Abstract
Bone morphogenetic proteins (BMPs) have multiple functions during vertebrate development. Previously, it was shown that BMP type I receptor ALK2 (also known as ACVRI, ActRI, or ActRIA) was important for normal mouse gastrulation by deleting exon 4 or exon 5 of Alk2. Recently, flanking exon 7 by loxP sites generated a conditional allele for Alk2. To assess whether the deletion of exon 7 causes functional null of ALK2, and does not produce a dominant negative form or a partially functional form of ALK2, we performed a comparative analysis between Alk2 homozygous mutant embryos with an exon 5 deletion (Alk2Δ5/Δ5) and embryos with an exon 7 deletion (Alk2Δ7/Δ7). Both Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutants showed identical morphological gastrulation defects. Histological examinations and molecular marker analyses revealed identical abnormal gastrulation phenotypes in Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutants. Although Fgf8 was expressed in the primitive streak of Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutants, Brachyury, Wnt3a, and Tbx6 were dramatically downregulated in Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutants. These results indicate that deletion of exon 7 for Alk2 leads to a functionally null mutation in vivo, and Alk2 is crucial for sustaining the proper gastrulation events in early mouse embryogenesis.
Keywords: Alk2, bone morphogenetic protein, gastrulation, mouse
INTRODUCTION
Genetic studies for the transforming growth factor-beta (TGF-β) superfamily have revealed various functions of family members in mammalian development (Roberts and Sporn, 1993; Wall and Hogan, 1994; ten Dijke et al., 2000). TGF-β superfamily signals including bone morphogenetic proteins (BMPs) are mediated through membrane-bound heteromeric complexes of type I and type II serine/threonine kinase receptors (Heldin et al., 1997; Kretzschmar and Massague, 1998; Whitman, 1998; Miyazono et al., 2000). Upon binding to a ligand, the type II receptors phosphorylate and activate associated type I receptors, which in turn transduce the signal by phosphorylating signaling pathway using SMADs, especially SMAD1/5/8 for BMP signaling cascades (Heldin et al., 1997; Whitman, 1998).
ALK2 (known as ACVRI, ActRI, or ActRIA) is one of the type I BMP receptors to bind BMPs in conjunction with corresponding type II receptors (Attisano et al., 1993; He et al., 1993; ten Dijke et al., 1993, 1994; Mishina, 2003; Kishigami and Mishina, 2005). During mouse embryogenesis, Alk2 is expressed in visceral endoderm at embryonic day (E) E6.5, and in both the visceral endoderm and mesoderm at E7.5 (Roelen et al., 1994; Gu et al., 1999). To understand the role of BMP signaling mediated by ALK2 in mammalian embryogenesis, two different types of conventional knockout mice were generated previously (Gu et al., 1999; Mishina et al., 1999). Exon 4, which encodes a transmembrane domain, or exon 5, which encodes a GS-domain (rich in Gly and Ser residues), were eliminated (Gu et al., 1999; Mishina et al., 1999). Despite different targeting strategies for Alk2, both Alk2-deficient mice showed a similar phenotype of early embryonic lethality with severe disruption of mesoderm formation (Gu et al., 1999; Mishina et al., 1999). The mutant embryos start the gastrulation, however their development is arrested at late streak stages. In addition, results of chimeric studies suggested that Alk2 is essential in the extraembryonic tissue at gastrulation for normal mesoderm formation (Gu et al., 1999; Mishina et al., 1999). Furthermore, it is reported that BMP signaling mediated by ALK2 in the visceral endoderm is necessary for the generation of primordial germ cells in the mouse embryo (de Sousa Lopes et al., 2004).
Recently, to address how ALK2-mediated BMP signaling is associated with later stages of mammalian development, an Alk2 conditional mouse line was generated by floxing exon 7 (Kaartinen and Nagy, 2001). Using this line, the importance of Alk2 for the normal cranial, cardiac, and neuronal development was revealed (Dudas et al., 2004; Kaartinen et al., 2004; Wang et al., 2005; Israelsson et al., 2006). However, exon 7 encodes the Smad interacting domain (L45 loop) and a part of the kinase domain (Kaartinen and Nagy, 2001). This raises concerns about the deletion of exon 7 and whether it might produce a dominant negative form, or a partially functional form of ALK2. Although in vitro analysis showed no induction for phosphorylation of Smad1 when co-transfected with Smad1 cDNA and a construct lacking sequences encoded by exon 7 into CHO cells (Dudas et al., 2004), it is still necessary to analyze the exon 7 deletion mutant in more detail in vivo. Therefore, we carefully compared Alk2 homozygous mutant embryos with an exon 5 deletion (Alk2Δ5/Δ5) to embryos with an exon 7 deletion (Alk2Δ7/Δ7).
Both Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutants showed similar gastrulation defects at the late streak stage. To examine the molecular cascade initiated by Alk2 in mouse gastrulation, we analyzed the expression patterns of anteroposterior axis marker genes. Interestingly, while Fgf8 was detected in the primitive streak of Alk2Δ5/Δ5 and Alk2Δ7/Δ7embryos, Brachyury, Wnt3a, and Tbx6 were dramatically downregulated in both Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutants, suggesting that Alk2 is involved in Brachyury and Wnt3a signaling cascades. These results indicate that deletion of exon 7 for Alk2 leads to the functionally null allele in vivo and, that Alk2 is essential for the mouse gastrulation procedures.
RESULTS AND DISCUSSION
The mouse Alk2 gene is encoded by 10 exons (Schmitt et al., 1995; Fig. 1A). We previously used conventional gene targeting to delete exon 5 of Alk2, which encodes a GS domain that is critical for ALK2 kinase activity (Mishina et al., 1999; Fig. 1B). In the present study, exon 7, which is a part of the kinase domain of ALK2, was floxed and removed as previously described (Kaartinen and Nagy, 2001). Genotype was confirmed by polymerase chain reaction (PCR) for both Alk2 exon 5 and exon 7 deletion (Fig. 1C).
Fig. 1.
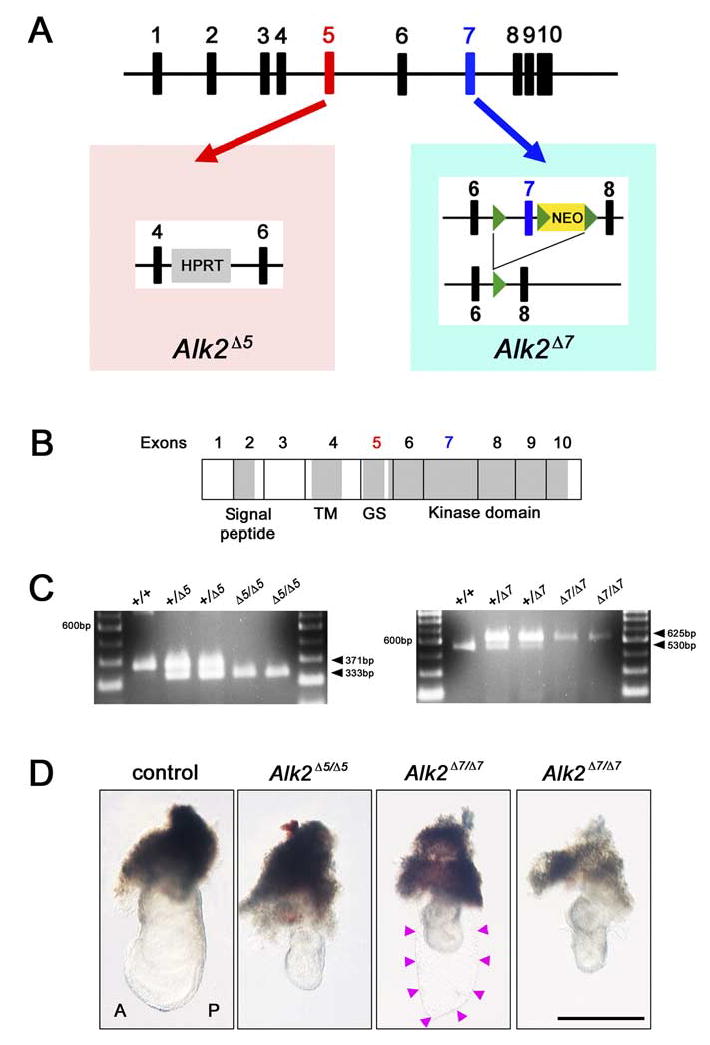
Targeted disruption of the Alk2 exon 5 and exon 7. A: Schematic illustration of the mouse Alk2 gene. The left panel shows the conventional Alk2 targeted exon 5 allele (Alk2Δ5), and the right panel shows the targeted mutation of the Alk2 exon 7 allele (Alk2Δ7). Black boxes represent Alk2 exons. Red and blue boxes show Alk2 exon 5 and exon 7, respectively. HPRT, hypoxanthine-guanine phosphori-bosyltransferase; NEO, neomycin. B: A schematic presentation of ALK2 protein and organization of Alk2 exons 5 and 7. Exon 1 is noncording. TM, transmembrane domain; GS, GS-domain rich in Gly and Ser. C: Genotyping results of the disruption for Alk2 exon 5 (wild-type; 371 bp, mutant; 333 bp) or Alk2 exon 7 (wild-type; 530 bp, mutant; 625 bp) by polymerase chain reaction analysis. D: Whole-mount view of control (wild-type or Alk2 heterozygous) and Alk2 homozygous for exon 5 (Alk2Δ5/Δ5) or exon 7 (Alk2Δ7/Δ7) embryos at embryonic day (E) 7.5. A, anterior; P, posterior. Scale bar = 500 μm.
At the onset of gastrulation at E6.5, Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutant embryos appeared morphologically indistinguishable from normal littermates (data not shown). However, we started to recover abnormal embryos around E7.0–E7.5 (Fig. 1D). Both Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutant embryos showed morphologically consistent phenotypes (N = 31/31 for Alk2Δ5/Δ5 mutant embryos, N = 30/30 for Alk2Δ7/Δ7 mutant embryos). Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutant embryos were much smaller and had formed empty sacs composed of parietal endoderm (Fig. 1D, arrowheads). Histological examination confirmed the presence of the thicker primitive streak forcing the posterior epiblast into the proamniotic cavity in Alk2Δ5/Δ5 mutants as previously reported (Gu et al., 1999; Mishina et al., 1999; Fig. 2F–H). As shown in Figure 2, Alk2Δ7/Δ7 mutants displayed similar abnormalities suggesting that as seen in the Alk2Δ5/Δ5 mutants mesoderm formation is initiated, but the subsequent development is arrested during the mid-late streak stages.
Fig. 2.
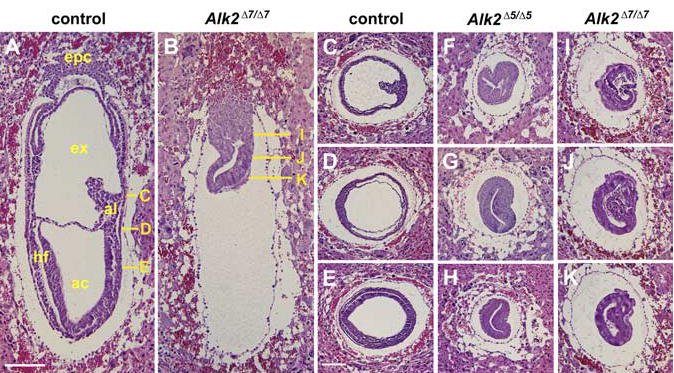
Histological analysis of Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutant embryos. A,B: Sagittal sections of embryonic day (E) 7.5 control (A) and Alk2Δ7/Δ7 mutant (B) embryos. C–K: Histological comparison of the control (C–E), Alk2Δ5/Δ5 mutant (F–H), and Alk2Δ7/Δ7 mutant (I–K) specimen by transverse section at E7.5. The solid yellow lines in A and B indicate the approximate position of the transverse sections in C–E and I–K, respectively. ac, amniotic cavity; al, allantois; epc, ectoplacental cone; ex, exocoelomic cavity; hf, head fold. Scale bar = 200 μm.
To confirm whether the mesoderm formation is initiated and develops normally in Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutants, we analyzed expression of markers for anterior tissues and primitive streak by in situ hybridization. Shh is normally expressed in the anterior mesendoderm (Lu and Robertson, 2004; Fig. 3A). However, the expression level of Shh was dramatically decreased in both Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutants (Fig. 3B,C). In contrast, Cer1, a marker for the anterior definitive endoderm (Shawlot et al., 1998; Fig. 3D), was expressed in the definitive endoderm with an expanded expression domain (Fig. 3E,F). An axial mesoderm marker, Foxa2 was also examined (Sasaki and Hogan, 1993; Fig. 3G). Foxa2 was expressed in ten of thirteen Alk2Δ5/Δ5 mutants and in five of five Alk2Δ7/Δ7 mutants. Interestingly, expression of Foxa2 was restricted in primitive streak like wild-type embryos at E6.5 (Fig. 3H,I). This suggests that development of Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutants is arrested at late streak stages, and does not proceed to headfold stage where the Foxa2 expression domain moves to anteriorly.
Fig. 3.
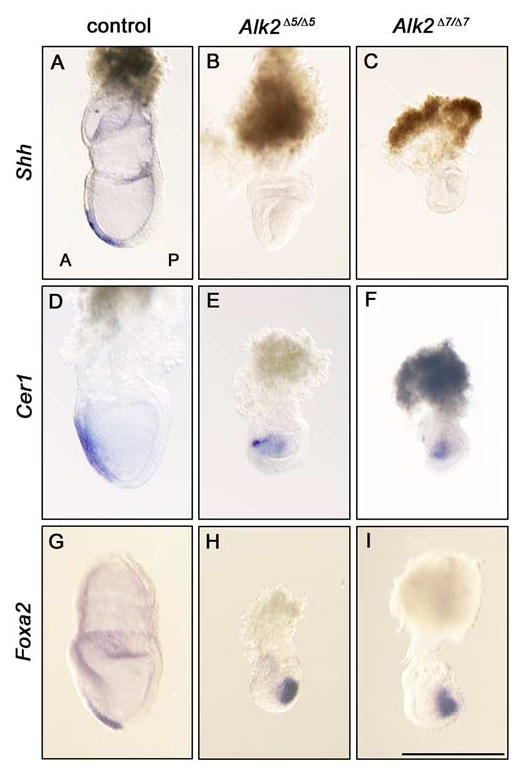
Anterior marker gene expression analysis of Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutant embryos. A–I: Marker gene expression pattern of Shh (A–C), Cer1 (D–F), and Foxa2 (G–I) at E7.5 in control (A,D,G), Alk2Δ5/Δ5 mutant (B,E,H), and Alk2Δ7/Δ7 (C,F,I) mutant embryos, respectively. A, anterior; P, posterior. Scale bar = 500 μm.
Next, to analyze the arrested phenotype of the primitive streak, we examined the expression of Brachyury, Fgf8, Wnt3a, and Tbx6 in Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutants. Brachyury is one of the earliest mesoderm markers and it is strongly expressed in the primitive streak (Wilkinson et al., 1990). Genetic and vertebrate embryological studies have also revealed a conserved role of Brachyury for maintenance, axis elongation, and the specification of posterior mesoderm populations (Smith, 1997). In Alk2Δ5/Δ5 mutants, Brachyury expression was repressed or undetectable (Fig. 4B and data not shown) as reported previously (Gu et al., 1999; Mishina et al., 1999). As shown in Figure 4C, Brachyury expression was not detected in Alk2Δ7/Δ7 mutants either. Fibroblast growth factor (FGF) signaling is crucial for mesoderm cell fate specification and required for Brachyury expression at gastrulation (Griffin et al., 1995; Ciruna and Rossant, 2001). Therefore, another primitive streak and nascent mesoderm marker, Fgf8 was analyzed (Sun et al., 1999). As shown in Figure 4E,F, Fgf8 was expressed in both Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutants. At late streak stages, Wnt3a is expressed in the primitive streak, which is known as a direct regulator of Brachyury (Yamaguchi et al., 1999). Tbx6 is also required for the specification of posterior paraxial mesoderm during late streak stages (Chapman et al., 1996). Interestingly, both Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutant embryos failed to express Wnt3a (Fig. 4H,I) and Tbx6 (Fig. 4K,L). These results suggest that ALK2 signaling associates with the expression of Brachyury, Wnt3a, and Tbx6 at late streak stages to complete appropriate gastrulation.
Fig. 4.
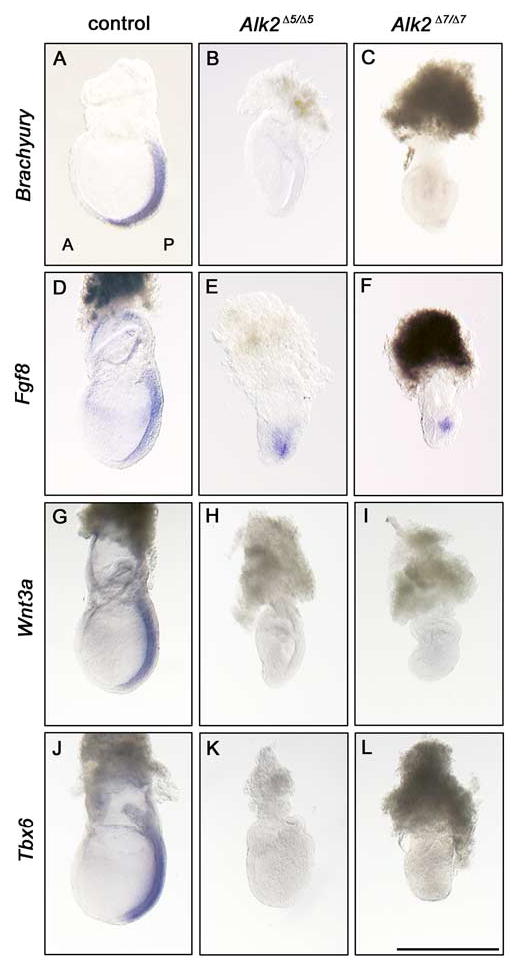
Posterior marker gene expression analysis of Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutant embryos. A–L: Marker gene expression pattern of Brachyury (A–C), Fgf8 (D–F), Wnt3a (G–I), and Tbx6 (J–L) at embryonic day (E) 7.5 in control embryos (A,D,G,J), Alk2Δ5/Δ5 mutant embryos (B,E,H,K), and Alk2Δ7/Δ7 (C,F,I,L) mutant embryos, respectively. A, anterior; P, posterior. Scale bar = 500 μm.
In this study, we analyzed Alk2 mutant embryos homozygous for an exon 5 deletion (Alk2Δ5/Δ5) and for an exon 7 deletion (Alk2Δ7/Δ7). Both Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutants showed a similar categorized phenotype. Targeted disruption of exon 4 for Alk2, which encodes a transmembrane domain of ALK2, blocks BMP signal transfer into the intracellular region through ALK2, because mutated ALK2 is unable to remain in the plasma membrane (Gu et al., 1999). Another conventional mutant targeted exon 5, which encodes the GS box of ALK2, displayed an identical phenotype (Mishina et al., 1999). Exon 7 encodes the Smad interacting domain (L45 loop) in part of the ALK2 kinase domain (Kaartinen and Nagy, 2001). Disruption of floxed exon 7 by Cre re-combinase is expected to produce a null allele of Alk2. However, it is possible that the deleted allele would produce a truncated ALK2, which still contains a ligand binding domain, a transmembrane domain, a GS domain, and a part of the kinase domain (Kaartinen and Nagy, 2001). This finding raises the possibility that deletion of exon 7 leads to a formation of a dominant negative form or a partially functional form of ALK2. Expression of mutant proteins that lack cytoplasmic regions are frequently used to block the specific signals, because these types of proteins can act as dominant negatives (Chen et al., 1998; Zhao et al., 2002). On the other hand, a mutation in the GS domain was reported recently to lead to a rare autosomal dominant disorder of skeletal malformations, fibrodysplasia ossificans progressiva (Shore et al., 2006). This finding would suggest that the truncated ALK2, which still has the GS domain, could act as a signaling molecule. Therefore, we believed that it was important to clarify whether the targeted mutation of exon 7 produces a functionally null allele as do the conventional Alk2 mutations, or whether it produces a dominant-negative form or a partially functional form of ALK2 in vivo. Our present study demonstrates that Alk2 targeted for exon 7 is functionally null, showing that the studies using the Alk2 floxed mouse reveal phenotypes that result from the null mutation of Alk2.
In conclusion, the comparative analysis of two different types of Alk2-deficient mutants showed an identical phenotype. Although the targeted exon was different from conventional Alk2 mutations, the Alk2 floxed mouse can be used to make the conditional Alk2 null mouse. The defects observed in Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutants reveal intriguing links between the Alk2, Brachyury, Wnt3a, and Tbx6 genetic pathways in primitive streak development. Because BMP signaling through ALK2 is involved in the function of extraembryonic region (Gu et al., 1999; Mishina et al., 1999; de Sousa Lopes et al., 2004), one possibility is that ALK2 signaling in the extraembryonic region induces unknown factors that act on the embryonic region to regulate the expression of Brachyury, Wnt3a, and Tbx6. Further analysis for the downstream target genes of Alk2 in the extraembryonic region will provide more detailed information about BMP signaling mediated by ALK2 in proper mouse gastrulation.
EXPERIMENTAL PROCEDURES
Animals
Generation of Alk2 exon 5 or exon 7 mutant mice were reported previously (Mishina et al., 1999; Kaartinen and Nagy, 2001). Both Alk2Δ5/Δ5 and Alk2Δ7/Δ7 mutants were maintained on a mixed background of 129/SvEv and C57BL/6J. All mouse experiments were performed in accordance with National Institute of Environmental Health Sciences (NIEHS) guidelines covering the humane care and use of animals in research.
Genotyping Analysis
The DNA from mouse embryos was analyzed by PCR. Primer sequences for exon 5-specific Alk2 mutant were 5′-ATG CTA GAC CTG GGC AGC CAT A-3′, 5′-CAT GCT AGC AGC TCG GAG AAA C-3′, 5′-GAG ACT AGT GAG ACG TGC TAC T-3′. The conditions for genotyping PCR were 94°C for 20 sec, 65°C for 20 sec, 72°C for 20 min, repeated 40 cycles for detecting of exon 5-specific Alk2 mutant allele. Genotyping PCR reaction yielded 371 bp for wild-type, or 333 bp for Alk2 exon 5 mutant DNA fragment. Primer sequences for exon7-specific Alk2 mutant were 5′-CCC CCA TTG AAG GTT TAG AGA GAC-3′, 5′-TGA GAT TGT TCT AGC ACT GCC C-3′, 5′-GAA TTG CTA GAA GCC CAT AGG C-3′. The conditions for genotyping PCR were 94°C for 20 sec, 60°C for 20 sec, 72°C for 20 min, repeated 40 cycles for detecting of exon 7-specific Alk2 mutant allele. Genotyping PCR reaction yielded 530 bp for wild-type or 625 bp for Alk2 exon 7 mutant DNA fragment.
Histological Analysis and In Situ Hybridization
Embryos were fixed in 4% paraformal-dehyde, embedded in paraffin, and stained with hematoxylin and eosin. In situ hybridization was performed with a digoxigenin-labeled RNA probe by standard procedures (Wilkinson and Nieto, 1993). Brachyury, Cer1, Fgf8, Foxa2, Shh, Tbx6, and Wnt3a probes were kindly provided by Drs. B.G. Herrmann, W. Shawlot, G.R. Martin, H. Sasaki, A.P. McMahon, D.L. Chapman, and T.P. Yamaguchi, respectively.
Acknowledgments
We thank Drs. B.G. Herrmann, W. Shawlot, G.R. Martin, H. Sasaki, A.P. McMahon, D.L. Chapman, and T.P. Yamaguchi for in situ probes. We also thank Ms. M. Kamikawa-Miyado for advice on histological analysis, Dr. C. Kimura-Yoshida for suggestion of in situ hybridization, and Drs. E.M. Eddy and M.K. Ray for critical comments. We are grateful to Ms. Li He and Ms. Chiaki Komatsu for encouragement. V.K. was funded by a grant from the NIH and Y.M. was funded by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (NIH/NIEHS).
Footnotes
Grant sponsor: NIH; Grant number: HL074862; Grant number: DE013085.
References
- Attisano L, Carcamo J, Ventura F, Weis FM, Massague J, Wrana JL. Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell. 1993;75:671–680. doi: 10.1016/0092-8674(93)90488-c. [DOI] [PubMed] [Google Scholar]
- Chapman DL, Agulnik I, Hancock S, Silver LM, Papaioannou VE. Tbx6, a mouse T-Box gene implicated in paraxial mesoderm formation at gastrulation. Dev Biol. 1996;180:534–542. doi: 10.1006/dbio.1996.0326. [DOI] [PubMed] [Google Scholar]
- Chen D, Ji X, Harris MA, Feng JQ, Karsenty G, Celeste AJ, Rosen V, Mundy GR, Harris SE. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol. 1998;142:295–305. doi: 10.1083/jcb.142.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruna B, Rossant J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell. 2001;1:37–49. doi: 10.1016/s1534-5807(01)00017-x. [DOI] [PubMed] [Google Scholar]
- de Sousa Lopes SM, Roelen BA, Monteiro RM, Emmens R, Lin HY, Li E, Lawson KA, Mummery CL. BMP signaling mediated by ALK2 in the visceral endoderm is necessary for the generation of primordial germ cells in the mouse embryo. Genes Dev. 2004;18:1838–1849. doi: 10.1101/gad.294004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudas M, Sridurongrit S, Nagy A, Okazaki K, Kaartinen V. Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech Dev. 2004;121:173–182. doi: 10.1016/j.mod.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Griffin K, Patient R, Holder N. Analysis of FGF function in normal and no tail zebrafish embryos reveals separate mechanisms for formation of the trunk and the tail. Development. 1995;121:2983–2994. doi: 10.1242/dev.121.9.2983. [DOI] [PubMed] [Google Scholar]
- Gu Z, Reynolds EM, Song J, Lei H, Feijen A, Yu L, He W, MacLaughlin DT, van den Eijnden-van Raaij J, Donahoe PK, Li E. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development. 1999;126:2551–2561. doi: 10.1242/dev.126.11.2551. [DOI] [PubMed] [Google Scholar]
- He WW, Gustafson ML, Hirobe S, Donahoe PK. Developmental expression of four novel serine/threonine kinase receptors homologous to the activin/transforming growth factor-beta type II receptor family. Dev Dyn. 1993;196:133–142. doi: 10.1002/aja.1001960207. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Israelsson C, Lewen A, Kylberg A, Usoskin D, Althini S, Lindeberg J, Deng CX, Fukuda T, Wang Y, Kaartinen V, Mishina Y, Hillered L, Ebendal T. Genetically modified bone morphogenetic protein signalling Alters traumatic brain injury-induced gene expression responses in the adult mouse. J Neurosci Res. 2006;84:47–57. doi: 10.1002/jnr.20856. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Nagy A. Removal of the floxed neo gene from a conditional knockout allele by the adenoviral Cre recombinase in vivo. Genesis. 2001;31:126–129. doi: 10.1002/gene.10015. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Dudas M, Nagy A, Sridurongrit S, Lu MM, Epstein JA. Cardiac outflow tract defects in mice lacking ALK2 in neural crest cells. Development. 2004;131:3481–3490. doi: 10.1242/dev.01214. [DOI] [PubMed] [Google Scholar]
- Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–278. doi: 10.1016/j.cytogfr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Massague J. SMADs: mediators and regulators of TGF-beta signaling. Curr Opin Genet Dev. 1998;8:103–111. doi: 10.1016/s0959-437x(98)80069-5. [DOI] [PubMed] [Google Scholar]
- Lu CC, Robertson EJ. Multiple roles for Nodal in the epiblast of the mouse embryo in the establishment of anterior-posterior patterning. Dev Biol. 2004;273:149–159. doi: 10.1016/j.ydbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Mishina Y. Function of bone morphogenetic protein signaling during mouse development. Front Biosci. 2003;8:d855–d869. doi: 10.2741/1097. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Crombie R, Bradley A, Behringer RR. Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Dev Biol. 1999;213:314–326. doi: 10.1006/dbio.1999.9378. [DOI] [PubMed] [Google Scholar]
- Miyazono K, ten Dijke P, Heldin CH. TGF-beta signaling by Smad proteins. Adv Immunol. 2000;75:115–157. doi: 10.1016/s0065-2776(00)75003-6. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor-beta (TGF-beta) Growth Factors. 1993;8:1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- Roelen BA, Lin HY, Knezevic V, Freund E, Mummery CL. Expression of TGF-beta s and their receptors during implantation and organogenesis of the mouse embryo. Dev Biol. 1994;166:716–728. doi: 10.1006/dbio.1994.1350. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Hogan BL. Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development. 1993;118:47–59. doi: 10.1242/dev.118.1.47. [DOI] [PubMed] [Google Scholar]
- Schmitt J, Mielke R, Schrewe H. Genomic organization of a mouse type I activin receptor. Biochem Biophys Res Commun. 1995;213:211–217. doi: 10.1006/bbrc.1995.2118. [DOI] [PubMed] [Google Scholar]
- Shawlot W, Deng JM, Behringer RR. Expression of the mouse cerberus-related gene, Cerr1, suggests a role in anterior neural induction and somitogenesis. Proc Natl Acad Sci U S A. 1998;95:6198–6203. doi: 10.1073/pnas.95.11.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- Smith J. Brachyury and the T-box genes. Curr Opin Genet Dev. 1997;7:474–480. doi: 10.1016/s0959-437x(97)80073-1. [DOI] [PubMed] [Google Scholar]
- Sun X, Meyers EN, Lewandoski M, Martin GR. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 1999;13:1834–1846. doi: 10.1101/gad.13.14.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P, Ichijo H, Franzen P, Schulz P, Saras J, Toyoshima H, Heldin CH, Miyazono K. Activin receptor-like kinases: a novel subclass of cell-surface receptors with predicted serine/threonine kinase activity. Oncogene. 1993;8:2879–2887. [PubMed] [Google Scholar]
- ten Dijke P, Yamashita H, Ichijo H, Franzen P, Laiho M, Miyazono K, Heldin CH. Characterization of type I receptors for transforming growth factor-beta and activin. Science. 1994;264:101–104. doi: 10.1126/science.8140412. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Miyazono K, Heldin CH. Signaling inputs converge on nuclear effectors in TGF-beta signaling. Trends Biochem Sci. 2000;25:64–70. doi: 10.1016/s0968-0004(99)01519-4. [DOI] [PubMed] [Google Scholar]
- Wall NA, Hogan BL. TGF-beta related genes in development. Curr Opin Genet Dev. 1994;4:517–522. doi: 10.1016/0959-437x(94)90066-c. [DOI] [PubMed] [Google Scholar]
- Wang J, Sridurongrit S, Dudas M, Thomas P, Nagy A, Schneider MD, Epstein JA, Kaartinen V. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev Biol. 2005;286:299–310. doi: 10.1016/j.ydbio.2005.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M. Smads and early developmental signaling by the TGFbeta superfamily. Genes Dev. 1998;12:2445–2462. doi: 10.1101/gad.12.16.2445. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Nieto MA. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Bhatt S, Herrmann BG. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990;343:657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Takada S, Yoshikawa Y, Wu N, McMahon AP. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev. 1999;13:3185–3190. doi: 10.1101/gad.13.24.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Harris SE, Horn D, Geng Z, Nishimura R, Mundy GR, Chen D. Bone morphogenetic protein receptor signaling is necessary for normal murine post-natal bone formation. J Cell Biol. 2002;157:1049–1060. doi: 10.1083/jcb.200109012. [DOI] [PMC free article] [PubMed] [Google Scholar]


