Abstract
In humans, lesions of contact eczema or atopic dermatitis can exhibit increases in epidermal nerves, but the mechanism resulting in such nerve elongation are not fully understood. We found that contact hypersensitivity reactions to oxazolone in mice were associated with significant increases in the length of nerves in the epidermis and dermis. Using genetically mast cell-deficient c-kit mutant mice selectively repaired of their dermal mast cell deficiency with either wild-type or tumor necrosis factor (TNF)-deficient mast cells, we found that mast cells, and mast cell-derived TNF, significantly contributed to the elongation of epidermal and dermal PGP 9.5+ nerves and dermal CGRP+ nerves, as well as to the inflammation observed at sites of contact hypersensitivity in response to oxazolone. Moreover, the percentage of mast cells in close proximity to dermal PGP 9.5+ nerve fibers was significantly higher in wild-type mice and in c-kit mutant mice repaired of their dermal mast cell deficiency by the adoptive transfer of wild-type mast cells than in TNF-deficient mice or in TNF−/− mast cell-engrafted c-kit mutant mice. These observations show that mast cells, and mast cell-derived TNF, can promote the elongation of cutaneous nerve fibers during contact hypersensitivity in the mouse.
Several lines of evidence indicate that sensory nerves can both be affected by and contribute to inflammatory responses, including those in the skin,1 the respiratory tract,2 and the gastrointestinal system.3–5 In the skin, cutaneous nerves are thought to participate importantly in the pathophysiology of a diverse spectrum of disorders, such as urticaria, contact hypersensitivity (CHS), atopic dermatitis (AD), and psoriasis.1 For example, the expression of certain CHS responses can be diminished in mice that have been depleted of cutaneous capsaicin-sensitive neurons.6 In addition, it has recently been reported that increases in sensory nerve fibers occur in both the epidermis and the dermis in a model of CHS in mice.7 Moreover, in humans, the length of epidermal nerve fibers can be significantly increased in patients with contact eczema8 or AD,9 and increases in both dermal and epidermal nerve fibers have been reported in other dermatological conditions as well, such as prurigo nodularis10 and notalgia paraesthetica.11
One resident cell of the dermis that may functionally interact with cutaneous nerves is the mast cell, and a number of findings suggest that interactions between mast cells and nerves may occur during certain skin disorders. For example, an increased number of instances of close proximity between sensory nerves and mast cells has been described in inflammatory skin lesions from patients with psoriasis12 or AD.13 In addition, dermal mast cells can be increased in number in several inflammatory dermatoses, including psoriasis and AD.14
In part because of their close anatomical association, it has been proposed that cutaneous sensory nerves and mast cells can represent a functional unit whereby stimulated nerve fibers may activate local mast cells, which in turn can control local nerve function.1,15 For example, neuropeptides that can be released by cutaneous nerves, such as calcitonin gene-related peptide (CGRP), substance P, vasoactive intestinal peptide, and somatostatin can induce mast cells to release histamine, tumor necrosis factor (TNF), and other inflammatory mediators.16–21 Afferent nerves express specific receptors for neuropeptides,22 prostaglandins,23 histamine,24 proteases,25 neurotrophins,26 and cytokines,27,28 and mast cells can also respond to many of these products.1,2,15,29,30 Moreover, certain adhesion molecules, such as N-cadherin31 and spermatogenic Ig superfamily/synaptic cell adhesion molecule,32 have been shown to be important in mediating interactions between nerves and mast cells.
These findings, and other evidence, suggest that potentially significant interactions may occur between mast cells and cutaneous nerves during a variety of skin disorders. Remarkably, however, relatively few studies have assessed to what extent such potential mast cell-nerve interactions actually are important in influencing specific features of individual cutaneous responses or defined mechanisms that might account for significant interactions between these two cell types. Therefore, we investigated whether a CHS reaction that has been shown in the mouse to be partly mast cell-dependent can be associated with significant changes in the length of epidermal or dermal nerve fibers and, if so, whether mast cells, and mast cell-derived TNF, can promote nerve fiber elongation in that setting.
There are several reasons why we chose this approach to test the hypothesis that mast cells, and mast cell-derived TNF, might contribute to elongation of dermal nerves during inflammatory responses. First, as noted above, the length of epidermal nerve fibers can be significantly increased in patients with contact eczema8 or AD,9 disorders in which mast cells have been implicated. Second, mast cells also are required for optimal expression of certain models of CHS in mice.33,34 Third, mast cells have been identified as an important potential source of TNF,35,36 and mast cell-derived TNF can contribute to the expression of certain specific features of CHS responses in mice, including tissue swelling and infiltration of polymorphonuclear leukocytes (PMNs).37,38 Finally, even though the effects of TNF on nerve cell biology are complex,39–43 TNF has been reported to have some effects that could contribute to elongation or functional properties of peripheral nerves.39,44
Using genetically mast cell-deficient c-kit mutant mice repaired of their dermal mast cell deficiency by the adoptive transfer of either wild-type or TNF-deficient mast cells, we found that a model of oxazolone (OX)-induced CHS in the mouse was indeed associated with an elongation of cutaneous nerves at the sites of hapten challenge and that mast cells, and mast cell-derived TNF, were required for optimal nerve elongation in this setting.
Materials and Methods
Mice
C57BL/6-TNF−/− mice were originally generated using C57BL/6J ES cells as described elsewhere.45 Mast cell-deficient KitW-sh/W-sh mice on the C57BL/6 background were generously provided by Dr. Peter Besmer (Memorial Sloan-Kettering Cancer Center, New York, NY).46 C57BL/6J mice were obtained from The Jackson Laboratories (Bar Harbor, ME). Female 6- to 8-week-old mice were used for all experiments. All mice were housed at the Animal Care Facilities at Stanford University Medical Center (Stanford, CA); were kept under standard temperature, humidity, and timed lighting conditions; and were provided mouse chow and water ad libitum. All experiments were performed in compliance with the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources, National Research Council, and published by the National Academy Press (revised 1996) and with the approval of the Stanford University Committee on Animal Welfare.
OX-Induced CHS
Mice were sensitized with 100 μl of 2% 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one (oxazolone; OX) (Sigma, St. Louis, MO) in ethanol to the shaved abdomen. Five days after sensitization with OX, mice were challenged with a total of 20 μl of vehicle (ethanol) alone to the right ear (10 μl to each side) and 1% OX in ethanol to the left ear (10 μl to each side). Ear thickness was measured before and at multiple intervals after OX challenge with an engineer’s microcaliper (Ozaki Mfg. Co., Ltd., Itabashi, Tokyo, Japan) at the time points indicated. Mice were sacrificed by CO2 inhalation 48 hours after OX challenge for immunohistological analysis of their ears to quantify numbers of mast cells and neutrophils (polymorphonuclear leukocytes, PMNs) (in 4-μm-thick sections) and length of nerve fibers (in 14-μm-thick sections) in ear skin.
Preparation of Bone Marrow-Derived, Cultured Mast Cells and Local Mast Cell Engraftment of Mast Cell-Deficient Mice
Bone marrow-derived, cultured mast cells (BMCMCs) were obtained by culturing bone marrow cells of C57BL/6J mice in WEHI-3 conditioned medium (containing interleukin-3) for 4 to 6 weeks, at which time >98% of the cells were identified as mast cells by Toluidine blue staining and by flow cytometry analysis for c-Kit and FcɛRI. For mast cell engraftment studies, BMCMCs (1.0 × 106 cells in 20 μl/ear) were injected intradermally into the dorsal surface of the center of the pinnae of 7-week-old KitW-sh/W-sh mice. Nine to 10 weeks after injection, the mice were used for CHS experiments, and the numbers and distribution of mast cells in the ear skin were assessed in Toluidine blue-stained sections obtained at 48 hours after vehicle or OX challenge. The suitability of such mast cell-engrafted KitW-sh/W-sh mice for studies of mast cell development and function has been described in detail.47
Immunohistochemistry and Measurement of Nerve Fiber Length
Immediately after sacrificing the mice as described above, naïve, OX-sensitized and challenged, or OX-sensitized and vehicle-challenged mice were fixed by intracardiac perfusion with a mixture of 4% paraformaldehyde and 14% saturated picric acid. Then, the ear skin was harvested and embedded in OCT compound as described elsewhere.48 For visualization of nerve fibers, cryostat sections (14 μm) were incubated with polyclonal rabbit anti-protein gene product 9.5 (PGP 9.5) IgG (Biogenesis, Poole, UK) or rabbit anti-calcitonin gene-related peptide (CGRP) IgG (Chemicon, Temcula, CA), or, as a control, rabbit IgG (Jackson ImmunoResearch, West Grove, PA) at room temperature overnight and then incubated with tetramethyl-rhodamine isothiocyanate (TRITC)-conjugated goat anti-rabbit IgG F(ab′)2 fragments (Jackson ImmunoResearch) as described elsewhere.49 For mast cell detection, sections then were incubated with the fluorescein isothiocyanate (FITC)-labeled avidin (Vector Laboratories, Burlingame, CA) in phosphate-buffered saline (PBS) for 1 hour at room temperature.
To detect and quantify close proximity between mast cells and nerve fibers, sections were incubated with a mixture of the FITC- and TRITC-labeled avidins. Sections were rinsed in PBS and mounted in fluorescent mounting medium (DAKO, Carpinteria, CA). This approach is useful for analyzing the proximity between nerve fibers and mast cells because PGP 9.5+ or CGRP+ nerve fibers are labeled with TRITC, and mast cells are identified with a mixture of FITC- and TRITC-labeled avidins, permitting the visualization of mast cells in two colors. This method takes advantage of the fact that avidin binds strongly to the highly charged glycosaminoglycans, and perhaps other constituents, of mast cell granules.49 FITC is used to identify mast cells alone because only mast cells are FITC-labeled, and then TRITC is used to visualize the proximity of these mast cells to TRITC-labeled nerve fibers in the same section.
The sections were assessed at ×400 magnification under an Olympus fluorescence microscope (Tokyo, Japan). Using NIH Image 1.63 software (Bethesda, MD), the length of profiles of single nerve fibers was measured in central areas of the ear pinna sections. The areas of ear pinna skin, dermis, and epidermis (mean ± SEM) analyzed in the various groups were as follows: naïve mice = 0.222 ± 0.012 mm2 of skin (consisting of 0.177 ± 0.010 mm2 of dermis and 0.042 ± 0.002 mm2 of epidermis); OX-sensitized, OX-challenged mice = 0.275 ± 0.013 mm2 of skin (consisting of 0.230 ± 0.012 mm2 of dermis and 0.049 ± 0.003 mm2 of epidermis); and OX-sensitized, vehicle-challenged mice = 0.254 ± 0.009 mm2 of skin (consisting of 0.212 ± 0.009 mm2 of dermis and 0.042 ± 0.002 mm2 of epidermis). The results are expressed as length of nerve fibers per mm of cartilage in the ear skin sections. The numbers of mast cells (and in some sections, PMNs) were also counted in the same areas of dermis, and the numbers of mast cells in close proximity to nerve fibers (defined as <2 μm) were recorded separately.
Statistics
The Student’s t-test (two-tailed) or, for analysis of groups of data that were not normally distributed, the Mann-Whitney U-test (two-tailed), were used for statistical evaluation of the results. Unless otherwise specified, all results are presented as mean + SEM.
Results
OX-Induced CHS Is Associated with Elongation of Cutaneous Nerves
CHS is a classic model of immune-mediated dermatitis in the mouse.50 For this study, we used a model of OX-induced CHS in which we and colleagues have demonstrated that mast cells are required for optimal expression of the response.34 Mice were sensitized with 2% OX in ethanol and then challenged with 1% OX in ethanol or vehicle alone. As shown in Figure 1A, OX challenge of OX-sensitized mice resulted in a marked increase in ear swelling, with the peak of the response at 24 hours after challenge with OX; by contrast, no significant increase in ear swelling was observed after challenge with vehicle alone in OX-sensitized mice. Substantial infiltration of PMNs also could be detected in the OX-challenged skin of OX-sensitized mice, even though the 48-hour time point was probably past the peak of that component of the response, but PMN infiltration was not observed in the vehicle-treated skin of such mice (data not shown).
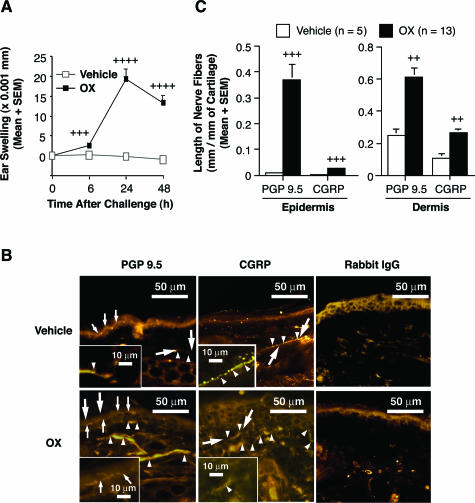
Figure 1.
A: Tissue-swelling responses to vehicle (open squares) or OX (closed squares) in C57BL/6 wild-type mice that had been sensitized to express CHS responses. B: Immunohistochemical staining with rabbit anti-PGP 9.5 IgG (PGP 9.5), rabbit anti-CGRP IgG (CGRP), or control IgG (rabbit IgG) in vehicle-treated ear skin (vehicle) or in OX-challenged ear skin (OX) of OX-sensitized mice at 48 hours after challenge. Large arrows in four of the panels indicate the areas that are shown at higher magnification in the insets; arrowheads and small arrows indicate profiles of dermal and epidermal nerve fibers, respectively, stained with antibodies against PGP 9.5 or CGRP. C: The length of PGP 9.5-positive or CGRP-positive nerve fibers in epidermis or dermis, respectively, in vehicle-treated ears (open columns) or OX-challenged ears (closed columns) of OX-sensitized mice 48 hours after challenge. In A and C, data are the pooled results from three independent experiments, which gave similar results (vehicle: n = 5, OX; n = 13). In A, +++, ++++ = P < 0.001, P < 0.0001 versus corresponding values for vehicle-treated ears; In C, ++, +++ = P < 0.01, P < 0.001 versus corresponding values for vehicle-treated ears (Student’s t-test, two-tailed). Original magnifications, ×400.
In the skin lesions of patients with AD, the length of sensory nerve fibers can increase, with some of them extending from the dermis into the epidermis.9 Similar findings recently have been reported in a model of contact allergy in mice.7 We therefore assessed the length of nerve fibers in the skin during OX-induced CHS responses. To detect nerve fibers, ear skin sections from OX- or vehicle-challenged OX-sensitized mice were stained with anti-PGP 9.5 Ab51 or with anti-CGRP Ab (to detect cutaneous nerve fibers that contain the neuropeptide, CGRP52) (Figure 1B). As has been reported by others,53,54 we found that the immunohistochemical staining of cutaneous CGRP-immunoreactive nerves revealed areas of variable staining intensity of individual CGRP+ nerve fibers (eg, see Figure 1B, inset of CGRP+ fibers in vehicle-challenged skin).
The length of both PGP 9.5+ and CGRP+ nerve fibers was significantly increased in both the epidermis and dermis in the OX-challenged skin at 48 hours after challenge compared with corresponding values in the vehicle-challenged skin (Figure 1C). These results show that this model of CHS in the mouse is associated with a significant increase in the length of nerve fibers both in the dermis, and, even more strikingly, in the epidermis. As has been reported by others based on analyses of human skin51 or mouse skin,54 we found that the length of nerves that stained with the antibody for the pan-neuronal marker, PGP 9.5 was greater than that for nerves that stained with the antibody for CGRP, which identifies only a subset of sensory nerves in the skin.
Mast Cells and Mast Cell-Derived TNF Are Required for Optimal Expression of OX-Induced CHS
Microscopic examination indicated that the numbers (Figure 2B) and distribution of mast cells in the central portion of the ear pinna dermis of wild-type or TNF−/− mice, or in wild-type- or TNF−/− BMCMC-engrafted KitW-sh/W-sh mice, were similar whereas the KitW-sh/W-sh mice remained devoid of dermal mast cells at sites of OX or vehicle challenge. Moreover, in confirmation of previously published data from our experiments with KitW/W-v mice on the WBxB6F1 background,34 we found that mast cells were required for optimal expression of the hapten-induced tissue swelling (Figure 2A) and PMN infiltration (Figure 2C) in this model of CHS, as assessed by comparing the responses of C57BL/6J wild-type mice, genetically mast cell-deficient C57BL/6J-KitW-sh/W-sh mice, and C57BL/6J-KitW-sh/W-sh mice that had been engrafted with in vitro-derived mast cells of C57BL/6J wild-type origin.
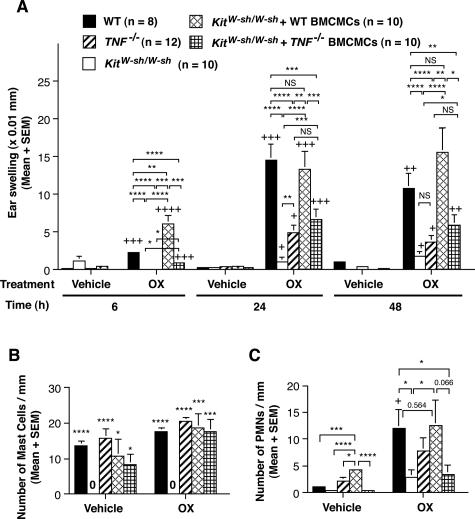
Figure 2.
A: Tissue swelling responses to vehicle or OX in OX-sensitized C57BL/6 wild-type mice (n = 8), TNF−/− mice (n = 12), mast cell-deficient KitW-sh/W-sh mice (n = 10), and KitW-sh/W-sh mice that had been selectively repaired of their mast cell deficiency by the intradermal injection of wild-type (n = 10) or TNF−/− (n = 10) BMCMCs. +, ++, +++, ++++ = P < 0.05, P < 0.01, P < 0.001, P < 0.0001 versus corresponding values for vehicle-treated ears; *, **, ***, **** = P < 0.05, P < 0.01, P < 0.001, P < 0.0001 for the comparisons indicated by brackets. NS, not significant (P > 0.05). B and C: Numbers of mast cells (B) and PMNs (C) in ear pinna dermis from vehicle-treated or OX-challenged ears (48 hours). In B, C57BL/6 wild-type mice (vehicle, n = 5; OX, n = 14), TNF−/− mice (vehicle, n = 5; OX, n = 10), mast cell-deficient KitW-sh/W-sh mice (vehicle, n = 5; OX, n = 10), and KitW-sh/W-sh mice that had been selectively repaired of their mast cell deficiency by the intradermal injection of wild-type (vehicle, n = 5; OX, n = 10) or TNF−/− (vehicle, n = 5; OX, n = 10) BMCMCs. *, ***, **** = P < 0.05, P < 0.001, P < 0.0001 versus values for corresponding vehicle- or OX-treated ears in KitW-sh/W-sh mice. In C, C57BL/6 wild-type mice (vehicle, n = 5; OX, n = 9), TNF−/− mice (vehicle, n = 5; OX, n = 10), mast cell-deficient KitW-sh/W-sh mice (vehicle, n = 5; OX, n = 8), and KitW-sh/W-sh mice that had been selectively repaired of their mast cell deficiency by the intradermal injection of wild-type (vehicle, n = 5; OX, n = 9) or TNF−/− (vehicle, n = 5; OX, n = 10) BMCMCs. + = P < 0.05 versus corresponding values for vehicle-treated ears; *, ***, **** = P < 0.05, P < 0.001, P < 0.0001 for the comparisons indicated by brackets. Data in A–C are the pooled results from two or three independent experiments, which gave similar results. A, B: Student’s t-test (two-tailed); C: Mann-Whitney U-test (two-tailed).
However, the tissue swelling and PMN infiltration associated with such CHS responses were reduced in C57BL/6J-TNF−/− mice or in C57BL/6J-KitW-sh/W-sh mice that had been engrafted with in vitro-derived mast cells of C57BL/6J-TNF−/− origin, compared with the responses in wild-type mice or in C57BL/6J-KitW-sh/W-sh mice that had been engrafted with in vitro-derived mast cells of C57BL/6J wild-type origin (Figure 2, A and C). The differences in the CHS responses of C57BL/6J-KitW-sh/W-sh mice that had been engrafted with in vitro-derived mast cells of C57BL/6J-TNF−/− or C57BL/6J-wild-type origin could not be attributed to differences in the numbers of mast cells that had successfully engrafted in the dermis of these mice because the numbers of mast cells/mm2 of dermis in the two groups was nearly identical (Figure 2B). Likewise, C57BL/6J-TNF−/− mice exhibited relatively weak CHS responses (Figure 2, A and C), even though the dermis of these mice contained slightly (although not significantly) higher numbers of mast cells/mm2 of dermis than did the dermis of the corresponding wild-type mice (Figure 2B). Our results thus confirm, in a different model of CHS, the findings of Biedermann and colleagues37 and Suto and colleagues,38 indicating that mast cell-derived TNF can contribute to PMN recruitment during CHS in the mouse.
Mast Cells and Mast Cell-Derived TNF Contribute to Nerve Fiber Elongation in CHS Responses
Next, we assessed the roles of mast cells, and mast cell-derived TNF, in the elongation of nerve fibers that occurred during these OX-induced CHS responses. Sections of ear skin of naïve mice, or of ear skin obtained 48 hours after challenge with OX or vehicle, were stained with both avidin (FITC), to identify mast cells,55 and anti-PGP 9.5 (rhodamine) antibody or anti-CGRP (rhodamine) antibody, to identify CGRP-containing nerves51,52 (Figure 3, and data not shown), and the lengths of the PGP 9.5+ or CGRP+ nerve fibers in the epidermis and dermis were quantified (Figure 4).
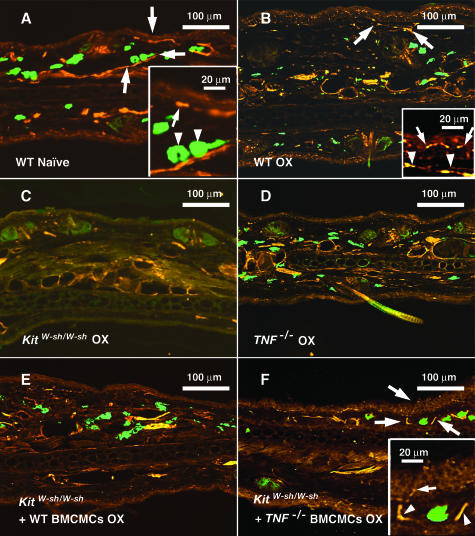
Figure 3.
A–F: Immunohistochemical staining of ear pinna from a naïve C57BL/6 wild-type mouse (A) and from the OX-challenged ears (B–F) (48 hours after challenge) of individual OX-sensitized C57BL/6 wild-type (B), mast cell-deficient KitW-sh/W-sh (C), TNF−/− (D), wild-type BMCMCs-engrafted KitW-sh/W-sh (E), and TNF−/− BMCMCs-engrafted KitW-sh/W-sh (F) mice. These mice are from the same groups shown in Figure 2. Nerve fibers were stained with rabbit anti-PGP 9.5 IgG and rhodamine anti-rabbit IgG, and mast cells were stained with FITC-conjugated avidin. Large arrows in A, B, and F indicate the areas that are depicted at higher magnification in the insets. In the inset in A, the arrow indicates a nerve profile near the dermal-epidermal junction, and the two arrowheads indicate two mast cells in close proximity to a dermal nerve. In the inset in B the two arrows indicate a nerve profile within the epidermis, and the two arrowheads indicate nerve profiles within the underlying dermis. In the inset in F, the arrow indicates a profile of a nerve fiber within the epidermis, and the two arrowheads indicate profiles of dermal nerves to the left and right of a mast cell (unlabeled).
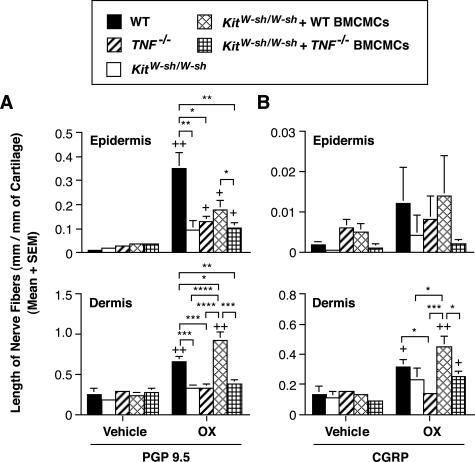
Figure 4.
A and B: The length of PGP 9.5-positive (A) or CGRP-positive (B) nerve fibers in ears examined 48 hours after hapten challenge in OX-sensitized C57BL/6 wild-type (vehicle, n = 5; OX, n = 10), TNF−/− mice (vehicle, n = 5; OX, n = 10), mast cell-deficient KitW-sh/W-sh mice (vehicle, n = 5; OX, n = 10), and KitW-sh/W-sh mice that had been selectively repaired of their mast cell deficiency by the intradermal injection of wild-type or TNF−/− BMCMCs (vehicle, n = 5; OX, n = 9 in each group). These mice are from the same groups shown in Figure 2. Data show the length of nerve fibers at 48 hours after OX or vehicle challenge in OX-sensitized mice. +, ++ = P < 0.05, P < 0.01 versus corresponding values for vehicle-treated ears; *, **, ***, **** = P < 0.05, P < 0.01, P < 0.001, P < 0.0001 for the comparisons indicated by brackets (Student’s t-test, two-tailed).
Consistent with our observations about the levels of ear swelling and neutrophil infiltration in this model of CHS, we found that the elongation of PGP 9.5+ nerve fibers in the epidermis and dermis that was detectable at 48 hours after OX challenge in this model of CHS was significantly decreased in mast cell-deficient KitW-sh/W-sh mice and in TNF−/− mice, compared with the corresponding values in wild-type mice (Figure 4A). Values for nerve length at sites of CHS in KitW-sh/W-sh mice that had been engrafted with wild-type mast cells, but not with TNF−/− mast cells, were significantly higher than those in the corresponding sites in mast cell-deficient KitW-sh/W-sh mice; in the case of the dermis, levels in wild-type mast cell-engrafted KitW-sh/W-sh mice were even higher than those in the wild-type mice (Figure 4A). Similar results were obtained when we quantified CGRP+ nerves, although the differences achieved statistical significance only in the case of dermal nerves (Figure 4B). These results indicate that mast cells and mast cell-derived TNF can contribute significantly to the elongation of nerve fibers in both the epidermis and the dermis during such OX-induced CHS responses.
CHS Is Associated with an Increased Frequency of Close Associations Between Mast Cells and Nerves
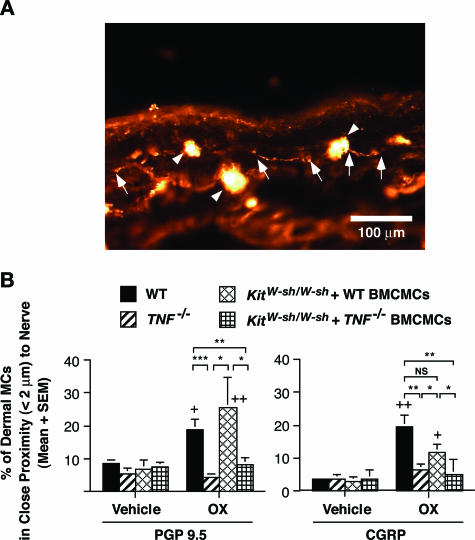
As shown in Figure 5A, we observed close associations between nerve fibers and mast cells in the dermis at sites of CHS in wild-type mice. When expressed as the percentage of dermal mast cells that occurred within <2 μm of the nearest nerve fiber, we found that the percentage of mast cells in close proximity with PGP 9.5+ and/or CGRP+ nerve fibers at 48 hours after OX or vehicle challenge was significantly increased in OX-challenged wild-type ear skin compared with vehicle-treated wild-type ear skin (Figure 5B). Notably, KitW-sh/W-sh mice engrafted with wild-type BMCMCs, but not with TNF−/− BMCMCs, also exhibited a significantly increased frequency of close associations of PGP 9.5+ and CGRP+ nerves and mast cells after OX challenge (Figure 5B). This was true even though the numbers of mast cells/mm2 of dermis were statistically indistinguishable in the assessed areas of dermis in the wild-type BMCMC- versus TNF−/− BMCMC-engrafted KitW-sh/W-sh mice (Figure 2B).
Figure 5.
A: Immunohistochemical staining of PGP 9.5+ nerves and mast cells in the ear pinna dermis 48 hours after hapten challenge in an OX-sensitized C57BL/6 wild-type mouse. Arrows indicate nerves, and arrowheads indicate mast cells. B: Percentage of mast cells in close proximity (<2 μm) to nerve fibers in ear pinna dermis 48 hours after hapten or vehicle challenge in OX-sensitized C57BL/6 wild-type mice (vehicle, n = 5; OX, n = 13), TNF−/− mice (vehicle, n = 5; OX, n = 9), and KitW-sh/W-sh mice (vehicle, n = 5; OX, n = 7), and in KitW-sh/W-sh mice that had been selectively repaired of their mast cell deficiency by the intradermal injection of wild-type or TNF−/− BMCMCs (vehicle, n = 5; OX, n = 7 in each group). These mice are from the same groups shown in Figure 2. Nerve fibers were stained with rabbit anti-PGP 9.5 IgG and rhodamine anti-rabbit IgG, and mast cells were stained with rhodamine- and FITC-conjugated avidin. Data in B are the pooled results from three independent experiments, which gave similar results. +, ++ = P < 0.05, P < 0.01 versus corresponding values for vehicle-treated ears; *, **, *** = P < 0.05, P < 0.01 or P < 0.001 for the comparisons indicated by brackets (Mann-Whitney U-test, two-tailed).
Discussion
We have shown that elongation of cutaneous nerves occurs during CHS reactions to OX in mice and that mast cells, and mast cell-derived TNF, are required for optimal development of this newly recognized feature of the CHS response to OX. The effects of mast cells, and mast cell-derived TNF, in CHS-associated skin nerve elongation achieved statistical significance for both PGP 9.5+ and CGRP+ nerves in the dermis and for PGP 9.5+ nerves in the epidermis. PGP 9.5 reactivity identifies all cutaneous nerves,51,56 whereas only a fraction of such nerves produce CGRP.52,56
Notably, the neuropeptide CGRP can induce degranulation of certain mast cell populations,16,19,20 including dermal mast cells in both humans16 and mice.19 Accordingly, CHS in response to OX represents a setting in which mast cells contribute to the elongation of nerves that can, in turn, produce a neuropeptide with the capacity to induce functional responses in dermal mast cells. Together with the evidence that nerve elongation in this model of CHS is associated with an increased proportion of dermal mast cells that occur in close proximity to cutaneous nerves, our findings support the hypothesis that functional interactions between nerves and mast cells can contribute to the pathophysiology of some of the other skin changes associated with CHS. However, verifying this hypothesis will require studies that can identify and characterize the effects of functional interactions between nerves and mast cells in such responses.
To our knowledge, this represents the first evidence derived from studies of genetically mast cell-deficient and mast cell-engrafted c-kit mutant mice showing that mast cells can contribute to nerve elongation in any tissue or disease model. Likewise, our observations are the first indicating that TNF is required for optimal nerve elongation in any setting in vivo.
However, it should be noted that the full extent of the c-kit-dependent differences between wild-type mice (C57BL/6-Kit+/+) and c-kit mutant C57BL/6-KitW-sh/W-sh mice is not yet completely understood, and some of these differences may influence nerve development and/or function. For example, it has been reported that the KIT ligand, stem cell factor, can induce outgrowth of KIT-positive neurites and can support the survival of KIT-positive neurons in the dorsal root ganglia of mouse embryos.57 Accordingly, it is possible that there are c-kit-dependent differences between the nerves of wild-type and C57BL/6-KitW-sh/W-sh mice that are unrelated to the profound mast cell deficiency of the C57BL/6-KitW-sh/W-sh mice. Nevertheless, our mast cell engraftment studies show that the abnormalities in nerve elongation observed during this model of CHS in C57BL/6-KitW-sh/W-sh mice can be largely corrected based on the contributions of adoptively transferred populations of wild-type, but not TNF-deficient, mast cells.
The mechanism(s) that promotes mast cell TNF production in CHS, and which links such mast cell TNF production with nerve elongation, remains to be fully defined. However, in speculating about the potential roles of TNF in this response, it is important to emphasize that prior work on the effects of TNF on nerve cell biology has identified apparently paradoxical effects of this cytokine. For example, there is evidence that TNF can either promote protection or induce death in particular nerve cells in various physiological and pathological conditions.43,58 Thus, TNF can enhance, through TNFR1 signaling, the nerve cell apoptosis induced by nerve growth factor (NGF) deprivation in vitro.41 Yet TNF also can promote proliferation of nerve cells such as oligodendrocytes through TNFR2-dependent, but not TNFR1-dependent, mechanisms,59 as well as protect against nerve cell apoptosis in vivo.42 Because both TNFR1 and TNFR2 are expressed on peripheral nerve cells,59 it is possible that mast cell-derived TNF has direct effects on nerves that can promote their elongation during CHS responses.
On the other hand, TNF also can induce NGF production by fibroblasts,39 suggesting an indirect mechanism that may contribute to mast cell- and TNF-dependent nerve elongation in CHS. We assessed additional potential mechanisms by which TNF might enhance NGF production but found no evidence by ELISA of NGF secretion by naïve, IgE-sensitized, or IgE+ antigen-stimulated mouse BMCMCs that had been maintained in vitro in the presence of various concentrations of (rm)TNF for 24 hours (Supplemental Figure 1A, see http://ajp.amjpathol.org)60 or 48 hours (data not shown). Moreover, bNGF mRNA was hardly detectable by RT-PCR in any of the BMCMCs tested (data not shown). As expected, we detected interleukin-6 production by the IgE/antigen-stimulated BMCMCs; however, the level of interleukin-6 secretion by BMCMCs was not influenced significantly by rmTNF. These results indicate that, at least under the in vitro conditions tested, exposure to TNF does not enhance NGF production by mouse mast cells. Quite different results were obtained when we tested keratinocytes derived from mouse ear skin. In keratinocytes incubated in vitro without TNF, NGF mRNA expression was below the detection limit by RT-PCR. By contrast, NGF mRNA expression was markedly increased in keratinocytes after TNF stimulation (Supplemental Figure 1B, see http://ajp.amjpathol.org). Based on these results, we speculate that mast cell-derived TNF might enhance NGF production by keratinocytes (our in vitro data), as well as by fibroblasts,61 during CHS responses and that keratinocyte- and/or fibroblast-derived NGF may in turn contribute to the nerve elongation observed in this setting.
We found that the frequency of close associations between nerves and mast cells increased in the dermis of normal mice or wild-type BMCMC-engrafted C57BL/6-KitW-sh/W-sh mice. Studies by others have shown that the frequency of close associations between mast cells and nerves can be significantly increased in inflammatory skin lesions from patients with psoriasis62 or palmoplantar pustulosis,63 as well as in the lesions in atherosclerotic coronary arteries.64 Whether mast cells can contribute to nerve elongation in these conditions, as well as in CHS in mice, remains to be determined.
However, in these settings, as well as in the CHS responses to OX analyzed in our study, it is difficult to exclude the possibility that the increased frequency of close associations between mast cells and nerve fibers reflects, at least in part, the increased numbers of nerve profiles and/or mast cells that can occur in such conditions. We made a similar point in a previous analysis of the close association of mast cells and nerves that can be observed in various anatomical sites in the rat: if two elements (eg, nerves and mast cells) occur together in a relatively small area, this by itself will favor, by chance alone, close associations between these two elements.5 For this reason, one should avoid drawing any conclusions about biological significance based on increased proximity of two elements in the absence of additional studies that can assess the functional significance of such increased proximity or that attempt to explain the association of the two cell types in mechanistic terms.
On the other hand, in wild-type mice, the frequency of close associations between mast cells and CGRP+ nerves in the dermis at sites of CHS was approximately fivefold that at the corresponding vehicle-challenged sites, whereas the ratio of nerve lengths in the same specimens was ∼2:1 (Figures 5B and 4B, respectively). Because numbers of dermal mast cells increased only slightly in the CHS reactions in wild-type mice versus the corresponding values for the vehicle-challenged sites (Figure 2B), these findings suggest that the CHS-associated increase in percentage of mast cells in close proximity to CGRP+ nerves in the dermis of wild-type mice was greater than would have been expected by chance alone. However, in the case of wild-type BMCMC-engrafted C57BL/6-KitW-sh/W-sh mice, the CHS-associated increase in the percentage of mast cells in close proximity to CGRP+ nerves in the dermis was approximately in proportion to the increased length of such nerves associated with the CHS responses. Whether the differences in the findings obtained for dermal mast cell/dermal CGRP+ nerve associations in the wild-type mice versus the wild-type BMCMC-engrafted C57BL/6-KitW-sh/W-sh mice are biologically significant is not clear.
Supplementary Material
Acknowledgments
We thank Peter Besmer (Molecular Biology Program, Memorial Sloan-Kettering Cancer Center and Cornell University Graduate School of Medical Sciences, New York, NY) for generously providing KitW-sh/W-sh mice on the C57BL/6 background, Jonathon D. Sedgwick (DNAX Research, Inc., Palo Alto, CA) for generously providing C57BL/6-TNF−/− mice, Eon Rios for technical assistance and suggestions, and members of the Galli laboratory for helpful discussions.
Footnotes
Address reprint requests to Stephen J. Galli, Department of Pathology, L-235, Stanford University School of Medicine, 300 Pasteur Dr., Stanford, CA 94305-5324. E-mail: sgalli@stanford.edu.
Supported by the National Institutes of Health (grants AI23990, CA72074, and HL67674, project 1 to S.J.G.) and the Uehara Memorial Foundation (fellowship to M.K.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Steinhoff M, Stander S, Seeliger S, Ansel JC, Schmelz M, Luger T. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol. 2003;139:1479–1488. doi: 10.1001/archderm.139.11.1479. [DOI] [PubMed] [Google Scholar]
- Bienenstock J, MacQueen G, Sestini P, Marshall JS, Stead RH, Perdue MH. Mast cell/nerve interactions in vitro and in vivo. Am Rev Respir Dis. 1991;143:S55–S58. doi: 10.1164/ajrccm/143.3_Pt_2.S55. [DOI] [PubMed] [Google Scholar]
- Stead RH, Tomioka M, Quinonez G, Simon GT, Felten SY, Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci USA. 1987;84:2975–2979. doi: 10.1073/pnas.84.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead RH, Dixon MF, Bramwell NH, Riddell RH, Bienenstock J. Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology. 1989;97:575–585. doi: 10.1016/0016-5085(89)90627-6. [DOI] [PubMed] [Google Scholar]
- Arizono N, Matsuda S, Hattori T, Kojima Y, Maeda T, Galli SJ. Anatomical variation in mast cell nerve associations in the rat small intestine, heart, lung, and skin. Similarities of distances between neural processes and mast cells, eosinophils, or plasma cells in the jejunal lamina propria. Lab Invest. 1990;62:626–634. [PubMed] [Google Scholar]
- Beresford L, Orange O, Bell EB, Miyan JA. Nerve fibres are required to evoke a contact sensitivity response in mice. Immunology. 2004;111:118–125. doi: 10.1111/j.1365-2567.2003.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Nour H, Lundeberg L, Boman A, Beck O, Harvima IT, Theodorsson E, Nordlind K. Study of innervation, sensory neuropeptides, and serotonin in murine contact allergic skin. Immunopharmacol Immunotoxicol. 2005;27:67–76. doi: 10.1081/iph-51617. [DOI] [PubMed] [Google Scholar]
- Kinkelin I, Motzing S, Koltenzenburg M, Brocker EB. Increase in NGF content and nerve fiber sprouting in human allergic contact eczema. Cell Tissue Res. 2000;302:31–37. doi: 10.1007/s004410000202. [DOI] [PubMed] [Google Scholar]
- Tobin D, Nabarro G, Baart de la Faille H, van Vloten WA, van der Putte SC, Schuurman HJ. Increased number of immunoreactive nerve fibers in atopic dermatitis. J Allergy Clin Immunol. 1992;90:613–622. doi: 10.1016/0091-6749(92)90134-n. [DOI] [PubMed] [Google Scholar]
- Abadia Molina F, Burrows NP, Jones RR, Terenghi G, Polak JM. Increased sensory neuropeptides in nodular prurigo: a quantitative immunohistochemical analysis. Br J Dermatol. 1992;127:344–351. doi: 10.1111/j.1365-2133.1992.tb00452.x. [DOI] [PubMed] [Google Scholar]
- Springall DR, Karanth SS, Kirkham N, Darley CR, Polak JM. Symptoms of notalgia paresthetica may be explained by increased dermal innervation. J Invest Dermatol. 1991;97:555–561. doi: 10.1111/1523-1747.ep12481889. [DOI] [PubMed] [Google Scholar]
- Naukkarinen A, Jarvikallio A, Lakkakorpi J, Harvima IT, Harvima RJ, Horsmanheimo M. Quantitative histochemical analysis of mast cells and sensory nerves in psoriatic skin. J Pathol. 1996;180:200–205. doi: 10.1002/(SICI)1096-9896(199610)180:2<200::AID-PATH632>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Sugiura H, Maeda T, Uehara M. Mast cell invasion of peripheral nerve in skin lesions of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1992;176:74–76. [PubMed] [Google Scholar]
- Harvima IT, Naukkarinen A, Harvima RJ, Aalto ML, Neittaanmaki H, Horsmanheimo M. Quantitative enzyme-histochemical analysis of tryptase- and chymase-containing mast cells in psoriatic skin. Arch Dermatol Res. 1990;282:428–433. doi: 10.1007/BF00402617. [DOI] [PubMed] [Google Scholar]
- Alving K, Sundstrom C, Matran R, Panula P, Hokfelt T, Lundberg JM. Association between histamine-containing mast cells and sensory nerves in the skin and airways of control and capsaicin-treated pigs. Cell Tissue Res. 1991;264:529–538. doi: 10.1007/BF00319042. [DOI] [PubMed] [Google Scholar]
- Piotrowski W, Foreman JC. Some effects of calcitonin gene-related peptide in human skin and on histamine release. Br J Dermatol. 1986;114:37–46. doi: 10.1111/j.1365-2133.1986.tb02777.x. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Kawakita K, Kiso Y, Nakano T, Kitamura Y. Substance P induces granulocyte infiltration through degranulation of mast cells. J Immunol. 1989;142:927–931. [PubMed] [Google Scholar]
- Yano H, Wershil BK, Arizono N, Galli SJ. Substance P-induced augmentation of cutaneous vascular permeability and granulocyte infiltration in mice is mast cell dependent. J Clin Invest. 1989;84:1276–1286. doi: 10.1172/JCI114295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niizeki H, Alard P, Streilein JW. Calcitonin gene-related peptide is necessary for ultraviolet B-impaired induction of contact hypersensitivity. J Immunol. 1997;159:5183–5186. [PubMed] [Google Scholar]
- Ottosson A, Edvinsson L. Release of histamine from dural mast cells by substance P and calcitonin gene-related peptide. Cephalalgia. 1997;17:166–174. doi: 10.1046/j.1468-2982.1997.1703166.x. [DOI] [PubMed] [Google Scholar]
- De Jonge F, De Laet A, Van Nassauw L, Brown JK, Miller HR, van Bogaert PP, Timmermans JP, Kroese AB. In vitro activation of murine DRG neurons by CGRP-mediated mucosal mast cell degranulation. Am J Physiol. 2004;287:G178–G191. doi: 10.1152/ajpgi.00528.2003. [DOI] [PubMed] [Google Scholar]
- Budai D, Larson AA. Role of substance P in the modulation of C-fiber-evoked responses of spinal dorsal horn neurons. Brain Res. 1996;710:197–203. doi: 10.1016/0006-8993(95)01384-9. [DOI] [PubMed] [Google Scholar]
- Oida H, Namba T, Sugimoto Y, Ushikubi F, Ohishi H, Ichikawa A, Narumiya S. In situ hybridization studies of prostacyclin receptor mRNA expression in various mouse organs. Br J Pharmacol. 1995;116:2828–2837. doi: 10.1111/j.1476-5381.1995.tb15933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic M, Hunt SP. Opiate and histamine H1 receptors are present on some substance P-containing dorsal root ganglion cells. Neurosci Lett. 1985;53:133–137. doi: 10.1016/0304-3940(85)90109-0. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, Luger TA, Schmelz M. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23:6176–6180. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AM, Bandtlow C, Heumann R, Korsching S, Rohrer H, Thoenen H. Timing and site of nerve growth factor synthesis in developing skin in relation to innervation and expression of the receptor. Nature. 1987;326:353–358. doi: 10.1038/326353a0. [DOI] [PubMed] [Google Scholar]
- Thier M, Marz P, Otten U, Weis J, Rose-John S. Interleukin-6 (IL-6) and its soluble receptor support survival of sensory neurons. J Neurosci Res. 1999;55:411–422. doi: 10.1002/(SICI)1097-4547(19990215)55:4<411::AID-JNR2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Stark B, Carlstedt T, Risling M. Distribution of TGF-beta, the TGF-beta type I receptor and the R-II receptor in peripheral nerves and mechanoreceptors; observations on changes after traumatic injury. Brain Res. 2001;913:47–56. doi: 10.1016/s0006-8993(01)02757-3. [DOI] [PubMed] [Google Scholar]
- Bauer O, Razin E. Mast cell-nerve interactions. News Physiol Sci. 2000;15:213–218. doi: 10.1152/physiologyonline.2000.15.5.213. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Suzuki R, Furuno T, Teshima R, Nakanishi M. N-cadherin plays a role in the synapse-like structures between mast cells and neurites. Biol Pharm Bull. 2004;27:1891–1894. doi: 10.1248/bpb.27.1891. [DOI] [PubMed] [Google Scholar]
- Furuno T, Ito A, Koma Y, Watabe K, Yokozaki H, Bienenstock J, Nakanishi M, Kitamura Y. The spermatogenic Ig superfamily/synaptic cell adhesion molecule mast-cell adhesion molecule promotes interaction with nerves. J Immunol. 2005;174:6934–6942. doi: 10.4049/jimmunol.174.11.6934. [DOI] [PubMed] [Google Scholar]
- Askenase PW, Van Loveren H, Kraeuter-Kops S, Ron Y, Meade R, Theoharides TC, Nordlund JJ, Scovern H, Gerhson MD, Ptak W. Defective elicitation of delayed-type hypersensitivity in W/Wv and SI/SId mast cell-deficient mice. J Immunol. 1983;131:2687–2694. [PubMed] [Google Scholar]
- Bryce PJ, Miller ML, Miyajima I, Tsai M, Galli SJ, Oettgen HC. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity. 2004;20:381–392. doi: 10.1016/s1074-7613(04)00080-9. [DOI] [PubMed] [Google Scholar]
- Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- Gordon JR, Galli SJ. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon RI. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J Exp Med. 1991;174:103–107. doi: 10.1084/jem.174.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann T, Kneilling M, Mailhammer R, Maier K, Sander CA, Kollias G, Kunkel SL, Hultner L, Rocken M. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J Exp Med. 2000;192:1441–1452. doi: 10.1084/jem.192.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto H, Nakae S, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cell-associated TNF promotes dendritic cell migration. J Immunol. 2006;176:4102–4112. doi: 10.4049/jimmunol.176.7.4102. [DOI] [PubMed] [Google Scholar]
- Hattori A, Hayashi K, Kohno M. Tumor necrosis factor (TNF) stimulates the production of nerve growth factor in fibroblasts via the 55-kDa type 1 TNF receptor. FEBS Lett. 1996;379:157–160. doi: 10.1016/0014-5793(95)01502-7. [DOI] [PubMed] [Google Scholar]
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- Barker V, Middleton G, Davey F, Davies AM. TNFalpha contributes to the death of NGF-dependent neurons during development. Nat Neurosci. 2001;4:1194–1198. doi: 10.1038/nn755. [DOI] [PubMed] [Google Scholar]
- Diem R, Meyer R, Weishaupt JH, Bahr M. Reduction of potassium currents and phosphatidylinositol 3-kinase-dependent AKT phosphorylation by tumor necrosis factor-(alpha) rescues axotomized retinal ganglion cells from retrograde cell death in vivo. J Neurosci. 2001;21:2058–2066. doi: 10.1523/JNEUROSCI.21-06-02058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SW, Dewhurst S, Bellizzi MJ, Gelbard HA. Tumor necrosis factor-alpha in normal and diseased brain: conflicting effects via intraneuronal receptor crosstalk? J Neurovirol. 2002;8:611–624. doi: 10.1080/13550280290101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Houwelingen AH, Kool M, de Jager SC, Redegeld FA, van Heuven-Nolsen D, Kraneveld AD, Nijkamp FP. Mast cell-derived TNF-alpha primes sensory nerve endings in a pulmonary hypersensitivity reaction. J Immunol. 2002;168:5297–5302. doi: 10.4049/jimmunol.168.10.5297. [DOI] [PubMed] [Google Scholar]
- Korner H, Cook M, Riminton DS, Lemckert FA, Hoek RM, Ledermann B, Kontgen F, Fazekas de St Groth B, Sedgwick JD. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur J Immunol. 1997;27:2600–2609. doi: 10.1002/eji.1830271020. [DOI] [PubMed] [Google Scholar]
- Duttlinger R, Manova K, Chu TY, Gyssler C, Zelenetz AD, Bachvarova RF, Besmer P. W-sash affects positive and negative elements controlling c-kit expression: ectopic c-kit expression at sites of kit-ligand expression affects melanogenesis. Development. 1993;118:705–717. doi: 10.1242/dev.118.3.705. [DOI] [PubMed] [Google Scholar]
- Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant KitW-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus R, Hofmann U, Eichmuller S, Czarnetzki BM. Distribution and changing density of gamma-delta T cells in murine skin during the induced hair cycle. Br J Dermatol. 1994;130:281–289. doi: 10.1111/j.1365-2133.1994.tb02922.x. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Eichmuller S, Peters EM, Pietsch P, Johansson O, Maurer M, Paus R. A simple immunofluorescence technique for simultaneous visualization of mast cells and nerve fibers reveals selectivity and hair cycle-dependent changes in mast cell-nerve fiber contacts in murine skin. Arch Dermatol Res. 1997;289:292–302. doi: 10.1007/s004030050195. [DOI] [PubMed] [Google Scholar]
- Grabbe S, Schwarz T. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Am J Contact Dermatol. 1996;7:238–246. [PubMed] [Google Scholar]
- Dalsgaard CJ, Rydh M, Haegerstrand A. Cutaneous innervation in man visualized with protein gene product 9.5 (PGP 9.5) antibodies. Histochemistry. 1989;92:385–390. doi: 10.1007/BF00492495. [DOI] [PubMed] [Google Scholar]
- Gibbins IL, Wattchow D, Coventry B. Two immunohistochemically identified populations of calcitonin gene-related peptide (CGRP)-immunoreactive axons in human skin. Brain Res. 1987;414:143–148. doi: 10.1016/0006-8993(87)91335-7. [DOI] [PubMed] [Google Scholar]
- Rice FL, Albers KM, Davis BM, Silos-Santiago I, Wilkinson GA, LeMaster AM, Ernfors P, Smeyne RJ, Aldskogius H, Phillips HS, Barbacid M, DeChiara TM, Yancopoulos GD, Dunne CE, Fundin BT. Differential dependency of unmyelinated and A delta epidermal and upper dermal innervation on neurotrophins, trk receptors, and p75LNGFR. Dev Biol. 1998;198:57–81. [PubMed] [Google Scholar]
- Lindenlaub T, Teuteberg P, Hartung T, Sommer C. Effects of neutralizing antibodies to TNF-alpha on pain-related behavior and nerve regeneration in mice with chronic constriction injury. Brain Res. 2000;866:15–22. doi: 10.1016/s0006-8993(00)02190-9. [DOI] [PubMed] [Google Scholar]
- Bergstresser PR, Tigelaar RE, Tharp MD. Conjugated avidin identifies cutaneous rodent and human mast cells. J Invest Dermatol. 1984;83:214–218. doi: 10.1111/1523-1747.ep12263584. [DOI] [PubMed] [Google Scholar]
- Karanth SS, Springall DR, Francavilla S, Mirrlees DJ, Polak JM. Early increase in CGRP- and VIP-immunoreactive nerves in the skin of streptozotocin-induced diabetic rats. Histochemistry. 1990;94:659–666. doi: 10.1007/BF00271994. [DOI] [PubMed] [Google Scholar]
- Hirata T, Morii E, Morimoto M, Kasugai T, Tsujimura T, Hirota S, Kanakura Y, Nomura S, Kitamura Y. Stem cell factor induces outgrowth of c-kit-positive neurites and supports the survival of c-kit-positive neurons in dorsal root ganglia of mouse embryos. Development. 1993;119:49–56. doi: 10.1242/dev.119.1.49. [DOI] [PubMed] [Google Scholar]
- Kassiotis G, Kranidioti K, Kollias G. Defective CD4T cell priming and resistance to experimental autoimmune encephalomyelitis in TNF-deficient mice due to innate immune hypo-responsiveness, J Neuroimmunol. 2001;119:239–247. doi: 10.1016/s0165-5728(01)00403-9. [DOI] [PubMed] [Google Scholar]
- Shubayev VI, Myers RR. Axonal transport of TNF-alpha in painful neuropathy: distribution of ligand tracer and TNF receptors. J Neuroimmunol. 2001;114:48–56. doi: 10.1016/s0165-5728(00)00453-7. [DOI] [PubMed] [Google Scholar]
- Nakae S, Naruse-Nakajima C, Sudo K, Horai R, Asano M, Iwakura Y. IL-1 alpha, but not IL-1 beta, is required for contact-allergen-specific T cell activation during the sensitization phase in contact hypersensitivity. Int Immunol. 2001;13:1471–1478. doi: 10.1093/intimm/13.12.1471. [DOI] [PubMed] [Google Scholar]
- Hattori A, Tanaka E, Murase K, Ishida N, Chatani Y, Tsujimoto M, Hayashi K, Kohno M. Tumor necrosis factor stimulates the synthesis and secretion of biologically active nerve growth factor in non-neuronal cells. J Biol Chem. 1993;268:2577–2582. [PubMed] [Google Scholar]
- Naukkarinen A, Harvima IT, Aalto ML, Harvima RJ, Horsmanheimo M. Quantitative analysis of contact sites between mast cells and sensory nerves in cutaneous psoriasis and lichen planus based on a histochemical double staining technique. Arch Dermatol Res. 1991;283:433–437. doi: 10.1007/BF00371778. [DOI] [PubMed] [Google Scholar]
- Hagforsen E, Nordlind K, Michaelsson G. Skin nerve fibres and their contacts with mast cells in patients with palmoplantar pustulosis. Arch Dermatol Res. 2000;292:269–274. doi: 10.1007/s004030000132. [DOI] [PubMed] [Google Scholar]
- Laine P, Naukkarinen A, Heikkila L, Penttila A, Kovanen PT. Adventitial mast cells connect with sensory nerve fibers in atherosclerotic coronary arteries. Circulation. 2000;101:1665–1669. doi: 10.1161/01.cir.101.14.1665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.