Abstract
Schistosoma mansoni egg-induced inflammation is accompanied by TH2 cell polarization and development of fibrotic granulomas in host tissue. The interleukin (IL)-4 receptor α (IL-4Rα), which mediates IL-4 and IL-13 signaling, is essential for granulomatous pathology through a putative CD4+ T-cell-dependent mechanism. In this study, we asked whether CD4+ T-cell-specific IL-4Rα-deficient mice (LckCreIL-4Rα−/lox) developed granulomas and egg-driven collagen production. Although eosinophilia and goblet cell hyperplasia were impaired in LckCreIL-4Rα−/lox mice, there was no reduction in size or collagen content of lung and liver granulomas. The lack of CD4+ T-cell IL-4Rα expression caused significant increases in interferon-γ-producing cells, inducible nitric-oxide synthetase production, and hepatic damage, compared with similarly infected wild-type mice. Interestingly, this TH1-associated liver injury did not lead to premature mortality in this strain. Instead, lower levels of serum endotoxin in LckCreIL-4Rα−/lox mice suggest that intestinal barrier function may be the dominant factor for survival during natural infection.
The inflammation and pathology caused by Schistosoma mansoni eggs lodged in host tissue is widely used for the study of TH2 cytokine-driven responses and granuloma formation.1–3 Interleukins (ILs)-4, -5, -9, and -13 are up-regulated in the local microenvironment surrounding parasite ova accompanied by an influx of eosinophils, goblet cell hyperplasia, and increased IgG1 and IgE production.4 Studies using both experimental infection with S. mansoni larvae and synchronous egg-induced lung granulomas have demonstrated critical, yet independent, roles for IL-4 and IL-13.5–7 However, the cell-specific requirements for IL-4/IL-13 responsiveness during egg-induced granuloma development in liver and lung tissue are unclear.
IL-4 and IL-13 functions are completely dependent on the expression of IL-4Rα,8 which is required for generation of the respective receptors. The IL-4Rα chain pairs either with the common gamma chain (γc) to form the type 1 IL-4R (for IL-4 binding) or with the IL-13Rα1 chain to form the type 2 IL-4R (for IL-4 and IL-13 binding). These cytokines can modulate the function of bone marrow-derived cells (T, B, NK, macrophage) and nonhematopoietic lineages, such as endothelium,9 intestinal epithelial cells,10 and smooth muscle.11 Indeed, nonbone marrow cell-derived IL-4Rα expression drives airway inflammation12 and is required for expulsion of gastrointestinal helminths.13,14
IL-4 signaling is critically important during natural infection with S. mansoni for the prevention of the severe lethal form of schistosomiasis, as shown by studies performed with mice deficient in IL-4, IL-4/IL-10, or IL-4/IL-13.15–17 These reports demonstrated a significant increase in the production of proinflammatory mediators [ie, NO, tumor necrosis factor-α, and interferon (IFN)-γ], accompanied by significant morbidity and mortality.16,18 Studies performed using S. mansoni-infected IL-4-deficient mice suggested a causative role for inducible nitric-oxide synthetase (NOS-2) in the severe liver damage that was observed.19 Indeed, vaccination of mice with soluble egg antigens (SEAs) and rIL-12 or with SEA with complete Freund’s adjuvant results in increased morbidity associated with an expansion of IFN-γ-secreting T cells, significant impairment of collagen production, and reduced production of type 2 antibodies.20–22 However, it is not clear from these studies whether TH1 polarization or the lack of IL-4/IL-13 responsiveness on specific cell types was controlling the phenotype of the granulomatous response.
The present study utilizes CD4+ T-cell-specific IL-4Rα-deficient mice (LckCreIL-4Rα−/lox) that were generated by an intercross between mice carrying a loxP-flanked IL-4Rα allele and mice that expressed Cre recombinase under the T-cell-specific promoter p56Lck (LckCre) (M. Radwanska, A. Cutler, M.S. Magez, C. Holscher, A. Bohms, B. Arendse, R. Kirsch, J. Alexander, J. Kaye, F. Brombacher, submitted for publication). Using both natural infection with S. mansoni larvae and the synchronized pulmonary granuloma model, we tested whether deletion of IL-4Rα signaling in the T-helper cell compartment would impair liver and/or lung granuloma formation, exacerbate liver damage, or abrogate fibrosis. Our results indicate that both liver and lung granuloma formation were slightly exacerbated by the CD4+ T-cell-specific IL-4Rα deletion in the context of a significantly increased TH1 cell number and elevated NOS-2 activity. Interestingly, type 1 responses did not inhibit fibrosis in liver or lung granulomas, nor did it lead to premature mortality of these mice, as was expected. The lower serum endotoxin levels in infected LckCreIL-4Rα−/lox mice compared with wild-type (WT) littermates may suggest that intestinal barrier disruption was a more dominant factor in disease outcome. Overall, this study demonstrates that CD4+ T-cell-specific IL-4Rα signaling is critical for T-helper polarization after exposure to S. mansoni eggs, but other IL-4Rα-expressing cells such as macrophages may be more important for host survival.
Materials and Methods
Animals and Infection Studies
LckCreIL-4Rα−/flox animals were generated through an intercross between LckCre (BALB/c) and IL-4Rαflox/flox (BALB/c) mice followed by mating of offspring with IL-4Rα-deficient (IL-4Rα−/−) animals to generate LckCreIL-4Rα−/flox mice. In all experiments transgenic-negative littermates (IL-4Rα−/flox) were used as WT controls. Genotyping of mice was performed as previously described.23 All mice were housed under specific pathogen-free barrier conditions in the University of Cape Town animal facility. For infection with S. mansoni, naïve sex-matched mice from 6 to 10 weeks of age were percutaneously infected with 70 to 80 live cercariae of a Puerto Rican strain of S. mansoni obtained from infected Biomphalaria glabrata (a generous gift from Adrian Mountford, York, UK). Mice were monitored for mortality and recorded weekly.
Induction of Pulmonary Granulomas
The induction of synchronous egg-induced granulomas was performed as previously described.3 In brief, S. mansoni eggs were extracted from the livers of infected mice and enriched for mature eggs. To induce pulmonary granulomas, mice sensitized to schistosome eggs by a prior intraperitoneal injection of 5000 live eggs on day −14 were intravenously injected with 5000 eggs on day 0. Animals were sacrificed on days 7, 14, 21, and 28 after challenge.
Enzyme-Linked Immunosorbent Assay (ELISA), ELISPOT, and ex Vivo Analysis
Splenocyte single cell suspensions were stimulated with 10 μg/ml of SEA in Iscove’s modified Dulbecco’s medium containing 10% fetal calf serum, 100 U of penicillin, 100 μg of streptomycin, and 0.2 mmol/L l-glutamine (all from Sigma Aldrich SA Pty Ltd., Johannesburg, South Africa) as previously described.23 Supernatants were stored at −80°C until used for ELISA. SEA-specific antibody ELISA was performed as previously described.23
ELISPOT plates (Millipore) were coated overnight at 4°C with anti-IFN-γ (clone An18KL6; concentration, 5 μg/ml) and then blocked for 1 hour at 37°C with 5% bovine serum albumin (Sigma). Splenocytes were added at a concentration of 5 × 105 cells per well and incubated at 37°C for 40 to 48 hours. Detection was then performed using 0.5 μg/ml biotinylated anti-IFN-γ (BD Pharmingen), alkaline phosphatase-labeled streptavidin (BD Pharmingen), and BCIP/NBT alkaline phosphatase substrate (Sigma). The plates were then washed with distilled water and air-dried. Spots were quantitated by two independent observers by examination under a dissecting microscope. Cytokines in supernatants and antigen-specific antibody isotypes in sera from infected animals were determined as previously described.24
Lung Homogenates for Cytokine Analysis
Lung samples were obtained and processed for cytokine analysis as previously described.23 Briefly, 5% (w/v) lung tissue suspensions were homogenized in lysis buffer containing 50 mmol/L Tris-HCl (pH 8.0), 0.5% Nonidet P-40, 1 mmol/L ethylenediaminetetraacetic acid, 150 mmol/L NaCl, 10% glycerol, 1 mmol/L aprotinin, and 1 mmol/L phenylmethyl sulfonyl fluoride with a protease inhibitor cocktail (Sigma). Extracts were frozen immediately in liquid nitrogen and stored at −80°C.
Hydroxyproline Assay
Hydroxyproline content as a measure of collagen production was determined as previously described.23
Histology
Lung and liver samples were fixed in neutral buffered formalin and processed, and 5-μm sections were stained with hematoxylin and eosin (H&E). The diameters of each granuloma containing a single egg were measured by means of a computerized morphometry analysis program (Scion Image, 1988; Scion Corp., Frederick, MD) by subtracting the egg diameter from the diameter of the whole granuloma. An average of 50 granulomas per mouse was included in the analysis. All histological examinations were scored by the same individual in a blinded manner to control for consistency. OCT-embedded liver tissue was cut into 7-μm frozen sections for immunohistochemistry. After acetone fixation, macrophages were detected using PE-anti-F4/80 monoclonal antibody (mAb) (Caltag, Burlingame, CA). A rabbit anti-NOS-2 mAb (a gift from J. Pfeilschifter, Frankfurt, Germany) was used to analyze macrophage function. Numbers of eosinophils in granulomas were also evaluated in the same sections used for granuloma measurements, whereas periodic acid-Schiff (PAS)-positive cells were evaluated from sections stained with PAS reagent. Granuloma eosinophils were counted under immersion oil at ×1000 magnification in four fields from each granuloma, and 25 granulomas were examined for each mouse.
Granuloma Cell Purification
Liver granuloma cells were isolated by collagenase digestion as previously described.25 Livers of 7.5-week infected mice were homogenized, and granulomas were collected by 1 × g sedimentation, washed in Iscove’s modified Dulbecco’s medium. Cell suspensions were incubated at 37°C with shaking in Iscove’s modified Dulbecco’s medium containing 0.5% collagenase. The dispersed granulomas were further disrupted by repeated suction and expulsion through a 20-ml syringe and then passed through sterile gauze and washed. Viability was determined by trypan blue exclusion.
Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
RNA from intestinal tissue was extracted, and the presence of contaminating genomic DNA was tested before cDNA synthesis by real-time PCR using β-actin primers that bind to genomic DNA (5′-TGGAATCCTGTGGCATCCAGAAAC-3′ and 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′). Data were analyzed using the fit points and standard curve method using β-2 microglubulin as the housekeeping gene.23 β2MG forward: 5′-TGACCGGCTTGTATGCTATC-3′, β2MG reverse: 5′-CAGTGTGAGCCAGGATATAG-3′, NOS-2 forward: 5′-AGCTCCTCCCAGGACCACAC-3′, and NOS-2 reverse: 5′-ACGCTGAGTACCTCATTGGC-3′.
Statistics
Values are given as mean ± SD and significant differences were determined using the unpaired two-tailed Student’s t-test or analysis of variance. All experiments shown were performed twice unless indicated, and a representative experiment is shown. *P < 0.05 compared with WT, **P < 0.01, and ***P < 0.001.
Results
CD4+ T-Cell IL-4Rα Expression Is Not Essential for Lung Granuloma Development or Fibrosis
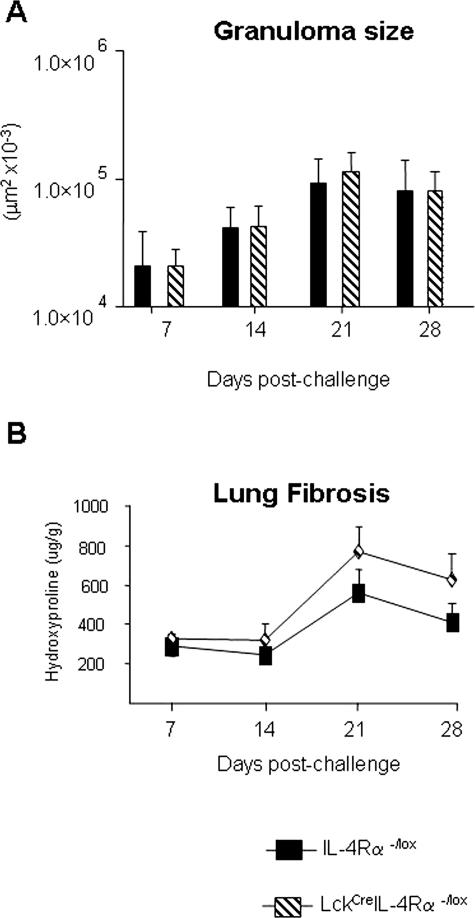
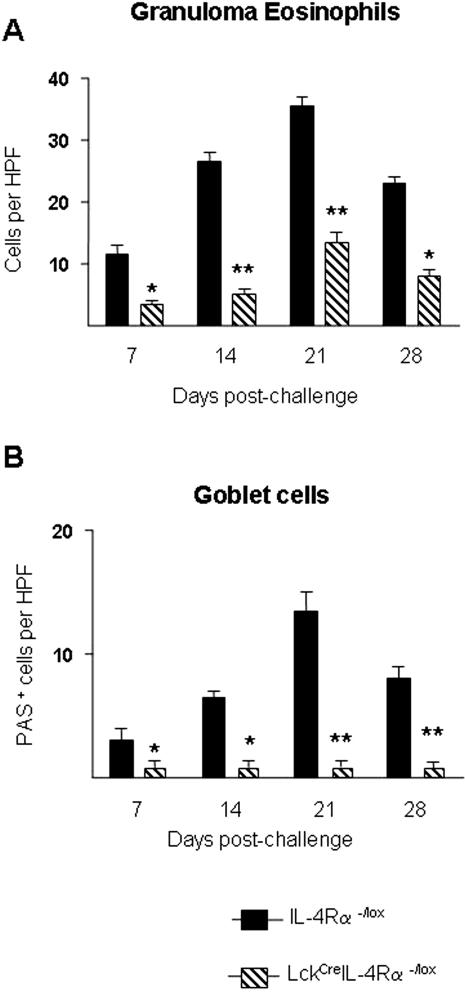
To address the requirement for CD4+ T-cell-specific IL-4Rα signaling in egg-induced lung granuloma formation and fibrosis, we analyzed granuloma formation 7, 14, 21, and 28 days after intravenous challenge in intraperitoneal egg-sensitized WT and LcKCreIL-4Rα−/− mice. Histological examination of granuloma development showed no significant differences in granuloma sizes between the two groups during the 4 weeks after challenge (Figure 1A). There was a slight but comparable decrease in granuloma sizes of both LcKCreIL-4Rα−/lox and WT mice on day 28 compared with day 21 after challenge. Consistent with these results, the analysis of collagen deposition within the lung, assessed by measurement of hydroxyproline levels, also peaked at day 21 in LcKCreIL-4Rα−/lox mice and was slightly reduced at day 28 after challenge (Figure 1B). Histological assessment of goblet cell hyperplasia was performed after periodic acid-Schiff (PAS) staining of formalin-fixed lung tissue from egg-challenged mice. Quantitation of PAS+ cells per high-power field (×100) revealed significantly fewer numbers of positive cells in LcKCreIL-4Rα−/lox mice compared with similarly challenged WT littermates (Figure 2B). In addition, the number of eosinophils counted per granuloma was significantly lower in the LcKCreIL-4Rα−/lox strain compared with WT (Figure 2A). These data indicate that CD4+ T-cell IL-4Rα expression is not required for S. mansoni egg-driven lung fibrosis or granuloma development but is essential for goblet cell hyperplasia and eosinophil recruitment.
Figure 1.
CD4+ T-cell-specific IL-4Rα expression is not required for S. mansoni egg-induced granuloma formation or collagen deposition in the lung. WT (closed bars) and LckCreIL-4Rα−/lox (hatched bars). A: Graph shown represents kinetic analysis of synchronized lung granuloma size after intravenous challenge with 5000 live S. mansoni eggs. Each time point represents five mice per group and 30 granulomas per mouse analyzed. B: Measurement of lung hydroxyproline content in experiment described in A. Whole lung tissues of five mice were analyzed per time point.
Figure 2.
Fewer granuloma eosinophils and abrogation of goblet cell hyperplasia in lungs of S. mansoni egg-challenged LckCreIL-4Rα−/lox mice. WT (closed bars) and LckCreIL-4Rα−/lox (hatched bars). A: Quantitation of granuloma eosinophils in H&E-stained lung sections from egg-challenged mice at the indicated time points. Five mice were analyzed and 30 granulomas examined per mouse. B: Airway goblet cell hyperplasia was quantitated by PAS staining of formalin-fixed egg-challenged lung tissue. Number of PAS+ cells per high-power field (×100) was determined by light microscopy. Data represent five mice per group. Statistical significance in all panels was determined using GraphPad Prism software by one-way analysis of variance with *P < 0.05 and **P < 0.01, compared with the WT value.
S. mansoni Egg-Challenged LcKCreIL-4Rα−/lox Mice Develop Systemic TH1 Responses
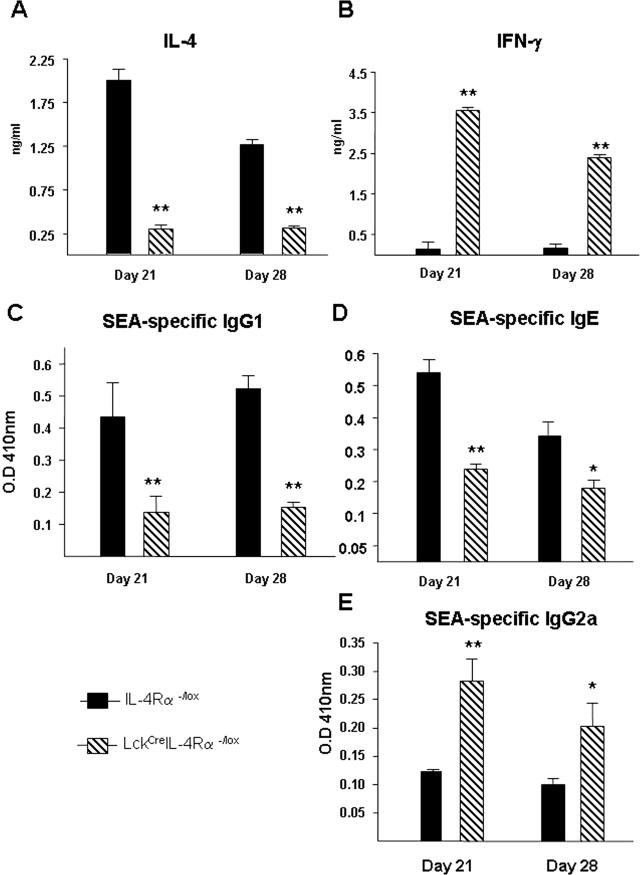
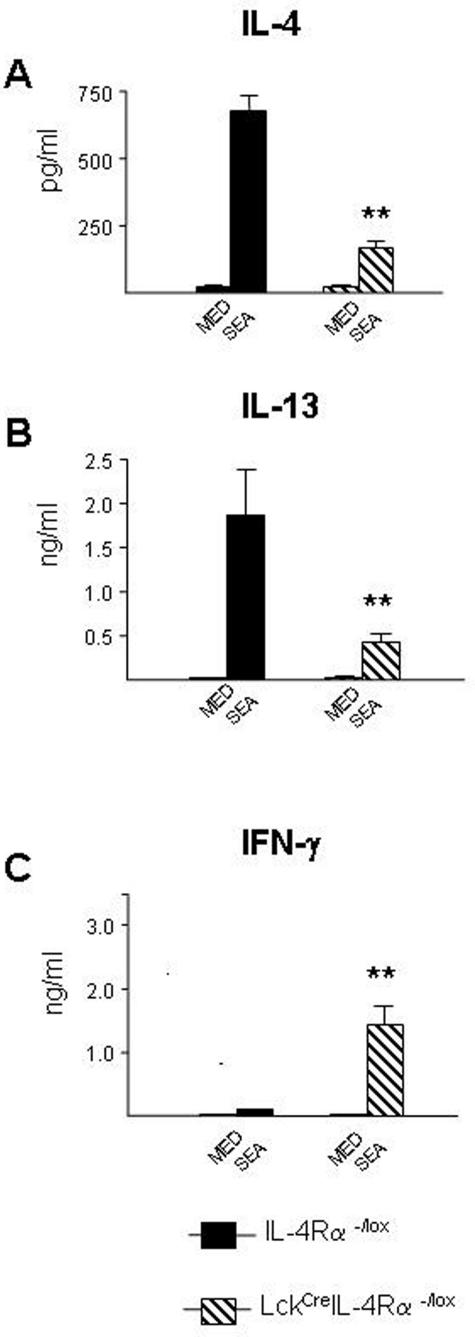
TH1 versus TH2 cytokine secretion and immunoglobulin production was examined in S. mansoni egg-challenged WT and LcKCreIL-4Rα−/flox mice. Measurement of the SEA-specific antibody response revealed a significant decrease of IgG1 and IgE levels in LcKCreIL-4Rα−/lox sera at days 21 and 28 compared with egg-challenged WT littermates (Figure 3, C and D). Similar trends were observed at days 7 and 14 (data not shown). Conversely, SEA-specific IgG2a levels were significantly elevated in LcKCreIL-4Rα−/lox sera at these same time points compared with WT mice (Figure 3E).
Figure 3.
Predominant TH1 response develops in S. mansoni egg-challenged CD4+ T-cell-specific IL-4Rα-deficient mice. WT (closed bars) and LckCreIL-4Rα−/lox (hatched bars). Measurement of IL-4 levels (A), IFN-γ levels (B), antigen-specific IgG1 response (C), antigen-specific IgE response (D), and antigen-specific IgG2a (E) in whole lung homogenates of S. mansoni egg-challenged lung. Five mice per group were analyzed. Data represent three experiments performed with similar results. Statistical significance in all panels was determined using GraphPad Prism software by one-way analysis of variance with *P < 0.05 and **P < 0.01, compared with the WT value.
We next examined whether IL-4 versus IFN-γ cytokine levels in the local microenvironment of egg granulomas reflected the phenotype of the systemic antibody response. IL-4 and IFN-γ levels in lung tissue homogenates could be detected by ELISA from as early as day 7 after egg embolization (not shown), but peak production of both cytokines were detected at day 21 after challenge. Whereas WT mice generated the expected IL-4 response, LcKCreIL-4Rα−/lox mice produced significantly lower amounts of IL-4 (Figure 3A), as well as significantly lower levels of IL-5 and IL-13 (data not shown). In contrast, IFN-γ levels were much higher in LcKCreIL-4Rα−/lox compared with WT mice (Figure 3B). These data show that CD4+ T-cell-specific IL-4Rα-deficient mice generate a predominant TH1 response to S. mansoni egg challenge in lung tissue at all time points examined.
TH1 Response Correlates with Enhanced Liver Damage and NOS-2 Activity
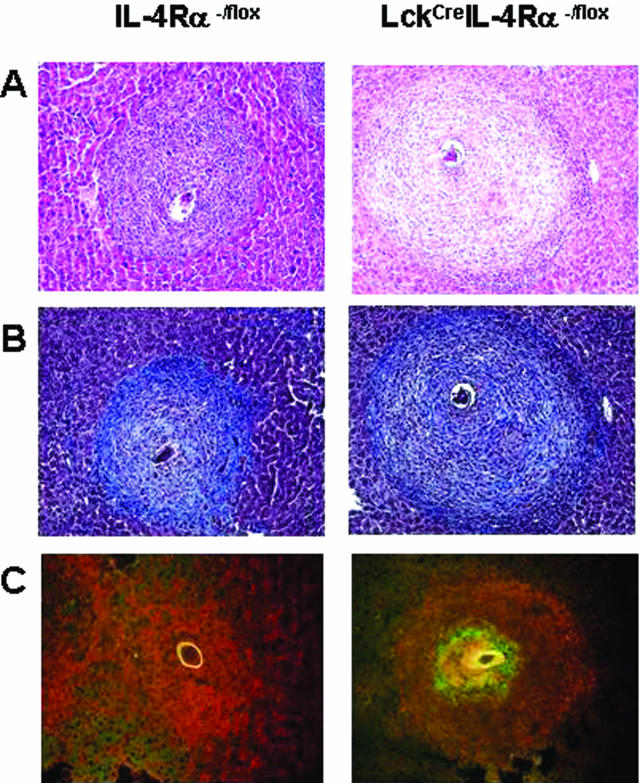
We sought to determine whether the TH1-dominant granulomatous response would also be evident in the S. mansoni natural infection model. Liver granuloma size and cellular composition were compared between strains to further characterize egg-driven immunopathology. Liver granulomas that developed in LckCreIL-4Rα−/lox mice (70 ± 23 μm2 × 10−3) were somewhat larger than WT lesions (54 ± 19 μm2 × 10−3) (P < 0.05) (Table 1). Macrophage composition, as determined by immunofluorescence staining for F4/80+ cells, did not reveal any obvious differences in total cellular recruitment between WT and LckCreIL-4Rα−/lox granulomas (Figure 4C). There was also no difference in the mean number of eosinophils per granuloma of WT and LckCreIL-4Rα−/lox strains (Table 1). Measurement of total hydroxyproline levels in granuloma-containing liver tissue was performed to indicate the extent of granuloma fibrosis, in addition to Chromotrope Aniline blue (CAB) staining of formalin-fixed liver sections. Neither approach revealed major differences in fibrosis, with a modest increase of hydroxyproline levels in LckCreIL-4Rα−/lox mice compared with WT littermates (Figure 4B, Table 1).
Table 1.
Liver Granuloma Size, Collagen Content, and Eosinophil Recruitment
| WT | LckCre IL-4Ra−/lox | |
|---|---|---|
| Granuloma area (μm2 × 10−3) | 54 ± 19 | 70 ± 23 |
| Hydroxyproline (μM/104 eggs) | 6.18 ± 0.31 | 8.95 ± 0.49 |
| Eosinophils (per granuloma) | 172 ± 25 | 146 ± 30 |
Measurement of liver granuloma area and hydroxyproline content of infected liver tissue at 7.5 weeks after infection was performed as previously described.23 Quantitation of granuloma eosinophils was performed by analysis of H&E-stained liver sections using a light microscope under oil immersion at ×100 magnification. Thirty granulomas from each mouse were examined with five mice per group in the analysis. Data represent the mean ± SD. The result represents one of four independent, but similar, analyses.
Figure 4.
Liver granulomatous pathology and fibrosis during natural infection is slightly increased in CD4+ T-cell-specific IL-4Rα-deficient mice. WT and LckCreIL-4Rα−/lox mice (n = 5) were infected with 70 to 80 cercariae and were examined at 7.5 weeks for analysis of granulomatous liver tissue. Significance in all panels was determined by one-way analysis of variance with *P < 0.05. A: Chromotrope aniline blue (CAB) staining of formalin-fixed liver tissue showing granulomas. B: H&E staining of formalin-fixed liver tissue showing granulomas. C: Immunofluorescence staining was performed on frozen sections of liver granulomas from the indicated strains. F4/80 (red) and NOS-2 (green). All granulomas represent 50 granulomas per mouse. Original magnifications, ×400.
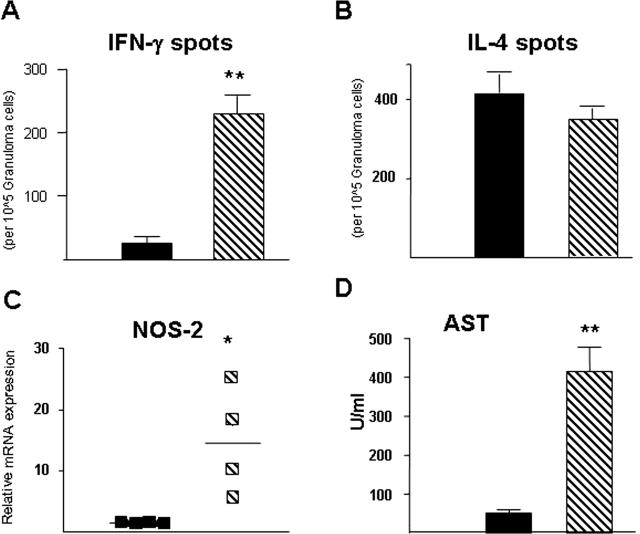
TH1 versus TH2 cytokine production from splenocytes was analyzed after the antigen-specific recall response to SEA. Figure 5, A and B, shows that LckCreIL-4Rα−/lox mice produced significantly less IL-4 and IL-13 than WT littermate controls. Conversely, there was a higher amount of IFN-γ produced in the LckCreIL-4Rα−/flox than the WT strain (Figure 5C). Similar to the lung-egg challenge model, levels of SEA-specific IgG1 were decreased and IgG2b increased in LcKCreIL-4Rα−/lox sera compared with WT littermates (data not shown). It was unclear whether this systemic response was reflected by the egg-driven cytokine response in the liver. Therefore, granuloma cells were isolated from livers of S. mansoni-infected WT and LcKCreIL-4Rα−/lox strains and the frequency of IL-4-positive versus IFN-γ-positive cells determined by ELISPOT. The number of IFN-γ-producing cells in LckCreIL-4Rα−/lox liver granulomas was 12-fold higher than in similarly infected WT mice (Figure 6A). In contrast, there were no differences in the frequency of IL-4-producing cells (Figure 6B).
Figure 5.
Significantly impaired antigen-specific TH2 development in CD4+ T-cell-specific IL-4Rα-deficient mice is accompanied by polarized TH1. Splenocyte stimulation with SEA (10 μg/ml) for 72 hours and measurement of cytokine levels, IL-4 (A), IL-13 (B), and IFN-γ (C), in supernatants as determined by ELISA. Data shown are mean ± SD from five individual mice and are representative of three independent experiments.
Figure 6.
Elevated numbers of IFN-γ-producing cells and NOS-2 production are associated with exacerbated liver damage in CD4+ T-cell-specific IL-4Rα-deficient mice. ELISPOT analysis of IFN-γ-producing (A) or IL-4-producing (B) cells from granuloma cell suspensions isolated from the liver of S. mansoni-infected mice. Experiment performed three times with similar results. C: mRNA levels of NOS-2 in liver tissue were determined by real-time PCR, normalized to β-2 microglobulin and expressed as fold increase over noninfected WT mice. Each symbol represents an individual mouse liver sample. Experiment performed three times with similar results. D: Serum levels of aspartate transaminase (AST), an indicator of hepatocellular damage expressed as mean ± SD of five individual samples per group. Results are representative of three experiments.
We next asked whether IFN-γ target genes such as NOS-2 were similarly up-regulated in LcKCreIL-4Rα−/lox mice and if so, whether this was associated with liver injury. A striking increase of NOS-2 activity was found by immunofluorescence staining of frozen liver sections from LcKCreIL-4Rα−/lox strain compared with the WT (Figure 4C). Quantitation of NOS-2 mRNA levels in granuloma-containing liver tissue over levels in naive WT controls revealed that the LcKCreIL-4Rα−/lox strain contained significantly more NOS-2 transcripts than infected WT mice (P < 0.001) (Figure 6C). Because of the reported association between NOS-2 and hepatic damage,18 we measured aspartate transaminase (AST) levels in serum at 7.5 weeks after infection. LckCreIL-4Rα−/lox mice produced eightfold higher levels of this enzyme than WT littermate controls (P = <0.001) (Figure 6D). These data agree with previous studies suggesting a link exists between NOS-2 activity and hepatocellular damage during acute schistosomiasis19 and confirm that IFN-γ production correlates with increased NOS-2 activity.
Absence of Gut Injury Correlates with Extended Survival in LckCreIL-4Rα−/lox Mice
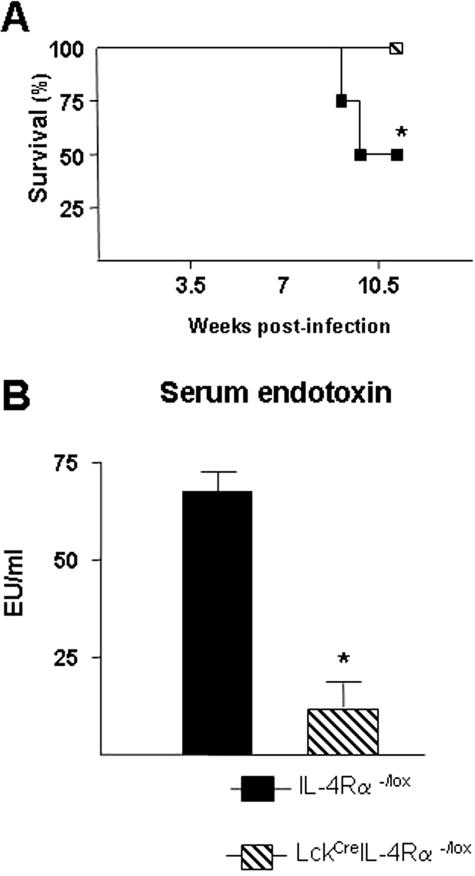
Survival studies were performed to determine whether LckCreIL-4Rα−/lox mice were more susceptible to death associated with S. mansoni infection. Naïve mice were given a high-dose infection (70 to 80 cercariae) and monitored weekly for signs of weight loss and mortality. Results of four independent experiments showed that greater than 95% of infected LckCreIL-4Rα−/lox mice survived beyond 11 weeks after infection (Figure 7A), whereas WT littermates showed only a 50 to 75% survival rate at this dose. We previously demonstrated that increased mortality during S. mansoni infection is associated with intestinal injury and systemic endotoxemia.23 Measurement of serum endotoxin levels in 7.5-week-infected LcKCreIL-4Rα−/lox and WT mice revealed that LckCreIL-4Rα−/lox mice had significantly lower levels of lipopolysaccharide than WT littermate controls (Figure 7B). This may suggest that increased mortality associated with high-dose S. mansoni infection in BALB/c mice correlates with gut injury rather than hepatic damage.
Figure 7.
Survival of CD4+ T-cell-specific IL-4Rα-deficient mice during high-dose S. mansoni infection correlates with less serum endotoxin. WT (closed bars) and LckCreIL-4Rα−/lox (hatched bars) infected with 70 to 80 cercariae were monitored for survival throughout 11 weeks. A: Survival of infected mice from the indicated strains. Data represent four independent experiments with similar results. Significance was determined by one-way analysis of variance with *P < 0.05 and **P < 0.01, compared with the WT group (n = 5). B: Serum levels of endotoxin at 7.5 weeks after infection. Data are expressed as mean ± SD of five individual samples per group. Representative of three independent experiments.
Discussion
It is well accepted that CD4+ T-helper 2 cell production of the hallmark cytokines IL-4 and IL-13 drives B-cell IgG1 and IgE class switching, eosinophil recruitment, fibrosis, and mucus hypersecretion.8,26 Such type 2 effector functions serve a host protective role against intestinal helminth infection14 but also damages the body’s own tissues in various types of allergic disease.27 IL-4 and IL-13 signaling in both mice and humans requires an intact IL-4 receptor α chain (IL-4Rα),8 but much is still unknown about its function in specific tissues and/or cell types during complex in vivo immune responses. The present study describes characterization of a novel mouse strain recently generated in our laboratory that contains the proximal lck promoter driving Cre recombinase expression combined with a floxed IL-4 receptor allele (LckCreIL-4Rα−/lox). These mice have been demonstrated to have abrogated CD4+ T-cell-specific IL-4Rα expression, partial deletion on the CD8+ T-cell population, but no alteration on B-cell or myeloid cell lineages (M. Radwanska, A. Cutler, M.S. Magez, C. Holscher, A. Bohms, B. Arendse, R. Kirsch, J. Alexander, J. Kaye, F. Brombacher, submitted for publication). Experiments were performed using two different models of exposure to S. mansoni egg antigens, in which the predominance of TH1 versus TH2 cells has critical influence on immunopathology and host survival.
The S. mansoni egg is a powerful inducer of type 2 inflammation and has been extensively studied for its ability to drive IL-4/IL-13-dependent granuloma formation in virtually any tissue in which it is lodged.28 Synchronous lung granuloma development can be studied after intravenous injection of eggs that become trapped in the small blood vessels of the airways. This model is characterized by a rapid influx of eosinophils, type 2 antibody production, mucous, goblet cell hyperplasia, and collagen deposition.3,5 Our experiments examined cohorts of mice at 7, 14, 21, and 28 days after challenge to determine what specific role was served by CD4+ T-cell IL-4Rα expression during granuloma maturation in the lung. Antibody-mediated IL-4 neutralization7 and IL-4 gene deletion have been shown to significantly decrease lung granuloma volume, but data in Figure 1, A and B, show that LckCreIL-4Rα−/lox mice had no impairment of granuloma size when compared with WT littermates at any of the time points examined. Because mouse CD4+ T cells only express the type 1 IL-4Rα and not the type 2 (IL-4Rα/IL-13Rα1),8 this result suggested that CD4+ T cells did not require an IL-4 signal to drive granuloma formation around S. mansoni eggs in the lung. This is a significant finding because fibrosis and granuloma size is dramatically reduced on IL-13 neutralization of IL-4-deficient mice,5 absence of T cells,29 STAT6 deficiency,30 or IL-4Rα-null mutation,23 greater than with IL-13 neutralization alone.5 Levels of IL-13 in lung tissue homogenates of LckCreIL-4Rα−/lox mice were lower than in WT controls but were still present (data not shown), which could have promoted the fibrotic response. The cell type(s) responsible for fibrosis in this model are unclear, but studies by Hesse and colleagues31 suggest that arginase induction after IL-4/IL-13-driven alternative macrophage activation was a major source of polyamines and hydroxyproline, the latter being important for collagen biosynthesis. This is unlikely because macrophage/neutrophil-specific IL-4Rα-deficient mice, impaired for alternative macrophage activation, also showed the formation of fibrotic granulomas similar to WT controls.23 Therefore, although the presence of CD4+ T cells is required, other resident or recruited cells, possibly fibroblast themselves, seem to be responsible for IL-4/IL-13-mediated collagen deposition.
Despite no significant impairment of fibrotic granuloma development, egg-challenged LckCreIL-4Rα−/lox mice had a clear impairment of lung eosinophil recruitment and goblet cell hyperplasia at all time points examined (Figure 2, A and B). The defective presence of such classic markers of TH2-driven lung inflammation was the first indication that CD4+ T cell IL-4 responsiveness was indeed required for some aspects of type 2 immunity. It was doubtful that the decrease of granuloma eosinophils and goblet cell hyperplasia was attributable to a direct deletion of the IL-4Rα on these cell types because the gene deletion was confirmed to be restricted to the T lymphocyte population (M. Radwanska, A. Cutler, M.S. Magez, C. Holscher, A. Bohms, B. Arendse, R. Kirsch, J. Alexander, J. Kaye, F. Brombacher, submitted for publication). Lung eosinophil recruitment is controlled by the combined actions of IL-5 and eotaxin,32 the latter being a STAT-6 target gene,33 and goblet cell hyperplasia is promoted by the TH2 cytokines IL-4, IL-9, and IL-13.34 This was a strong indication that perhaps some alteration in T-helper cell polarization occurred in the LckCreIL-4Rα−/lox mice that did not occur in the WT littermates.
Investigation of cytokine milieu in the egg microenvironment was performed by ELISA measurement of IL-4 and IFN-γ levels in lung homogenates from egg-challenged mice. Results shown in Figure 3, A and B, demonstrate a striking contrast between WT and LckCreIL-4Rα−/lox mice in the levels of these two major opposing cytokines. As expected, WT mice produced significant IL-4 in the lung and mediastinal lymph nodes (not shown), whereas LckCreIL-4Rα−/lox lung tissue was dominated by elevated IFN-γ. LckCreIL-4Rα−/lox mice also had significantly lower amounts of IL-5 in lung homogenates compared with WT mice (not shown), which provided at least a partial explanation for the fewer eosinophils in the lung of the former strain. CD4+ IL-4Rα expression was therefore required to maintain high IL-4 levels in the lung during the egg-induced response. It is known that STAT-6/IL-4Rα activation is nonessential for TH2 differentiation;24,35–37 but STAT-6 is an important antagonist for TH1 cell differentiation.25 Moreover, the short half-life of phosphorylated STAT-6 requires constant reactivation via IL-4 receptor signaling38 and provides an explanation for the deficiency of TH2 memory cells in STAT-6-deficient mice.33
Because the WT and LckCreIL-4Rα−/lox mice in our experiments were presensitized with eggs before the induction of lung granulomas, it is likely that our ELISA measurements reflected a memory/recall response that was strongly affected by the T-cell-specific IL-4Rα deletion. Also influenced was the SEA-specific antibody response, which revealed that LckCreIL-4Rα−/lox mice produced primarily an antigen-specific IgG2a response (Figure 3E) and had significantly lower levels of SEA-specific IgE compared with the WT (Figure 3D). It is generally accepted that T cell-B cell collaboration for antibody production is essential for appropriate affinity maturation and the qualitative nature of the antigen-specific antibody response. Combined, these data in the lung model demonstrated that when IL-4 signaling was prohibited in CD4+ T cells, the resultant phenotype of the type 2 response was dramatically impaired, affecting B-cell class switching, eosinophil recruitment, and goblet cell hyperplasia.
Based on these findings, we asked whether this skewed TH1 immune response after exposure to eggs in the lungs of LckCreIL-4Rα−/lox mice also occurred during natural infection. In this model, a low-level IFN-γ response predominates from 0 to 5 weeks after infection as worms develop into mature adults.39 However, on oviposition, parasite eggs released in mesenteric veins induce robust IL-4 production as they travel to liver and intestine where granulomas develop. Examination of the SEA-specific recall response of splenocytes at 7.5 weeks after infection showed an impairment of IL-4 and IL-13 production in LckCreIL-4Rα−/lox mice that was significant compared with that of WT littermates (Figure 5, A and B). This was accompanied by significantly increased IFN-γ release in LckCreIL-4Rα−/lox mice (Figure 5C). ELISPOT analysis was performed to determine whether such systemic recall responses actually reflected the cytokine milieu of the egg microenvironment. Figure 6A shows that there was a striking increase in the frequency of IFN-γ-producing liver granuloma cells in LckCreIL-4Rα−/lox mice, but in contrast to the splenocyte response, the number of IL-4-secreting cells was similar in WT and LckCreIL-4Rα−/lox mice (Figure 6B). Several reports have indicated that CD4+ T cells are not the only source of cytokines in the Schistosoma liver granuloma40 and eosinophils may be a primary source of TH2 cytokines in granulomas of this organ.41 In contrast to the lung model, the number of eosinophils in liver granulomas of LckCreIL-4Rα−/lox mice was only slightly decreased compared with WT mice (Table 1). However, whether eosinophils actually contributed to our IL-4 ELISPOT results was not determined. Despite this discrepancy between lung and liver models, the increase in IFN-γ-producing cells in LckCreIL-4Rα−/lox mice was a very consistent finding. Therefore, CD4+ T-cell-specific IL-4Rα deletion resulted in an expansion of antigen-specific IFN-γ effectors, both systemically and in the local microenvironment of parasite eggs.
S. mansoni infection of mice deficient for IL-4,18 IL-4/IL-13,16 or IL-4/IL-1015 causes rapid death through a seemingly common mechanism involving enhanced production of IFN-γ, NOS-2, tumor necrosis factor, and hepatocyte damage. This prompted us to investigate whether the TH1 predominance in the granuloma was accompanied by characteristic IFN-γ-driven effector functions. NOS-2, an IFN-γ target gene, is responsible for many of the anti-microbial effects of this cytokine.42 Measurement of NOS-2 activity at 7.5 weeks after infection revealed that LckCreIL-4Rα−/lox mice had significantly increased NOS-2 protein and mRNA levels when compared with the WT (Figures 4C and 6A). Curiously, NOS-2-positive staining was in close apposition to the parasite egg and overlapped with F4/80 staining (Figure 4C). NOS-2 production during murine schistosomiasis has been directly associated with the severity of hepatocellular damage and is thought to cause premature death in S. mansoni-infected IL-4-deficient mice.19 Our data agree partially with such conclusions, in that high serum levels of aspartate transaminase were detected in LckCreIL-4Rα−/lox mice (Figure 6D), but these mice did not experience enhanced mortality during infection (Figure 7A). This was paradoxical because TH1-driven IFN-γ responses are associated with a severe lethal form of acute schistosomiasis,43,44 and the severity of hepatic immunopathology during S. mansoni infection bears a strong genetic linkage to IFN-γ.22,43,44 However, LckCreIL-4Rα−/lox mice were no more susceptible than WT BALB/c mice, and in the experiments performed LckCreIL-4Rα−/lox mice were consistently more resistant to S. mansoni infection.
The mechanistic explanation for this resistance is at present unclear, but may be related to the immunobiology of this parasite. During natural infection of the mammalian host, eggs containing live larvae (miracidia) traverse the intestinal architecture as they pass into the gut lumen for completion of the life cycle.44 How overt septicemia is prevented while eggs exit into the fecal stream is poorly understood, but it does require an intact immune system.45 Previously we demonstrated that alternatively activated macrophages are essential in the egg passage event because mice deficient for IL-4Rα specifically on macrophages experienced heightened mortality rates associated with increased NOS-2 activity and sepsis.23 Alternatively activated macrophages were still present in LckCreIL-4Rα−/lox mice, albeit at lower levels (data not shown), but, interestingly, the serum endotoxin levels in LckCreIL-4Rα−/lox strain were significantly lower than WT littermates (Figure 7B). Mice in the BALB/c background are somewhat more susceptible to high-dose S. mansoni infection than other inbred mouse strains, and death is often accompanied by colitis-type pathology with bloody stool (unpublished observations). This implies the existence of additional cell populations or factor(s) that contribute to protection of the murine host from excessive intestinal injury as eggs pass into the gut lumen. One possible hypothesis is that LckCreIL-4Rα−/lox mice produce a heightened level of regulatory cytokines (IL-10, TGF-β) as a compensatory mechanism to counteract the increased numbers of IFN-γ precursors,46 which in turn maintains the intestinal barrier better than in the WT setting. In support of this hypothesis, we detected increased levels of TGF-β, known for its protective effects in the gut,47 in intestinal homogenates of LckCreIL-4Rα−/lox mice compared with the WT (manuscript in preparation).
In conclusion, exposure of CD4+ T-cell-specific IL-4Rα-deficient mice to S. mansoni eggs or to the complete life cycle uncovered the distinct role of this receptor in T helper cells. There were certain aspects of the TH2 response and TH1 cell expansion that were greatly dysregulated, but this was not sufficient for lethal disease. The novel mouse strain described here may provide a valuable tool in other infectious disease models in which disruption of TH1 versus TH2 polarization could be attained, while IL-4/IL-13 responsiveness on other lymphoid, myeloid, and nonbone marrow cell-derived lineages is preserved.
Acknowledgments
We thank Marilyn Tyler and Zoe Lotz for expert assistance with histology; Marquard Simpson, Reagon Peterson, Erica Smit, and Wendy Green for expert technical assistance; and Dr. Tony Cutler for critical reading of this manuscript.
Footnotes
Address reprint requests to Frank Brombacher, Institute of Infectious Disease and Molecular Medicine, Division of Immunology, Health Science Faculty, University of Cape Town, Werhner Beit South, Cape Town, South Africa. E-mail: fbrombac@uctgsh1.uct.ac.za.
Supported by the Wellcome Trust and Royal Society, UK; the Medical Research Council; and the National Research Foundation of South Africa.
M.L. and D.R.H. contributed equally to this article.
References
- Sandor M, Weinstock JV, Wynn TA. Granulomas in schistosome and mycobacterial infections: a model of local immune responses. Trends Immunol. 2003;24:44–52. doi: 10.1016/s1471-4906(02)00006-6. [DOI] [PubMed] [Google Scholar]
- Stavitsky AB. Regulation of granulomatous inflammation in experimental models of schistosomiasis. Infect Immun. 2004;72:1–12. doi: 10.1128/IAI.72.1.1-12.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Eltoum I, Cheever AW, Lewis FA, Gause WC, Sher A. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J Immunol. 1993;151:1430–1440. [PubMed] [Google Scholar]
- Sabin EA, Kopf MA, Pearce EJ. Schistosoma mansoni egg-induced early IL-4 production is dependent upon IL-5 and eosinophils. J Exp Med. 1996;184:1871–1878. doi: 10.1084/jem.184.5.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaramonte MG, Schopf LR, Neben TY, Cheever AW, Donaldson DD, Wynn TA. IL-13 is a key regulatory cytokine for Th2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansoni eggs. J Immunol. 1999;162:920–930. [PubMed] [Google Scholar]
- Jankovic D, Kullberg MC, Noben-Trauth N, Caspar P, Ward JM, Cheever AW, Paul WE, Sher A. Schistosome-infected IL-4 receptor knockout (KO) mice, in contrast to IL-4 KO mice, fail to develop granulomatous pathology while maintaining the same lymphokine expression profile. J Immunol. 1999;163:337–342. [PubMed] [Google Scholar]
- Eltoum IA, Wynn TA, Poindexter RW, Finkelman FD, Lewis FA, Sher A, Cheever AW. Suppressive effect of interleukin-4 neutralization differs for granulomas around Schistosoma mansoni eggs injected into mice compared with those around eggs laid in infected mice. Infect Immun. 1995;63:2532–2536. doi: 10.1128/iai.63.7.2532-2536.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- Schnyder B, Schnyder-Candrian S, Panski A, Bommel H, Heim M, Duschl A, Moser R. Phytochemical inhibition of interleukin-4-activated Stat6 and expression of VCAM-1. Biochem Biophys Res Commun. 2002;292:841–847. doi: 10.1006/bbrc.2002.6754. [DOI] [PubMed] [Google Scholar]
- Madden KB, Yeung KA, Zhao A, Gause WC, Finkelman FD, Katona IM, Urban JF, Jr, Shea-Donohue T. Enteric nematodes induce stereotypic STAT6-dependent alterations in intestinal epithelial cell function. J Immunol. 2004;172:5616–5621. doi: 10.4049/jimmunol.172.9.5616. [DOI] [PubMed] [Google Scholar]
- Zhao A, McDermott J, Urban JF, Jr, Gause W, Madden KB, Yeung KA, Morris SC, Finkelman FD, Shea-Donohue T. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol. 2003;171:948–954. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]
- Kelly-Welch AE, Melo ME, Smith E, Ford AQ, Haudenschild C, Noben-Trauth N, Keegan AD. Complex role of the IL-4 receptor alpha in a murine model of airway inflammation: expression of the IL-4 receptor alpha on nonlymphoid cells of bone marrow origin contributes to severity of inflammation. J Immunol. 2004;172:4545–4555. doi: 10.4049/jimmunol.172.7.4545. [DOI] [PubMed] [Google Scholar]
- Urban JF, Jr, Noben-Trauth N, Schopf L, Madden KB, Finkelman FD. Cutting edge: IL-4 receptor expression by non-bone marrow-derived cells is required to expel gastrointestinal nematode parasites. J Immunol. 2001;167:6078–6081. doi: 10.4049/jimmunol.167.11.6078. [DOI] [PubMed] [Google Scholar]
- Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF., Jr Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol. 2000;164:2585–2591. doi: 10.4049/jimmunol.164.5.2585. [DOI] [PubMed] [Google Scholar]
- Brunet LR, Finkelman FD, Cheever AW, Kopf MA, Pearce EJ. IL-4 protects against TNF-alpha-mediated cachexia and death during acute schistosomiasis. J Immunol. 1997;159:777–785. [PubMed] [Google Scholar]
- Patton EA, La Flamme AC, Pedras-Vasoncelos JA, Pearce EJ. Central role for interleukin-4 in regulating nitric oxide-mediated inhibition of T-cell proliferation and gamma interferon production in schistosomiasis. Infect Immun. 2002;70:177–184. doi: 10.1128/IAI.70.1.177-184.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Flamme AC, Patton EA, Bauman B, Pearce EJ. IL-4 plays a crucial role in regulating oxidative damage in the liver during schistosomiasis. J Immunol. 2001;166:1903–1911. doi: 10.4049/jimmunol.166.3.1903. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Eltoum I, Oswald IP, Cheever AW, Sher A. Endogenous interleukin 12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J Exp Med. 1994;179:1551–1561. doi: 10.1084/jem.179.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse M, Cheever AW, Jankovic D, Wynn TA. NOS-2 mediates the protective anti-inflammatory and antifibrotic effects of the Th1-inducing adjuvant, IL-12, in a Th2 model of granulomatous disease. Am J Pathol. 2000;157:945–955. doi: 10.1016/S0002-9440(10)64607-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutitzky LI, Hernandez HJ, Yim YS, Ricklan DE, Finger E, Mohan C, Peter I, Wakeland EK, Stadecker MJ. Enhanced egg-induced immunopathology correlates with high IFN-gamma in murine schistosomiasis: identification of two epistatic genetic intervals. J Immunol. 2005;174:435–440. doi: 10.4049/jimmunol.174.1.435. [DOI] [PubMed] [Google Scholar]
- Herbert DR, Holscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann H, Claussen B, Forster I, Brombacher F. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminum hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol. 1999;163:6448–6454. [PubMed] [Google Scholar]
- Metwali A, Blum A, Elliott DE, Weinstock JV. Interleukin-4 receptor alpha chain and STAT6 signaling inhibit gamma interferon but not Th2 cytokine expression within schistosome granulomas. Infect Immun. 2002;70:5651–5658. doi: 10.1128/IAI.70.10.5651-5658.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- Cheever AW, Lenzi JA, Lenzi HL, Andrade ZA. Experimental models of Schistosoma mansoni infection. Mem Inst Oswaldo Cruz. 2002;97:917–940. doi: 10.1590/s0074-02762002000700002. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Linette GP, Doughty BL, Byram JE, Von Lichtenberg F. In vivo T cell depletion regulates resistance and morbidity in murine schistosomiasis. J Immunol. 1987;139:919–926. [PubMed] [Google Scholar]
- Kaplan MH, Whitfield JR, Boros DL, Grusby MJ. Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J Immunol. 1998;160:1850–1856. [PubMed] [Google Scholar]
- Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, Pearce EJ, Wynn TA. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol. 2001;167:6533–6544. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- Mattes J, Yang M, Mahalingam S, Kuehr J, Webb DC, Simson L, Hogan SP, Koskinen A, McKenzie AN, Dent LA, Rothenberg ME, Matthaei KI, Young IG, Foster PS. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J Exp Med. 2002;195:1433–1444. doi: 10.1084/jem.20020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann N, Hogan SP, Mishra A, Brandt EB, Bodette TR, Pope SM, Finkelman FD, Rothenberg ME. Murine eotaxin-2: a constitutive eosinophil chemokine induced by allergen challenge and IL-4 overexpression. J Immunol. 2000;165:5839–5846. doi: 10.4049/jimmunol.165.10.5839. [DOI] [PubMed] [Google Scholar]
- Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, Schad GA, Scott P, Wu GD. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci USA. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noben-Trauth N, Shultz LD, Brombacher F, Urban JF, Jr, Gu H, Paul WE. An interleukin 4 (IL-4)-independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc Natl Acad Sci USA. 1997;94:10838–10843. doi: 10.1073/pnas.94.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic D, Kullberg MC, Noben-Trauth N, Caspar P, Paul WE, Sher A. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J Immunol. 2000;164:3047–3055. doi: 10.4049/jimmunol.164.6.3047. [DOI] [PubMed] [Google Scholar]
- Mohrs M, Holscher C, Brombacher F. Interleukin-4 receptor alpha-deficient BALB/c mice show an unimpaired T helper 2 polarization in response to Leishmania major infection. Infect Immun. 2000;68:1773–1780. doi: 10.1128/iai.68.4.1773-1780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews RP, Ericksen MB, Cunningham CM, Daines MO, Hershey GK. Analysis of the life cycle of stat6. Continuous cycling of STAT6 is required for IL-4 signaling. J Biol Chem. 2002;277:36563–36569. doi: 10.1074/jbc.M200986200. [DOI] [PubMed] [Google Scholar]
- Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- Metwali A, de Andres B, Blum A, Elliott D, Li J, Qadir K, Sandor M, Weinstock J. Th2-type granuloma development in acute murine schistosomiasis is only partly dependent on CD4+ T cells as the source of IL-4. Eur J Immunol. 2002;32:1242–1252. doi: 10.1002/1521-4141(200205)32:5<1242::AID-IMMU1242>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Rumbley CA, Sugaya H, Zekavat SA, El Refaei M, Perrin PJ, Phillips SM. Activated eosinophils are the major source of Th2-associated cytokines in the schistosome granuloma. J Immunol. 1999;162:1003–1009. [PubMed] [Google Scholar]
- Shi L, Jung YJ, Tyagi S, Gennaro ML, North RJ. Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc Natl Acad Sci USA. 2003;100:241–246. doi: 10.1073/pnas.0136863100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutitzky LI, Hernandez HJ, Stadecker MJ. Th1-polarizing immunization with egg antigens correlates with severe exacerbation of immunopathology and death in schistosome infection. Proc Natl Acad Sci USA. 2001;98:13243–13248. doi: 10.1073/pnas.231258498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever AW, Hoffmann KF, Wynn TA. Immunopathology of schistosomiasis mansoni in mice and men. Immunol Today. 2000;21:465–466. doi: 10.1016/s0167-5699(00)01626-1. [DOI] [PubMed] [Google Scholar]
- Dunne DW, Hassounah O, Musallam R, Lucas S, Pepys MB, Baltz M, Doenhoff M. Mechanisms of Schistosoma mansoni egg excretion: parasitological observations in immunosuppressed mice reconstituted with immune serum. Parasite Immunol. 1983;5:47–60. doi: 10.1111/j.1365-3024.1983.tb00722.x. [DOI] [PubMed] [Google Scholar]
- Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195:1499–1505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]