigated the effect of melanin on 4-TBP-induced cytotoxicity. Sensitivity to 4-TBP did not correlate with melanin content in normal human melanocyte lines (Figure 4). To exclude the effects of genetic variation on susceptibility, we tested sensitivity to 4-TBP on one cell line, SK-MEL188 melanoma cells, which had been stimulated to increase melanin synthesis. Cells were cultured in RPMI-based as opposed to DMEM-based media, thereby reducing the concentration of l-tyrosine from 0.4 mmol/L in DMEM to 0.07 mmol/L in RPMI (30% dilution of RPMI). Melanin production was thus reduced due to decreased availability of this precursor. These cultures were then supplemented with increasing concentrations of tyrosine (0.090, 0.129, 0.249, and 1.249 mmol/L tyrosine) such that four individual cultures with varying melanin content resulted (18.5, 25.7, 40.9, and 186.2 μg of melanin/mg of protein) (Figure 7). Cells were then cultured for 3 days under identical conditions to allow gene expression to equilibrate before determining cell viability in the presence of 4-TBP. No significant difference was found in viability between the variously pigmented cultures.
Figure 7.
Melanin content does not alter sensitivity to 4-TBP. SK-MEL188 melanoma cells were cultured in growth medium with reduced tyrosine concentrations. When melanin content had been reduced approximately 10-fold, cells were passaged and treated with increasing concentrations of tyrosine to promote melanin synthesis. After 72 hours, the tyrosine-supplemented medium was replaced with media low in tyrosine and serum (4%), and cells were cultured for a further 72 hours to normalize gene expression. A melanin assay was then performed to verify differences in melanin content. Cells were dosed with increasing concentrations of 4-TBP, and a viability assay was performed at 72 hours. The error bars represent SD in four wells of one experiment. The experiment was done in triplicate, and a representative experiment is shown. No significant difference was found between melanoma cells with different melanin contents.
Tyrp1 Expression Modulates Sensitivity to 4-TBP
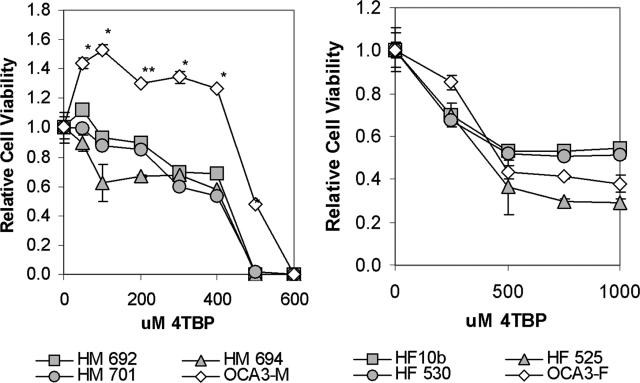
Expression of MITF increases melanin synthesis, and this increase is mediated by increased expression of the enzymes that catalyze the conversion of tyrosine to melanin. We have shown previously that tyrosinase activity14 has no effect on sensitivity to 4-TBP. We therefore investigated the effect of the melanogenic protein Tyrp1, which is regulated by MITF,18 on sensitivity to 4-TBP. We compared sensitivity of normal human melanocytes with those from an individual with oculocutaneous albinism type 3 (OCA3), which results from autosomal recessive mutations at the Tyrp1 locus.33,34 Dose-response curves demonstrate that melanocytes from the individual with OCA3 were significantly more resistant (P < 0.01) to 4-TBP than normally pigmented melanocytes (Figure 8, left panel). To exclude the possibility that non-melanocyte proteins contributed to the resistance of OCA3 melanocytes, we compared the sensitivity of fibroblasts from the affected individual with those from normally pigmented individuals and found no significant difference (Figure 8, right panel). Melanoma cells that express Tyrp1 were also found to be more sensitive to 4-TBP (Figure 5).
Figure 8.
Melanocytes that lack functional Tyrp1 are less sensitive to 4-TBP compared with normal melanocytes. Human melanocyte and fibroblast cultures were established from foreskin of three normally pigmented individuals (HM/HF) and one null for Tyrp1 (OCA3 mol/L/OCA3F). Left: Melanocytes were plated in 96-well plates. After 24 hours, the culture medium was replaced with medium containing 4-TBP. Viability was determined after 72 hours. There was a significant difference in sensitivity to 4-TBP between melanocytes isolated from normally pigmented individuals compared with melanocytes from an individual with OCA3 (*P < 0.01, **P < 0.05). Right: Fibroblasts were plated and allowed to attach overnight. HF10b is the unaffected twin of the individual with OCA3.33 Cells were dosed with 4-TBP and viability determined after 72 hours. Viability is shown relative to cells treated with vehicle alone. There is no significant difference in sensitivity to 4-TBP between fibroblasts isolated from normally pigmented individuals compared with fibroblasts from an individual with OCA3. Error bars indicate SD. The experiment was performed in triplicate, and a representative experiment is shown.
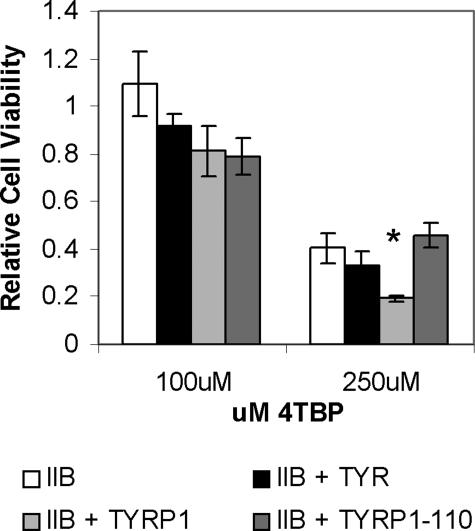
To confirm the effect of Tyrp1 expression on sensitivity to 4-TBP, IIB melanoma cells, which lack expression of functional Tyrp1 protein, were transfected with an expression plasmid carrying either a wild-type or mutant (Tyrp1-b110) Tyrp1 sequence. Tyrp1-b110 was generated by site-directed mutagenesis and is equivalent to the mouse brown-null mutation.30 IIB melanoma cells overexpressing wild-type Tyrp1 were found to have a significantly increased sensitivity to 4-TBP (P < 0.01), whereas expression of the mutant plasmid had no effect (Figure 9). Cells were also transfected with an expression plasmid encoding wild-type tyrosinase. Overexpression of tyrosinase did not confer increased sensitivity to 4-TBP.
Figure 9.
Expression of Tyrp1 correlates with increased melanoma sensitivity to 4-TBP. IIB melanoma cells, which lack functional Tyrp1, were transfected with an empty vector (IIB), functional Tyrp1 (IIB-Tyrp1), mutant Tyrp1 (IIB-Tyrp1–110), and functional tyrosinase (IIB-Tyr). Stable lines were generated and expression confirmed. Cells were then treated with either 100 or 250 μmol/L 4-TBP for 48 hours and the viability relative to cells treated with vehicle alone determined. Although there was no statistical difference in viability of cells exposed to 100 μmol/L 4-TBP, overexpression of Tyrp1 causes significantly increased sensitivity to 250 μmol/L 4-TBP compared with overexpression of either tyrosinase or the mutant form of Tyrp1 (Student’s t-test between lines treated with 250 μmol/L 4-TBP, P < 0.01, indicated by *). Error bars indicate SD.
Reduction of Tyrp1 Protein Following Treatment with 4-TBP
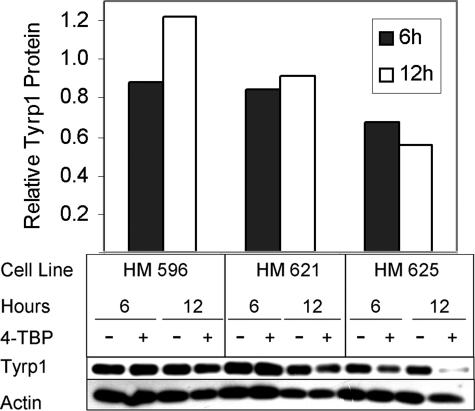
Reduction in Tyrp1 expression following exposure to 4-TBP and subsequent to down-regulation of MITF was confirmed. Normal human melanocytes were subjected to treatment with 4-TBP for varying time points, and the change in Tyrp1 expression determined by Western blot analysis. We found that Tyrp1 protein levels were reduced 6 to 12 hours after the addition of 4-TBP to the media (Figure 10).
Figure 10.
Expression of Tyrp1 is reduced in the presence of 4-TBP. Normal human melanocytes were treated with 250 μmol/L 4-TBP and harvested at 0, 6, and 12 hours, normalized for protein content, and subjected to Western blot analysis and densitometric analysis. Expression of Tyrp1 (Mel5) is shown relative to actin, the loading control. Tyrp1 protein was found to be reduced 6 to 12 hours after exposure to 4-TBP; however, the extent of the reduction varied between cell lines.
Discussion
Understanding the mechanism(s) by which 4-TBP causes melanocyte death may elucidate the pathology underlying contact vitiligo in specific and idiopathic vitiligo in general. 4-TBP is converted to quinones,12 which undergo redox cycling within the cell, thereby generating ROS35 and inducing oxidative stress. Thus, melanocyte susceptibility to oxidative stress may play a role in the pathogenesis of vitiligo.
Evidence of oxidative stress has been demonstrated in both the skin36 and blood37 of individuals with active vitiligo. Hydrogen peroxide accumulates in the epidermis of these individuals, concomitant with reduced levels of the antioxidant catalase.36 Epidermal levels of additional antioxidants, including ubiquinol, vitamin E, and reduced glutathione were also found to be significantly reduced in active vitiligo.38 Altered antioxidant levels, including catalase, glutathione and superoxide dismutase, were found to extend to the sera of patients,39,40 whereas lipid peroxidation, a hallmark of ROS-induced damage, was observed in erythrocytes derived from vitiligo patients,40 all suggesting systemic oxidative stress in individuals with vitiligo. In this study, we show that 4-TBP induces oxidative stress in melanocytes (Figure 1) and that melanocytes from individuals with vitiligo are more sensitive to 4-TBP compared with normal melanocytes. This sensitivity can be reduced significantly by inclusion of an antioxidant in the culture medium (Figure 2). Thus, vitiligo may result from an insufficient response to oxidative stress induced by exposure to 4-TBP.
Oxidative stress induced by hydrogen peroxide causes down-regulation of MITF in melanoma cells.21 This transcription factor, which is most active when phosphorylated,19 regulates both melanocyte proliferation and expression of proteins required for melanin synthesis. We have shown previously that MSH, which stimulates MITF expression and phosphorylation, increases sensitivity of melanocytes to 4-TBP.15 We now show that a second cytokine ET-1, which also increases expression and phosphorylation of MITF,31 caused an increase in melanocyte sensitivity to 4-TBP-induced cytotoxicity (Figure 4) and that MITF expression is sensitive to the redox state of cells (Figure 3).21 The first mouse model for vitiligo was an Mitf mutant41; however, mutations at the MITF locus do not result in human vitiligo.42 We therefore propose that disruption of the pathway/s that regulate MITF expression and/or pathways regulated by MITF itself play a role in the etiology of this condition. Disruption of these pathways mimics the effect of 4-TBP on normal melanocytes treated with MSH. Pathways involved in MITF regulation have been shown to be defective in vitiligo43 and may contribute to the increased risk of melanocyte death.
A number of events known to trigger vitiligo also result in increased melanogenesis, for example severe sunburn and pregnancy.6 Likewise, normal melanocytes stimulated to increase melanin synthesis by either MSH or ET-1 are more sensitive to 4-TBP than untreated cells. To identify how MSH, ET-1, and MITF expression alter 4-TBP toxicity, we investigated the effects of MSH on the melanocyte and their contribution to sensitivity to 4-TBP. We have shown previously that neither proliferation rate15 nor tyrosinase activity14 has an effect on sensitivity to 4-TBP. We now demonstrate that despite the ability to remove ROS from the cellular environment, melanin content per se has no effect on melanocyte sensitivity to 4-TBP (Figures 4 and 7). However, expression of functional Tyrp1 leads to increased sensitivity (Figures 8 and 9) and normal melanocytes challenged with 4-TBP respond by down-regulating expression of Tyrp1 expression (Figure 10). Although its precise function is not known, Tyrp1 has been shown to have several catalytic activities, including tyrosine hydroxylase activity.44,45 Tyrp1 may thus use 4-TBP as a substrate and promote ROS formation by catalyzing quinone production. Therefore, reduction in expression of MITF and subsequently Tyrp1 will protect cells against further oxidative stress. Abnormal expression of Tyrp1 has been observed in melanocytes from individuals with vitiligo.22 Sustained expression or overexpression of Tyrp1 following exposure to 4-TBP may induce melanocyte death in vitiligo, similar to melanoma cells transfected with the Tyrp1 expression plasmid (Figure 9).
Reduced expression of MITF would decrease expression of Tyrp1 and additional genes that promote apoptosis during oxidative stress. Expression of the pink-eyed dilution (p) protein, which is also regulated by MITF,46 increases sensitivity to chemicals that are detoxified by glutathione-dependent processes.47 It has been proposed that the p protein alters the availability of glutathione, thus reducing the capacity of the melanocyte to remove chemotoxins and combating oxidative stress.
MITF also regulates B-cell lymphoma 2 protein (BclII), thioredoxin reductase, and mitochondrial stress-70 protein (GRP75/mortalin/mthsp70/pbp74).48 Thioredoxin reductase and mitochondrial stress-70 protein are targets of quinones. Thioredoxin reductase is a key antioxidant in mammalian cells; however, quinones can alter the properties of this enzyme such that it acts as an nicotinamide adenine dinucleotide phosphate-oxidase capable of generating superoxide.49 GRP75 is also targeted by quinones, which form covalent bonds with the protein.50 GRP75 is a member of the HSP70 family of proteins and seems to have numerous functions in the cell including a role as a chaperone in the endoplasmic reticulum and as a p53 inhibitor.51 Binding of quinones to GRP75 allows them to remain in the cell, preventing redox balance; in addition, depletion of functional GRP75 may promote p53 activity and thereby increase the probability that apoptosis is initiated. Thus, several proteins (Tyrp1, p protein, thioredoxin reductase, and GRP75) regulated by MITF can potentiate the toxicity of chemotoxins that induce oxidative stress.
Furthermore, a recent study has demonstrated that MITF is a target of caspases in both melanocytes and melanoma cells. The C terminus product of this digestion can promote apoptosis in melanoma cells. Thus, expression levels of MITF may have a significant impact on both survival and initiation of apoptosis in melanocytes.20
We therefore propose that in normal human melanocytes, exposure to 4-TBP induces oxidative stress, which triggers down-regulation of MITF. This in turn reduces expression of Tyrp1 as well as other genes regulated by MITF that may exacerbate the oxidative stress, such as the p gene and thioredoxin reductase. Reduced melanogenesis facilitates the return to intracellular redox balance by decreasing the generation of intermediate quinones. Once oxidative stress is dissipated, MITF expression is restored. We propose that dysregulation of this system in melanocytes contributes to the pathophysiology of vitiligo. Although MITF and Tyrp1 may be key components, additional factors such as oxidative stress response pathways may also contribute to the onset of vitiligo. This is evident in the observations that melanocyte sensitivity is not directly proportional to MITF expression (Figure 6) and that melanoma cells that express extremely low levels of MITF and Tyrp1 are only less sensitive to 4-TBP rather than completely resistant to the compound (Figure 5).
In vitiligo, melanocytes are in a continuous state of oxidative stress. Cell death may result following a further increase in ROS due either to a failure of cellular antioxidants and/or failure of MITF regulation of Tyrp1. Inclusion of antioxidants in combination with current treatment modalities may increase the efficiency with which vitiligo is remedied.
Acknowledgments
We thank Zalfa Abdel-Malek for helpful discussions during the preparation of this manuscript and for providing the adult-skin derived melanocytes and melanocytes lacking functional MC1R.
Footnotes
Address reprint requests to Dr. Raymond Boissy, Department of Dermatology, University of Cincinnati, PO Box 670592, Cincinnati, OH 45267-0592. E-mail: boissyre@ucmail.uc.edu.
Supported in part by National Institutes of Health grant AR046115 (R.E.B.), the Vitiligo Foundation (R.E.B., P.M.), and the Dermatology Foundation (P.M.).
References
- Arrunategui A, Arroyo C, Garcia L, Covelli C, Escobar C, Carrascal E, Falabella R. Melanocyte reservoir in vitiligo. Int J Dermatol. 1994;33:484–487. doi: 10.1111/j.1365-4362.1994.tb02860.x. [DOI] [PubMed] [Google Scholar]
- Lerner AB. On the etiology of vitiligo and gray hair. Am J Med. 1971;51:141–147. doi: 10.1016/0002-9343(71)90232-4. [DOI] [PubMed] [Google Scholar]
- Nordlund JJ. The pigmentary system and inflammation. Pigment Cell Res. 1992;5:362–365. doi: 10.1111/j.1600-0749.1992.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Le Poole C, Boissy RE. Vitiligo. Semin Cutan Med Surg. 1997;16:3–14. doi: 10.1016/s1085-5629(97)80030-2. [DOI] [PubMed] [Google Scholar]
- Nath SK, Majumder PP, Nordlund JJ. Genetic epidemiology of vitiligo: multilocus recessivity cross-validated. Am J Hum Genet. 1994;55:981–990. [PMC free article] [PubMed] [Google Scholar]
- Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208–214. doi: 10.1111/j.1600-0749.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- Cummings MP, Nordlund JJ. Chemical leukoderma: fact or fancy. Am J Contact Dermatitis. 1995;6:122–127. [Google Scholar]
- Gellin GA, Maibach H. Chemically induced depigmentation. Maibach H, Lowe N, editors. Basel: Karger,; Models in Dermatology. 1985:pp 443–459. [Google Scholar]
- James O, Mayes RW, Stevenson CJ. Occupational vitiligo induced by p-tert-butylphenol: a systemic disease? Lancet. 1977;2:1217–1219. doi: 10.1016/s0140-6736(77)90451-2. [DOI] [PubMed] [Google Scholar]
- Gellin GA, Possick PA, Perone VB. Depigmentation from 4-tertiary butyl catechol—an experimental study. J Invest Dermatol. 1970;55:190–197. doi: 10.1111/1523-1747.ep12280700. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lopez JN, Fenoll LG, Penalver MJ, Garcia-Ruiz PA, Varon R, Martinez-Ortiz F, Garcia-Canovas F, Tudela J. Tyrosinase action on monophenols: evidence for direct enzymatic release of o-diphenol. Biochim Biophys Acta. 2001;1548:238–256. doi: 10.1016/s0167-4838(01)00237-0. [DOI] [PubMed] [Google Scholar]
- Thorneby-Andersson K, Sterner O, Hansson C. Tyrosinase-mediated formation of a reactive quinone from the depigmenting agents, 4-tert-butylphenol and 4-tert-butylcatechol. Pigment Cell Res. 2000;13:33–38. doi: 10.1034/j.1600-0749.2000.130107.x. [DOI] [PubMed] [Google Scholar]
- Yang F, Boissy RE. Effects of 4-tertiary butylphenol on the tyrosinase activity in human melanocytes. Pigment Cell Res. 1999;12:237–245. doi: 10.1111/j.1600-0749.1999.tb00756.x. [DOI] [PubMed] [Google Scholar]
- Yang F, Sarangarajan R, Le Poole IC, Medrano EE, Boissy RE. The cytotoxicity and apoptosis induced by 4-tertiary butylphenol in human melanocytes are independent of tyrosinase activity. J Invest Dermatol. 2000;114:157–164. doi: 10.1046/j.1523-1747.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- Yang F, Abdel-Malek Z, Boissy RE. Effects of commonly used mitogens on the cytotoxicity of 4-tertiary butylphenol to human melanocytes. In Vitro Cell Dev Biol Anim. 1999;35:566–570. doi: 10.1007/s11626-999-0094-5. [DOI] [PubMed] [Google Scholar]
- Suzuki I, Cone RD, Im S, Nordlund J, Abdel-Malek ZA. Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis. Endocrinology. 1996;137:1627–1633. doi: 10.1210/endo.137.5.8612494. [DOI] [PubMed] [Google Scholar]
- Widlund HR, Fisher DE. Microphthalamia-associated transcription factor: a critical regulator of pigment cell development and survival. Oncogene. 2003;22:3035–3041. doi: 10.1038/sj.onc.1206443. [DOI] [PubMed] [Google Scholar]
- Fang D, Setaluri V. Role of microphthalmia transcription factor in regulation of melanocyte differentiation marker TRP-1. Biochem Biophys Res Commun. 1999;256:657–663. doi: 10.1006/bbrc.1999.0400. [DOI] [PubMed] [Google Scholar]
- Xu W, Gong L, Haddad MM, Bischof O, Campisi J, Yeh ET, Medrano EE. Regulation of microphthalmia-associated transcription factor MITF protein levels by association with the ubiquitin-conjugating enzyme hUBC9. Exp Cell Res. 2000;255:135–143. doi: 10.1006/excr.2000.4803. [DOI] [PubMed] [Google Scholar]
- Larribere L, Hilmi C, Khaled M, Gaggioli C, Bille K, Auberger P, Ortonne JP, Ballotti R, Bertolotto C. The cleavage of microphthalmia-associated transcription factor, MITF, by caspases plays an essential role in melanocyte and melanoma cell apoptosis. Genes Dev. 2005;19:1980–1985. doi: 10.1101/gad.335905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Cervantes C, Martinez-Esparza M, Perez C, Daum N, Solano F, Garcia-Borron JC. Inhibition of melanogenesis in response to oxidative stress: transient downregulation of melanocyte differentiation markers and possible involvement of microphthalmia transcription factor. J Cell Sci. 2001;114:2335–2344. doi: 10.1242/jcs.114.12.2335. [DOI] [PubMed] [Google Scholar]
- Jimbow K, Chen H, Park JS, Thomas PD. Increased sensitivity of melanocytes to oxidative stress and abnormal expression of tyrosinase-related protein in vitiligo. Br J Dermatol. 2001;144:55–65. doi: 10.1046/j.1365-2133.2001.03952.x. [DOI] [PubMed] [Google Scholar]
- Schallreuter KU, Moore J, Wood JM, Beazley WD, Peters EM, Marles LK, Behrens-Williams SC, Dummer R, Blau N, Thony B. Epidermal H(2)O(2) accumulation alters tetrahydrobiopterin (6BH4) recycling in vitiligo: identification of a general mechanism in regulation of all 6BH4-dependent processes? J Invest Dermatol. 2001;116:167–174. doi: 10.1046/j.1523-1747.2001.00220.x. [DOI] [PubMed] [Google Scholar]
- Yildirim M, Baysal V, Inaloz HS, Kesici D, Delibas N. The role of oxidants and antioxidants in generalized vitiligo. J Dermatol. 2003;30:104–108. doi: 10.1111/j.1346-8138.2003.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Abdel-Malek Z, Swope VB, Pallas J, Krug K, Nordlund JJ. Mitogenic, melanogenic, and cAMP responses of cultured neonatal human melanocytes to commonly used mitogens. J Cell Physiol. 1992;150:416–425. doi: 10.1002/jcp.1041500226. [DOI] [PubMed] [Google Scholar]
- Le Poole IC, Boissy RE, Sarangarajan R, Chen J, Forristal JJ, Sheth P, Westerhof W, Babcock G, Das PK, Saelinger CB. PIG3V, an immortalized human vitiligo melanocyte cell line, expresses dilated endoplasmic reticulum. In Vitro Cell Dev Biol Anim. 2000;36:309–319. doi: 10.1290/1071-2690(2000)036<0309:PAIHVM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Kanto H, Kavanagh R, Abdel-Malek ZA. Significance of the melanocortin 1 receptor in regulating human melanocyte pigmentation, proliferation, and survival. Ann NY Acad Sci. 2003;994:359–365. doi: 10.1111/j.1749-6632.2003.tb03200.x. [DOI] [PubMed] [Google Scholar]
- Guerra L, Mordoh J, Slavutsky I, Larripa I, Medrano EE. Characterization of IIB-MEL-J: a new and highly heterogenous human melanoma cell line. Pigment Cell Res. 1989;2:504–509. doi: 10.1111/j.1600-0749.1989.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Zhao H, Eling DJ, Medrano EE, Boissy RE. Retroviral infection with human tyrosinase-related protein-1 (TRP-1) cDNA upregulates tyrosinase activity and melanin synthesis in a TRP-1-deficient melanoma cell line. J Invest Dermatol. 1996;106:744–752. doi: 10.1111/1523-1747.ep12345799. [DOI] [PubMed] [Google Scholar]
- Zdarsky E, Favor J, Jackson IJ. The molecular basis of brown, an old mouse mutation, and of an induced revertant to wild type. Genetics. 1990;126:443–449. doi: 10.1093/genetics/126.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadekaro AL, Kavanagh R, Kanto H, Terzieva S, Hauser J, Kobayashi N, Schwemberger S, Cornelius J, Babcock G, Shertzer HG, Scott G, Abdel-Malek ZA. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005;65:4292–4299. doi: 10.1158/0008-5472.CAN-04-4535. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Kavanagh RJ, Wakamatsu K, Ito S, Pipitone MA, Abdel-Malek ZA. Cutaneous photobiology. The melanocyte vs. the sun: who will win the final round? Pigment Cell Res. 2003;16:434–447. doi: 10.1034/j.1600-0749.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- Boissy RE, Zhao H, Oetting WS, Austin LM, Wildenberg SC, Boissy YL, Zhao Y, Sturm RA, Hearing VJ, King RA, Nordlund JJ. Mutation in and the lack of expression of tyrosinase-related protein-1 (TRP-1) in melanocytes from an individual with brown oculocutaneous albinism: a new subtype of albinism classified as “OCA3”. Am J Hum Genet. 1996;58:1145–1156. [PMC free article] [PubMed] [Google Scholar]
- Manga P, Kromberg JG, Box NF, Sturm RA, Jenkins T, Ramsay M. Rufous oculocutaneous albinism in southern African Blacks is caused by mutations in the TYRP1 gene. Am J Hum Genet. 1997;61:1095–1101. doi: 10.1086/301603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien PJ. Molecular mechanisms of quinone cytotoxicity. Chem Biol Interact. 1991;80:1–41. doi: 10.1016/0009-2797(91)90029-7. [DOI] [PubMed] [Google Scholar]
- Schallreuter KU, Moore J, Wood JM, Beazley WD, Gaze DC, Tobin DJ, Marshall HS, Panske A, Panzig E, Hibberts NA. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J Investig Dermatol Symp Proc. 1999;4:91–96. doi: 10.1038/sj.jidsp.5640189. [DOI] [PubMed] [Google Scholar]
- Beazley WD, Gaze D, Panske A, Panzig E, Schallreuter KU. Serum selenium levels and blood glutathione peroxidase activities in vitiligo. Br J Dermatol. 1999;141:301–303. doi: 10.1046/j.1365-2133.1999.02980.x. [DOI] [PubMed] [Google Scholar]
- Passi S, Grandinetti M, Maggio F, Stancato A, De Luca C. Epidermal oxidative stress in vitiligo. Pigment Cell Res. 1998;11:81–85. doi: 10.1111/j.1600-0749.1998.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Koca R, Armutcu F, Altinyazar HC, Gurel A. Oxidant-antioxidant enzymes and lipid peroxidation in generalized vitiligo. Clin Exp Dermatol. 2004;29:406–409. doi: 10.1111/j.1365-2230.2004.01524.x. [DOI] [PubMed] [Google Scholar]
- Agrawal D, Shajil EM, Marfatia YS, Begum R. Study on the antioxidant status of vitiligo patients of different age groups in Baroda. Pigment Cell Res. 2004;17:289–294. doi: 10.1111/j.1600-0749.2004.00149.x. [DOI] [PubMed] [Google Scholar]
- Lamoreux ML, Boissy RE, Womack JE, Nordlund JJ. The vit gene maps to the mi (microphthalmia) locus of the laboratory mouse. J Hered. 1992;83:435–439. doi: 10.1093/oxfordjournals.jhered.a111247. [DOI] [PubMed] [Google Scholar]
- Tripathi RK, Flanders DJ, Young TL, Oetting WS, Ramaiah A, King RA, Boissy RE, Nordlund JJ. Microphthalmia-associated transcription factor (MITF) locus lacks linkage to human vitiligo or osteopetrosis: an evaluation. Pigment Cell Res. 1999;12:187–192. doi: 10.1111/j.1600-0749.1999.tb00512.x. [DOI] [PubMed] [Google Scholar]
- Kitamura R, Tsukamoto K, Harada K, Shimizu A, Shimada S, Kobayashi T, Imokawa G. Mechanisms underlying the dysfunction of melanocytes in vitiligo epidermis: role of SCF/KIT protein interactions and the downstream effector, MITF-M. J Pathol. 2004;202:463–475. doi: 10.1002/path.1538. [DOI] [PubMed] [Google Scholar]
- Jimenez-Cervantes C, Garcia-Borron JC, Valverde P, Solano F, Lozano JA. Tyrosinase isoenzymes in mammalian melanocytes. 1. Biochemical characterization of two melanosomal tyrosinases from B16 mouse melanoma. Eur J Biochem. 1993;217:549–556. doi: 10.1111/j.1432-1033.1993.tb18276.x. [DOI] [PubMed] [Google Scholar]
- Zhao H, Zhao Y, Nordlund JJ, Boissy RE. Human TRP-1 has tyrosine hydroxylase but no dopa oxidase activity. Pigment Cell Res. 1994;7:131–140. doi: 10.1111/j.1600-0749.1994.tb00040.x. [DOI] [PubMed] [Google Scholar]
- Ancans J, Flanagan N, Hoogduijn MJ, Thody AJ. P-locus is a target for the melanogenic effects of MC-1R signaling: a possible control point for facultative pigmentation. Ann NY Acad Sci. 2003;994:373–377. doi: 10.1111/j.1749-6632.2003.tb03202.x. [DOI] [PubMed] [Google Scholar]
- Staleva L, Manga P, Orlow SJ. The pink-eyed dilution protein modulates arsenic sensitivity and intracellular glutathione metabolism. Mol Cell. 2002;13:1406–1420. doi: 10.1091/mbc.E02-05-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill GG, Horstmann M, Widlund HR, Du J, Motyckova G, Nishimura EK, Lin YL, Ramaswamy S, Avery W, Ding HF, Jordan SA, Jackson IJ, Korsmeyer SJ, Golub TR, Fisher DE. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell. 2002;109:707–718. doi: 10.1016/s0092-8674(02)00762-6. [DOI] [PubMed] [Google Scholar]
- Cenas N, Nivinskas H, Anusevicius Z, Sarlauskas J, Lederer F, Arner ES. Interactions of quinones with thioredoxin reductase: a challenge to the antioxidant role of the mammalian selenoprotein. J Biol Chem. 2004;279:2583–2592. doi: 10.1074/jbc.M310292200. [DOI] [PubMed] [Google Scholar]
- Lame MW, Jones AD, Wilson DW, Segall HJ. Protein targets of 1,4-benzoquinone and 1,4-naphthoquinone in human bronchial epithelial cells. Proteomics. 2003;3:479–495. doi: 10.1002/pmic.200390062. [DOI] [PubMed] [Google Scholar]
- Wadhwa R, Yaguchi T, Hasan MK, Mitsui Y, Reddel RR, Kaul SC. Hsp70 family member, mot-2/mthsp70/GRP75, binds to the cytoplasmic sequestration domain of the p53 protein. Exp Cell Res. 2002;274:246–253. doi: 10.1006/excr.2002.5468. [DOI] [PubMed] [Google Scholar]