Abstract
PURPOSE.
Lens connexins undergo proteolytic cleavage of their C termini during fiber maturation. Although the functional significance of this is unknown, cleavage has been correlated with changes in channel-gating properties. This study evaluates the functional consequences of this endogenous truncation by characterizing the properties of a C-terminal truncated Cx50 protein.
METHODS.
Murine and human Cx50 were truncated at amino acids 290 and 294, respectively, before expression in paired Xenopus oocytes or mammalian cells. Protein expression was evaluated by immunocytochemistry. Dual whole-cell voltage clamp techniques were used to analyze macroscopic and single-channel conductance, voltage-gating properties, and kinetics; pH gating sensitivity was measured by superfusion with 100% CO2-saturated media.
RESULTS.
Cx50tr290 channels exhibited an 86% to 89% reduction in mean macroscopic conductance compared with full-length Cx50. Heterotypic channels formed functional gap junctions, displayed an intermediate level of coupling, and exhibited unaltered voltage-gating properties. C-terminal truncation did not alter single-channel gating characteristics or unitary conductance. Interestingly, truncated and full-length Cx50 channel conductances were reversibly blocked by cytoplasmic acidification.
CONCLUSIONS.
C-terminal truncation of Cx50 did not inhibit the formation of homotypic or heterotypic channels. However, a significant decrease in conductance was observed for truncated channels, a phenomenon independent of alterations in voltage-gating sensitivity, kinetics, or chemical gating. These results provide a plausible explanation for the 50% decrease in junctional coupling observed during lens fiber maturation. (Invest Ophthalmol Vis Sci. 2006;47:4474-4481) DOI:10.1167/iovs.05-1582
Gap junctions are transmembrane channels that facilitate the intercellular transport of essential ions, metabolites, secondary messengers, and other small molecules.1-4 Gap junctions result from the alignment of hemichannels in the extracellular spaces of adjacent cells. Hemichannels, in turn, are formed by the oligomerization of six connexin proteins within the cell membrane.5,6 Intercellular channels can be either homotypic—the association of two identical hemichannels— or heterotypic—a combination of two hemichannels, each containing a different connexin.7 Connexins (Cxs) are integral membrane proteins containing four membrane-spanning domains, two extracellular loops, a cytoplasmic loop, and cytoplasmic amino and carboxyl termini.8,9 The connexin gene family consists of 22 members,10,11 all varying in amino acid sequence, molecular weight, and length of cytoplasmic domains. Additionally, gap junctions formed by different connexin subunits have unique physiologic properties.1,2,12,13
The lens is an avascular, spherical organ suspended between the aqueous and vitreous humors of the eye. The mammalian lens depends on three specific connexins—Cx43, Cx46, and Cx50 —to facilitate intercellular communication between the metabolically active cells of the lens epithelium (Cx43 and Cx50) and the quiescent lens core (Cx46 and Cx50).11,14,15 Throughout life, lens epithelial cells proliferate, migrate to the lens equator, and elongate by stretching from anterior to posterior poles, forming lens fibers.16 These cells then undergo a series of specialized differentiation processes, including increased synthesis of crystallin proteins,15,17 degradation of all intracellular organelles,18 and proteolytic cleavage of the C termini of fiber cell connexins.19,20 Although the functional relevance of this endogenous truncation remains unknown, cleavage coincides with junctional plaque reorganization and protein stabilization.21-23
Several lines of evidence indicate that the carboxyl terminus may play an important role in channel gating and permeability.24-28 Two calpain cleavage sites have been identified at amino acids 290 and 300 within the carboxyl terminus of Cx5020; however, conflicting results on the relationship between Cx50 truncation and pH gating have been published. Some reports have shown greatly reduced pH sensitivity after truncation of the C terminus,24,26 whereas other data have shown the persistence of pH gating after cleavage.25 This study uses the paired Xenopus oocyte system in conjunction with transfected N2A cells to clarify the functional differences in voltage and pH gating between gap junctions composed of full-length Cx50 and its naturally occurring C-terminal truncation.
MATERIALS AND METHODS
Molecular Cloning
Murine Cx5029 was cloned between the XhoI and XbaI sites of the pCS2+ expression vector. Cx50tr290 was prepared by inserting a stop codon (TGA) after amino acid 290 by PCR amplification, subcloned into the pCS2+ vector, and sequenced on both strands. Cx50 and Cx50tr290 were also subcloned into the pIRES2-EGFP expression vector (Clontech, Palo Alto, CA). Human Cx50tr294 was prepared by inserting a stop codon (TGA) after amino acid 294 of human Cx50 by PCR amplification, subcloned into pCS2+ between the EcoRI and XhoI sites, and sequenced on both strands.
In Vitro Transcription, Oocyte Microinjection, and Pairing
Cx50, Cx50tr290, and human Cx50tr294 constructs were linearized using NotI and were transcribed (SP6 mMessage mMachine kit; Ambion, Austin, TX). Adult Xenopus females were anesthetized, and ovaries were removed in a manner that fully conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Stage V to VI oocytes were collected after ovarian lobes were defolliculated in a solution containing 50 mg/mL collagenase B and 50 mg/mL hyaluronidase in modified Barths (MB) medium without Ca2+. Cells were cultured in MB medium at room temperature. Cells were first injected with 10 ng antisense Xenopus Cx38 oligonucleotide to eliminate endogenous intercellular channels. Twenty-four hours later, oocytes were reinjected with full-length Cx50 cRNA (20 ng/cell), Cx50tr290 cRNA (80 ng/cell), human Cx50tr294 cRNA (80 ng/cell), or H2O as a negative control. Vitelline envelopes were removed in a hypertonic solution (200 mM aspartic acid, 10 mM HEPES, 1 mM MgCl2, 10 mM EGTA, and 20 mM KCl at pH 7.4), and oocytes were manually paired.
Dual Whole-Cell Voltage Clamp
Gap junctional coupling between oocyte pairs was measured using dual-whole cell voltage clamp.30,31 Electrodes (1.2-mm diameter, omega dot; Glass Company of America, Millville, NJ) were pulled to a resistance of 1 to 2 MΩ with a vertical puller (Narishige, Tokyo, Japan) and filled with 3 M KCl, 10 mM EGTA, and 10 mM HEPES, pH 7.4. Voltage clamping of oocyte pairs was performed with two amplifiers (GeneClamp 500; Axon Instruments, Foster City, CA) controlled by a PC-compatible computer through interface (Axon Instruments). Software (pCLAMP 8.0; Axon Instruments) was used to program stimulus and data collection paradigms.
For measurements of junctional conductance (Gj), both cells of a pair were initially clamped at -40 mV, and alternating pulses of ±20 mV were applied to one cell. Junctional current (Ij) was divided by the voltage to yield the conductance: Gj = Ij/(V1-V2). To analyze voltage gating, transjunctional potentials (Vj) of opposite polarity were generated by hyperpolarizing or depolarizing one cell in 20-mV steps (over a range of ±120 mV). Steady state conductance was calculated at the end of the voltage pulse, normalized to the values determined at ±20 mV, plotted against Vj, and fit to a Boltzmann relation of the form: Gjss = (Gjmax-Gjmin)/1 + exp[A(Vj-V0)] + Gjmin, where Gjss is the steady state junctional conductance, Gjmax (normalized to unity) is the maximum conductance, Gjmin is the residual conductance at large values of Vj, and V0 is the transjunctional voltage at which Gjss = (Gjmax-Gjmin)/2. The constant A (= nq/kT) represents the voltage sensitivity in terms of gating charge as the equivalent number (n) of electron charges (q) moving through the membrane, k is the Boltzmann constant, and T is the absolute temperature.
To examine pH sensitivity, Gj measurements were taken in 30-second intervals by applying voltage steps of 10 mV (1-second duration) to one cell while the voltage in cell two was held constant at -40 mV. The oocyte chamber was perfused at a constant flow rate with MB medium containing Ca2+ and saturated with 100% CO2 for a period of 5 minutes, followed by washout with normal MB solution for 12 minutes.
Preparation of Oocyte Samples for Western Blot Analysis and Quantification
Oocytes were collected in 1 mL buffer containing 5 mM Tris pH 8.0, 5 mM EDTA, and protease inhibitors29 and were lysed with a series of mechanical passages through needles of diminishing caliber (20, 22, and 26 gauge). Extracts were centrifuged at 1000g at 4°C for 5 minutes. The supernatant was then centrifuged at 100,000g at 4°C for 30 minutes. Membrane pellets were resuspended in SDS sample buffer (2 μL per oocyte), and samples were separated on 10% SDS gels and transferred to nitrocellulose membranes. Blots were blocked with 5% BSA in 1× PBS with 0.02% NaN3 for 1 hour and were probed with a polyclonal Cx50 antibody at a 1:1000 dilution.29 This was followed by incubation with alkaline phosphatase-conjugated anti-rabbit secondary antibody (Jackson Laboratories, Bar Harbor, ME). Band intensities were quantified (1D Image Analysis software; Eastman Kodak, Rochester, NY). Values from four independent experiments were normalized to the mean value of band intensity of the full-length Cx50 sample.
Transient Transfection and Immunocytochemical Localization
HeLa cells were plated on 22-mm square coverslips, grown to 50% confluence, and transiently transfected with 1 μg connexin DNA subcloned into the pIRES2-EGFP vector (Lipofectamine 2000; Gibco BRL, Gaithersburg, MD). After overnight incubation, cells were fixed for 15 minutes with 1% paraformaldehyde in PBS and blocked with 5% BSA in PBS with 0.1% Tx-100 and 0.02% NaN3 for 1 hour. Cells were stained with a polyclonal Cx50 antibody at a 1:1000 dilution,29 followed by incubation with a Cy3-conjugated fluorescent goat anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories). Cells were viewed and photographed on a microscope (BX51; Olympus, Hamburg, Germany) with digital camera (MagnaFire; Optronics, Goleta, CA).
Transient Transfection and Dual Whole-Cell Patch-Clamp Electrophysiology
N2A cells were transiently transfected with Cx50 or Cx50tr290 with reagent (Lipofectamine 2000; Gibco BRL) and were plated at low density onto glass coverslips. Coverslips were transferred to the stage of a microscope (Diaphot; Nikon, Tokyo, Japan) and were bathed in a external solution containing 140 mM NaCl, 2 mM CsCl, 2 mM CaCl2,1 mM MgCl2, 5 mM HEPES, 4 mM KCl, 5 mM dextrose, 2 mM pyruvate, and 1 mM BaCl2, pH 7.2. Each cell of a cell pair was voltage clamped with patch pipettes pulled on a micropipette puller (Flaming/Brown; Sutter P87; Sutter Instrument, Novato, CA) with patch-clamp amplifiers (Axopatch1D; Axon Instruments). Patch electrodes had resistances from4to7MΩ when filled with internal solution containing 130 mM CsCl, 10 mM EGTA, 0.5 mM CaCl2, and 10 mM HEPES, pH 7.2. All current recordings were filtered at 0.2 to 0.5 kHz and were sampled at 2 to 5 kHz. Data were acquired (pCLAMP 8.0 software; Axon Instruments) and analyzed with that or another software (Origin 6.1; OriginLab Corp., Northampton, MA). Pairs were initially held at a common holding potential of 0 mV. To evaluate junctional coupling and macroscopic channel gating, depolarizing and hyperpolarizing pulses to various voltages were applied to one cell to establish a Vj gradient, and Ij was measured in the second cell (held at 0 mV). Single-channel currents were measured between weakly coupled cell pairs. All-point amplitude histograms were constructed, and data were fitted to Gaussian functions to determine the mean and variance of the baseline and open-channel current.
RESULTS
Expression of Connexin Proteins in Xenopus Oocytes
Translation of connexin cRNAs was quantified by immunoblot analysis of oocytes injected with full-length Cx50, Cx50tr290, or water using a polyclonal Cx50 antibody raised against an epitope in the central cytoplasmic loop of mouse Cx50.29 The antibody recognized a 63-kDa band corresponding to full-length Cx50 (◀) and a 32-kDa band corresponding to Cx50tr290 (▷) in oocyte samples injected with their respective cRNAs (Fig. 1A). The antibody also detected a nonspecific band in all three samples (denoted by the asterisk). Expression levels were quantified by measuring band densitometry, and a plot of the mean band density values (Fig. 1B) showed no significant difference in full-length Cx50 and Cx50tr290 protein expression (P > 0.05; Student t test). Thus both proteins were synthesized at equal levels, and any alteration in channel function caused by truncation could not be attributed to differences in protein expression.
FIGURE 1.
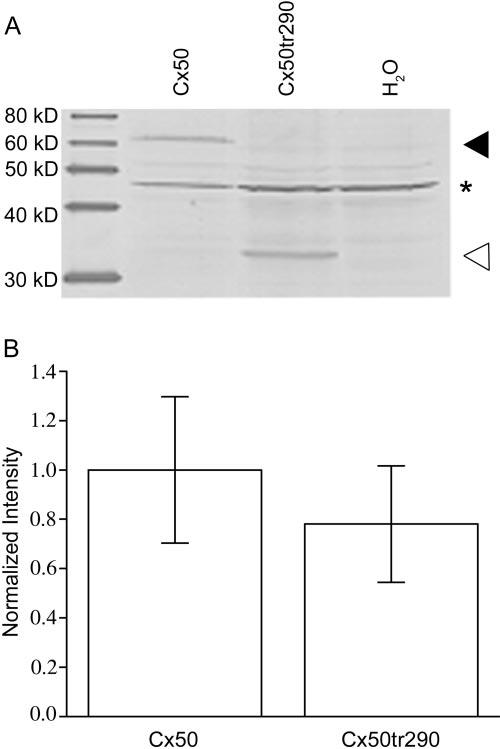
Western blot analysis of Xenopus oocyte extracts indicates similar expression of full-length (Cx50) and truncated (Cx50tr290) proteins. (A) Oocytes were collected and membrane fractions were prepared. Equal amounts of the preparations were loaded into each lane for each condition. Western blots were probed with a polyclonal rabbit anti-mouse Cx50 antiserum. Full-length (◀) and truncated (◁) Cx50 proteins were efficiently translated. *Antibody detection of a nonspecific band. (B) Quantitation of protein expression by densitometry showed equivalent levels of connexin expression. Levels of synthesis of full-length and truncated Cx50 proteins were not statistically different (P > 0.05). Densitometry values are the mean ± SE of four independent experiments that were normalized to the mean band intensity of the full-length sample.
Truncated Cx50 Channel Formation in Xenopus Oocytes
The ability to form intercellular channels was tested by whole-cell voltage clamp of paired Xenopus oocytes (Fig. 2). As expected, full-length Cx50 pairs formed functional channels with a mean intercellular conductance of 23.8 μS, a value several hundred-fold higher than the water-injected negative control pairs (0.06 μS). Cells injected with Cx50tr290 cRNA exhibited a mean conductance value of 3.4 μS, which was approximately 50-fold higher than background but was significantly decreased compared with full-length Cx50 (86% reduction; P < 0.05). Heterotypic cell pairs in which one cell contributed full-length Cx50 and the other expressed Cx50tr290 displayed a mean conductance of 6.2 μS. This 100-fold increase over background was intermediate between the homotypic full-length and truncated measurements. The level of heterotypic coupling was significantly decreased compared with full-length Cx50 (P < 0.05) but not Cx50tr290 homotypic channels (P > 0.05). These results show that Cx50tr290 forms functional gap junctions in homotypic and heterotypic pairs, but the mean gap junctional conductance was significantly decreased in both channel configurations.
FIGURE2.

Truncated Cx50 forms functional intercellular channels. Junctional conductance values were measured between oocyte pairs injected with full-length Cx50, Cx50tr290, or water. Full-length Cx50 and Cx50tr290 channels exhibited mean conductance values 400- or 50-fold greater than water-injected controls. Heterotypic channels formed by pairing two oocytes— one expressing full-length Cx50, the other injected with truncated Cx50 cRNA— displayed an intermediate level of coupling that was 100-fold higher than background. Columns are the mean ± SE. Data points within each column represent the individual conductance values for each condition tested.
Voltage-Gating Behavior of Full-Length and Truncated Cx50 Channels in Xenopus Oocytes
To characterize the functional properties of full-length and truncated Cx50 proteins, voltage-gating was examined by subjecting oocyte pairs to a series of hyperpolarizing and depolarizing transjunctional voltages (Vj). Figure 3 shows a comparison of representative traces of junctional currents (Ij) for oocyte pairs expressing homotypic Cx50 (Fig. 3A), homotypic Cx50tr290 (Fig. 3B), or heterotypic Cx50/Cx50tr290 (Fig. 3C) gap junctions. Ij decreased symmetrically with time in a voltage-dependent manner for all channel types, with a more rapid decline in current at greater transjunctional potentials. To analyze channel-closure kinetics, the initial 200-ms current decay was plotted against time and was fit to a mono-exponential function to determine the time constant tau (τ). Figure 3 shows representative current decays after application of a +80 mV voltage step for truncated (Fig. 3D) and for full-length (Fig. 3E) and heterotypic (Figs. 3F, 3G) Cx50 channels. All channels types exhibited a mean τ of 44 to 46 ms at this Vj step, and no significant changes in channel closure kinetics resulted from truncation of the C terminus of Cx50 (P > 0.05). The steady state voltage-gating behavior of these channels was examined by plotting Vj against Gj (normalized to the values obtained at ±20 mV) and fitting the data to the Boltzmann equation (Fig.3H). Equilibrium voltage conductance was similar; Boltzmann parameters are listed in Table 1. These data show that C-terminal truncation of Cx50 did not alter voltage gating in homotypic or heterotypic channels.
FIGURE 3.

Voltage-gating properties of full-length, truncated, and heterotypic Cx50 channels. Junctional currents (Ij) induced by transjunctional voltages (Vj) were plotted as a function of time. For homotypic channels composed of full-length Cx50 (A), Cx50tr290 (B), and heterotypic (C) channels, rapid current decay was seen at large voltages (±80 -120 mV), whereas modest voltages (±20-60 mV) exhibited slower decline in junctional current. No qualitative changes in channel voltage gating were seen among the three channel types. Channel kinetics were analyzed by fitting the initial 200 ms of current decay recorded at +80 mV to a monoexponential model for cell pairs expressing truncated (D), full-length (E), and heterotypic channels (F, G). Channel closure kinetics were not affected by C-terminal truncation. Tau (τ) values represent the mean ± SE of six independent Ij traces. Equilibrium-gating properties were analyzed by plotting normalized steady state conductance against transjunctional voltage and were fit to the Boltzmann equation (H). Comparison between full-length and truncated channels showed similar voltage sensitivity. Boltzmann parameters are given in Table 1.
TABLE 1.
Boltzmann Parameters for Full-length and Truncated Cx50 Channels
| Vj | V0 | Gjmin | A | |
|---|---|---|---|---|
| Xenopus oocytes | ||||
| Cx50 | + | 34 | 0.16 | 0.06 |
| Cx50 | - | -40 | 0.20 | 0.09 |
| Cx50tr290 | + | 28 | 0.14 | 0.11 |
| Cx50tr290 | - | -30 | 0.16 | 0.12 |
| N2A cells | ||||
| Cx50* | + | 38 | 0.21 | 0.16 |
| Cx50* | - | -37 | 0.22 | 0.15 |
| Cx50tr290 | + | 35 | 0.24 | 0.11 |
| Cx50tr290 | - | -35 | 0.29 | 0.22 |
Gjmin represents the minimum conductance value,V0 indicates the voltage measured midway through the Gj decline, and A denotes the cooperativity constant, reflecting the number of charges moving through the transjunctional field. Plus and minus signs for Vj indicate transjunctional membrane potential polarity.
Data taken from Srinivas et al.32
Localization and Functional Expression of Cx50tr290 in Mammalian Cells
The expression and intracellular targeting of connexin proteins was further analyzed in HeLa cells transiently transfected with full-length Cx50 or Cx50tr290 DNA. Immunofluorescent images revealed qualitatively similar levels of connexin expression for full-length (Figs. 4A, 4C) and truncated (Figs. 4B, 4D) Cx50 proteins. Punctate staining (Fig. 4, arrows) indicated that Cx50 and Cx50tr290 were properly targeted to the plasma membrane, especially at regions of cell-to-cell apposition. Thus, truncated Cx50 proteins were expressed and successfully trafficked to the cell membrane in a manner indistinguishable from that of full-length Cx50. The ability of Cx50tr290 to form functional channels in mammalian cells was tested by dual whole-cell patch-clamp measurements of transiently transfected N2A cell pairs (Fig. 5). All green fluorescence protein (GFP)-positive cell pairs expressing full-length Cx50 were electrically coupled, with a mean conductance of 17.5 nS. Most (68%) GFP-labeled cell pairs expressing Cx50tr290 also formed functional channels, though with a mean level of coupling an order of magnitude lower (1.9 nS) than the full-length Cx50 value. This 89% reduction in mean Gj was statistically significant (P < 0.05) and correlated well with the 86% coupling decrease recorded between Xenopus oocyte pairs (Fig. 2). These results indicate that cell pairs expressing truncated Cx50 have significantly decreased coupling even though expression or localization of the protein was unaltered.
FIGURE 4.

Immunofluorescence imaging of Cx50 in transfected HeLa cells. Transiently transfected HeLa cells expressing full-length Cx50 (A, C) or Cx50tr290 (B, D) proteins were immunostained and examined by fluorescence microscopy. Merged images taken at ×100 (A, B) and ×60 (C, D) exhibit Cy3 staining of connexins (red) and DAPI staining of cell nuclei (blue). All images showed that full-length and truncated Cx50 were efficiently translated and localized to the membrane specifically at areas of cell-to-cell apposition (arrows).
FIGURE 5.

Cx50tr290 subunits form functional intercellular channels with reduced coupling in N2A cell pairs. Gj was measured using dual whole-cell patch clamp on transiently transfected N2A cells expressing either full-length or truncated Cx50 proteins Full-length Cx50 channels displayed a mean conductance of 17.5 nS, a significantly higher level of coupling than the 1.9 nS exhibited by truncated Cx50 channels (P < 0.05). Columns are the mean ± SE. Symbols within each column represent individual conductance values. Uncoupled pairs are represented on the x-axis.
Electrophysiological Properties of Truncated Cx50 Channels in Mammalian Cells
Voltage-gating characteristics of Cx50tr290 channels expressed in N2A cells were determined with dual whole-cell patch clamp. A recording of junctional currents from an N2A cell pair expressing truncated Cx50 channels (Fig. 6A) indicated that Ij decreased symmetrically over time in a voltage-dependent manner, with a swifter current decay at larger transjunctional voltage applications. Examination of Cx50tr290 gating kinetics revealed a τ of 44 ms after an 80-mV potential was applied (Fig. 6B). Analysis of the steady state Gj/Vj relationship (Fig. 6C) showed that truncated channels displayed voltage gating similar to that previously reported for full-length Cx50,32 as indicated by the Boltzmann parameters provided in Table 1. Taken together, these data suggested that C-terminal truncation did not alter voltage-dependent gating in transfected mammalian cells, a finding consistent with our data from paired Xenopus oocytes (Fig. 3).
FIGURE 6.

Voltage dependence and gating kinetics of truncated Cx50 channels in transfected cells. Representative current traces recorded from transiently transfected N2A cell pairs expressing Cx50tr290 subunits (A). Junctional currents were recorded during a series of transjunctional voltage applications ranging from +120 to-120 mV in 20-mV intervals and were plotted as a function of time. Gating properties were symmetrical at positive and negative polarities. Cx50tr290 channel closure kinetics were analyzed at a transjunctional potential of 80 mV by fitting to a monoexponential decay (B) and were similar to full-length and truncated Cx50 channels expressed in oocytes. Normalized steady state conductance was plotted against transjunctional voltage and analyzed using the Boltzmann equation (C), whose parameters are given in Table 1. Voltage gating sensitivity of Cx50tr290 channels was largely unchanged when compared with full-length Cx50 junctions.
Unitary Conductance and Single-Channel Gating of Cx50tr290
To examine the functional role of the carboxyl tail of Cx50 in single-channel conductance and gating, current traces were recorded from paired N2A cells transfected with full-length or Cx50tr290 cDNA using dual whole-cell patch clamp. Representative Cx50tr290 and full-length Cx50 single-channel current traces are illustrated in Figure 7. Amplitude histograms for the single-channel currents recorded at the Vj s of -25 mV (Fig. 7A) and -50 mV (Fig. 7B) had distinct peaks that corresponded to the open-channel levels at 5.2 and 10.4 pA, respectively, and yielded a unitary conductance of 210 pS, a value similar to that found for full-length Cx50 channel currents (see Fig 7E for a recording of Cx50 channels at a Vj of -40 mV). Gating of Cx50 channels was also unaffected by cleavage. The Cx50tr290 channels were primarily in the fully open state at the smaller Vj pulse of -25 mV but transitioned to a subconductance state at the larger driving voltage (-50 mV), as previously described for full-length Cx50 channels.32 Additionally, Figure 7C shows that during a 100-second recording of a single Cx50tr290 channel at a Vj of -30 mV, the truncated channels spent long periods of time in the fully open state, with only brief transitions into subconducting or nonconducting states. An expanded portion of the 100-second record (Fig. 7D) showed that the truncated channel spent 88% of the time in the fully open state, similar to full-length Cx50 channels.32 Therefore, truncation did not affect the single channel conductance or gating properties of Cx50.
FIGURE 7.

Cx50tr290 and full-length Cx50 have identical single-channel properties. Junctional currents were recorded from single channels composed of Cx50tr290 at three different transjunctional voltages. Cx50tr290 channels were primarily open at -25 mV (A) but transitioned to lower conductance states at -50 mV (B) and showed a unitary conductance of 210 pS. During extended recording of a single Cx50tr290 channel at -30 mV (C), the truncated channels remained in the fully open state, with only brief transitions into lower conducting states. An expanded portion of the record (D) shows only two brief transitions (asterisk) to substates. Full-length Cx50 single-channel currents recorded at -40 mV had a peak at 8.4 pA, corresponding to a unitary conductance of 210 pS (E). All-point histograms are plotted on the right.
pH Gating Sensitivity of Truncated Cx50 Channels
The functional significance of the Cx50 carboxyl tail on pH gating was examined by perfusing Xenopus oocyte pairs with 100% CO2-saturated external solution. Full-length and Cx50tr290-injected oocyte pairs exhibited a rapid decline in Gj over time on perfusion with 100% CO2 (Fig. 8A). Junctional coupling recovered on switching the extracellular medium back to the original MB medium. These data indicated that C-terminal truncation of murine connexin50 at amino acid 290 did not inhibit the chemical gating properties of gap junction channels in paired Xenopus oocytes.
FIGURE 8.

pH gating sensitivity of full-length and truncated Cx50 channels. (A) Full-length and Cx50tr290 channels rapidly closed during cytoplasmic acidification, indicating that C-terminal truncation did not eliminate pH gating. On washout, transjunctional conductance recovered. Human truncated Cx50 formed pH-sensitive intercellular channels. Human Cx50tr294 channels exhibited a mean conductance of 5.9 μS, a value 100-fold higher than water-injected controls (B). Human Cx50tr294 channels reversibly closed during cytoplasmic acidification indicating that, like murine Cx50, C-terminal truncation did not abolish pH gating (C). Data are the mean ± SE of 3 to 10 pairs.
Human Cx50 was reported to be insensitive to cytosolic acidification on truncation,26 a discrepancy with our data that might have resulted from the use of an ammonium exchange technique to produce a calculated reduction in intracellular pH26 or from intrinsic differences between the human and murine truncated Cx50 proteins. To distinguish between these two possibilities, we made a human Cx50tr294 construct and expressed it in Xenopus oocytes. Like its mouse counterpart, human Cx50tr294 induced coupling between paired oocytes with a mean conductance of 5.9 μS (Fig. 8B). Perfusion of human truncated Cx50 pairs with 100% CO2-saturated media resulted in a greater than 90% reduction in coupling that recovered on washout of CO2 (Fig. 8C). These data show that truncation of human Cx50 did not abolish pH sensitivity.
DISCUSSION
We have mimicked the naturally occurring C-terminal cleavage of Cx50 and characterized the functional properties of the resultant channels in vitro to further elucidate the role of truncation in lens fiber maturation. Immunoblotting confirmed that full-length and truncated Cx50 expression levels were not statistically different, and immunocytochemistry showed that truncated proteins targeted to sites of cell-cell contact. Interestingly, intercellular conductance measurements showed that Cx50tr290 proteins formed functional gap junctions that exhibited an 86% to 89% reduction in junctional conductance from that of the full-length level, a phenomenon that has not been reported. This reduction in coupling was not a result of decreased unitary conductance, improper targeting, or dramatic alterations in voltage or chemical gating.
Previous studies of truncated lens connexins have shown that cleavage of the C termini did not abolish channel-forming ability,24-26,28 a finding that coincides with the data described in this study. However, we found that the macroscopic gap junctional conductance produced by Cx50tr290 was significantly decreased when compared with full-length channels in two different expression functional systems. Furthermore, we observed a 210-pS unitary conductance for Cx50tr290 channels, a value very close to the 220-pS conductance previously reported for full-length mouse Cx50 channels.32 Thus, C-terminal cleavage caused a reduction of macroscopic coupling with-out affecting unitary conductance, a phenomenon that could link the endogenous cleavage of Cx50 to the observed 50% decrease in junctional coupling at the differentiating-to-mature fiber transition during lens fiber maturation.33,34 This idea is further supported by coupling measurements in Cx46 and Cx50 knockout lenses that have shown deletion of Cx46 eliminated coupling in the mature fibers, whereas knockout of Cx50 produced only a marginal reduction.35,36 These data were interpreted as linking the 50% drop in coupling during fiber maturation to a loss of functional Cx50 channels. The large reduction in macroscopic coupling exhibited by Cx50tr290 is consistent with the idea that the endogenous cleavage of Cx50 could negatively regulate channel activity, possibly through a reduction in the number of functional channel subunits.
No role for the carboxyl terminus in voltage gating had been identified by previous studies of cleaved lens fiber connexins.24-26,28 In agreement with this earlier work, we found that channel gating and kinetics were nearly identical when full-length and truncated electrophysiology data were compared. Transjunctional current recordings collected at different transjunctional potentials revealed indistinguishable symmetrical traces for both channel sets, supporting the conclusion that the endogenous C-terminal truncation did not affect voltage-dependent gating. This was further confirmed by studies of heterotypic channels that displayed no asymmetry in their junctional currents, suggesting that Cx50tr290 can interact with full-length Cx50 without alteration of gating properties.
In marked contrast to voltage gating, previous studies of truncated lens connexins have reached conflicting conclusions regarding the role of the C terminus in chemical gating of junctional conductance by pH.24-26,28 The intracellular pH in the lens core is more acidic than that in the cortex, and impedance studies have shown that junctional coupling in the mature fibers of the core is less sensitive to pH.14 One hypothesis was that cleavage of lens fiber connexins reduced sensitivity to pH gating. This was supported by some studies showing that cleavage of the carboxyl terminus of Cx50 abolished pH gating in vitro.24,26 However, others have found that truncated lens connexins retained their sensitivity to cytosolic acidification and that pH gating was largely unaffected by carboxyl terminal cleavage.25,28
One source of variation in the aforementioned studies could be the Cx50 isoform being assayed. We found that the murine and human truncated Cx50 retained pH sensitivity, a finding that agreed with Stergiopoulos et al.,25 who also tested mouse Cx50. In contrast, the human and ovine isoforms were both reported to be insensitive to the effects of cytosolic acidification on truncation.24,26 Although the Cx50 orthologs are highly conserved, the exact sites of truncation vary between species. In mice, the endogenous truncation sites were at amino acids 290 and 300, which corresponded to positions 284 and 294 in human Cx50. Our data suggest that truncated Cx50 retained pH sensitivity regardless of species and imply that differences in experimental design underlie the conflicting results. In this context, it is worth noting that early reports of pH insensitivity of chick lens connexins37 were subsequently shown to be premature on further analysis.38 A greater source of variability could come from the methodology used to reduce intracellular pH. Xu et al.26 used an ammonium exchange technique; the other studies relied on superfusion of CO2-saturated solution,24,25 and the efficiency and degree of acidification could vary between methods. Although we did not directly verify the acidification of the cytoplasm, our results are in good agreement with those of Stergiopoulos et al.,25 the only study that directly measured intracellular pH, and found that truncated Cx50 was still pH gated, though with a modest shift in pKa.
The greatest consequence of Cx50 truncation was a significant reduction in macroscopic conductance. This correlated well with the approximately 50% drop in gap junctional conductance observed in vivo during fiber maturation. Further experimentation on the functional significance of C-terminal cleavage may benefit from the generation and analysis of genetically engineered animals expressing forms of Cx50 that are either permanently truncated or that have mutated calpain cleavage sites that prevent truncation.
Footnotes
Supported by National Institutes of Health Grants EY013163 and EY013869.
Disclosure: A.M. DeRosa, None; R. Mui, None; M. Srinivas, None; T.W. White, None
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
References
- 1.Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- 2.Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 3.White TW, Paul DL. Genetic diseases and gene knockouts reveal diverse connexin functions. Annu Rev Physiol. 1999;61:283–310. doi: 10.1146/annurev.physiol.61.1.283. [DOI] [PubMed] [Google Scholar]
- 4.Evans WH, Martin PE. Gap junctions: structure and function (review) Mol Membr Biol. 2002;19:121–136. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- 5.Sosinsky GE. Molecular organization of gap junction membrane channels. J Bioenerg Biomembr. 1996;28:297–309. doi: 10.1007/BF02110106. [DOI] [PubMed] [Google Scholar]
- 6.Unger VM, Kumar NM, Gilula NB, Yeager M. Three-dimensional structure of a recombinant gap junction membrane channel. Science. 1999;283:1176–1180. doi: 10.1126/science.283.5405.1176. [DOI] [PubMed] [Google Scholar]
- 7.White TW, Bruzzone R. Multiple connexin proteins in single intercellular channels: connexin compatibility and functional consequences. J Bioenerg Biomembr. 1996;28:339–350. doi: 10.1007/BF02110110. [DOI] [PubMed] [Google Scholar]
- 8.Milks LC, Kumar NM, Houghten R, Unwin N, Gilula NB. Topology of the 32-kd liver gap junction protein determined by site-directed antibody localizations. EMBO J. 1988;7:2967–2975. doi: 10.1002/j.1460-2075.1988.tb03159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makowski L, Caspar DL, Phillips WC, Goodenough DA. Gap junction structures, II: analysis of the x-ray diffraction data. J Cell Biol. 1977;74:629–645. doi: 10.1083/jcb.74.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willecke K, Eiberger J, Degen J, et al. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 11.Gerido DA, White TW. Connexin disorders of the ear, skin, and lens. Biochim Biophys Acta. 2004;1662:159–170. doi: 10.1016/j.bbamem.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 12.White TW. Nonredundant gap junction functions. News Physiol Sci. 2003;18:95–99. doi: 10.1152/nips.01430.2002. [DOI] [PubMed] [Google Scholar]
- 13.Donaldson PJ, Dong Y, Roos M, Green C, Goodenough DA, Kistler J. Changes in lens connexin expression lead to increased gap junctional voltage dependence and conductance. Am J Physiol. 1995;269:C590–C600. doi: 10.1152/ajpcell.1995.269.3.C590. [DOI] [PubMed] [Google Scholar]
- 14.Mathias RT, Rae JL, Baldo GJ. Physiological properties of the normal lens. Physiol Rev. 1997;77:21–50. doi: 10.1152/physrev.1997.77.1.21. [DOI] [PubMed] [Google Scholar]
- 15.Donaldson P, Kistler J, Mathias RT. Molecular solutions to mammalian lens transparency. News Physiol Sci. 2001;16:118–123. doi: 10.1152/physiologyonline.2001.16.3.118. [DOI] [PubMed] [Google Scholar]
- 16.Menko AS. Lens epithelial cell differentiation. Exp Eye Res. 2002;75:485–490. doi: 10.1006/exer.2002.2057. [DOI] [PubMed] [Google Scholar]
- 17.Piatigorsky J. Lens differentiation in vertebrates: a review of cellular and molecular features. Differentiation. 1981;19:134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 18.Bassnett S. Lens organelle degradation. Exp Eye Res. 2002;74:1–6. doi: 10.1006/exer.2001.1111. [DOI] [PubMed] [Google Scholar]
- 19.Kistler J, Schaller J, Sigrist H. MP38 contains the membrane-embedded domain of the lens fiber gap junction protein MP70. J Biol Chem. 1990;265:13357–13361. [PubMed] [Google Scholar]
- 20.Lin JS, Fitzgerald S, Dong Y, Knight C, Donaldson P, Kistler J. Processing of the gap junction protein connexin50 in the ocular lens is accomplished by calpain. Eur J Cell Biol. 1997;73:141–149. [PubMed] [Google Scholar]
- 21.Gruijters WT, Kistler J, Bullivant S, Goodenough DA. Immunolocalization of MP70 in lens fiber 16-17-nm intercellular junctions. J Cell Biol. 1987;104:565–572. doi: 10.1083/jcb.104.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zampighi GA, Simon SA, Hall JE. The specialized junctions of the lens. Int Rev Cytol. 1992;136:185–225. doi: 10.1016/s0074-7696(08)62053-7. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs MD, Soeller C, Sisley AM, Cannell MB, Donaldson PJ. Gap junction processing and redistribution revealed by quantitative optical measurements of connexin46 epitopes in the lens. Invest Ophthalmol Vis Sci. 2004;45:191–199. doi: 10.1167/iovs.03-0148. [DOI] [PubMed] [Google Scholar]
- 24.Lin JS, Eckert R, Kistler J, Donaldson P. Spatial differences in gap junction gating in the lens are a consequence of connexin cleavage. Eur J Cell Biol. 1998;76:246–250. doi: 10.1016/s0171-9335(98)80002-2. [DOI] [PubMed] [Google Scholar]
- 25.Stergiopoulos K, Alvarado JL, Mastroianni M, Ek-Vitorin JF, Taffet SM, Delmar M. Hetero-domain interactions as a mechanism for the regulation of connexin channels. Circ Res. 1999;84:1144–1155. doi: 10.1161/01.res.84.10.1144. [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Berthoud VM, Beyer EC, Ebihara L. Functional role of the carboxyl terminal domain of human connexin 50 in gap junctional channels. J Membr Biol. 2002;186:101–112. doi: 10.1007/s00232-001-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peracchia C, Chen JT, Peracchia LL. CO(2) sensitivity of voltage gating and gating polarity of gap junction channelsconnexin40 and its COOH-terminus-truncated mutant. J Membr Biol. 2004;200:105–113. doi: 10.1007/s00232-004-0697-4. [DOI] [PubMed] [Google Scholar]
- 28.Eckert R. pH gating of lens fibre connexins. Pflugers Arch. 2002;443:843–851. doi: 10.1007/s00424-001-0760-2. [DOI] [PubMed] [Google Scholar]
- 29.White TW, Bruzzone R, Goodenough DA, Paul DL. Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein MP70. Mol Biol Cell. 1992;3:711–720. doi: 10.1091/mbc.3.7.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruzzone R, Veronesi V, Gomes D, et al. Loss-of-function and residual channel activity of connexin26 mutations associated with non-syndromic deafness. FEBS Lett. 2003;533:79–88. doi: 10.1016/s0014-5793(02)03755-9. [DOI] [PubMed] [Google Scholar]
- 31.Spray DC, Harris AL, Bennett MV. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981;77:77–93. doi: 10.1085/jgp.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srinivas M, Costa M, Gao Y, Fort A, Fishman GI, Spray DC. Voltage dependence of macroscopic and unitary currents of gap junction channels formed by mouse connexin50 expressed in rat neuroblastoma cells. J Physiol. 1999;517(pt 3):673–689. doi: 10.1111/j.1469-7793.1999.0673s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathias RT, Riquelme G, Rae JL. Cell to cell communication and pH in the frog lens. J Gen Physiol. 1991;98:1085–1103. doi: 10.1085/jgp.98.6.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baldo GJ, Mathias RT. Spatial variations in membrane properties in the intact rat lens. Biophys J. 1992;63:518–529. doi: 10.1016/S0006-3495(92)81624-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong X, Baldo GJ, Kumar NM, Gilula NB, Mathias RT. Gap junctional coupling in lenses lacking alpha3 connexin. Proc Natl Acad Sci USA. 1998;95:15303–15308. doi: 10.1073/pnas.95.26.15303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldo GJ, Gong X, Martinez-Wittinghan FJ, Kumar NM, Gilula NB, Mathias RT. Gap junctional coupling in lenses from alpha(8) connexin knockout mice. J Gen Physiol. 2001;118:447–456. doi: 10.1085/jgp.118.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rup DM, Veenstra RD, Wang HZ, Brink PR, Beyer EC. Chick connexin-56, a novel lens gap junction protein: molecular cloning and functional expression. J Biol Chem. 1993;268:706–712. [PubMed] [Google Scholar]
- 38.Jiang JX, White TW, Goodenough DA. Changes in connexin expression and distribution during chick lens development. Dev Biol. 1995;168:649–661. doi: 10.1006/dbio.1995.1109. [DOI] [PubMed] [Google Scholar]


