Abstract
The objective of this study was to assess the type and frequency of cardiac dysrhythmias occurring after routine ovariohysterectomy or orchidectomy in young, healthy dogs by using 2 anesthetic protocols (group I: propofol and isoflurane; group II: thiopental and halothane). Fifty dogs under 2 years of age, judged to be clinically normal by physical examination and standard electrocardiography, were evaluated by using 24-hour ambulatory electrocardiography. The most common dysrhythmias in the postoperative period were 2nd degree atrioventricular block (44%), ventricular premature complexes (44%), and atrial premature complexes (32%). For study purposes, more than 100 ventricular or atrial premature complexes per 24 hours, or any occurrence of R-on-T phenomenon, ventricular or atrial tachycardia were classified as clinically significant arrhythmias. Significant arrhythmias were observed in 9 dogs in the postoperative period, 5 of which were in group I and 4 in group II. All of these dogs were under 1 year of age. The R-on-T phenomenon occurred in 4 dogs in group II and 1 dog in group I. Results from this study show that significant arrhythmias, including R-on-T phenomenon, can occur in the perioperative period in young, healthy dogs undergoing routine surgeries with both protocols used.
Résumé
Prévalence des arythmies périopératoires chez 50 jeunes chiens en santé. L’objectif de cette étude était d’évaluer la variété et la fréquence des dysrythmies cardiaques survenant après une ovario-hystérectomie ou une orchidectomie chez de jeunes chiens en santé anesthésiés selon 2 protocoles (groupe I : propofol et isoflurane; groupe II : thiopental et halothane). Cinquante chiens âgés de moins de 2 ans, jugés cliniquement normaux après examen physique et électrocardiographie standard, ont été évalués pendant 24 heures par électrocardiographie ambulatoire. Les dysrythmies les plus fréquentes dans la période postopératoire étaient un blocage atrioventriculaire du 2e degré (44 %) et des extrasystoles auriculaires (32 %). Pour les besoins de l’étude, on a classé comme arythmie cliniquement significative plus de 100 extrasystoles ventriculaires ou auriculaires par 24 heures, n’importe quelle apparition de phénomène R/T de Smirk et Palmer et la tachycardie ventriculaire ou auriculaire. Des arythmies significatives ont été observées chez 9 chiens pendant la période postopératoire dont 5 étaient dans le groupe I et 4 dans le groupe II. Tous ces chiens avaient moins d’un an. Le phénomène R/T de Smirk et Palmer est survenu chez 4 chiens du groupe II et 1 chien du groupe I. Les résultats de cette étude montrent que des arythmies significatives, comprenant le phénomène R/T de Smirk et Palmer, peuvent survenir pendant la période postopératoire chez de jeunes chiens en santé soumis à des chirurgies de routine selon les 2 protocoles utilisés.
(Traduit par Docteur André Blouin)
Introduction
Cardiac arrhythmias are a complication often associated with general anesthesia in small animal patients (1,2). In a recent study, cardiac dysrhythmias were observed in 2.5% of anesthetized patients (3). Causes for anesthesia-induced arrhythmias include autonomic imbalances, anesthetic agents used, surgical stimulation, electrolyte and acid-base disturbances, alterations in blood pressure, or temperature and low oxygen delivery (4,5). Arrhythmias can lead to hypoxia, hypotension, compromised organ perfusion, and cardiac arrest (6). Fatal cardiac arrest during the recovery period in animals undergoing orchidectomy or ovariohysterectomy has been reported (2,3). Anecdotal reports of death occurring in the postoperative period after discharge from the hospital may also be related to cardiac dysrhythmias.
Postoperative cardiac arrhythmias are frequently encountered and monitored in veterinary patients after splenectomy, gastric-dilatation volvulus, and trauma (7–10). Recently, ambulatory electrocardiography (AECG), also known as Holter monitoring, has become a frequently used tool in veterinary medicine for protracted recording of cardiac electrical activity. This technique allows a noninvasive, continuous recording during normal activity in contrast to an electrocardiogram (ECG), which only records up to a few minutes and precludes normal activity. Holter monitoring has been used in veterinary medicine to detect transient arrhythmias, evaluate antiarrhythmic therapy, and screen for subclinical cardiomyopathy (11–14). Few studies have been performed to derive baseline AECG data for healthy dogs during normal activity (15–17). In 3 studies, continuous cardiac monitoring was used in the postoperative period of healthy dogs undergoing surgery (18–20). In 1 study, a variety of surgeries were performed, including thoracic, and various anesthetic protocols were used (19). Furthermore, serial routine ECG strips were recorded, so the information may not have been complete. Ambulatory electrocardiography was used in 2 more recent studies (18,20). One study evaluated patients undergoing orthopedic surgery under 2 different anesthetic protocols (18): one group was premedicated with levomethadone, induced with diazepam, and maintained on isoflurane; the other group was induced and maintained with propofol. The other study used Holter monitoring to assess heart rate characteristics after pre-medication with acepromazine or medetomidine in combination with butorphanol in dogs undergoing ovariohysterectomy (20). Dogs of all ages were used for all 3 studies.
The aim of this study was to quantify and characterize cardiac dysrhythmias observed in young, healthy dogs during the postoperative period following elective orchidectomy or ovariohysterectomy for which they were anesthetized with 2 commonly used anesthetic protocols. The null hypothesis was that young, healthy dogs would not show any clinically significant arrhythmias (SA).
Materials and methods
Animal recruitment
Fifty dogs were recruited to the study from the population presented to the Veterinary Teaching Hospital, Small Animal Clinic, Western College of Veterinary Medicine, University of Saskatchewan for orchidectomy or ovariohysterectomy. Inclusion criteria were that the dogs had to be under 2 y of age and without any previous medical history of cardiac disease. Doberman pinschers and boxers were excluded from the study because of their predisposition for cardiac disease and arrhythmias (21). Animals were judged to be clinically normal by physical examination, standard 6-lead ECG, and the following baseline tests: blood urea nitrogen, blood glucose, blood-packed cell volume, and blood total protein. Animals with any cardiovascular abnormalities were excluded from the study.
Dogs were randomly assigned to a group by drawing an anesthetic protocol sheet out of a box containing 25 sheets of each protocol. The research protocol was approved by the University of Saskatchewan Animal Care Committee.
Holter monitoring
A standard 6-lead ECG (Cardiofax; Nihon Kohden, Foothill Ranch, California, USA) was recorded with the dog in right lateral recumbency before Holter placement. Before anesthetic induction, a 3-lead Holter monitor (DL-700; LET Medical Systems, Miami Lakes, Florida, USA) was fitted to the dog, as previously described (13). After a recording period of at least 20 h, the Holter monitor was removed and the data transferred to a computer for evaluation.
Surgical treatment and anesthesia
All dogs were fasted overnight and deprived of water on the morning of surgery. A combination of acepromazine (Atravet; Ayerst, Guelph, Ontario), 0.05 mg/kg body weight (BW), hydromorphone (Hydromorphone; Sabex, Boucherville, Quebec), 0.1 mg/kg BW, and glycopyrrolate (Glycopyrrolate; Sabex), 0.01 mg/kg BW, was administered, IM, approximately 30 min prior to induction of anesthesia for both anesthetic protocols. For group I, general anesthesia was induced with propofol (Rapinovet; Schering-Plough Animal Health, Point Claire, Quebec), max 4 mg/kg BW, IV, to allow intubation of the trachea. Isoflurane (IsoFlo; Abbott Laboratories, Saint-Laurent, Quebec) in oxygen was administered to maintain anesthesia. For group II, anesthetic induction was accomplished with thiopental (Pentothal; Abbott Laboratories), max 10 mg/kg BW, IV, to allow intubation of the trachea and general anesthesia was maintained with halothane (Halothane BP; MTC Pharmaceuticals, Cambridge, Ontario) in oxygen. All dogs were allowed to ventilate spontaneously and were maintained at a surgical plane of anesthesia. Intravenous fluids (Normosol R; Abbott Laboratories), approximate rate 10 mL/kg BW/h, were administered during surgery. Standard techniques were used to evaluate anesthetic depth (eye position, palpebral reflexes), and indirect blood pressure was measured by using a Doppler probe placed on a distal artery with a sphygmomanometer and cuff. If necessary, dogs received a bolus of colloids (Dextran 75; 80 mg/mL, Abbott Laboratories), approximate rate 5 mL/kg BW, IV, to treat hypotension (defined as systolic pressure lower than 80 mm Hg). Meloxicam (Metacam; Boehringer Ingelheim, Burlington, Ontario), 0.2 mg/kg BW, SC, was administered to every animal during recovery. Dogs were regularly assessed for effective analgesia, and additional hydromorphone or oxymorphone was administered if deemed necessary.
Routine orchidectomy or ovariohysterectomy was performed, as previously described (22,23), by veterinarians employed by the WCVM or by senior veterinary students under direct supervision by these veterinarians.
Ambulatory electrocardiography analysis
The digital Holter recordings for each dog were evaluated for dysrhythmias by 2 observers (FD, AC) who were blinded to the protocol group. The data were analyzed semiautomatically (Holter Win P-V, version 5.60; Advanced Biosensor, Columbia, South Carolina, USA) and, additionally, all complexes were visually inspected as displayed on a monitor by the first author.
Electrocardiographic abnormalities and questionable complexes were marked and evaluated by an experienced electrocardiographer (AC). Where abnormalities were not consistent with a specific arrhythmia, complexes were excluded from further analysis. The recordings were analyzed to identify the following electrocardiographic abnormalities (see Table 1 for detailed criteria of identification): 2nd degree atrioventricular (AV) blocks (AVB), sinus pauses (SP), ventricular premature complexes (VPC, 2 or 3 consecutive VPC are referred to as VPC2/3), ventricular escape complexes (VEsC), ventricular tachycardia (VT), R-on-T phenomenon (RT), atrial premature complexes (APC, 2 or 3 consecutive APC are referred to as APC2/3), atrial tachycardia (AT), and advanced AVB (aAVB). The time between Holter placement and administration of premedication was defined as the preoperative period. The perioperative period was defined as the time between premedication and extubation, and the postoperative period was defined as the time from extubation until removal of the Holter. If single cardiac arrhythmias exceeded at least 200 events per period, the manual count was stopped and recorded as greater than 200.
Table 1.
Criteria for arrhythmia identification
| Arrhythmia | Criteria for identification |
|---|---|
| Second degree AV block (AVB) | Absence of QRST complexes following P wave, without complete AV-dissociation |
| Advanced AVB (aAVB) | More than one consecutive blocked atrial depolarization |
| Sinus pause (SP) | Intervals greater than two seconds between consecutive sinus complexes |
| Ventricular premature complexes (VPC) | Abnormal QRS complex that is bizarre, premature, large, and not associated with a P wave |
| Ventricular escape complexes (VEsC) | Abnormal QRS complex that follows a pause that is equal or longer than the preceding R-R interval, bizarre, large and not associated with a P wave |
| Ventricular tachycardia (VT) | Runs of more than 3 ventricular premature complexes occurring in succession at a rate of greater than 160 beats per minute |
| R-on-T phenomenon (RT) | Ventricular premature complex that falls on preceding beat’s T wave (RTs were also counted as VPC) |
| Atrial premature complexes (APC) | Normal QRS complex that follows a premature P wave, which is configured differently than sinus P waves. The prematurity exceeds the maximum observed heart rate after glycopyrrolate administration |
| Atrial tachycardia (AT) | Runs of more than 3 atrial premature complexes |
For the purpose of the study, clinically significant arrhythmias (SA) were defined as 1 or more of the following: more than 100 VPC or APC (including VPC2/3 or APC2/3) per 24 h (equalling a rate of 4.17/h) or any presence of RT, VT, or AT.
Statistical analysis
Descriptive and comparative statistics were computed by using analytical software (SPSS 12.0; SPSS, Chicago, Illinois, USA). Odds ratios and 95% confidence intervals were used to compare the relative proportions of each arrhythmia for animals in group II relative to group I. A single dichotomous variable was created indicating the presence or absence of SA. The relative proportions of dogs with SA under different anesthetic protocols and their sex were evaluated by using odds ratios (OR) and 95% confidence intervals. Mean surgery time for animals with and without SA, stratified by gender, was compared by using a t-test. A chi-squared test was used to compare the frequency of SA for dogs that did or did not receive additional pain medications.
Results
The study population consisted of various standard breed and mongrel dogs, including 24 females (12 per group) and 26 males (13 per group). Dogs weighed between 3.9 and 43 kg (mean: 17.7, s = 9.87). All dogs were between 5 mo and 2 y of age; 39 dogs were < 1 y (group I: 21; group II: 18), and 11 dogs were > 1 y (group I: 4; group II: 7). All dogs were monitored by using AECG during the peri- and postoperative periods; 40 dogs (group I: 19; group II: 21) were also monitored pre-operatively. The mean overall monitoring period, was 23.27 h (19.57 to 24.29 h). Data available for analysis ranged from 0.1 to 5.5 h (mean: 2.7, s = 1.2) for the preoperative period, 1.3 to 5.0 h (mean: 2.7, s = 0.9) for the perioperative period and 9.0 to 22.8 h (mean: 18.79, s = 2.5) for the postoperative period.
No significant complications (cardiac arrest, severe hypotension, or blood loss) occurred during the study period. Most dogs were required to wear an Elizabethan collar to prevent damage to the Holter monitor. Several dogs were subjectively considered stressed by the Elizabethan collar and hospitalization. Fifteen dogs (6 animals in group I; 9 in group II) were given additional pain control medication during the recovery period, consisting of 1 or 2 additional doses (0.05 or 0.1 mg/kg BW, IM or SC) of either hydromorphone or oxymorphone (Oxymorphone; Sabex), depending on the clinician’s preference. No statistical significance (P = 0.094) in the frequency of SA was found when dogs that did or did not receive additional pain control medications were compared.
Mean surgery time was significantly different between gender (P < 0.001) with females under general anesthesia for a mean of 115.1 min (s = 38.7), while males were anesthetized for a mean of 45.3 min (s = 21.2); further analysis of surgery time was stratified by gender. Mean surgery time for females (P = 0.65) or males (P = 0.30) experiencing SA in the postoperative period was not statistically different from that for animals not experiencing SA.
Frequencies of dysrhythmias for dogs in each group are presented in Table 2. Clinically significant arrhythmias were identified in 9 dogs. All dogs with SA were < 1 y. Of the 9 animals with SA, 5 were in group I and 4 in group II. Seven animals were females and 2 were males. No statistically significant difference in the OR of animals having SA during the postoperative period was observed between groups II and I (OR 0.76, 95% CI 0.18, 3.25), dogs < 1 y and > 1y (OR 3.87, 95% CI 0.45, 33.59), or females and males (OR 4.94, 95% CI 0.91, 26.77). Dogs in group II were approximately 3 times more likely to develop VPC (OR 2.71, 95% CI 0.85, 8.57), VPC2–3 (OR 2.88, 95% CI 0.50, 16.48), VT (OR 3.25, 95% CI 0.32, 33.41), and RT (OR 3.27, 95% CI 0.32, 33.84) than dogs in group I; however, these results were not statistically significant.
Table 2.
Arrhythmia prevalence for all dogs
| Preoperative
|
Perioperative
|
Postoperative
|
Statistical significance
|
|||||
|---|---|---|---|---|---|---|---|---|
| Arrhythmia | Group I n = 19 | Group II n = 21 | Group I n = 25 | Group II n = 25 | Group I n = 25 | Group II n = 25 | ORa | CI (95%) |
| AVB | 1 | 0 | 12 | 14 | 10 | 12 | 1.39 | 0.45, 4.25 |
| aAVB | 0 | 0 | 1 | 4 | 1 | 1 | 1 | 0.06, 16.93 |
| SP | 1 | 1 | 1 | 1 | 9 | 8 | 0.84 | 0.26, 2.70 |
| VPC | 1 | 3 | 3 | 2 | 8 | 14 | 2.71 | 0.85, 8.57 |
| VPC 2/3 | 0 | 0 | 1 | 0 | 2 | 5 | 2.88 | 0.50, 16.48 |
| VEsC | 0 | 0 | 0 | 2 | 3 | 3 | 1 | 0.18, 5.51 |
| VT | 0 | 0 | 0 | 0 | 0 | 2 | 3.25 | 0.32, 33.41 |
| RT | 1 | 1 | 0 | 1 | 1 | 3 | 3.27 | 0.32, 33.84 |
| APC | 1 | 0 | 3 | 2 | 9 | 7 | 0.69 | 0.21, 2.29 |
| APC 2/3 | 0 | 0 | 0 | 0 | 2 | 0 | 0.31 | 0.03, 3.16 |
| AT | 0 | 0 | 1 | 0 | 2 | 0 | 0.31 | 0.03, 3.16 |
Comparison of arrhythmia prevalence for group II in respect to group I in the postoperative period
AVB = second degree atrioventricular (AV) block; aAVB = advanced AVB; SP = sinus pause; VPC = ventricular premature complex; VPC 2/3 = 2 or 3 consecutive VPCs; VEsC = ventricular escape complex; VT = ventricular tachycardia; RT = R-on-T phenomenon; APC = atrial premature complex; APC 2/3 = 2 or 3 consecutive APCs; AT = atrial tachycardia
Heart rate was recorded continuously for all animals (n = 50) by the Holter monitor. Maximum heart rates were 214 to 281 (mean 247) beats/min (bpm); minimum overall heart rates were observed during the postoperative period and ranged from 33 to 78 (mean 49) bpm for all dogs. The maximum heart rate was reached by 1 dog during the preoperative period (group II), by 5 dogs during the perioperative period (4 in group I, 1 in group II), and by the remaining dogs (n = 44) in the postoperative period.
Sinus pauses were observed mainly in the postoperative period (total: 17/50, 34%). Only 1 dog developed more than 200 SP (during the postoperative period). This dog was in group I and also had the longest SP (4.7 s) and several SP in the pre- and perioperative periods.
Second degree AV blocks were most commonly identified during the peri- and postoperative periods (total peri: 26/50, 52%; total post: 22/50). Nine dogs (1 in group I, 8 in group II) had more than 200 AVB in the perioperative period. Most of the AVB in the perioperative period were observed within 20 min of administration of the premedicament drugs. Four dogs (3 in group I, 1 in group II) had more than 200 AVB in the postoperative period. Advanced AVB were infrequently observed in 5 dogs in the perioperative period (total: 5/40) and 2 dogs in the postoperative period (total: 2/50, 4%).
Ventricular premature complexes were most commonly observed in the postoperative period (total: 22/50, 44%). All dogs with VPC in the preoperative period (n = 4) continued to have this electrocardiographic abnormality in the postoperative period. Seven dogs had 20 or more VPC in the postoperative period. Six of these dogs were in group II. The only dog (#12) in group I was also the only dog that developed more than 200 VPC in the postoperative period. This dog also showed bigeminy and trigeminy in the postoperative periods (see Table 3 for other abnormalities). Three dogs showed VPC only in the postoperative period, 2 in all 3 periods, and 1 only in the pre- and postoperative periods. Four dogs also had further ventricular abnormalities (VT, RT). Only 2 dogs (#5, #23) both in group II, showed more than 2 VPC2/3 in the postoperative period and both animals also showed VT, RT.
Table 3.
Detailed results for all dogs with significant arrthymias
| Dog Number | 1 | 5 | 8 | 12 | 23 | 32 | 47 | 48 | 49 |
|---|---|---|---|---|---|---|---|---|---|
| Sex | female | Female | female | Female | male | female | male | female | female |
| Weight | 3.90 | 21.50 | 11.80 | 25.90 | 20.00 | 24.60 | 26.50 | 11.60 | 18.40 |
| Age | < 1y | < 1y | < 1y | < 1y | < 1y | < 1y | < 1y | < 1y | < 1y |
| Protocol | Isoflurane | Halothane | Halothane | Isoflurane | Halothane | Isoflurane | Halothane | Isoflurane | Isoflurane |
| Complications | none | none | hypotension | none | none | none | none | none | none |
| Sx time (min) | 90 | 100 | 75 | 120 | 30 | 120 | 30 | 120 | 140 |
| Add. drugs | A, C | A | A, D | none | none | A | none | none | B |
| Timea (min) | 0/153/1265 | 5/175/1235 | 170/178/1050 | 0/150/1260 | 20/140/795 | 0/240/1140 | 150/85/1135 | 185/98/1040 | 130/160/1070 |
| Max HR | 231 | 233 | 256 | 214 | 266 | 251 | 275 | 260 | 270 |
| Min HR | 46 | 58 | 73 | 52 | 78 | 40 | 59 | 50 | 53 |
| AVBa | -/47/4 | 0/0/0 | 0/maxb/99 | -/0/0 | 0/0/0 | -/1/0 | 0/0/0 | 10/max/max | 0/13/0 |
| VPCa | -/0/2 | 0/0/178 | 0/0/3 | -/154/max | 4/5/152 | -/0/0 | 55/5/40 | 0/0/7 | 08/01/10 |
| VPC 2-3a | -/0/0 | 0/0/60 | 0/0/0 | -/2/0 | 0/0/57 | -/0/0 | 0/0/1 | 0/0/0 | 0/0/0 |
| VTa | -/0/0 | 0/0/61c | 0/0/0 | -/0/0 | 0/0/30d | -/0/0 | 0/0/0 | 0/0/0 | 0/0/0 |
| RTa | -/0/0 | 0/0/1 | 0/0/2 | -/0/0 | 0/0/4 | -/0/0 | 20/05/16 | 0/0/0 | 1/0/1 |
| VEsCa | -/0/0 | 0/20/27 | 0/0/0 | -/0/0 | 0/0/0 | -/0/0 | 0/0/61 | 0/0/161 | 0/0/0 |
| APCa | -/1/77 | 0/1/7 | 0/0/0 | -/0/0 | 0/0/0 | -/0/2 | 0/0/0 | 95/4/max | 0/0/82 |
| APC 2-3a | -/0/24 | 0/0/0 | 0/0/0 | -/0/0 | 0/0/0 | -/0/0 | 0/0/0 | 0/0/0 | 0/0/34 |
| ATa | -/0/36e | 0/0/0 | 0/0/0 | -/0/0 | 0/0/0 | -/1f/0 | 0/0/0 | 0/0/0 | 0/0/16f |
A = Additional dose of hydromorphone at 0.05 mg/kg BW postoperatively; B = Additional dose of hydromorphone at 0.1 mg/kg BW postoperatively; C = Additional dose of glycopyrrolate at 0.01 mg/kg BW perioperatively; D = Dextran perioperatively; max = more than 200; AVB = second degree AV block; VPC = ventricular premature complex; VPC 2-3 = 2 or 3 consecutive VPCs; VT = ventricular tachycardia; RT = R-on-T phenomenon; VEsC = ventricular escape complex; APC = atrial premature complex; APC 2-3 = 2 or 3 consecutive APCs; AT = atrial tachycardia
Available ambulatory electrocardiography data for the pre-/peri-/and postoperative period
Advanced atrioventricular block
Longest duration: 28 s, max HR: 178
Longest duration: 11 s, max HR: 206
Max number of beats: 15
Paroxysmal AT
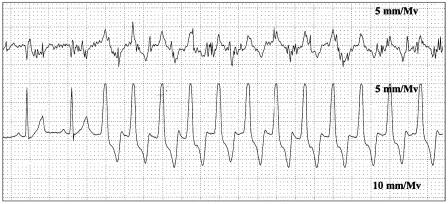
Ventricular tachycardia was identified exclusively in the postoperative period. Both dogs showing this abnormality were under < 1 y and in group II. One dog (#23) had 30 episodes of VT with a maximum heart rate of 206 and the longest run was 11 s (see Figure 1). The other dog (#5) had 61 episodes of VT with a maximum heart rate of 178 and the longest run was 28 s.
Figure 1.
Ventricular tachycardia (dog # 23) Paper speed: 25 mm/s, sensitivity: 5 mm/mv
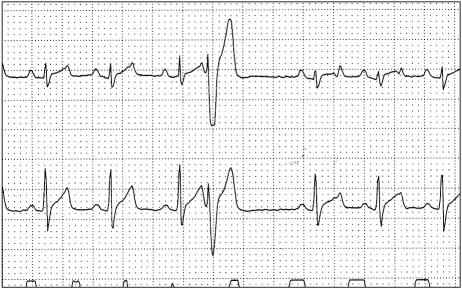
The R-on-T phenomenon was observed in 5 dogs. All 5 dogs with RT were < 1 y; 2 were males, 3 were females. The dog with the highest number of RT (#47) was in group II and had 20, 5, and 16 RT, respectively, in the pre-, peri-, and postoperative periods (see Figure 2). The only dog in group I that showed RT (#49) did so once in the pre- and postoperative period.
Figure 2.
R-on-T phenomenon (dog #47) Paper speed: 25 mm/s, sensitivity: 5 mm/mv
Ventricular escape complexes were only identified in the peri- and postoperative periods (total peri: 2/50, 4%; total post: 6/50, 12%). Only 1 dog showed VEsC as the only ventricular abnormality. Four dogs had more than 20 VEsC in the postoperative period; they were divided equally among groups I and II.
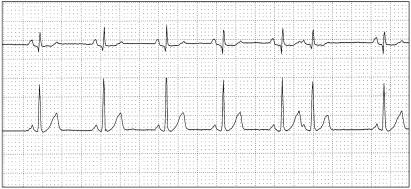
Atrial premature complexes were commonly observed in the postoperative period (total: 16/50, 32%). Only 1 dog (#48) showed APC in the preoperative period. This dog also had APC (see Figure 3) in the peri- and postoperative periods. None of the dogs (n = 5) with APC in the perioperative period had more than 4 APC. Five dogs developed more than 20 APC in the postoperative period. Two dogs (#1, #49) developed APC2/3 in the postoperative period. Both dogs were in group I and they also developed AT.
Figure 3.
Atrial premature complex (dog # 48) Paper speed: 25 mm/s, sensitivity: 5 mm/mv
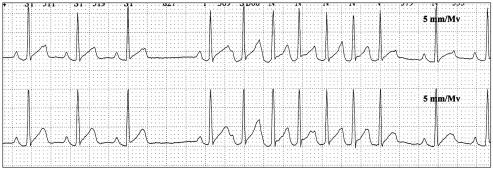
Atrial tachycardia was observed in 3 dogs. One dog (#1) had 36 episodes in the postoperative period. The maximum number of beats in 1 run was 15. This dog received a 2nd dose of glycopyrrolate in the perioperative period. Another dog (#49) had 16 episodes of AT (see Figure 4) in the postoperative period with a maximum of 6 beats. The last dog (#32) showed paroxysmal AT in the perioperative period.
Figure 4.
Atrial tachycardia (dog # 49) Paper speed: 25 mm/s, sensitivity: 5 mm/mv
Discussion
In this study, clinically significant arrhythmias (SA) were frequently, and only, observed in dogs < 1 y. The cause for the high frequency of postoperative arrhythmias remains unknown. Possible reasons include the anesthetic protocol used, age of the dogs, cardiac disease, electrolyte and acid-base imbalance, hypoxia, hypercapnia, pain, and increased catecholamine levels due to stress or excitement.
Previous studies evaluating healthy dogs in the postoperative period included dogs of varying ages, but predominantly adults, and the results were not stratified by age (18,20,24). Single VPC were commonly reported; however, RT and AT were not observed. Ventricular tachycardia was reported in only 1 study (18). In only 1 of the previous studies was VT noted; however, no details regarding the age of the dogs with VT are available. A recent study evaluated dogs between 2 and 7 y undergoing routine ovariohysterectomy (20). No VT, RT, or AT were observed in any of the dogs. Relative to these studies our population was comprised of markedly younger dogs. No statistical significance was found between dogs over and under 1 y; however, the wide confidence intervals indicate a lack of power. The conclusion that a higher frequency of arrhythmias may exist in younger dogs cannot be made based on our data; however, further investigation into the true prevalence of arrhythmias in dogs < 1 y is warranted.
The anesthetic protocol for our study varied with respect to induction and maintenance drugs. The same medications were used for premedication in both groups. That no statistical significance was identified when anesthetic protocols were compared may be due to lack of power or because the observed dysrhythmias were related to the drugs used for premedication. A variety of dysrhythmias, including VPC, AVB, bradycardia, and sinoatrial arrest, may be related to the combination of morphine, acepromazine, and glycopyrrolate used for premedication (25–28). In previous studies evaluating healthy dogs in the postoperative period with AECG, a variety of anesthetic pre-medications were used, including acepromazine, medetomidine, butorphanol, levomethadone, and diazepam (18,20); however, glycopyrrolate was not used. Glycopyrrolate has been reported to result in bradycardia and AVB, and may cause VPC (27,28). Because all dogs in this study received the same protocol, the anesthetic premedication could not be evaluated statistically.
Stress may have been associated with the Elizabethan collar and hospitalization. However, 1 study reported that an Elizabethan collar does not affect AECG results (29), but this study only evaluated heart rate and spontaneous activity. Two of the dogs categorized as SA on the basis of VPC occurrence had multiple VPC preoperatively. This suggests that observed postoperative arrhythmias may not be exclusively due to surgery, anesthesia, or choice of anesthetic drugs, but to individual predisposition. All dogs categorized as SA because of supraventricular arrhythmias were < 1 y. One study evaluating maturational changes in canine hearts suggested that the immature heart might be more prone to certain supraventricular arrhythmias (30). Other causes for dysrhythmias, such as cardiac disease, electrolyte and acid-base imbalance, hypoxia, hypercapnia, and pain, however, cannot be completely ruled out.
The definition of SA was based on previously published normative data and guidelines for treatment of cardiac arrhythmias (31). Existing AECG data during regular activity suggest that some VPC should be considered normal for healthy animals. In 1 study, 28/228 clinically normal beagles had between 1 and 9 VPC per day, 2 dogs showed 66 and 70 complexes, and 1 female showed VT (16). In another study that included 2 boxers, 10 out of 16 dogs showed occurrence of VPC in a 24-hour period, 3 dogs showed more than 10 VPC, and the maximum number observed was 52 (17). Meurs et al (32) evaluated 50 dogs and found 16 dogs had VPC with a maximum daily number of 24. Based on these results, we arbitrarily picked a number for VPC (100/24h) and any occurrence of VT as a cut-off for classification of dogs into the SA category because of ventricular arrhythmias. Very few data are available for the frequency of APC in normal dogs. In 1 study (18), 12 out of 60 dogs developed APC 5 d after orthopedic surgery. Only 1 dog out of the 60 developed more than 10 APC. None of the dogs showed evidence of AT. Because of the lack of normative data for this type of arrhythmia in veterinary medicine, we arbitrarily picked the same cut-off as for ventricular arrhythmias for classification of dogs into the SA category because of APC (100/24h) or AT (any occurrence). Dogs with RT were classified into the SA group because this arrhythmia is believed to imply electrical instability and an indication for treatment (31). Furthermore, in none of the previously performed studies was any occurrence of RT reported.
One of the most common arrhythmias in the postoperative period was VPC. Ventricular ectopic complexes were primarily observed in the postoperative period. Not surprisingly, dogs receiving the thiopental/halothane protocol were approximately 3 times more likely to develop VPC, VT, and RT than were dogs receiving the propofol/isoflurane protocol. Thiobarbiturates and halothane have the potential to trigger ventricular arrhythmias by cardiac sensitization (6,33–38). In some instances, however, ventricular arrhythmias did not occur shortly after surgery but were observed in the 2nd half of the monitoring period, several hours after surgery. The reason for this remains unclear, but potentiation of halothane-induced arrhythmias by thiopental has been described to extend well beyond the clinical effects (38,39). On the other hand, ventricular ectopy has been reported following IV injection of glycopyrrolate (27). Ventricular premature complex occurrence after administration of propofol has also been reported in 1 dog that received diazepam as well (40). Increased catecholamine levels due to stress or excitement triggering ventricular arrhythmias (4,41) could also be a reason for the observed arrhythmias. In our study, 44% of the dogs developed VPC in the postoperative period, but only 16% had more than 10 VPC. Five dogs were categorized as SA because of ventricular ectopic complexes. Five dogs showed RT and 2 of these dogs also had VT. Four out of the 5 dogs with RT were in group II. Interestingly, 2 dogs (including the only dog in group I) showed RT in the preoperative period, implying no relationship to anesthesia or surgery. None of the dogs showed any clinical signs or abnormalities on routine cardiac auscultation and ECG assessment. Because no further diagnostic tests were pursued, primary cardiac disease cannot be completely excluded in any of the dogs.
Supraventricular arrhythmias were the second most common arrhythmias in this study. Atrial premature complexes are often associated with heart disease, sepsis, neoplasia, and drug toxicity, but they can also be detected in normal dogs (42). In an experimental study, halothane has been shown to sensitize the heart to epinephrine-induced atrial dysrhythmias (43). In another study, the effects of halothane and isoflurane on induction of atrial fibrillation in anesthetized dogs were evaluated, and it was concluded that isoflurane provides more antifibrillatory effects in atrial tissue (44). Conversely, in our study, all dogs but 1 in the SA category that showed APC or AT were in group I. One dog in our study was found to have sustained paroxysmal AT throughout the whole monitoring period. This dog received an additional dose of glycopyrrolate in the perioperative period. Because the AT persisted until the end of the monitoring period, an association with this medication seems unlikely but cannot be completely ruled out.
Because surgery time was significantly different between sexes, a stratified analysis was performed to evaluate the relationship between surgery time and the presence of SA in the postoperative period. Surgery time for either gender did not differ significantly between those individuals experiencing SA and those that did not. Of the 9 animals showing SA, 7 were females. Even though no statistical difference was found between male and female dogs, a potential female predisposition could be related to a greater amount of soft tissue destruction with ovariohysterectomy compared with orchidectomy, resulting in a higher pain level. Furthermore, ventricular arrhythmias are reported to be a frequently encountered abnormality during and after anesthesia in dogs undergoing other abdominal surgeries, such as for splenectomy or for gastric dilatation-volvulus (6,8,9). Alternatively, longer duration of anesthesia in females relative to males may predispose to postoperative SA.
The heart rate variation and frequency of sinus pauses observed in our study are comparable with those seen in previous studies and are thought to be related to changes in vagosympathetic tone during the wake-sleep cycle (16,17). It is important to note that heart rate measurements were obtained only by the Holter monitor; since analysis software is designed for cardiac evaluation in humans, interpretation errors are possible when it is used for veterinary patients (45). Second degree AV block was observed in 44% of the animals in our study. Second degree AV block has been associated with increased vagal tone and has been reported to occur frequently (up to 100%) even in normal dogs (46). The high frequency of this conduction defect in the perioperative period could also be related to the premedicament drugs. Morphine and acepromazine have both been shown to enhance vagal tone (25,26). Second degree AV block immediately after glycopyrrolate administration was a common finding in 2 studies (27,28). In our study, a close relationship between the occurrence of AVB and the administration of the premedicament drugs was observed during analysis. Therefore, it was concluded that many of the AVB observed in the perioperative period were induced by 1, or a combination, of the drugs used for premedication.
The emphasis of this study was on the postoperative period because normative data for arrhythmia prevalence in healthy dogs during regular activity already exist (16,17,32). In retrospect, after observation of the high frequency of SA, it would have been useful to obtain a full 24-hour AECG before surgery to obtain normative data for each dog. These data would have been helpful to determine whether the frequency of cardiac arrhythmias was truly higher after anesthesia and surgical intervention or whether it was related to the age of the dogs used for this study. It also might have been helpful to discharge animals from the hospital to exclude environmental factors, such as increased stress during hospitalization. No statistical significance was identified for any comparison between groups, implying that the anesthetic regime used for induction and maintenance may not be the reason for the observed arrhythmias. This implication is supported by the even distribution of dogs with SA between the 2 protocols. On the other hand, confidence intervals around many estimates were wide, suggesting a lack of power. Although drugs used for group I did not appear to change the overall frequency of SA, more serious ventricular arrhythmias were observed in dogs in group II. This should be considered when selecting the anesthetic protocol, especially in a patient with pre-existing arrhythmias. No significant complications occurred in any of the study dogs and no antiarrhythmic treatment was instituted.
Clinicians should be aware of the potential for the occurrence of serious cardiac arrhythmias after routine surgery in young, healthy dogs with both of the anesthetic protocols used in this study. CVJ
Footnotes
Dr. Duerr’s current address is Department of Clinical Sciences, Veterinary Medical Center, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, 300 West Drake Road, Fort Collins, Colorado 80523-1678, USA.
This study was supported by the Companion Animal Health Fund, Western College of Veterinary Medicine.
References
- 1.Dodman NH, Lamb LA. Survey of small animal anesthetic practice in Vermont. J Am Anim Hosp Assoc. 1992;28:439–445. [Google Scholar]
- 2.Dyson DH, Maxie MG, Schnurr D. Morbidity and mortality associated with anesthetic management in small animal veterinary practice in Ontario. J Am Anim Hosp Assoc. 1998;34:325–335. doi: 10.5326/15473317-34-4-325. [DOI] [PubMed] [Google Scholar]
- 3.Gaynor JS, Dunlop CI, Wagner AE, Wertz EM, Golden AE, Demme WC. Complications and mortality associated with anesthesia in dogs and cats. J Am Anim Hosp Assoc. 1999;35:13–17. doi: 10.5326/15473317-35-1-13. [DOI] [PubMed] [Google Scholar]
- 4.Kushner LI, Calvert CA. Perianesthetic arrhythmias. Compend Contin Educ Vet Pract. 2000;22:61–72. [Google Scholar]
- 5.Atlee JL. Perioperative cardiac dysrhythmias: Diagnosis and management. Anesthesiology. 1997;86:1397–1424. doi: 10.1097/00000542-199706000-00026. [DOI] [PubMed] [Google Scholar]
- 6.Cohen RB, Tilley LP. Cardiac arrhythmias in the anesthetized patient. Vet Clin North Am Small Anim Pract. 1979;9:155–167. doi: 10.1016/s0195-5616(79)50027-8. [DOI] [PubMed] [Google Scholar]
- 7.Snyder PS, Cooke KL, Murphy ST, Shaw NG, Lewis DD, Lanz OI. Electrocardiographic findings in dogs with motor vehicle-related trauma. J Am Anim Hosp Assoc. 2001;37:55–63. doi: 10.5326/15473317-37-1-55. [DOI] [PubMed] [Google Scholar]
- 8.Whitney WO. Complications associated with the medical and surgical management of gastric dilatation-volvulus in the dog. Probl Vet Med. 1989;1:268–280. [PubMed] [Google Scholar]
- 9.Marino DJ, Matthiesen DT, Fox PR, Lesser MB, Stamoulis ME. Ventricular arrhythmias in dogs undergoing splenectomy: A prospective study. Vet Surg. 1994;23:101–106. doi: 10.1111/j.1532-950x.1994.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 10.Brourman JD, Schertel ER, Allen DA, Birchard SJ, DeHoff WD. Factors associated with perioperative mortality in dogs with surgically managed gastric dilatation-volvulus: 137 cases (1988–1993) J Am Vet Med Assoc. 1996;208:1855–1858. [PubMed] [Google Scholar]
- 11.Calvert CA, Pickus CW, Jacobs GJ. Efficacy and toxicity of tocainide for the treatment of ventricular tachyarrhythmias in Doberman pinschers with occult cardiomyopathy. J Vet Intern Med. 1996;10:235–240. doi: 10.1111/j.1939-1676.1996.tb02055.x. [DOI] [PubMed] [Google Scholar]
- 12.Calvert CA, Jacobs G, Pickus CW, Smith DD. Results of ambulatory electrocardiography in overtly healthy Doberman pinschers with echocardiographic abnormalities. J Am Vet Med Assoc. 2000;217:1328–1332. doi: 10.2460/javma.2000.217.1328. [DOI] [PubMed] [Google Scholar]
- 13.Ware WA. Practical use of Holter monitoring. Compend Contin Educ Vet Pract. 1998;20:167–177. [Google Scholar]
- 14.Goodwin JK. Holter monitoring and cardiac event recording. Vet Clin North Am Small Anim Pract. 1998;28:1391–1407. doi: 10.1016/s0195-5616(98)50128-3. [DOI] [PubMed] [Google Scholar]
- 15.Meurs KM, Spier AW, Wright NA, Hamlin RL. Comparison of in-hospital versus 24-hour ambulatory electrocardiography for detection of ventricular premature complexes in mature Boxers. J Am Vet Med Assoc. 2001;218:222–224. doi: 10.2460/javma.2001.218.222. [DOI] [PubMed] [Google Scholar]
- 16.Ulloa HM, Houston BJ, Altrogge DM. Arrhythmia prevalence during ambulatory electrocardiographic monitoring of beagles. Am J Vet Res. 1995;56:275–281. [PubMed] [Google Scholar]
- 17.Hall LW, Dunn JK, Delaney M, Shapiro LM. Ambulatory electrocardiography in dogs. Vet Rec. 1991;129:213–216. doi: 10.1136/vr.129.10.213. [DOI] [PubMed] [Google Scholar]
- 18.Buhl K, Kersten U, Kramer S, Mischke R, Fedrowitz M, Nolte I. Incidence of post-anaesthetic arrhythmias in dogs. J Small Anim Pract. 2005;46:131–138. doi: 10.1111/j.1748-5827.2005.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 19.Buss DD, Hess RE, Webb AI, Spencer KR. Incidence of post-anaesthetic arrhythmias in the dog. J Small Anim Pract. 1982;23:399–404. [Google Scholar]
- 20.Vaisanen MA, Vainio OM, Raekallio MR, Hietanen H, Huikuri HV. Results of 24-hour ambulatory electrocardiography in dogs undergoing ovariohysterectomy following premedication with medetomidine or acepromazine. J Am Vet Med Assoc. 2005;226:738–745. doi: 10.2460/javma.2005.226.738. [DOI] [PubMed] [Google Scholar]
- 21.Broschk C, Distl O. (Dilated cardiomyopathy (DCM) in dogs — pathological, clinical, diagnosis and genetic aspects) Dtsch Tierärztl Wochenschr. 2005;112:380–385. [PubMed] [Google Scholar]
- 22.Stone EA. Reproductive system — Ovary and uterus — Ovariohysterectomy. In: Slatter D, ed. Textbook of Small Animal Surgery. 3rd ed. vol 2. Philadelphia: WB Saunders, 2003:1495–1498.
- 23.Boothe HW. Reproductive system — Testes and epididymides — Orchidectomy. In: Slatter D, ed. Textbook of Small Animal Surgery. 3rd ed. vol 2. Philadelphia: WB Saunders, 2003:1527–1529.
- 24.Buss DD, Hess RE, Webb AI, Spencer KR. Incidence of post-anaesthetic arrhythmias in the dog. J Small Anim Pract. 1982;23:399–404. [Google Scholar]
- 25.DeSilva RA, Verrier RL, Lown B. Protective effect of the vagotonic action of morphine sulphate on ventricular vulnerability. Cardiovasc Res. 1978;12:167–172. doi: 10.1093/cvr/12.3.167. [DOI] [PubMed] [Google Scholar]
- 26.Popovic NA, Mullane JF, Yhap EO. Effects of acetylpromazine male-ate on certain cardiorespiratory responses in dogs. Am J Vet Res. 1972;33:1819–1824. [PubMed] [Google Scholar]
- 27.Richards DLS, Clutton RE, Boyd C. Electrocardiographic findings following intravenous glycopyrrolate to sedated dogs: A comparison with atropine. J Assoc Vet Anaesth. 1989;16:46–50. [Google Scholar]
- 28.Dyson DH, James-Davies R. Dose effect and benefits of glycopyrrolate in the treatment of bradycardia in anesthetized dogs. Can Vet J. 1999;40:327–331. [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada M, Tokuriki M. Effects of a canine Elizabethan collar on ambulatory electrocardiogram recorded by a Holter recording system and spontaneous activities measured continuously by an accelerometer in Beagle dogs. J Vet Med Sci. 2000;62:549–552. doi: 10.1292/jvms.62.549. [DOI] [PubMed] [Google Scholar]
- 30.Pickoff AS, Singh S, Flinn CJ, Torres E, Ezrin AM, Gelband H. Maturational changes in ventriculoatrial conduction in the intact canine heart. J Am Coll Cardiol. 1984;3:162–168. doi: 10.1016/s0735-1097(84)80444-1. [DOI] [PubMed] [Google Scholar]
- 31.Moise NS. Diagnosis and management of canine arrhythmias. In: Fox PR, Sisson D, Moise NS, eds. Textbook of Canine and Feline Cardiology. 2nd ed. Philadelphia: WB Saunders, 1999:338–386.
- 32.Meurs KM, Spier AW, Wright NA, Hamlin RL. Use of ambulatory electrocardiography for detection of ventricular premature complexes in healthy dogs. J Am Vet Med Assoc. 2001;218:1291–1292. doi: 10.2460/javma.2001.218.1291. [DOI] [PubMed] [Google Scholar]
- 33.Christensen LQ, Bonde J, Kampmann JP. Drug interactions with inhalational anaesthetics. Acta Anaesthesiol Scand. 1993;37:231–244. doi: 10.1111/j.1399-6576.1993.tb03707.x. [DOI] [PubMed] [Google Scholar]
- 34.Joas TA, Stevens WC. Comparison of the arrhythmic doses of epinephrine during forane, halothane, and fluroxene anesthesia in dogs. Anesthesiology. 1971;35:48–53. doi: 10.1097/00000542-197107000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Bednarski RM, Muir WW., 3rd Ventricular arrhythmogenic dose of epinephrine in dogs and cats anesthetized with tiletamine/zolazepam and halothane. Am J Vet Res. 1990;51:1468–1470. [PubMed] [Google Scholar]
- 36.Bufalari A, Miller SM, Giannoni C, Short CE. The use of propofol as an induction agent for halothane and isoflurane anesthesia in dogs. J Am Anim Hosp Assoc. 1998;34:84–91. doi: 10.5326/15473317-34-1-84. [DOI] [PubMed] [Google Scholar]
- 37.Muir WW. Thiobarbiturate-induced dysrhythmias: The role of heart rate and autonomic imbalance. Am J Vet Res. 1977;38:1377–1381. [PubMed] [Google Scholar]
- 38.Bednarski RM, Majors LJ, Atlee JL. Epinephrine-induced ventricular arrhythmias in dogs anesthetized with halothane: Potentiation by thiamylal and thiopental. Am J Vet Res. 1985;46:1829–1831. [PubMed] [Google Scholar]
- 39.Atlee JL, 3rd, Malkinson CE. Potentiation by thiopental of halothane — epinephrine-induced arrhythmias in dogs. Anesthesiology. 1982;57:285–288. doi: 10.1097/00000542-198210000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Smith JA, Gaynor JS, Bednarski RM, Muir WW. Adverse effects of administration of propofol with various preanesthetic regimens in dogs. J Am Vet Med Assoc. 1993;202:1111–1115. [PubMed] [Google Scholar]
- 41.Maze M, Smith CM. Identification of receptor mechanism mediating epinephrine-induced arrhythmias during halothane anesthesia in the dog. Anesthesiology. 1983;59:322–326. doi: 10.1097/00000542-198310000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Miller MS, Tilley LP, Smith FWK, Fox PR. Electrocardiography. In: Fox PR, Sisson D, Moise NS, eds. Textbook of Canine and Feline Cardiology. 2nd ed. Philadelphia: WB Saunders, 1999:94.
- 43.Woehlck HJ, Vicenzi MN, Bosnjak ZJ, Atlee JL., 3rd Anesthetics and automaticity of dominant and latent pacemakers in chronically instrumented dogs. I. Methodology, conscious state, and halothane anesthesia: Comparison with and without muscarinic blockade during exposure to epinephrine. Anesthesiology. 1993;79:1304–1315. doi: 10.1097/00000542-199312000-00022. [DOI] [PubMed] [Google Scholar]
- 44.Freeman LC, Ack JA, Fligner MA, Muir WW., 3rd Atrial fibrillation in halothane- and isoflurane-anesthetized dogs. Am J Vet Res. 1990;51:174–177. [PubMed] [Google Scholar]
- 45.Ware AW. Practical use of Holter monitoring. Compend Contin Educ Pract Vet. 1998;20:167–177. [Google Scholar]
- 46.Branch CE, Robertson BT, Williams JC. Frequency of second-degree atrioventricular heart blocks in dogs. Am J Vet Res. 1975;36:925–929. [PubMed] [Google Scholar]