Abstract
Purpose
A common treatment for motility disorders of the extraocular muscles (EOMs) is a resection procedure in which there is surgical shortening of the muscle. This procedure results in rotation of the globe toward the resected muscle, increased resting tension across the agonist–antagonist pair, and stretching of the elastic components of the muscles. In the rabbit, due to orbital constraints and limited rotation, resection results in more significant stretch of the surgically treated muscle than the antagonist. This surgical preparation allows for the examination of the effects of surgical shortening of one rectus muscle and passive stretch of its ipsilateral antagonist.
Methods
The insertional 6 mm of the superior rectus muscles of adult rabbits were resected and reattached to the original insertion site. After 7 and 14 days, the animals were injected intraperitoneally with bromodeoxyuridine (BrdU) every 2 hours for 12 hours, followed by a 24-hour BrdU-free period. All superior and inferior rectus muscles from both globes were examined for BrdU incorporation, MyoD expression, neonatal and developmental myosin heavy chain (MyHC) isoform expression, and myofiber cross-sectional area alterations.
Results
In the resected muscle and in the passively stretched antagonist muscle, there was a dramatic increase in the number of myofibers positive for neonatal MyHC and in the number of BrdU- and MyoD-positive satellite cells. The addition of BrdU-positive myonuclei increased from 1 per 1000 myofibers in cross sections of control muscles to 2 to 3 per 100 myofibers in the resected muscles. Single myofiber reconstructions showed that multiple BrdU-positive myonuclei were added to individual myofibers. Addition of new myonuclei occurred in random locations along the myofiber length of single fibers. There was no correlation between myofibers with BrdU-positive myonuclei and neonatal MyHC isoform expression.
Conclusions
Both active and passive stretch of the rectus muscles produced by strabismus surgery dramatically upregulated the processes of satellite cell activation, integration of new myonuclei into existing myofibers, and concomitant up-regulation of immature myosin heavy chain isoforms. Understanding the effects of strabismus surgery on muscle cell biological reactions and myofiber remodeling may suggest new approaches for improving surgical outcomes.
Skeletal muscle is a complex tissue composed of thousands of individual multinucleated myofibers. It is considered postmitotic, in that the myonuclei residing within these myofibers do not divide in adult muscle.1,2 Residing outside the sarcolemma but within the basal lamina of individual myofibers is a population of cells that are responsible for muscle repair and regeneration, the satellite cells.3 When a muscle is injured, this specialized cell population becomes activated, divides, and either repairs damaged fibers or forms new myofibers.
There are a number of factors that initiate myofiber remodeling in adult skeletal muscles. Among these are hypertrophy induced by synergist ablation,4 low-frequency stimulation,5 and eccentric exercise.6 These manipulations result in satellite cell activation and proliferation, which in turn are responsible for muscle repair and regeneration. Irradiation treatment, which eliminates satellite cells from these muscles, prevents the compensatory hypertrophy in these types of experiments.4
Another stimulus that activates satellite cells is stretching of the muscle.7–9 In fact, passive stretching alone can activate limb muscle satellite cells.10 Even more compelling is a recent study that subjected individual quiescent satellite cells isolated from adult limb muscle to stretching in vitro, and showed that direct stretching of the satellite cells themselves results in activation and bromodeoxyuridine (BrdU) incorporation.11 In a similar study, stretching was shown to activate satellite cells on individually isolated myofibers in vitro that had been prepared by using a method that maintains satellite cell quiescence.12 In animal models that induce significant amounts of stretching, particularly limb lengthening procedures that result in distraction-induced muscle stretching, muscle growth is a critical factor in the success of these procedures clinically.13,14
Strabismus surgery is a common procedure used to restore binocular alignment. One procedure typically used for an underacting muscle is surgical resection of the affected extraocular muscle (EOM). Resection procedures do not significantly alter the axial position of the globe in the orbit. Instead, resection rotates the eye in the direction of the resected muscle around a relatively fixed axis of rotation. This increases the resting tension and therefore stretching of both the resected muscle and its antagonist. In a previous study, resection of an extraocular rectus muscle induced fiber hypertrophy—presumably a response to the increased load created by the surgery.15 However, the mechanism of fiber hypertrophy in resected EOM is unknown and cannot be inferred directly from limb muscle studies because of significant differences known to exist between limb muscle and the EOMs.16 The effect of resection of the EOMs on satellite cell activation, myonuclear addition, and myosin heavy chain (MyHC) isoform expression has not been examined. This study was designed to determine the effects of this common strabismus treatment on the EOM of adult rabbits.
MATERIALS AND METHODS
Adult New Zealand White rabbits were obtained from Bakkon Rabbitry (Viroqua, WI) and housed in the University of Minnesota AALAC-approved animal facility. All procedures were approved by the Animal Care and Use Committee at the University of Minnesota and adhered to the guidelines for use of animals in research prepared by the National Institutes of Health and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Surgical resection of EOMs was performed on 12 normal adult rabbits. The rabbits were anesthetized with an intramuscular injection of ketamine and xylazine (1:1; 10 mg/kg to 2 mg/kg, respectively). One randomly selected superior rectus muscle in each animal was detached from the sclera. A 5- to 6-mm section was removed, and the muscle was sutured to the original insertion using Vicryl 7-0 (polyglactin 910; Ethicon, Somerville, NJ). Because of the size of the resection, the resected segment included both insertional tendon and distal muscle and involved both the orbital and global layers. Although some myofibers would be cut by this method, many myofibers do not run tendon-to-tendon in the EOM. This means that most of the myofibers would not be physically sectioned by this method. The resection resulted in significant muscle stretching in the resected muscle. As is typical of rabbits, minimal rotation of the globe occurred in the surgically treated eyes despite the large resection. As a consequence, there was less substantial stretch of the antagonist inferior rectus muscle than that which was induced in the resected superior rectus muscle. This allowed us to compare the effects of differing amounts of stretch in the same eye. One week after surgery, eight rabbits received intra-peritoneal injections of BrdU in sterile saline at a dose of 50 mg/kg body weight. The rabbits received an injection every 2 hours for 12 hours, followed by a 24 hour BrdU-free period. Two weeks after surgery, an additional four rabbits received the same series of BrdU injections. The muscles from four additional normal rabbits labeled similarly with BrdU were also used as control specimens. After the BrdU-free period the rabbits were reanesthetized, and the muscles were prepared for immunohistochemical examination of myonuclei and satellite cells that were positive for BrdU. The superior and inferior rectus muscles were removed from both orbits, embedded in tragacanth gum, and frozen in methylbutane chilled to a slurry on liquid nitrogen. The muscles were serially sectioned at 12 μm. The odd-numbered sections of all the muscles were immunostained for expression of BrdU (1:100; Chemicon, Temecula, CA) and dystrophin (1:20; Novocastra-Vector Laboratories, Burlingame, CA) to ascertain whether the labeled nuclei were within the muscle sarcolemma and therefore were myonuclei. Every 10th even-numbered section was immunostained for both BrdU and laminin (1:20; Sigma-Aldrich, St. Louis, MO), to quantify BrdU-labeled nuclei in the satellite cell position. Every 30th section was stained with hematoxylin and eosin for counting centrally placed nuclei. Of the sections that remained, every second, fourth, sixth, or eighth one was immunostained for one of the following: neonatal myosin, developmental myosin, insulin-like growth factor (IGF)-1, or MyoD. For double-labeling experiments, tissue sections were quenched with hydrogen peroxide, incubated with blocking serum followed by biotin-avidin blocking reagent (Vector Laboratories) and incubated in primary antibody to either dystrophin or laminin. The sections were rinsed in phosphate-buffered saline (PBS), followed by incubation, using the peroxidase ABC kit (Vectastain; Vector Laboratories). The peroxidase was developed with 3,3′-diaminobenzidine (DAB) and hydrogen peroxide without heavy metal intensification. The sections were rinsed in PBS. For the BrdU localization, the sections were incubated in 2 N HCl for 1 hour at 37°C, followed by neutralization in borate buffer and a PBS rinse. The sections were incubated in the primary antibody to BrdU. The sections were rinsed in PBS, incubated using reagents from the alkaline phosphatase ABC kit (Vector Laboratories) and reacted with the alkaline phosphatase black substrate kit. All BrdU myonuclear counts were performed on BrdU-dystrophin–labeled sections. The dystrophin or laminin immunostained brown and the BrdU-positive nuclei were black. BrdU labeling and the number of myofibers were quantified with the aid of a morphometry system (Nova Prime; BioQuant Inc., Nashville, TN).
Immunostaining for visualization of neonatal and developmental myosin heavy chain (MyHC), MyoD, and IGF-1 were performed as described for the dystrophin procedure, except that the antibodies were prepared as follows: neonatal and developmental MyHC at 1:20 (Novocastra-Vector Laboratories), MyoD at 1:25 (Dako Corp., Carpinteria, CA), and IGF-1 at 1:50 (R&D Systems, Minneapolis, MN). Alterations in the expression patterns of the neonatal and developmental myosin heavy chain isoforms, IGF-1, and MyoD in these muscles were examined. The total number of BrdU- or MyoD-labeled nuclei was determined as a percentage of the total number of myofibers in the microscopic fields counted. The percentages of centrally nucleated, neonatal MyHC, developmental MyHC, and MyoD-positive cells were determined as the number positive compared with the number of myofibers counted per cross section. Cross-sectional areas were determined on the BrdU-dystrophin–stained sections by manual tracing of myofibers using morphometry software (Nova Prime; BioQuant). For all measurements, counts and area measurements were made in a minimum of four random fields within both the orbital and global layers of four rectus muscles and four control muscles from a minimum of four rabbits for the resected treated muscles. Sections were chosen to include both the insertional tendon and the midbelly region of the analyzed muscles. In addition, the tissue sections were examined for inflammatory cell infiltrate by using hematoxylin and eosin staining and immunostaining for cd11b (1:10; Serotec, Raleigh, NC), which identified neutrophils, macrophages and lymphocytes. All data are presented as the mean ± SEM.
The percentage positive was compared between the orbital and global layers and analyzed for statistical significance using either an unpaired two-tailed t-test or an analysis of variance (ANOVA) and the Dunn multiple-comparison tests (Prism and Statmate software; GraphPad, San Diego, CA). An F-test was used to verify that the variances were not significantly different. Data were considered significantly different at P < 0.05.
A second set of sections was prepared from both the stretched and control superior rectus muscles in which all the even sections were immunostained with either developmental MyHC or neonatal MyHC. This second set of sections allowed us to determine how many BrdU-positive myonuclei were found within single myofibers and also whether myofibers with BrdU-positive myonuclei were positive or negative for one of the immature myosin heavy chain isoforms. Thus, single myofibers from the rectus muscles were reconstructed from serial cross-sections. A myofiber with a BrdU-positive myonucleus was located. These were selected to be randomly located in the muscle length and cross-section. The locations of BrdU-positive myonuclei and BrdU-positive satellite cells and whether the fiber was positive or negative for expression of developmental or neonatal MyHC were recorded along the length of each reconstructed myofiber by using morphology analysis software (Nova Prime; BioQuant). Three-dimensional reconstructions were built in a second program (Topographer Program; BioQuant).
Western blot analysis were performed on the nuclear fraction obtained from resected and normal EOMs. The muscles were weighed and homogenized with buffer from a kit (Buffer C; Compartmental Protein Extraction Kit; Chemicon). The samples were spun at 110,000 rpm for 20 minutes at 4°C. The pellets were placed in buffer W and spun a second time at 110,000 rpm for 20 minutes at 4°C. The pellets were placed in buffer N, rotated, and spun again as just described. Aliquots of the supernatant containing nuclear proteins were removed to determine protein concentration using the bicinchoninic acid (BCA) assay (Pierce Biotechnology, Rockford, IL). Nuclear proteins were separated on 4% to 20% Tris-HCl mini-gels (Bio-Rad, Hercules, CA) run at 120 V. After approximately 1 hour, the gels were removed into 80% Tris, glycine, SDS transfer buffer and 20% methanol and transferred onto nitrocellulose membranes at 350 mA for approximately 1 hour. Duplicate gels with the same amount of protein loaded were run and stained with Coomassie blue solution to monitor consistency in protein loading. The nitrocellulose membranes were placed overnight into blocking solution consisting of 0.015% NaN3, 1 × Tris–buffered saline (TBS) and 20% goat serum. The membranes were incubated with a primary antibody to MyoD (1:200, Santa Cruz Biotechnology, Santa Cruz, CA), washed three times in TBS containing 0.05% Tween-20, and incubated in secondary antibody at a 1:1000 dilution (Sigma-Aldrich). They were washed again and reacted with a reaction substrate consisting of the 5-bromo-4-chloro-3-indoyl phosphate/nitroblue tetrazolium (BCIP/NBT) substrate system for membranes (B3804; Sigma-Aldrich). To determine the relative content of MyoD in the immunoblots, densitometric analysis was performed (SigmaScan; SPSS Science, Chicago, IL). Preliminary experiments determined that 1 to 20 μg of protein produced a linear signal. Thus, 10 μg protein was used to load the gels for semiquantitative Western blot analysis. All lanes were compared to a lane of purified MyoD protein (Santa Cruz Biotechnology) included in each Western blot.
RESULTS
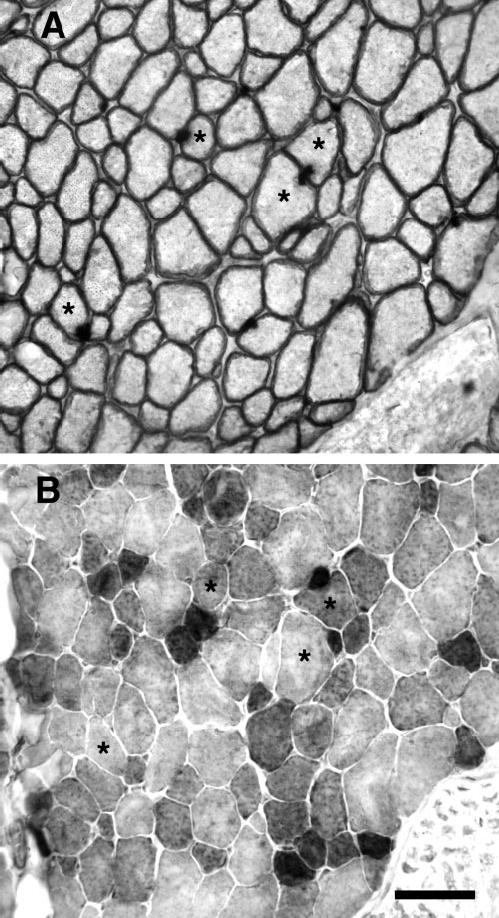
Resection resulted in remodeling of the operated superior rectus muscles. One week after surgery, a large number of myofibers of small cross-sectional areas was seen microscopically, a number of which had centrally located nuclei (Figs. 1A, 2, 3). Myofibers with extremely large cross-sectional areas also were evident (Fig. 1A), and in some cases the dystrophin staining was disrupted around the sarcolemmal periphery. These were most often localized to the lateral edges of the resected muscle. In cross section, the percentage of myofibers with central myonuclei was 11.9% ± 3.2% (P < 0.05) and 5.7% ± 1.5% (P < 0.05) in the orbital and global layers of the stretched muscles at 7 days after stretching compared with 0.3% ± 0.08% and 0.15% ± 0.03% in the orbital and global layers of the control muscles. The presence of myofibers with centrally located nuclei remained elevated at 2 weeks, with 7.39% ± 1.6% and 5.04% ± 1.5% in the orbital and global layers.
Figure 1.
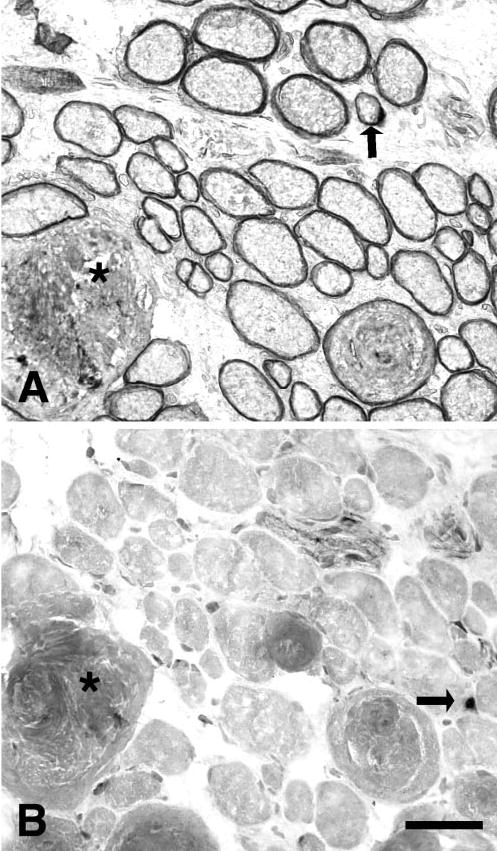
(A) Cross section from a superior rectus 1 week after resection induced stretch immunostained for dystrophin. Although most myofibers were within normal cross-sectional area ranges, myofibers with extremely large cross-sectional areas were present occasionally, and in some cases the dystrophin staining was disrupted around their sarcolemmal periphery (*). Arrow: A BrdU-positive nucleus. (B) Cross section from a superior rectus 1 week after resection immunostained for MyoD. (*) Same fiber in (A). Arrow: A MyoD positive nucleus. Bar, 50 μm.
Figure 2.
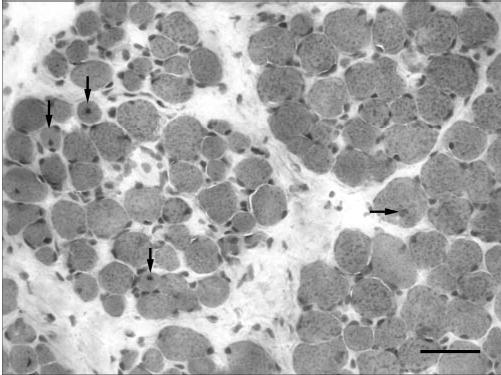
Cross section of the orbital and global layers of a resected superior rectus muscle 2 weeks after resection. Arrows: myofibers with centrally located nuclei. Bar, 50 μm.
Figure 3.
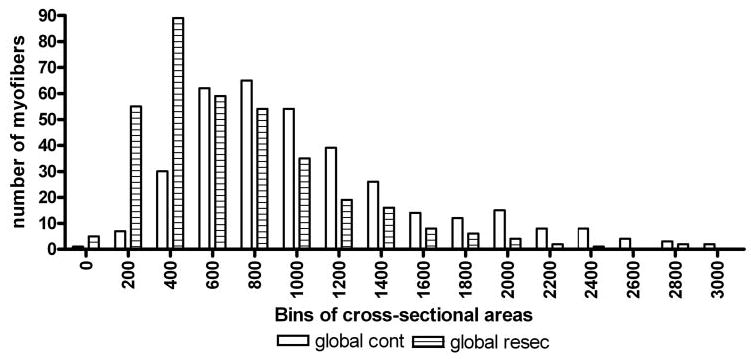
Cross-sectional areas of global fibers from resected and control superior rectus muscles. Cross-sectional areas of myofibers from the global layer of a resected superior rectus muscle (▤) and a control superior rectus muscle (□) separated into cross-sectional area bins 200 μm in size.
BrdU was used to follow addition of new myonuclei into the stretched myofibers. In the resected superior rectus muscles, an average of 1.23% ± 0.3% and 2.1% ± 0.4% of the myofibers in orbital and global layer cross sections, respectively, contained BrdU-positive myonuclei 1 week after stretch (Fig. 4). This represents a 10- and 200-fold increase over control superior rectus muscles, where approximately 0.18% ± 0.06% and 0.01% ± 0.01% had BrdU-positive myonuclei. New myonuclei were found in both layers, contrary to their preferential addition in the orbital layer myofibers in the normal EOM.17 Individual myofibers were reconstructed from the serially stained rectus muscles, and, in all cases, multiple BrdU-positive myonuclei were found within these myofibers after this 24-hour period after BrdU-labeling (Fig. 5, Table 1). Thus, surgically induced stretch not only increased the overall number of BrdU-positive myonuclei added to the muscles, but significantly increased the number of myonuclei added per individual myofiber. In all myofibers reconstructed from the resected superior rectus muscles, the BrdU-positive myonuclei were found in apparently random locations along the length of the individual myofibers.
Figure 4.
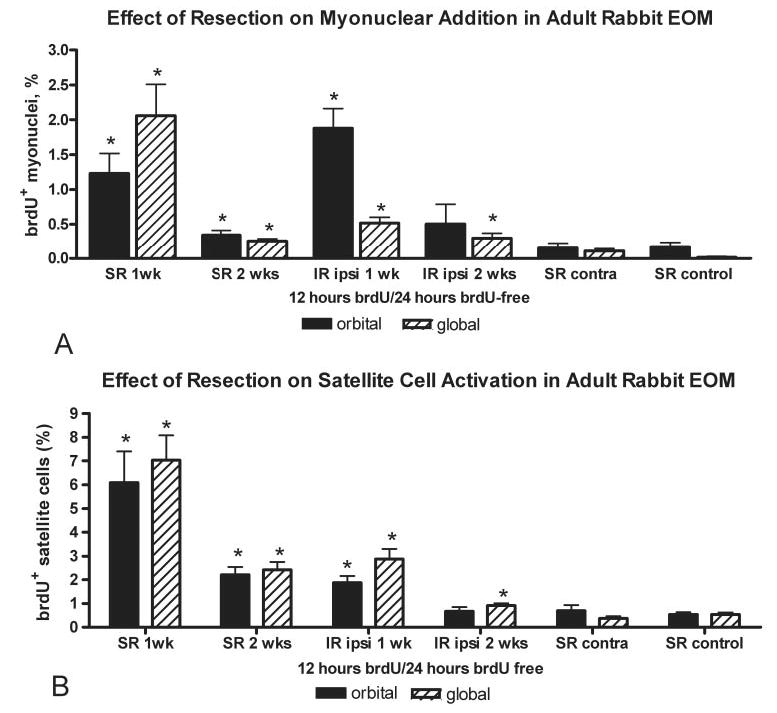
Effect of resection on myonuclear addition and satellite cell division. Quantification of (A) BrdU-positive myonuclei and (B) satellite cells 1 and 2 weeks after resection of the superior rectus (SR), the inferior rectus ipsilateral to the resected superior rectus muscle (IR), the superior rectus from the contralateral side to the surgical site (SR contra), and from the superior rectus of control animals, which did not undergo surgery (SR control). *Statistical significance compared with normal control levels (P < 0.05).
Figure 5.
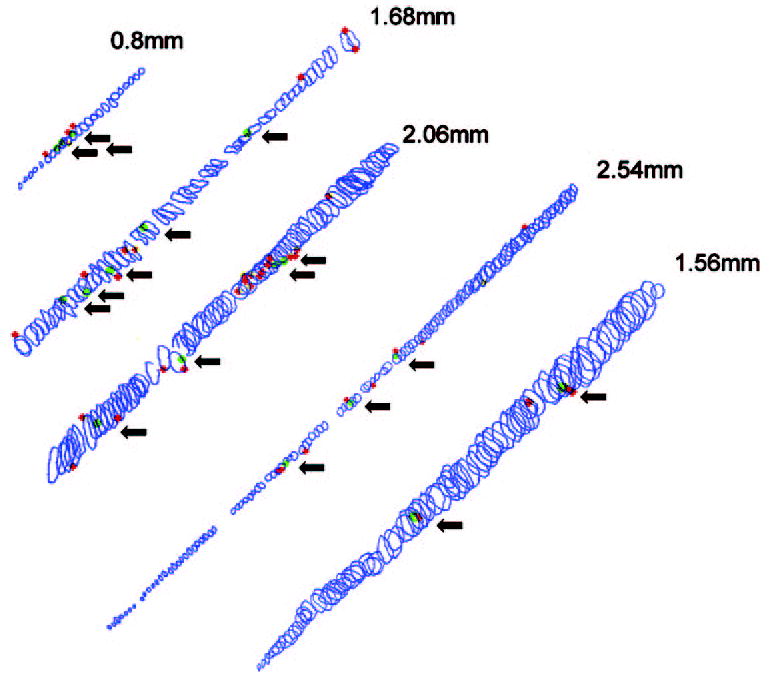
Reconstructions of five myofibers from serially sectioned (12 μm) resected muscle immunostained for BrdU and dystrophin. Each circle represents a single cross-sectional area. Red: BrdU-positive satellite cells; green: BrdU-positive myonuclei. Arrows: each BrdU-positive myonucleus. The total length is indicated for each reconstructed myofiber.
Table 1.
Reconstructions of Individual Myofibers after Stretch
| Myofiber Length (mm) | Number of BrdU-Labeled Myonuclei | Number of BrdU-Labeled Satellite Cells |
|---|---|---|
| 2.544 | 3 | 8 |
| 1.56 | 2 | 3 |
| 0.852 | 3 | 1 |
| 2.064 | 4 | 17 |
| 0.624 | 3 | 4 |
| 2.688 | 4 | 7 |
The unoperated, passively stretched inferior rectus muscle ipsilateral to the resected superior rectus muscle showed increases over control to that seen in the resected muscle 1 week after surgery. There was a significant increase in BrdU-positive myonuclei in the orbital and global layers, 1.89% ± 0.3% and 0.52% ± 0.08%, respectively, compared with the normal control muscles (Fig. 4).
In the resected superior rectus muscle, 2 weeks after resection, the number of BrdU-positive myonuclei remained elevated, with an average of 0.34% ± 0.07% and 0.3% ± 0.03% of the myofibers in orbital and global layer cross sections, respectively, of the surgically treated superior rectus muscle containing BrdU-positive myonuclei—still a significant increase over the control muscles. By 2 weeks after surgery, only the global layer showed a significant increase in myonuclear addition in the passively stretched inferior rectus.
The number of BrdU-positive satellite cells both 1 and 2 weeks after surgery was significantly increased over that in the normal adult EOMs. One week after resection, 6.1% ± 1.3% and 7.0% ± 1.0% of the myofibers in the orbital and global layer cross sections of the resected muscles, respectively, were associated with a BrdU-positive satellite cell. This upregulation of BrdU-positive satellite cells in the resected superior rectus muscle remained significantly elevated at 2 weeks, with increases of 2.2% ± 0.3% and 2.4% ± 0.3% in the orbital and global layers, respectively (Fig. 4). In the inferior rectus muscle ipsilateral to the resected superior rectus muscle 1 week after surgery, the number of BrdU-positive satellite cells was elevated over control levels at 1 week, and this significant elevation was maintained only in the global layer at 2 weeks after the passive stretch associated with resection of the superior rectus. Myofibers in both orbital and global layers in control muscles had 0.5% ± 0.08% of myofibers with BrdU-positive satellite cells associated with them. The number of BrdU-positive satellite cells within the resected muscles was fourfold higher than the number of BrdU-positive myonuclei.
The number of satellite cells positive for the myogenic regulatory factor MyoD in the resected muscles was determined (Figs. 1B, 6A). Both 1 and 2 weeks after resection, the number of MyoD-positive satellite cells was significantly elevated over control levels in both the surgically stretched superior rectus (Fig. 6A) and the passively stretched inferior rectus in the same orbit. In the resected superior rectus muscles, 8.17% ± 1.5% of the myofibers in the orbital layer and 11.43% ± 1.9% of the myofibers in the global layer were associated with a MyoD-positive satellite cells, compared with 4.27% ± 0.9% and 3.1% ± 0.6% in orbital and global layers of the control EOMs, respectively. In the inferior rectus muscle ipsilateral to the resected superior rectus muscle 1 week after surgery, there was a threefold increase in MyoD-positive satellite cells compared with the normal control: 15.27% ± 0.9% and 10.3% ± 1.4% in the orbital and global layers, respectively. These increases remained significantly increased over the control at 2 weeks for the resected superior rectus muscles and for the orbital layer of the inferior rectus. This differential increase of MyoD expression compared with control EOMs was semiquantified by using Western blot analysis at 1 week after resection. The resected muscles showed significantly elevated levels of MyoD compared with normal adult EOMs (Figs. 6B, 6C). Leg muscle is essentially MyoD-negative.18
Figure 6.
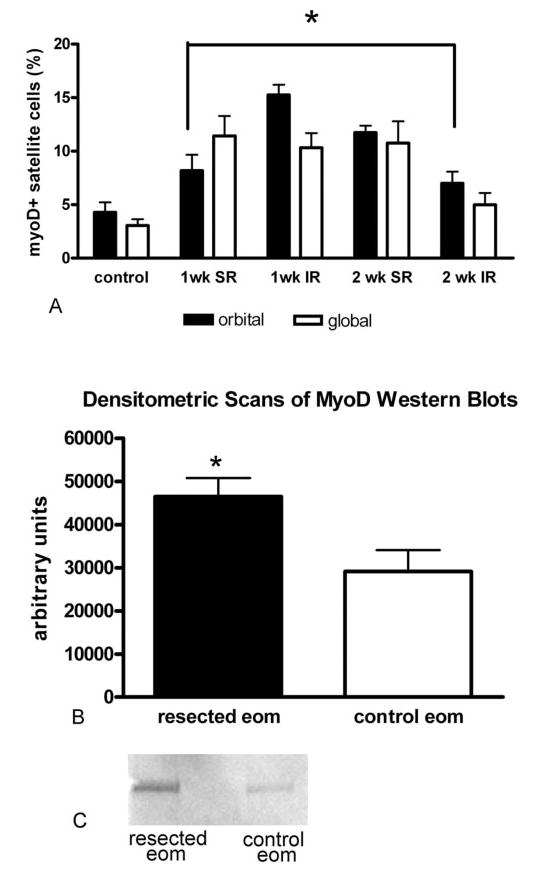
Effect of resection on MyoD expression. (A) Quantitative analysis indicates that resection of the superior rectus muscle resulted in an increased number of MyoD-positive satellite cells 1 week (SR) and 2 weeks after resection compared with normal superior rectus muscle (SR control). In the inferior rectus ipsilateral to the resected muscle (IR), there was also a significant increase in MyoD-positive satellite cells compared with the control *Statistically significant compared with control levels (P < 0.05). (B) Densitometric scans of Western blots prepared from resected superior rectus muscles and contralateral nonsurgical control muscles. The y-axis represents arbitrary units. *Statistical significance compared to control levels (n = 5; P < 0.05). (C) Representative Western blot of total MyoD expression in a resected muscle 1 week after surgery and in the contralateral control muscle from the same rabbit. 50, molecular mass marker (kDa).
In the resected EOM muscles, there were significant increases in the percentage of myofibers positive for the neonatal MyHC isoform in both orbital and global layers and the developmental MyHC isoform in the global layers of the resected superior rectus muscles (Fig. 7). The orbital layers are almost completely developmental MyHC–positive in the rabbits, and no change was seen in expression of this isoform with this surgical intervention. The passively stretched inferior rectus muscle on the operated globe did not show increased expression of either neonatal or developmental MyHC by 1 week, and only in neonatal expression 2 weeks after this passive stretch. The presence of a BrdU-positive myonucleus did not appear to be predictive of expression of neonatal MyHC (Fig. 8), where approximately 40% ± 10% of the myofibers were positive for neonatal MyHC and BrdU-positive myonuclei.
Figure 7.
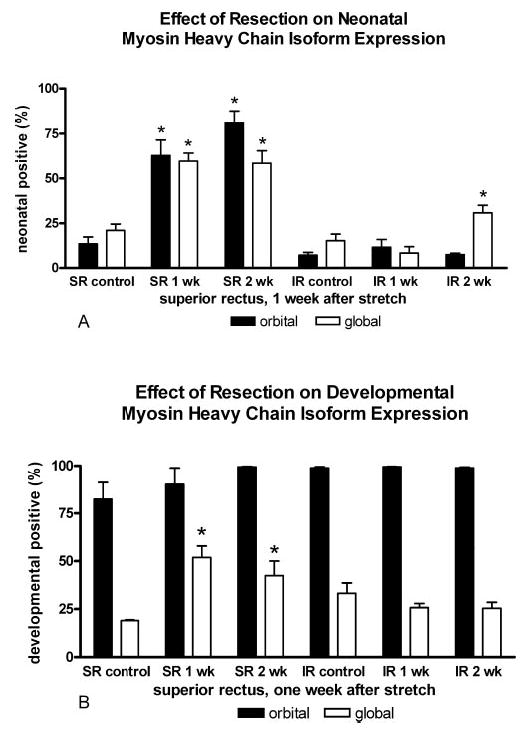
Effect of resection on neonatal and developmental MyHC expression. (A) Effect of resection on myofiber expression of the neonatal MyHC isoform in the orbital and global layers of control and resected superior rectus (SR) and inferior rectus (IR) muscles from surgically altered orbits. (B) Effect of resection on myofiber expression of developmental MyHC isoform in the orbital and global layers of control and resected superior rectus (SR) and inferior rectus (IR) muscles. *Significant difference from control levels (P < 0.05).
Figure 8.
(A) Cross section of a superior rectus muscle 1 week after resection, immunostained for BrdU and dystrophin. {*) Myofibers with BrdU-positive myonuclei. (B) Serial section of the same superior rectus muscle immunostained for neonatal myosin heavy chain isoform. *The same fibers indicated in (A), with BrdU-positive myonuclei. Bar, 50 μm.
The muscles were examined for inflammatory cell infiltrate using CD11b, an antibody that identifies neutrophils, monocytes, and macrophages. None of the resected muscles showed evidence for inflammatory cell infiltrate using this antibody at any of the postsurgical intervals (not shown).
The effect of resection on expression of IGF-1 was quite striking. Normally, the cytoplasm of a large percentage of the myofibers is IGF-1-positive (Fig. 9). After stretching caused by resection, the IGF decreased its cytoplasmic localization and appeared to have moved to the basal laminar/sarcolemmal boundary. Current studies are under way to clarify the changes in IGF-1 localization with resection.
Figure 9.
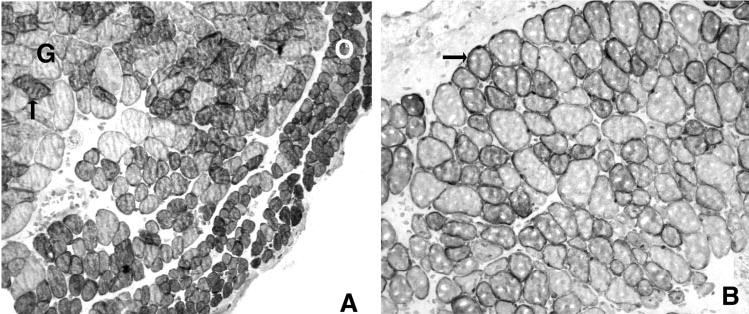
(A) Immunohistochemical staining for the presence of IGF-1 in myofibers from control a superior rectus muscle. O, orbital layer; G, global layer. Arrow: Example of a positive myofiber in the global layer. (B) Immunohistochemical staining for the presence of IGF-1 in myofibers from a superior rectus muscle 1 week after resection. Arrow: positive myofiber. Bar, 50 μm.
DISCUSSION
EOM resection significantly increased both satellite cell activation and apparent fusion of these cells into existing myofibers, as evidenced by increased BrdU-positive myonuclei in individual myofibers within the resected superior rectus muscles. In addition, evidence for myofiber degeneration and regeneration was present, as there was an increased number of myofibers of small cross–sectional area with centrally located nuclei. These changes in satellite cell activation and addition of myonuclei are not simply the result of surgical trauma, as increased numbers of both activated satellite cells and BrdU-positive myonuclei are present in the inferior rectus muscle ipsilateral to the resected superior rectus. This muscle was subjected to passive stretching due to rotation of the globe from shortening of its antagonist muscle. This passive stretch resulted in significant upregulation of myonuclei, albeit less pronounced than that in the resected muscle. In addition, a generalized inflammatory reaction did not develop, as evidenced by the lack of inflammatory cell infiltrate in hematoxylin and eosin and cd11b immunostaining.
Unlike limb skeletal muscles, the normal EOM in adults maintain a population of activated satellite cells.17,18 These activated satellite cells appear to fuse into existing myofibers in adult EOMs, and the myofiber population undergoes a continuous process of myonuclear addition throughout life.17,18 This process continues even in the EOM of elderly individuals.19 Using the same 12-hour BrdU exposure followed by a 24-hour BrdU-free period as was used in the present study, we found in an earlier study that approximately 1 per 1000 myofibers had BrdU-positive myonuclei.17 After resection of the superior rectus muscle, there were 20 per 1000 myofibers in cross section with BrdU-positive myonuclei. In addition, in individually reconstructed myofibers from normal adult EOMs, only one new myonucleus was found in those myofibers with BrdU-positive myonuclei. In contrast, after resection, multiple BrdU-positive myonuclei were found in the reconstructed myofibers. The apparently random location of the BrdU-positive myonuclei, which presumably represent satellite cells that have fused into existing myofibers within the stretched muscle,20,21 occurs after other muscle injuries that result in repair of existing myofibers. For example, limb lengthening by standard orthopedic methods resulted in BrdU-positive nuclei randomly along the length of desmin-positive leg muscle fibers.13 This type of segmental repair of existing myofibers occurs in muscular dystrophy as well.22,23
In an earlier study, approximately 0.54% ± 0.08% of myofiber profiles in cross section in normal, uninjured EOMs were associated with a BrdU-positive satellite cell 24 hours after a 12-hour BrdU-labeling regimen.17 The results of the present study indicate that, after resection, this was increased 10-fold in the resected muscle to 6.1% ± 1.3% and threefold in the passively stretched ipsilateral antagonist muscle, to 1.88% ± 0.28%. This upregulation in satellite cell activation occurs in other models of stretching, including wing-weighting8 and mechanical stretching of single fibers in vitro.11,12 In fact, even a single eccentric contraction can result in a measurable, albeit short-lived, increase in satellite cell activation.24 Single myofibers exposed to stretch and labeled with BrdU showed that this activation stimulus does not trigger activation of the entire population of satellite cells along that stretched fiber.12 This implies that the control of this process is very local along the fiber length. There is strong evidence of local gene regulation in both myonuclei and satellite cells along the length of individual myofibers, despite uniform protein composition.25,26 In addition, there is a great deal of evidence that suggests significant heterogeneity in the satellite cell population itself.27,28
The stretched muscles showed an increased heterogeneity in myofiber cross-sectional area, with very small and very large myofibers present, presumably representing both new fibers and hypertrophied fibers. Heterogeneity in cross-sectional area occurs in limb muscle subjected to eccentric exercise, where cross-sectional areas can reach three times their normal size.29,30 These large muscle fibers were thought to represent segmental areas of muscle fiber hypercontraction, and these fibers became more pronounced as the time after eccentric contraction injury increased. This was certainly true in the present study, as these were more prominent by 14 days after stretching than by 7 days. In addition, these large myofibers showed disruption in dystrophin staining of their sarcolemma.
The presence of increased numbers of myofibers with centrally located myonuclei and small cross-sectional areas in the resected muscles can be explained by several very distinct mechanisms. However, it should be noted that very few of the centrally located myonuclei were BrdU-positive. Centrally located nuclei could indicate that (1) new myofibers were being formed de novo, (2) stretched myofibers in the process of elongation by addition of new myonuclei have less cytoplasm, or (3) disruption of the internal myofibrillar order results in myonuclear translocation. Interestingly, an increased number of centrally located myonuclei in stretched hindlimb muscle was found in regions of myofibrillar disruption.31 Other studies have correlated myonuclear translocation to alterations in desmin after a stretch injury.32 It appears that resection may turn on both fiber regeneration and fiber repair mechanisms, and passive stretch in the antagonist muscle in the same orbit increases the number of myonuclei and satellite cell activation, albeit to a lesser extent.
Resection resulted in increased expression of MyoD in both satellite cells and myonuclei in the stretched EOMs. Stretch-overloaded limb skeletal muscle in quails increased their expression of both MRF4 and MyoD mRNA,33 although in this case the effect was independent of satellite cell proliferation.34 In a study of eccentric contraction in limb skeletal muscle, myostatin, MyoD, and myogenin transcripts were rapidly and transiently elevated. Although cellular localization of these myogenic regulatory factors was not performed, eccentric contraction induced expression of these myogenic and differentiation pathways in limb muscle.35 Particularly interesting is the fact that passive stretching of the soleus muscle resulted in significant increases in MyoD, myf5, and MRF4 along the entire muscle length.10
In the resected superior rectus muscles, there was a significant increase in the number of myofibers expressing the neonatal MyHC isoform in orbital and global layers and in developmental MyHC isoform in the global layer compared with normal adult EOMs. These changes were not confined just to the myotendinous regions. The passively stretched inferior rectus muscle on the surgical globe did not show a compensatory change in expression of either of these two isoforms. Several studies have examined the effect of stretch and/or eccentric contractions on MyHC isoform switching. In an in vitro stretch paradigm of single–skinned fibers from limb muscles, type 1 and 2 MyHC isoform switching was observed.36 Expression of embryonic MyHC was not examined. Eccentric exercise of the rat hindlimb resulted in a significant increase in the expression of the embryonic MyHC isoform.35 It appears that although changes in myofiber size may correlate with increases in immature MyHC expression, the two processes are most likely under different mechanisms of control. The lack of correlation between expression of neonatal MyHC expression and BrdU-positive myonuclei in the stretched EOM supports this hypothesis.
The alteration in IGF-1 localization after resection suggests that IGF-1 may be involved in signaling after resection. Further studies are needed to confirm or refute this hypothesis. However, one well known regulator of satellite cell activation and muscle hypertrophy induced by stretch is mechanogrowth factor, a splice variant of IGF-1.37 Both IGF-R and IGF-1 continue to be expressed in normal adult EOMs.38,39 In a stretch model of muscle injury, increases in IGF isoforms correlate with increases in MyoD expression and satellite cell activation.40 Locally produced IGF-1 is increased in both stretch-activated muscles40,41 and in functionally overloaded muscles.42 The role of IGF in the initiation and/or control of eye muscle responses to resection is currently being studied.
Strabismus is a disorder of binocular alignment that affects 3% to 5% of the children in the United States. A common treatment for this disorder is surgical resection, a procedure that involves removing several millimeters of the insertional end of the muscle and then resuturing the muscle back to the sclera. The goal of resection is to strengthen an underacting muscle and to alter the rotational position of the globe. However, the surgical failure rate of this surgery is high and is dependent on the original ocular deviation. Failure rates in patients with strabismus who have large angle deviations, as reported in the literature, range from 11% to 70%.43,44 The failure rates increase as the postsurgical period increases, such that by 3 years after surgery, more than 55% are no longer adequately aligned.45 The increasing failure rate over time suggests, in part, that some ongoing process occurs within the strabismic muscles to counteract the surgical shortening. It is certainly reasonable to hypothesize that in EOMs, the regenerative response to muscle stretch and its ongoing ability to remodel individual myofibers throughout life may play some role in surgical outcomes for strabismus. If true, pharmacological manipulation or immunomodulation of myonuclear turnover and satellite cell activation could be used as a therapeutic intervention to improve long- and short-term results of surgery in patients with strabismus.
Footnotes
Disclosure: S.P. Christiansen, None; L.K. McLoon, None
Supported by Grants EY13979, EY15313, and EY11375 from the National Eye Institute; the Minnesota Medical Foundation; the Minnesota Lions and Lionesses; and an unrestricted grant to the Department of Ophthalmology from Research to Prevent Blindness, Inc.
References
- 1.Holtzer H, Marshall JM, Finck H. An analysis of myogenesis by use of fluorescent antimyosin. J Biophys Biochem Cytol. 1957;3:705–724. doi: 10.1083/jcb.3.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmalbruch H, Lewis DM. Dynamics of nuclei of muscle fibers and connective tissue cells in normal and denervated rat muscles. Muscle Nerve. 2000;23:617–626. doi: 10.1002/(sici)1097-4598(200004)23:4<617::aid-mus22>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 3.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenblatt JD, Parry DJ. Gamma irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J Appl Physiol. 1992;73:2538–2543. doi: 10.1152/jappl.1992.73.6.2538. [DOI] [PubMed] [Google Scholar]
- 5.Putman CT, Dusterhoft S, Pette D. Satellite cell proliferation in low frequency-stimulated fast muscle of hypothyroid rat. Am J Physiol. 2000;279:C682–C690. doi: 10.1152/ajpcell.2000.279.3.C682. [DOI] [PubMed] [Google Scholar]
- 6.Smith HK, Maxwell L, Rodgers CD, McKee NH, Phyley MJ. Exercise-enhanced satellite cell proliferation and new myonuclear addition in rat skeletal muscle. J Appl Physiol. 2001;90:1407–1414. doi: 10.1152/jappl.2001.90.4.1407. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy JM, Eisenberg BR, Reid SK, Sweeney LJ, Zak R. Nascent muscle fiber appearance in overloaded chicken slow-tonic muscle. Am J Anat. 1988;181:203–215. doi: 10.1002/aja.1001810209. [DOI] [PubMed] [Google Scholar]
- 8.Winchester PK, Davis ME, Alway SE, Gonyea WJ. Satellite cell activation in the stretch-enlarged anterior latissimus dorsi muscle of the adult quail. Am J Physiol. 1991;260:C202–C212. doi: 10.1152/ajpcell.1991.260.2.C206. [DOI] [PubMed] [Google Scholar]
- 9.McCormick KM, Schultz E. Mechanisms of nascent fiber formation during avian skeletal muscle hypertrophy. Dev Biol. 1992;150:319–324. doi: 10.1016/0012-1606(92)90245-c. [DOI] [PubMed] [Google Scholar]
- 10.Zador E, Dux L, Wuytack F. Prolonged passive stretch of rat soleus muscle provokes an increase in the mRNA levels of the muscle regulatory factors distributed along the entire length of the fibers. J Muscle Res Cell Motil. 1999;20:395–402. doi: 10.1023/a:1005541522599. [DOI] [PubMed] [Google Scholar]
- 11.Tatsumi R, Sheehan SM, Iwasaki H, Hattori A, Allen RE. Mechanical stretch induces activation of skeletal muscle satellite cells in vitro. Exp Cell Res. 2001;267:107–114. doi: 10.1006/excr.2001.5252. [DOI] [PubMed] [Google Scholar]
- 12.Wozniak AC, Pilipowicz O, Yablonka-Reuveni Z, et al. C-Met expression and mechanical activation of satellite cells on cultured muscle fibers. J Histochem Cytochem. 2003;51:1437–1445. doi: 10.1177/002215540305101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day CS, Moreland MS, Floyd SS, Huard J. Limb lengthening promotes muscle growth. J Orthop Res. 1997;15:227–234. doi: 10.1002/jor.1100150211. [DOI] [PubMed] [Google Scholar]
- 14.Caiozzo VJ, Utkan A, Chou R, et al. Effects of distraction on muscle length: mechanisms involved in sarcomerogenesis. Clin Orthop. 2002;403(suppl):S133–S145. doi: 10.1097/00003086-200210001-00016. [DOI] [PubMed] [Google Scholar]
- 15.Christiansen S, Madhat M, Baker L, Baker R. Fiber hypertrophy in rat extraocular muscle following lateral rectus resection. J Pediatr Ophthalmol Strabismus. 1988;25:167–171. doi: 10.3928/0191-3913-19880701-05. [DOI] [PubMed] [Google Scholar]
- 16.Porter JD, Baker RS. Muscles of a different ‘color’: the unusual properties of the extraocular muscles may predispose or protect them in neurogenic and myogenic disease. Neurology. 1996;46:30–37. doi: 10.1212/wnl.46.1.30. [DOI] [PubMed] [Google Scholar]
- 17.McLoon LK, Rowe J, Wirtschafter JD, McCormick KM. Rates of myonuclear turnover and evidence of apoptosis in normal uninjured myofibers from extraocular muscles of adult rabbits. Muscle Nerve. 2004;29:707–715. doi: 10.1002/mus.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLoon LK, Wirtschafter JD. Continuous myonuclear addition to single extraocular myofibers in uninjured adult rabbits. Muscle Nerve. 2002;25:348–358. doi: 10.1002/mus.10056. [DOI] [PubMed] [Google Scholar]
- 19.McLoon LK, Wirtschafter JD. Activated satellite cells in extraocular muscles of normal adult monkeys and humans. Invest Ophthalmol Vis Sci. 2003;44:1927–1932. doi: 10.1167/iovs.02-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schultz E, McCormick KM. Skeletal muscle satellite cells. Rev Physiol Biochem Pharmacol. 1994;123:213–257. doi: 10.1007/BFb0030904. [DOI] [PubMed] [Google Scholar]
- 21.Goldring K, Partridge T, Watt D. Muscle stem cells. J Pathol. 2002;197:457–467. doi: 10.1002/path.1157. [DOI] [PubMed] [Google Scholar]
- 22.Blaveri K, Heslop L, Yu DS, et al. Patterns of repair of dystrophic mouse muscle: studies on isolated fibers. Dev Dyn. 1999;216:244–256. doi: 10.1002/(SICI)1097-0177(199911)216:3<244::AID-DVDY3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Kong J, Anderson JE. Dynamic restoration of dystrophin to dystrophin-deficient myotubes. Muscle Nerve. 2001;24:77–88. doi: 10.1002/1097-4598(200101)24:1<77::aid-mus9>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 24.Aarimma V, Rantanen J, Best T, Schultz E, Corr D, Kalimo H. Mild eccentric stretch injury in skeletal muscle causes transient effects on tensile load and cell proliferation. Scand J Med Sci Sports. 2004;14:367–372. doi: 10.1111/j.1600-0838.2004.403.x. [DOI] [PubMed] [Google Scholar]
- 25.Newlands S, Levitt LK, Robinson CS, et al. Transcription occurs in pulses in muscle fibers. Genes Dev. 1998;12:2748–2758. doi: 10.1101/gad.12.17.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wozniak AC, Kong J, Block E, Pilipowicz O, Anderson JE. Signaling satellite cell activation in skeletal muscle: markers, models, stretch and potential alternate pathways. Muscle Nerve. 2005;31:283–300. doi: 10.1002/mus.20263. [DOI] [PubMed] [Google Scholar]
- 27.Sherwood RI, Christensen JL, Conboy IM, et al. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 28.Olguin HC, Olwin BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol. 2004;15:375–388. doi: 10.1016/j.ydbio.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lieber RL, Friden J. Selective damage of fast glycolytic muscle fibers with eccentric contraction of the rabbit tibialis anterior. Acta Physiol Scand. 1988;133:587–588. doi: 10.1111/j.1748-1716.1988.tb08446.x. [DOI] [PubMed] [Google Scholar]
- 31.Dix DJ, Eisenberg BR. Redistribution of myosin heavy chain mRNA in the midregion of stretched muscle fibers. Cell Tissue Res. 1991;263:61–69. doi: 10.1007/BF00318400. [DOI] [PubMed] [Google Scholar]
- 30.Friden J, Lieber RL. Segmental muscle fiber lesions after repetitive eccentric contractions. Cell Tissue Res. 1998;293:165–171. doi: 10.1007/s004410051108. [DOI] [PubMed] [Google Scholar]
- 32.Lieber RL, Shah S, Friden J. Cytoskeletal disruption after eccentric contraction-induced muscle injury. Clin Orthop. 2002;403(suppl):S90–S99. doi: 10.1097/00003086-200210001-00011. [DOI] [PubMed] [Google Scholar]
- 33.Lowe DA, Lund T, Alway SE. Hypertrophy-stimulated myogenic regulatory factor mRNA increases are attenuated in fast muscle of aged quails. Am J Physiol. 1998;275:C155–C162. doi: 10.1152/ajpcell.1998.275.1.C155. [DOI] [PubMed] [Google Scholar]
- 34.Lowe DA, Alway SE. Stretch-induced myogenin, MyoD, and MRF4 expression and acute hypertrophy in quail slow-tonic muscle are not dependent upon satellite cell proliferation. Cell Tissue Res. 1999;296:531–539. doi: 10.1007/s004410051314. [DOI] [PubMed] [Google Scholar]
- 35.Peters DP, Barash IA, Burdi M, et al. Asynchronous functional, cellular, and transcriptional changes after a bout of eccentric exercise in the rat. J Physiol. 2003;553:947–957. doi: 10.1113/jphysiol.2003.048462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galler S, Schmitt TL, Hilber K, Pette D. Stretch activation and isoforms of myosin heavy chain and troponin-T of rat skeletal muscle fibers. J Muscle Res Cell Motil. 1997;18:555–561. doi: 10.1023/a:1018615302548. [DOI] [PubMed] [Google Scholar]
- 37.Yang S, Alnaqeeb M, Simpson H, Goldspink G. Cloning and characterization of an IGF-1 isoform expressed in skeletal muscle subjected to stretch. J Muscle Res Cell Motil. 1996;17:487–495. doi: 10.1007/BF00123364. [DOI] [PubMed] [Google Scholar]
- 38.Fischer MD, Gorospe JR, Felder E, et al. Expression profiling reveals metabolic and structural components of extraocular muscles. Physiol Genomics. 2002;9:71–84. doi: 10.1152/physiolgenomics.00115.2001. [DOI] [PubMed] [Google Scholar]
- 39.McLoon LK, Christiansen SP. A novel approach to treatment of strabismus: increasing extraocular muscle strength in rabbits with insulin growth factor II. Invest Ophthalmol Vis Sci. 2003;44:3866–3872. doi: 10.1167/iovs.03-0223. [DOI] [PubMed] [Google Scholar]
- 40.Hill M, Goldspink G. Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite cell activation following local tissue damage. J Physiol. 2003;549:409–418. doi: 10.1113/jphysiol.2002.035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldspink G. Changes in muscle mass and phenotype and the expression of autocrine and systemic growth factors by muscle in response to stretch and overload. J Anat. 1999;194:323–334. doi: 10.1046/j.1469-7580.1999.19430323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCall GE, Allen DL, Haddad F, Baldwin KM. Transcriptional regulation of IGF-1 expression in skeletal muscle. Am J Physiol. 2003;285:C831–C839. doi: 10.1152/ajpcell.00047.2003. [DOI] [PubMed] [Google Scholar]
- 43.Mims JL. Outcome of 5 mm resection of one medial rectus extraocular muscle for recurrent exotropia. Binocul Vis Strabismus Q. 2003;18:143–150. [PubMed] [Google Scholar]
- 44.Currie ZI, Shipman T, Burke JP. Surgical correction of large-angle exotropia in adults. Eye. 2003;17:334–339.13. doi: 10.1038/sj.eye.6700347. [DOI] [PubMed] [Google Scholar]
- 45.Esswein MB, von Noorden GK, Coburn A. Comparison of surgical methods in the treatment of dissociated vertical deviation. Am J Ophthalmol. 1992;15:287–290. doi: 10.1016/s0002-9394(14)71580-6. [DOI] [PubMed] [Google Scholar]


