Abstract
The presence of scattered tumor cells at the invading front of several carcinomas has clinical significance. These cells differ in their protein expression from cells in central tumor regions as recently shown for the EGF-TM7 receptor CD97. To understand the impact of such heterogeneity on tumor invasion, we investigated tumor cells with modified CD97 expression in vitro and in vivo. Applying an individual cell-based computer model approach, we linked specific cell properties of these cells to tumor invasion characteristics. CD97 overexpression promoted tumor growth in scid mice, stimulated single cell motility, increased proteolytic activity of matrix metalloproteinases, and secretion of chemokines in vitro in an isoform-specific manner. We demonstrated by computer simulation studies that these effects of CD97 can increase the invasion capacity of tumors. Furthermore, they can cause the appearance of scattered tumor cells at the invasion front. We identified local tumor environment interactions as triggers of these multiple capabilities. Experimentally, our simulation results are supported by the finding that CD97 expression in tumor cells is regulated by their environment. Our combined experimental-theoretical analysis provides novel insight to how variations of individual cell properties can be linked to individual patterns of tumor cell invasion.
The progression of carcinomas is associated with a loss of epithelial differentiation and gain of mesenchyme-like capabilities of scattered tumor cells at the invasive front. This transition is associated with alterations of the expression of molecules involved in cell-cell and/or cell-extracellular matrix interactions, such as motility-promoting molecules.1–3 Furthermore, the secretion of cytokines and extracellular matrix-degrading proteinases are altered.4,5 For colorectal carcinomas it has been shown that in metastases such alterations are reversed and the molecules show a similar expression pattern or level compared with the central region of the primary tumor.6,7 Consequently, Brabletz and co-workers6,7 suggested an active role of the tumor environment in malignant tumor progression. A tumor microenvironment invasion model was suggested by Condeelis and co-workers.8
It is a challenge to understand how the complex tumor invasion behavior emerges from collective interactions on the molecular and cellular level. Although the effects of molecular changes on the individual cell behavior can be quantified directly in experiments, dynamic links between the changes at the cellular level and the characteristics of the tumor invasion process are hard to assess. Here, we exclusively focus on that problem. A major insight into the link between individual cell behavior and tissue dynamics can be gained by computer modeling approaches. They permit investigation of the potential role of generic organization principles in specific tissues. We chose individual cell-based models (IBMs9,10) to approach the challenge.
Considering the growth of tumor cell populations, a number of IBMs of the growth of tumor spheroids have been established.11,12 Tumor invasion into stroma has been approached by cellular automatons13 and lattice variants of IBMs.14 Here, we introduce a novel class of lattice-free IBMs of the growth and collective invasion of carcinoma into stroma after breakdown of the basal membrane. On one hand, the approach enables us to analyze the impact of different cellular alterations on growth and invasion dynamics. On the other hand, we can study several assumptions about the origin of these alterations. We apply this approach to understand observations related to CD97 expression.
CD97 is a member of the EGF-7TM subfamily of class B G-protein-coupled receptors.15,16 It consists of a variable number of N-terminally located EGF domains, a long extracellular stalk, the 7-transmembrane (7TM) domain, and a short intracellular C-terminal region. Alternative splicing results in three isoforms containing three (EGF 1,2,5), four (EGF 1,2,3,5), or five EGF domains (EGF 1-5). Several lines of evidence suggest a role of CD97 in tumor migration and invasiveness. CD97 shows stronger expression at scattered tumor cells of the invasion front of nearly half of colorectal carcinomas than in solid formations of the same tumor.17 Normal colorectal epithelium is CD97-negative or only slightly positive. Furthermore, CD97 expression levels correlate with the in vitro migration and invasion capacity (chemotaxis) of colorectal tumor cell lines and model tumor clones.17 In thyroid carcinomas, CD97 is related to dedifferentiation and tumor stage.18
Motivated by these results and further directed by the presented studies on isoform-specific tumor growth in vivo, we used CD97-transfected cells to investigate the effect of CD97 (EGF 1,2,5), C-terminal-truncated CD97 (EGF 1,2,5/TM1) and CD97 (EGF 1-5) (Figure 1A) on in vitro random cell motility, proteolytic activity, and chemokine secretion. We relate the results in the framework of our IBM, directly demonstrating the relevance of the observed effects in tumor invasion and the impact of the tumor environment in the process. We discuss our results with an emphasis on colorectal carcinomas.
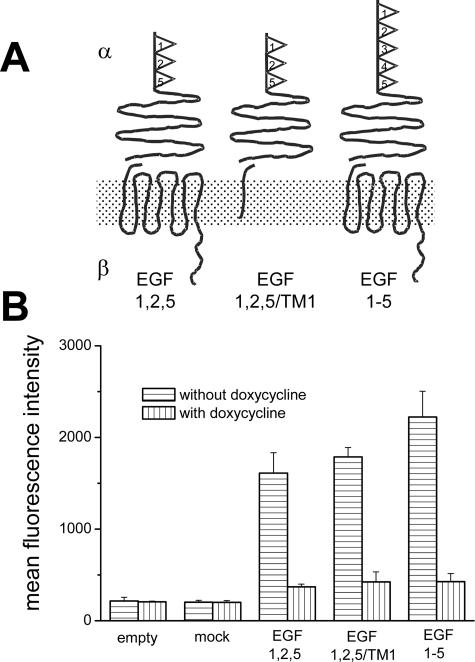
Figure 1.
CD97 clones. A: Schematic structure of stably expressed CD97 molecules in HT1080 cells. CD97 consists of an α and β chain that are noncovalently bound. The CD97 (EGF 1,2,5) and CD97 (EGF 1-5) isoforms differ in their number of extracellular EGF domains. CD97 (EGF 1,2,5/TM1) is truncated within the first transmembrane domain. B: In vitro CD97 expression levels of the HT1080 clones cultured with and without doxycycline determined by flow cytometry (mean ± SEM, n = 3).
Materials and Methods
CD97-Expressing Cells
Tet-off HT1080 clones stably expressing CD97 (EGF 1,2,5), CD97 (EGF 1-5), or C-terminal-truncated CD97 (EGF 1,2,5/TM1) were generated as described.17 Control cells contain only the empty plasmid or CD97 (EGF 1,2,5) mock. To induce the expression of the respective molecules, cells were cultured without doxycycline (Figure 1B). All clones additionally express enhanced green fluorescence protein (EGFP). Clones derived from wild-type Widr colorectal carcinoma cells were generated by stable transfection with the empty pEGFP-N1 vector (BD Biosciences, Heidelberg, Germany) or the plasmid encoding CD97 (EGF 1,2,5). Two clones for each transfected cDNA and cell type were selected out of 10 to 40 examining CD97 and EGFP expression by flow cytometry. Results of one clone are shown. Those of the other one were identical. The human tumor cell lines HT1080, Widr, DLD-1, Caco-2, and SW480 were obtained from the American Type Culture Collection (Rockville, MD).
Cell Cultures
For two-dimensional tumor colony monitoring, 1 × 103 cells were seeded in a six-well plate. For three-dimensional tumor colony monitoring, 1 × 103 cells were seeded into 0.25% agar/medium/20% fetal calf serum in a 24-well plate. Formed colonies were analyzed every 24 hours throughout at least 14 days using digital light microscopy. In two-dimensional colonies cell doubling times were observed by analyzing the initial exponential growth phase. In selected colonies, proliferation was determined after 14 days using the BrdU Labeling and Detection Kit II (Roche Diagnostics GmbH, Mannheim, Germany). The effect of cell density on CD97 expression in two-dimensional cultures was determined by fluorescence-activated cell sorting analysis in nonconfluent single cell and 90% confluent cultures after 48 hours.
Characterization of the Single Cell Motility
Cells (5 × 102 cells/cm2) were seeded in 96-well plates. In nine independent series, the position of 30 cells was detected at several times (t) using digital light microscopy starting 4 hours after plating. Within the time between the measurements (dt), a migrating cell changes its position and thus changes its distance to all cells of the selected group. We calculated the change of the distances Δdij = dij(t) − dij(t + dt) between all pairs i,j of cells in a group. For cells undergoing an independent Brownian random walk, it turns out that <(Δdij)2>, the average of (Δdij)2, grows linearly with time. We confirmed that time dependence and derived a migration coefficient D by calculating D = <(Δdij)2>/8dt for dt = 12 hours.
Interleukin (IL)-8 Enzyme-Linked Immunosorbent Assay and Sodium Dodecyl Sulfate-Substrate Gel Electrophoresis (Zymography)
Supernatants of 1 × 105 cells cultured in 500 μl of OPTI-MEM (Invitrogen, Karlsruhe, Germany) without and with tumor necrosis factor (TNF)-α (5 ng/ml; ImmunoTools, Friesoythe, Germany) were assayed for IL-8 (ELISA; Bender MedSystems, Vienna, Austria). Gelatin zymography of the supernatants was performed as described.19 Specific MMP activity was checked by adding 10 mmol/L ethylenediaminetetraacetic acid to the developing buffer.
Scid Mice
HT1080 tumor cells (1.5 × 106) in 150 μl of phosphate-buffered saline were injected subcutaneously between the scapulae of the BALB/c severe combined immunodeficient scid/scid (scid) mice, 5 to 6 weeks old. Each HT1080 clone was injected into two groups of eight animals (Table 1). To maintain down-regulation of CD97 in one of these groups, doxycycline (0.01 mg/g body weight) was injected subcutaneously every third day. The mice were controlled every second day for tumor growth and state of health and were sacrificed when the tumor had reached maximal growth (∼10% of the actual body weight of the animal or more then 2 cm3), started to ulcerate, or 100 days after tumor cell injection. Tumors were cryopreserved in liquid nitrogen. Expression of CD97 in the tumors was verified by immunohistology.
Table 1.
Scid Mice Groups
| Group | HT1080 cells, stably transfected | Number of animals | Doxycycline treatment |
|---|---|---|---|
| 1 | Empty plasmid | 8 | − |
| 2 | Empty plasmid | 8 | + |
| 3 | CD97 (EGF 1,2,5) | 8 | − |
| 4 | CD97 (EGF 1,2,5) | 8 | + |
| 5 | CD97 (EGF 1,2,5/TM1) | 8 | − |
| 6 | CD97 (EGF 1,2,5/TM1) | 8 | + |
| 7 | CD97 (EGF 1-5) | 8 | − |
| 8 | CD97 (EGF 1-5) | 8 | + |
All animals underwent a subcutaneous injection with different tet-off HT1080 stably transfected clones. Every second animal group received doxycycline every third day to maintain down-regulation of CD97.
Immunohistology and Flow Cytometry
The CD97 monoclonal antibody (mAb) CLB-CD97/3 detects an epitope within the stalk region (CD97stalk).15 For immunohistology, serial sections were cut to 6 μm and fixed in ice-cold methanol for 10 minutes. The sections were incubated with the primary antibody (Ab) (4°C, overnight). Bound Abs were detected with a supersensitive detection system (Vector Laboratories, Burlingame, CA). Microvessel density was analyzed by anti-CD31 (Santa Cruz Biotechnology, Heidelberg, Germany) staining as described.20
In flow cytometry, cells were phenotyped with the desired Ab by indirect immunofluorescence using F(ab′)2 goat anti-mouse immunoglobulin-phycoerythrin or streptavidin-phycoerythrin (DAKO, Hamburg, Germany) in the case of a biotinylated Ab. The antigen levels were determined as mean fluorescence intensity of stained cells in comparison to cells stained with an isotype-matched but irrelevant mAb.
Results
CD97 Clones
Tet-off HT1080 cells stably containing the empty plasmid or CD97 (EGF 1,2,5) mock showed basal CD97 expression in flow cytometry (Figure 1). The established clones CD97 (EGF 1,2,5), CD97 (EGF 1-5), or C-terminal truncated CD97 (EGF 1,2,5/TM1) showed a 6- to 10-fold overexpression of the respective CD97 forms compared with CD97 (EGF 1,2,5) mock clones. CD97 overexpression in CD97 (EGF 1,2,5), CD97 (EGF 1-5), or C-terminal truncated CD97 (EGF 1,2,5/TM1) clones was significantly reduced by doxycycline (Figure 1B).
CD97 (EGF 1,2,5) Alters Tumor Growth in Scid Mice
The induced tumors were found to grow confined within the subcutis. CD97 expression in tumors was confirmed by immunohistology. CD97 (EGF 1,2,5), (EGF 1-5), and (EGF 1,2,5/TM1) tumors were CD97stalk-positive. Tumors of mice treated with doxycycline and all tumors derived from empty cells were CD97stalk-negative or only slightly positive because of the basal CD97 expression in wild-type tet-off HT1080 cells (not shown).
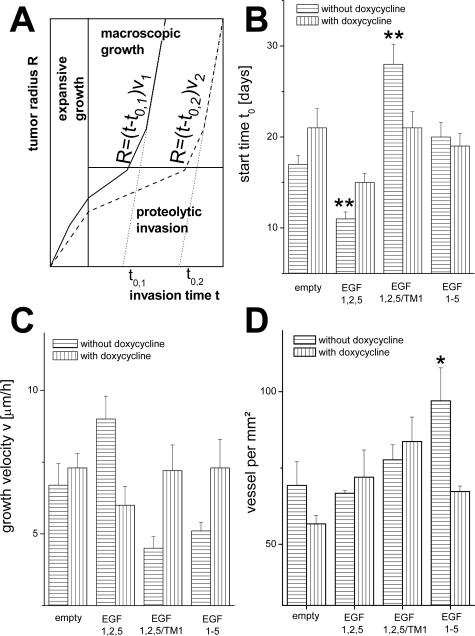
Assuming a linear growth of the radius R of tumor spheroids,12 we analyzed the macroscopic tumor growth quantitatively, calculating the extrapolated start time (t0) and the growth velocity (v) (Figure 2A). We found that CD97 (EGF 1,2,5) overexpression propagated tumor growth compared with controls, whereas CD97 (EGF, 1,2,5/TM1) reduces it (Figure 2, B and C). Decrease of CD97 expression by doxycycline reduces the observed effects depending on the CD97 iso- or truncated forms. Because tumor vascularization can promote growth and invasion, we analyzed vascularization by CD31 staining. We found an increased microvessel density in tumors induced with HT1080 CD97 (EGF 1-5) cells, whereas the density was equal between CD97 (EGF 1,2,5), CD97 (EGF 1,2,5/TM1), and the control cells (Figure 2D). Thus, the observed tumor growth was not determined by vascularization.
Figure 2.
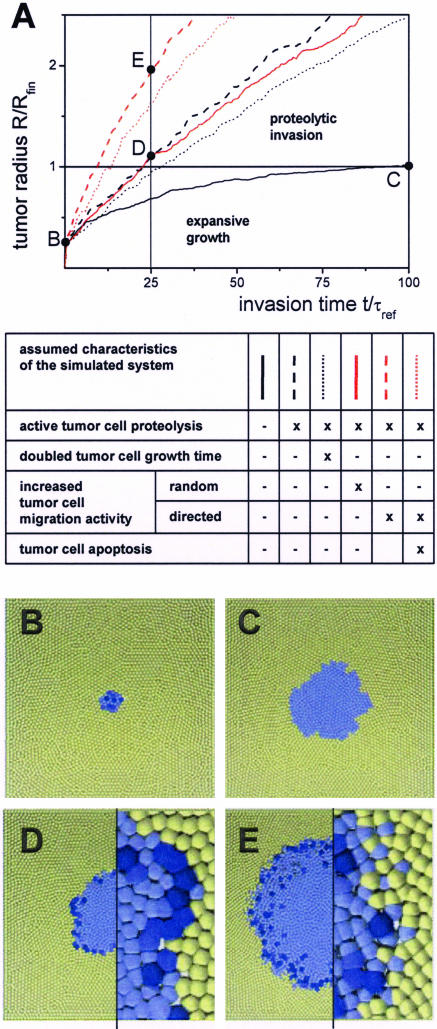
In vivo tumor growth. A: Sketch of a simplified model of tumor propagation in scid mice. An initial expansive growth phase is followed by a phase of proteolytic invasion. After releasing strong tissue confinements, a phase of rapid macroscopic growth starts. The phases are shown for two tumors (tumors 1 and 2), in which tumor 1 (solid line) is more invasive. B and C: Estimated tumor growth parameters. B: Starting time t0; C: growth velocity v derived from a linear fit to the tumor radius R = (t − t0)v in the phase of macroscopic growth (n = 8, mean ± SEM). D: Microvessel density determined by CD31 staining in histological sections of various HT1080 tumors (n = 8, mean ± SEM). Significance of a change compared with the empty control system: *P < 0.05, **P < 0.01; Westfall-Young test.42
Multiple Effects of CD97 on Tumor Cell Properties
Although we found no significant differences in the doubling times of HT1080 clones in vitro [empty: 23 ± 3 hours; CD97 (EGF 1,2,5) mock: 24 ± 4 hours; CD97 (EGF 1,2,5): 23 ± 3 hours; CD97 (EGF 1,2,5/TM1): 21 ± 3 hours; CD97 (EGF 1-5): 23 ± 4 hours; mean ± SD, n = 7, cultivation without doxycycline], we observed molecule-specific changes of cell properties regarding single cell motility, proteolytic activity, and chemokine secretion. The effects presented in the following were confirmed by measurements of cells precultured with doxycycline to decrease CD97. The decrease of the respective molecules by doxycycline reduced the effects gained by their overexpression. Furthermore, all effects induced by CD97 (EGF 1,2,5) were confirmed in Widr clones (not shown).
CD97 (EGF 1,2,5) Promotes Single Cell Motility
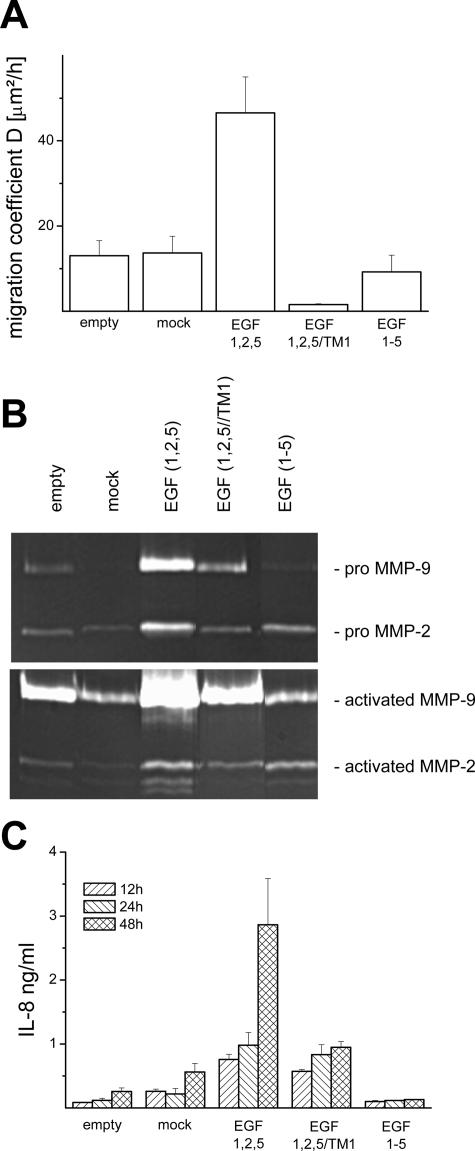
In vitro single cells of all clones investigated showed a Brownian-like random migration. We found an increase of the cell migration coefficient (D) by a factor of ∼3 to 4 in CD97 (EGF 1,2,5) HT1080 cells compared with empty control or CD97 (EGF 1,2,5) mock cells (Figure 3A). In contrast, CD97 (EGF 1-5) cells showed a nearly unaffected and CD97 (EGF 1,2,5/TM1) cells even a reduced motility.
Figure 3.
In vitro properties of the HT-1080 clones. A–C: Single cell properties of HT1080 clones cultured without doxycycline. CD97 (EGF 1,2,5) stimulates single cell motility and increases proteolytic activity of matrix metalloproteinases and chemokine secretion. A: Single cell migration coefficients calculated for a time span of 12 hours (n = 9, mean ± SEM). B: Gelatin zymograms of supernatants of unstimulated (top row) and TNF-α-stimulated (bottom row) HT1080 clones. C: Time-dependent basal IL-8 secretion (n = 3, mean ± SEM).
CD97 (EGF 1,2,5) Stimulates Proteolytic Activity
Considering that MMPs are hardly regulated at posttranscriptional level by cytokines, tissue inhibitors of MMPs (TIMPs) and cleaving to generate the active forms, we estimated proteolytic activity by zymography. In unstimulated cultures, the highest activity was found in CD97 (EGF 1,2,5) cells (Figure 3B). After stimulation with TNF-α, CD97 (EGF 1,2,5) cells especially secreted elevated levels of pro-MMP9 (92 kd) and its active 82-kd form. In addition, the active glycosylated 62-kd and unglycosylated 59-kd forms of pro-MMP2 (72 kd) could be found. No bands were visible after adding ethylenediaminetetraacetic acid to the gels (not shown).
CD97 (EGF 1,2,5) Increases Chemokine Secretion
IL-8 expression by colorectal carcinoma cells was shown to increase during tumor progression5 and to improve cell growth.21 We measured IL-8 secretion in vitro. CD97 (EGF 1,2,5) cells showed the highest basal secretion and time-dependent increase of IL-8 (Figure 3C). Because CD97 (EGF 1,2,5) and CD97 (EGF 1-5) cells differ in their extracellular domain only, IL-8 secretion may be triggered by this part of the molecule. This assumption is supported by cells expressing truncated CD97 (EGF 1,2,5/TM1), which also showed high IL-8 levels. TNF-α stimulated IL-8 secretion in all HT1080 clones. The highest effect was again found in CD97 (EGF 1,2,5) cells (not shown).
Modeling the Growth of Tumor Cell Colonies
We investigated the impact of the multiple cellular alterations induced by CD97 (EGF 1,2,5) on tumor invasion by applying a computational approach based on the IBM of growth regulation of epithelial cell populations recently introduced by us.9 In this model the cells are represented by elastic objects. They are able to move, to grow, and to divide and can form adhesive contacts with neighbor cells and matrix components. The loss of cell-matrix contacts induces programmed cell death and impedes proliferation. Cell compression results in quiescence via contact inhibition of growth. Details can be found in the online supplemental material (see http://ajp.amjpathol.org).
Parameterization
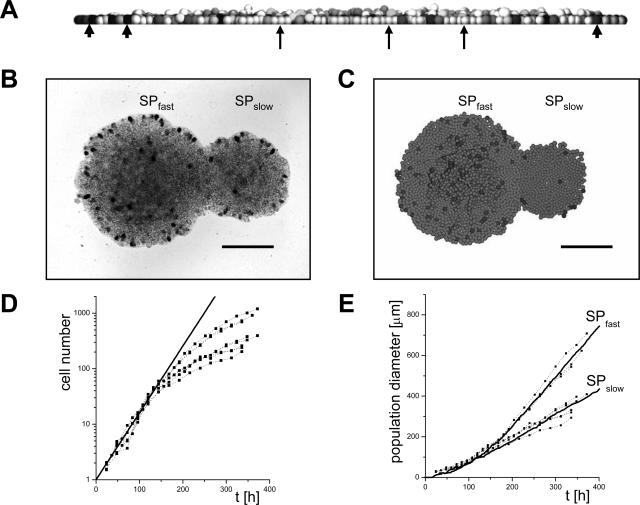
The computer model cells are characterized by a set of experimentally accessible biomechanical and cell-biological parameters. We derived the majority of these parameters from literature.9 The remaining ones were determined in growth experiments of tumor cell clones, forcing the model to reproduce the colony growth dynamics of Widr control cells. Two phases can be distinguished during colony growth in vitro9: an initial phase in which the number of cells grows exponentially, allowing derivation of the cell doubling times, and a subsequent phase in which the colony diameter grows linearly, ie, with a constant spreading velocity. In the computer model we used the cell doubling times as intrinsic cell growth time τ. Experimentally, we found two morphologically identical subpopulations (SP), SPfast and SPslow, of Widr control cells. Their doubling times were the same (21 ± 3 hours, mean ± SD, n = 7), but SPfast spread faster than SPslow. We assumed that the subpopulations are characterized by different capabilities to escape contact inhibition of growth.22 In our computer model cells undergo contact inhibition of growth if their volume is compressed by their neighbor cells below the threshold volume Vinh (Figure 4A). We assumed a smaller value of Vinh for the subpopulation SPfast compared with SPslow (see supplemental material, Table S1, at http://ajp.amjpathol.org), ie, we assumed that SPfast is less sensitive to contact inhibition of growth. This results in a quantitative description of the colony growth even regarding the distribution of proliferation events (Figure 4, B and C).
Figure 4.
Experimental and computer-simulated colony growth. A: Vertical section through a growing computer model population. The gray value of the cells is a marker of the cell volume. Black cells (arrowheads) are in the act of starting division; white cells (arrows) underwent contact inhibition of growth; ie, they are quiescent because of compression by neighboring cells. B: Top view on two cultured Widr empty control populations after 14 days stained for proliferation by BrdU. They grow from cells of different subpopulations (SPfast, SPslow). C: Computer simulation results related to B. D: Cell number of CD97 (EGF 1,2,5)-transfected Widr cell colonies versus time t. The thick line indicates exponential growth according to a cell doubling time of 25 hours. E: Diameter of the colonies shown in D. Lines are computer simulation results applying the parameter sets of Widr empty control cells for SPfast and SPslow and considering the doubling time of the transfected cells. Scale bars = 200 μm.
Regulation I: Contact Inhibition of Migration
We have shown that CD97 (EGF 1,2,5) transfectants are characterized by an increased single cell random migration coefficient D. However, in a growing population spatially restricted activation of cytoskeleton reorganization could cause a directed migration.23 Thus, we considered two cases in our model: a fully random migration of the cells and a locally directed migration at the colony boundary, where each migration step is assumed to point out of the colony. In computer simulations, we found that an increased random migration had negligible effects on the spreading of a colony, whereas a stimulated directed migration significantly promoted that process (not shown).
Analyzing the colony growth behavior of the CD97 (EGF 1,2,5) clones in vitro without stroma, we found no promoting effect on spreading. The spreading of the CD97 (EGF 1,2,5) clones could be described by that of clones of control cells, considering the slightly different doubling time of the transfected cells (25 ± 3 hours, mean ± SD, n = 7) only (Figure 4, D and E). The result implies that the cells either perform an enhanced random migration or are subjected to contact inhibition of migration. This is supported by the formation of smooth colony boundaries as found for controls (compare Figure 4B).
Regulation II: Contact Inhibition of Growth
A model of tumor invasion requires a representation of the tumor stroma. For that purpose, we introduced so-called stroma cells, which represent both cell and matrix components of the stroma. We expand a population of these cells until it fills a closed box inducing a persistent within-tissue pressure. This pressure keeps the stroma cells quiescent because of contact inhibition of growth. An initial tumor is generated selecting a few cells at the center of the box and changing their parameters. We used the parameter sets of Widr empty control cells as reference sets. For the tumor cells we used the set of subpopulation SPfast and for the stroma cell that of SPslow, assuming for tumor cells a decreased sensitivity to contact inhibition of growth.24 This growth advantage enables them to expand under the initial conditions. All other parameters are identical, facilitating further comparative analysis.
Tumor spheroids in a stable polymer matrix stop growing at a certain diameter that corresponds to a threshold pressure acting on the population and inducing a defined compression.25 In in vitro experiments, we found that tumor spheroids of both CD97-transfected and control Widr cells stopped growth at a diameter of ∼200 ± 20 μm (mean ± SD, n = 30, not shown). This indicates that in these cells contact inhibition of growth is identically regulated.
Modeling Collective Tumor Cell Invasion
Subsequent to tumor growth we investigated the impact of tumor cell motility, proteolytic activity, growth, and survival on collective tumor invasion. For specific tumor-stroma interaction parameters (see below), we assumed reasonable values ensuring that the invasion velocity is significantly smaller than the growth velocity of an unconfined tumor cell colony.
Loss of Contact Inhibition and Proteolytic Activity of Tumor Cells Are Prerequisites of Invasion
In our computer model reference tumors stop growth if their expansion results in a pressure in the system high enough to induce contact inhibition of growth in the tumor cells (Figure 5, A–C). To escape that kind of growth inhibition, tumor cells have to degrade their surrounding stroma. Thus, we assumed that stroma cells can be degraded by adjacent, proteolytically active tumor cells with a defined rate wprot per cell-cell contact. Changes of the proteolytic activity of tumor cells are modeled changing this rate wprot.
Figure 5.
Computer model results on tumor invasion. A: Effects of tumor cell properties on invasion dynamics. The tumor radius R is scaled with the finite tumor radius Rfin obtained without stroma degradation (black solid line); the time t with the reference cell growth time τref of 21 hours. B–E: Snapshots of computer-simulated tumors at the selected times points indicated in A. Tumor cells are blue; stroma cells are yellow. Color saturation indicates immanent cell division. Without proteolytic activity of the tumor cells (A, black solid line) tumor expansion starting from a small initial tumor (B) stops because of contact inhibition of growth after ∼100 τref (C). Proteolytic activity of the tumor cells (A, black dashed line) enables persistent tumor invasion. Thus, the tumor radius reaches the value of Rfin already after ∼25 τref (D). The invasion is only moderately slowed, doubling the tumor cell growth time τ (A, black dotted line). In contrast to enhanced random migration (A, red solid line), enhanced directed migration in the presence of active proteolysis (A, red dashed line) strongly promotes invasion by roughening the proteolytic active interface (compare magnifications in D and E). Regardless of that large interface, stroma contact-induced tumor cell apoptosis has only weak effects on invasion (A, red dotted line). Box height: 800 μm (B–E); magnifications; 160 μm (D, E).
In simulations we found that considering proteolysis the initial growth phase is followed by an invasion phase (Figure 5, A and D). During proteolytic invasion we found an approximately linear increase of the tumor radius R with time that is proportional to the degradation rate wprot. The process requires the assumed partial loss of contact inhibition in tumor cells. Without that growth advantage, proteolytic tumor invasion is inhibited by stroma self-regeneration (not shown).
Tumor Cell Growth Rates and Survival Have Minor Relevance for Invasion
As a complementary process to stroma degradation, the tumor environment may affect tumor cell growth and survival. Thus, we studied effects of changed tumor cell growth time τ and assumed that stroma cell contacts may induce tumor cell apoptosis with a rate wapop per cell-cell contact. An increase of the growth time τ of all tumor cells decreases the tumor invasion speed. However, because the invasion on large time scales is determined by the local stroma degradation, changes of τ have only moderate effects on the invasion dynamics (Figure 5A). The slower the tumor cells cycle, the more of them escape contact inhibition and start growing and expanding into the available space. In an analogous way, apoptosis of tumor cells at the invasion front with rate wapop is compensated by stimulated tumor cell proliferation and consequently has only moderate effects on the invasion dynamics (Figure 5A).
Increased Tumor Cell Motility Can Promote Collective Invasion
Although CD97 (EGF 1,2,5) overexpression in Widr does not induce a directed migration at the population boundary in vitro, we studied the impact of both elevated random and directed cell migration on tumor invasion assuming that the cell polarization required for directed migration is caused by specific tumor-stroma interactions. In computer simulations, we found that elevated random migration has negligible effects on invasion (Figure 5A). Assuming an orientation of the migration at the tumor invasion front causes a front morphology characterized by scattered cells and protruding cell clusters (Figure 5E). Thus, in case of proteolytically active tumor cells, directed migration increases the degradation capacity of the tumor by increasing the tumor-stroma interface and consequently strongly promotes invasion (Figure 5, A and E).
On the Origin of CD97 Expression in Vivo
The studies presented above demonstrate that all CD97 (EGF 1,2,5)-induced effects can promote invasion. It remains an open question, whether the observed CD97 staining in colorectal carcinomas results from single cell mutations or an environmental regulation process.
Clones of Highly Motile Cells Expand Rapidly
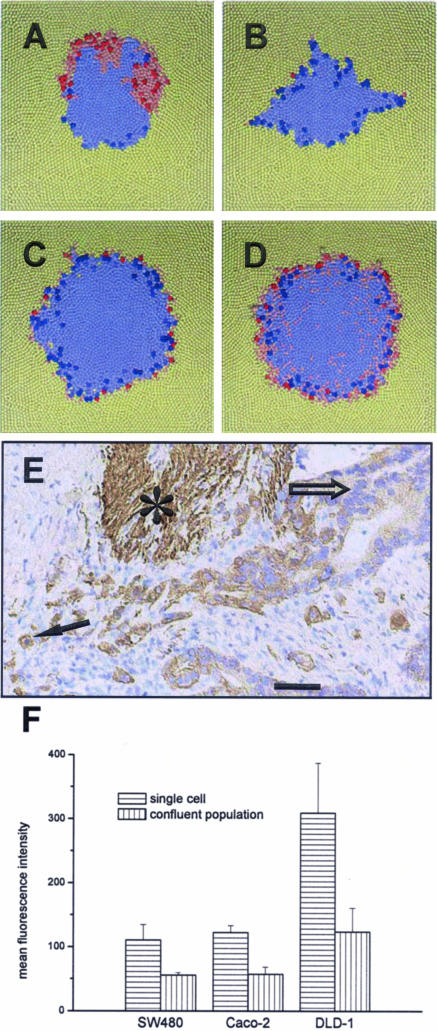
To mimic a mutation of selected tumor cells independent of their environment, we assumed that these cells are characterized by a modified parameter set. Focusing on cell migration we assumed for mutated cells a four times increased migration coefficient and directed migration induced by tumor-stroma interactions. Remarkably, the clone of a single mutated cell at the tumor front can spread within a short time throughout the front (Figure 6A). Computer simulations showed that although mutated cells that bud into the stroma rarely proliferate, these cells immediately start efficiently cycling if they become encapsulated by other tumor cells. We found that the clones of mutated cells spread faster than those of unaltered cells. Thus, they determine the entire tumor with time. Such a conversion was not found for tumor cells strongly stained for CD97. Consequently, we introduced an alternative approach.
Figure 6.
On the origin of increased CD97 expression in scattered tumor cells at the invasion front of colorectal carcinoma. Top view on computer model systems after an invasion time of 25 reference cell growth times of 21 hours. Altered cells (red) are characterized by a four times increased migration coefficient and a directed migration at the tumor invasion front. Saturated color indicates imminent cell division. A: Invasion after a mutation (stable alteration) of a single cell. The clone of that cell has spread partially throughout the tumor front. B–D: Invasion considering regulated alteration. Tumor cells become altered if the number of tumor cell neighbors falls below two (B), four (C), and six (D), respectively. Accordingly, altered cells occur at the tumor front. This corresponds to the expression of CD97 found in colorectal carcinoma (E). Stronger expression of CD97 is found in scattered tumor cells or tumor cell groups (arrow) surrounded by stroma compared with tumor cells located in tumor glands or solid tumor trabecula (open arrow). Smooth muscle cells (asterisk) also express CD97.43 F: CD97 expression in colorectal carcinoma cell lines, here shown for SW480, Caco-2, and DLD-1 cells, forming confluent layers is significantly decreased compared with that in single cell cultures of the same cell type as shown by flow cytometry (mean ± SEM, n = 3). The result does not depend on absolute CD97 expression levels. Box height, 800 μm (A–D). Scale bar = 50 μm.
Environmentally Regulated Tumor Cell Motility
We assumed that all tumor cells are regulated by their local environment. We modeled such regulation assuming the parameter set of each tumor cell to reversibly depend on the number of its tumor cell neighbors. We introduce a threshold number of tumor cell neighbors below which the parameter set of the tumor cell changes; ie, we assume a density-dependent regulation. We again focused on altered migration. In a series of simulations, we observed that altered migration (four times increased migration coefficient, directed migration) as mutated migration significantly strengthens invasion. Interestingly, we again found that altered cells at the tumor front rarely proliferate. The assumed environmental regulation generates an invasion front where only front cells exhibit altered cell properties (Figure 6, B–D). For a threshold number of approximately four to six in a layer, this distribution approaches that observed in colorectal carcinomas for cells strongly stained for CD97 (Figure 6E). This suggests a related mechanism in vivo.
CD97 (EGF 1,2,5) in Tumor Cell Lines Is Density Regulated
To confirm our hypothesis, we examined CD97 expression in wild-type colorectal carcinoma cell lines during their growth to confluence by flow cytometry. We found that in all cell types CD97 expression was significantly reduced in cells forming a confluent layer compared with nonconfluent, single cell cultures (Figure 6F). This result was independent of the absolute expression levels of CD97. This indicates that CD97 is regulated in a density-dependent manner, at least in vitro.
Discussion
We demonstrated a migration- and tumor growth-promoting effect of CD97. However, this effect was seen only for CD97 (EGF 1,2,5), not for CD97 (EGF 1-5) or the C-terminally truncated CD97 (EGF 1,2,5). The specificity of the regulation suggests that the extracellular as well as the transmembrane domains and/or C-terminal region of the protein are involved. Regarding the scid mice experiments, our results exclude vascularization as a promoting factor of tumor growth, which we found most pronounced for CD97 (EGF 1-5), in agreement with Wang and co-workers.26 The observed stimulation of random cell motility by CD97 (EGF 1,2,5) may explain why the molecule promotes cell migration and invasion in Boyden chamber assays as found by us.17 We expect comparable results for any common chemoattractant of the considered colorectal tumor cells.
In several studies tumor budding, ie, the occurrence of scattered tumor cells at the tumor invasion front or host interface, was discussed as prognostic marker for reduced overall survival.27,28 Here, we demonstrate that budding in carcinoma is a consequence of locally directed migration of highly motile tumor cells. Notably, the weak proliferation found in scattered tumor cells in colorectal carcinoma7,29 and skin cancer30 is reproduced by our model without additional assumptions. In the model, it arises as a consequence of the high motility of these cells modifying the cell-environment interaction and thus affecting cell compression. Our simulations demonstrate that the budding process does not require stable, long-range chemogradients. However, CD97-related budding requires stroma signaling to induce a directed migration at the tumor front. In colorectal carcinoma, the CD97 ligand CD5531,32 or non-CD97-specific ligands as accumulating collagen I6 may act as trigger. Note that this not implies a strong adhesive tumor-stroma interaction as often assumed to be required for invasion.13 In the case that long-range chemogradients arise in the stroma, the highly motile scattered cells may very efficiently follow them and spread to distant places.33 Thus, these cells in principle indicate a high risk of metastasis and postoperative recurrence. According to the density-dependent regulation, one would expect that metastases of colorectal carcinomas show the same staining of CD97 as the primary tumor as found for other molecules.6,7
The proteolytic activity of tumor cells was frequently used as a marker to predict tumor recurrence in several types of tumors.34,35 Here, we demonstrated that in spatially homogeneous carcinoma increased proteolytic activity promotes tumor expansion. However, we expect that an imbalance in the quantitative expression of the proteases rather than an overall change of their expression may affect prognosis. In this case, tumor invasion could be hampered by nondegradable stroma components that accumulate at the tumor front. As a consequence the tumor cells would be forced to adapt their invasion mechanisms.36
Previous studies in several carcinomas have reported that an increased rate of proliferation is correlated with a less favorable prognosis.37,38 However, low proliferation was also demonstrated to indicate reduced response to treatment, suggesting that tumors with elevated proliferation would have better response.39 Moreover, according to recent studies,40,41 in colorectal carcinoma a high Ki-67 value even constitutes a prognostic marker for low recurrence. We here provide an explanation for this apparent contradiction demonstrating that during proteolytic invasion the fraction of cycling cells depends on the balance between tumor cell proliferation, apoptosis, and stroma degradation. Thus, for fixed degradation rates a large fraction refers either to slow cycling times of the tumor cells or to their apoptosis. Consequently, in this specific case a high fraction of proliferating tumor cells signals a low capability to spread to distant places or a good response to treatment in agreement with results in colorectal carcinoma.40,41 The finding of poor prognosis37,38 may be related to an increased proteolytic activity of the tumor.
Applying our IBM, we derived a number of predictions about the impact of individual cell properties on tumor invasion dynamics. Assuming reasonable values for the tumor-stroma interaction parameters, these predictions regard the qualitative behavior. Quantitative models require a detailed specification of the heterogeneous stroma. This will be part of future model extensions. In summary, our experimental and theoretical results give a straight forward explanation why cells strongly stained for CD97 scatter at the invasion front of colorectal carcinoma and why the occurrence of these cells is related to poor prognosis. The higher expression of CD97 at the invasion front found in nearly half of colorectal carcinomas is suggested to be a result of an environmentally regulated, reversible expression of that molecule. More generally, our simulation results suggest that proteolytic activity at the tumor front in conjunction with elevated and directed cell motility are key steps to aggressive tumor invasion. High tumor cell proliferation rates in this context are not the cause but a consequence of invasion. Our findings may have major implications for the understanding of tumor invasion and prognostic factor analysis.
Supplementary Material
Acknowledgments
We thank J. Hamann (Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands) for providing the CD97 (1,2,5/TM1) plasmid and the CLB-CD97/3 mAb; and U. Schumacher and S. Feldhaus (Institute of Anatomy, University Hospital Eppendorf, Hamburg, Germany) for first screening experiments with scid mice.
Footnotes
Address reprint requests to Joerg Galle, IZBI, Haertelstr. 16-18, 4107 Leipzig Germany. E-mail: galle@izbi.uni-leipzig.de.
Supported by the Deutsche Forschungsgemeinschaft (DFG; project AU 132/3-1 and grant BIZ-6 1/1 to J.G.) and by the Interdisciplinary Center of Clinical Research at the Faculty of Medicine of the University Leipzig (project D6).
E.W. is a fellow of the Interdisciplinary Center of Clinical Research Leipzig.
Supplementary material for this article can be found on http://ajp.amjpathol.org.
References
- Thiery JP. Epithelial-mesenchymal transitions in tumor progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Bates RC, Mercurio AM. The epithelial-mesenchymal transition (EMT) and colorectal cancer progression. Cancer Biol Ther. 2005;4:365–370. doi: 10.4161/cbt.4.4.1655. [DOI] [PubMed] [Google Scholar]
- LaGamba D, Nawshad A, Hay ED. Microarray analysis of gene expression during epithelial-mesenchymal transformation. Dev Dyn. 2005;234:132–142. doi: 10.1002/dvdy.20489. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- Bates RC, DeLeo MJ, III, Mercurio AM. The epithelial-mesenchymal transition of colon carcinoma involves expression of IL-8 and CXCR-1-mediated chemotaxis. Exp Cell Res. 2004;299:315–324. doi: 10.1016/j.yexcr.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Brabletz T, Spaderna S, Kolb J, Hlubek F, Faller G, Bruns CJ, Jung A, Nentwich J, Duluc I, Domon-Dell C, Kirchner T, Freund JN. Down-regulation of the homeodomain factor Cdx2 in colorectal cancer by collagen type I: an active role for the tumor environment in malignant tumor progression. Cancer Res. 2004;64:6973–6977. doi: 10.1158/0008-5472.CAN-04-1132. [DOI] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA. 2001;98:10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Goswami S, Sahai E, Wyckoff JB, Segall JE, Condeelis JS. Tumor cells caught in the act of invading: their strategy for enhanced cell motility. Trends Cell Biol. 2005;15:138–145. doi: 10.1016/j.tcb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Galle J, Loeffler M, Drasdo D. Modelling the effect of deregulated proliferation and apoptosis on the growth dynamics of epithelial cell populations in vitro. Biophys J. 2005;88:62–75. doi: 10.1529/biophysj.104.041459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galle J, Aust G, Schaller G, Beyer T, Drasdo D. Individual cell-based models of the spatio-temporal organisation of multicellular systems—achievements and limitations. Cytometry A. 2006;69:704–710. doi: 10.1002/cyto.a.20287. [DOI] [PubMed] [Google Scholar]
- Schaller G, Meyer-Hermann M. Multicellular tumor spheroid in an off-lattice Voronoi/Delaunay cell model. Phys Rev E. 2005;71:051910. doi: 10.1103/PhysRevE.71.051910. [DOI] [PubMed] [Google Scholar]
- Drasdo D, Höhme S. Individual-based approaches to birth and death in avascular tumors. Math Comput Model. 2003;37:1163–1175. [Google Scholar]
- Alacron T, Byrne HM, Maini PK. A cellular automaton model for tumour growth in inhomogeneous environment. J Theor Biol. 2003;225:257–274. doi: 10.1016/s0022-5193(03)00244-3. [DOI] [PubMed] [Google Scholar]
- Turner S, Sherratt JA. Intercellular adhesion and cancer invasion: a discrete simulation using the extended Potts model. J Theor Biol. 2002;216:85–100. doi: 10.1006/jtbi.2001.2522. [DOI] [PubMed] [Google Scholar]
- Kwakkenbos MJ, Kop EN, Stacey M, Matmati M, Gordon S, Lin HH, Hamann J. The EGF-TM7 family: a postgenomic view. Immunogenetics. 2004;55:655–666. doi: 10.1007/s00251-003-0625-2. [DOI] [PubMed] [Google Scholar]
- Bjarnadottir TK, Fredriksson R, Hoglund PJ, Gloriam DE, Lagerstrom MC, Schioth HB. The human and mouse repertoire of the adhesion family of G-protein-coupled receptors. Genomics. 2004;84:23–33. doi: 10.1016/j.ygeno.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Steinert M, Wobus M, Boltze C, Schutz A, Wahlbuhl M, Hamann J, Aust G. Expression and regulation of CD97 in colorectal carcinoma cell lines and tumor tissues. Am J Pathol. 2002;161:1657–1667. doi: 10.1016/S0002-9440(10)64443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A, Laue S, Rost AK, Scherbaum WA, Aust G. mRNA levels of membrane-type 1 matrix metalloproteinase (MT1-MMP), MMP-2, and MMP-9 and of their inhibitors TIMP-2 and TIMP-3 in normal thyrocytes and thyroid carcinoma cell lines. Thyroid. 1998;8:203–214. doi: 10.1089/thy.1998.8.203. [DOI] [PubMed] [Google Scholar]
- Aust G, Hofmann A, Laue S, Rost A, Kohler T, Scherbaum WA. Human thyroid carcinoma cell lines and normal thyrocytes: expression and regulation of matrix metalloproteinase-1 and tissue matrix metalloproteinase inhibitor-1 messenger-RNA and protein. Thyroid. 1997;7:713–724. doi: 10.1089/thy.1997.7.713. [DOI] [PubMed] [Google Scholar]
- de Jong JS, van Diest PJ, Baak JP. Hot spot microvessel density and the mitotic activity index are strong additional prognostic indicators in invasive breast cancer. Histopathology. 2000;36:306–312. doi: 10.1046/j.1365-2559.2000.00850.x. [DOI] [PubMed] [Google Scholar]
- Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- Rubin H. Selected cell and selective microenvironment in neoplastic development. Cancer Res. 2001;61:799–807. [PubMed] [Google Scholar]
- Etienne-Manneville S. Cdc42—the centre of polarity. J Cell Sci. 2004;117:1291–1300. doi: 10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]
- Trosko JE, Ruch RJ. Cell-cell communication in carcinogenesis. Front Biosci. 1998;3:d208–d236. doi: 10.2741/a275. [DOI] [PubMed] [Google Scholar]
- Helmlinger G, Netti PA, Lichtenbeld HC, Melder RJ, Jain RK. Solid stress inhibits the growth of multicellular tumor spheroids. Nat Biotechnol. 1997;15:778–783. doi: 10.1038/nbt0897-778. [DOI] [PubMed] [Google Scholar]
- Wang T, Ward Y, Tian L, Lake R, Guedez L, Stetler-Stevenson WG, Kelly K. CD97, an adhesion receptor on inflammatory cells, stimulates angiogenesis through binding integrin counterreceptors on endothelial cells. Blood. 2005;105:2836–2844. doi: 10.1182/blood-2004-07-2878. [DOI] [PubMed] [Google Scholar]
- Park KJ, Choi HJ, Roh MS, Kwon HC, Kim C. Intensity of tumor budding and its prognostic implications in invasive colon carcinoma. Dis Colon Rectum. 2005;48:1597–1602. doi: 10.1007/s10350-005-0060-6. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Ochiai A, Kato H, Tachimori Y, Igaki H, Hirohashi S. Clinicopathological significance of tumor nest configuration in patients with esophageal squamous cell carcinoma. Cancer. 2001;91:1114–1120. doi: 10.1002/1097-0142(20010315)91:6<1114::aid-cncr1107>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Palmqvist R, Rutegard JN, Bozoky B, Landberg G, Stenling R. Human colorectal cancers with an intact p16/cyclin D1/pRb pathway have up-regulated p16 expression and decreased proliferation in small invasive tumor clusters. Am J Pathol. 2000;157:1947–1953. doi: 10.1016/S0002-9440(10)64833-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson S, Nilsson K, Ringberg A, Landberg G. Invade or proliferate? Two contrasting events in malignant behavior governed by p16(INK4a) and an intact Rb pathway illustrated by a model system of basal cell carcinoma. Cancer Res. 2003;63:1737–1742. [PubMed] [Google Scholar]
- Li L, Spendlove I, Morgan J, Durrant LG. CD55 is over-expressed in the tumour environment. Br J Cancer. 2001;84:80–86. doi: 10.1054/bjoc.2000.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann J, Vogel B, van Schijndel GM, van Lier RA. The seven-span transmembrane receptor CD97 has a cellular ligand (CD55, DAF). J Exp Med. 1996;184:1185–1189. doi: 10.1084/jem.184.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J, Segall JE. Intravital imaging of cell movement in tumors. Nat Rev Cancer. 2003;3:921–930. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- Sato H, Takino T, Miyamori H. Roles of membrane-type matrix metalloproteinase-1 in tumor invasion and metastasis. Cancer Sci. 2005;96:212–217. doi: 10.1111/j.1349-7006.2005.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihinen P, Kahari VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer. 2002;99:157–166. doi: 10.1002/ijc.10329. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K. Tumor-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- Trihia H, Murray S, Price K, Gelber RD, Golouh R, Goldhirsch A, Coates AS, Collins J, Castiglione-Gertsch M, Gusterson BA. International Breast Cancer Study Group. Ki-67 expression in breast carcinoma: its association with grading systems, clinical parameters, and other prognostic factors—a surrogate marker? Cancer. 2003;97:1321–1331. doi: 10.1002/cncr.11188. [DOI] [PubMed] [Google Scholar]
- Fernandez-Figueras MT, Puig L, Musulen E, Gilaberte M, Ferrandiz C, Lerma E, Ariza A. Prognostic significance of p27Kip1, p45Skp2 and Ki67 expression profiles in Merkel cell carcinoma, extracutaneous small cell carcinoma, and cutaneous squamous cell carcinoma. Histopathology. 2005;46:614–621. doi: 10.1111/j.1365-2559.2005.02140.x. [DOI] [PubMed] [Google Scholar]
- Imdahl A, Jenkner J, Ihling C, Ruckauer K, Farthmann EH. Is MIB-1 proliferation index a predictor for response to neoadjuvant therapy in patients with esophageal cancer? Am J Surg. 2000;179:514–520. doi: 10.1016/s0002-9610(00)00386-x. [DOI] [PubMed] [Google Scholar]
- Allegra CJ, Paik S, Colangelo LH, Parr AL, Kirsch I, Kim G, Klein P, Johnston PG, Wolmark N, Wieand HS. Prognostic value of thymidylate synthase, Ki-67, and p53 in patients with Dukes’ B and C colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project collaborative study. J Clin Oncol. 2003;21:241–250. doi: 10.1200/JCO.2003.05.044. [DOI] [PubMed] [Google Scholar]
- Garrity MM, Burgart LJ, Mahoney MR, Windschitl HE, Salim M, Wiesenfeld M, Krook JE, Michalak JC, Goldberg RM, O’Connell MJ, Furth AF, Sargent DJ, Murphy LM, Hill E, Riehle DL, Meyers CH, Witzig TE. North Central Cancer Treatment Group: prognostic value of proliferation, apoptosis, defective DNA mismatch repair, and p53 overexpression in patients with resected Dukes’ B2 or C colon cancer: a North Central Cancer Treatment Group Study. J Clin Oncol. 2004;22:1572–1582. doi: 10.1200/JCO.2004.10.042. [DOI] [PubMed] [Google Scholar]
- Eszlinger M, Krohn K, Berger K, Lauter J, Kropf S, Beck M, Fuhrer D, Paschke R. Gene expression analysis reveals evidence for increased expression of cell cycle-associated genes and Gq-protein-protein kinase C signaling in cold thyroid nodules. J Clin Endocrinol Metab. 2005;90:1163–1170. doi: 10.1210/jc.2004-1242. [DOI] [PubMed] [Google Scholar]
- Aust G, Wandel E, Blotze C, Sittig D, Schutz A, Horn L-C, Wobus M. Diversity of CD97 in smooth muscle cells. Cell Tissue Res. 2006;324:139–147. doi: 10.1007/s00441-005-0103-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.