Abstract
Although initial reports linked the costimulatory molecule ICOS preferentially with the deelopment of Th2 cells, there is evidence that it is not required for protective type 2 immunity to helminths and that it contributes to Th1 and Th2 responses to other parasites. To address the role of ICOS in the development of infection-induced polarized Th cells, ICOS-/- mice were infected with Trichuris muris or Toxoplasma gondii. Wild-type mice challenged with T. muris developed Th2 responses and expelled these helminths by day 18 postinfection, whereas ICOS-/- mice failed to clear worms and produced reduced levels of type 2 cytokines. However, by day 35 postinfection, ICOS-/- mice were able to mount an effective Th2 response and worms were expelled. This delay in protective immunity was associated with a defect in infection-induced increases in the number of activated and proliferating CD4+ T cells. Similarly, following challenge with T. gondii ICOS was required for optimal proliferation by CD4+ T cells. However, the reduced number of activated CD4+ T cells and associated defect in the production of IFN-γ did not result in increased susceptibility to T. gondii, but rather resulted in decreased CNS pathology during the chronic phase of this infection. Taken together, these data are consistent with a model in which ICOS is not involved in dictating polarity of the Th response but rather regulates the expansion of these subsets.
Successful activation of T cells requires a primary signal mediated by MHC/TCR interactions and a secondary signal provided by costimulation. Probably the best studied second signal is provided by the B7/CD28 interaction that promotes T cell production of IL-2, proliferation, and survival. However, it is clear that there are CD28-independent pathways of T cell activation and costimulatory properties have been ascribed to many molecules, including the inducible costimulatory molecule (ICOS). This member of the CD28 family can act as a positive costimulator of T cell production of IFN-γ, IL-4, and IL-10 (1, 2). Its ligand, B7RP-1 (ICOS-L), is constitutively expressed on a wide range of professional and nonprofessional APC, but ICOS is only present on activated T cells, suggesting a role distinct from that of CD28 (for review, see Ref. 3).
Originally, ICOS was associated with the preferential differentiation of Th2 cells (4-7), which is consistent with reports that resting Th2 cells express more ICOS than Th1 cells (4, 8) and that this costimulatory molecule regulates c-Maf expression (9) and GATA-3 induction (10). In addition, blockade of ICOS during CD4+ T cell differentiation in vitro led to increased levels of IFN-γ and reduced IL-4 and IL-10 production (4, 11, 12). However, there are also reports that Th2 responses occur in the absence of ICOS in models of allergy, graft vs host disease, and helminth infection models (13-17). Paradoxically, other studies identified a requirement for ICOS in the generation of Th1 responses (18) and seems to play an important role in IL-10 production (8) and for the inhibitory mechanisms of T regulatory cells (19-21).
Although there are conflicting reports on the requirement for ICOS/B7RP-1 interactions in T cell differentiation, there is a general consensus that ICOS, due to its role in up-regulating CD40L on T cells and thus for class switching and germinal center formation, is critical for T cell-dependent B cell help (1, 4, 5, 22, 23). The clinical importance of this finding is illustrated in humans by reports of patients with a homozygous deletion of ICOS associated with common variable immunodeficiency characterized by defects in Ab production and susceptibility to bacterial infections (24). Moreover, T cells from these patients display no defects in cytokine production or proliferation when stimulated ex vivo and, similar to the studies in murine models, the role of ICOS during the generation of T cell responses in humans has been harder to define (25-28).
To test the requirement of ICOS for CD4+ T cells in vivo, studies were undertaken using two complimentary models of polarized T cell responses. Resistance to the gastrointestinal helminth Trichuris muris is dependent on the generation of a Th2 response (for review, see Refs. 29 and 30), whereas the protozoan, Toxo-plasma gondii, stimulates a polarized Th1 response to control parasite replication (for review, see Refs. 31-33). The experiments presented here indicate that the role of ICOS during infection is not influencing the differentiation of polarized CD4+ T cells but rather for the expansion of Th1 and Th2 cells in vivo.
Materials and Methods
Animals
BALB/c ICOS-/- mice were originally generated by Tak Mak (6) and gifted by Amgen and bred at Charles River Laboratories. Control BALB/c mice were purchased from Charles River Laboratories. BALB/c bicistronic IL-4 reporter mice were generated as previously described and originally provided by M. Mohrs (Trudeau Institute, Saranac Lake, NY) (34, 35). Animals were maintained in a specific-pathogen free environment and tested negative for pathogens in routine screening. All experiments were conducted following the guidelines of the University of Pennsylvania Institutional Animal Care and Use Committee.
Parasites, Ags, and infections
Trichuris muris was maintained in genetically susceptible or immunocompromised animals. Isolation of Trichuris excretory-secretory Ag and eggs was conducted as described previously (36). Mice were infected on day 0 with 150-200 embryonated eggs, and parasite burdens were assessed on various days postinfection. In some cases, mice were treated i.p. with PBS sham or 500 μg of anti-IFN-γ, as previously described (37-39), every 4 days between day 4 and 20 postinfection. mAbs against IFN-γ (XMG-6) were purified from ascites by ammonium sulfate precipitation and extensively dialyzed against PBS. The Me49 strain of T. gondii was maintained in infected Swiss Webster, and CBA/CaJ mice and cysts were prepared from brains of donor mice as described previously (40). Experimental mice were infected with 20 cysts by i.p. injection in 200 μl of PBS. Experiments were performed on 6- to 8-wk-old female mice. Soluble toxoplasma Ag (sTAg)3 was prepared from RH strain tachyzoites as described previously (41). The activity of sTAg was titrated to determine the optimal concentration for splenocyte proliferation and cytokine production (20-25 μg/ml)
Analysis of type 2 responses
Serum was analyzed by ELISA for Trichuris-specific IgG1 and IgG2a as described previously (36). Total serum IgE was analyzed with an OptEIA IgE ELISA kit, following the manufacturer’s recommendations (BD Pharmingen). Segments of cecum were removed, washed in sterile PBS, and fixed for 24 h in 4% paraformaldehyde. Tissues were processed routinely and paraffin embedded using standard histological techniques. For detection of intestinal goblet cells, 5-μm sections were cut and stained with Alcian blue-periodic acid Schiff’s reagent. For quantification purposes, the number of goblet cells in 20 random crypts from serial sections were counted. Isolation of proteins from stool samples was performed as described previously (36). Equal amounts of protein were analyzed by SDS-PAGE and immunoblotted for resistin-like molecule β (RELMβ) with a polyclonal rabbit anti-murine RELMβ Ab (PeproTech).
Analysis of T. gondii-specific responses
For histological analysis of brains during T. gondii infection, brains were removed from each mouse and fixed overnight in 10% Formalin neutral-buffered solution (Sigma-Aldrich) and embedded in paraffin. Then, 5-μm paraffin sections were stained with H&E for visualization of pathological changes. To score pathological changes, a blinded analysis was performed using a score of 0 for no pathological changes; 1 for mild disease characterized by few lymphocytic infiltrates, no perivascular cuffs, and no meningitis; 2 for widespread lymphocytic infiltration with localized perivascular cuffs and meningitis; 3 for widespread lymphocytic infiltration, perivascular cuffing and meningitis, local gliosis, occasional necrosis, and neutrophils; and 4 for inflammation throughout the brain with prominent perivascular cuffs and meningitis, widespread areas of necrosis, large numbers of neutrophils, and a prominent gliosis. Slides were graded blindly by a single individual. For brain mononuclear cell (BMNC) preparation, following sacrifice, brains were perfused with 50-60 ml of ice-cold PBS to remove peripheral blood as described previously (42). Two mice were pooled for each BMNC sample and the brains removed, placed in 4 ml of complete RPMI 1640 medium (Invitrogen Life Technologies) supplemented with 10% FCS, 1% sodium pyruvate, 1% nonessential amino acids, 0.1% 2-ME, 100 U/ml penicillin, and 100 μg/ml streptomycin (all Invitrogen Life Technologies). Tissues were then passed multiple times through an 18-gauge needle, and the suspension was then incubated with 100 μl of collagenase/dispase (10 mg/ml) (Roche Diagnostics) for 45 min at 37°C and then an additional 45 min at 37°C with 300 μl of DNase (10 mg/ml) (Sigma-Aldrich). The suspension was then passed through a 70-μm cell strainer, resuspended in 40 ml of complete RPMI 1640 medium, and pelleted at 2000 rpm 10 min at 4°C. Cells were resuspended in 60% isotonic Percoll (Amersham Biosciences) solution and overlaid with 30%. The Percoll gradient was centrifuged at 1000 rpm for 25 min at 25°C without brakes, and the top myelin layer was removed before harvesting BMNC at the 60 and 30% interphase layer. Cells were washed with complete RPMI 1640 medium before additional analysis and resuspended at 1 × 106/ml.
Cell culture and cytokine analysis
At necropsy, the mesenteric LN (mLN) (T. muris infection), peritoneal exudates cells (PEC), spleen, and BMNC (T. gondii infection) were harvested, and single-cell suspensions were prepared in complete RPMI 1640 medium. Cells were plated at 1 × 106/ml in medium alone or in the presence of T. muris excretory-secretory Ag (50 μg/ml) or sTAg (25 μg/ml). Cell-free supernatants were harvested after 48 h and analyzed for cytokine secretion by sandwich ELISA.
FACS analysis
Whole splenocytes, following RBC lysis, or BMNC were resuspended in FACS buffer (1× PBS, 0.2% BSA fraction V, and 4 mM sodium azide) to a final concentration of 107/ml. Then, 200 μl of cells was preincubated with a saturating solution of Fc Block for 20 min on ice and then stained with various conjugated Abs against CD4, CD8, CD44, or L-selectin (CD62L) (eBioscience) for 20 min on ice. For intracellular cytokine staining cells were fixed with 4% paraformaldehyde for 10 min on ice, washed with FACS buffer, and permeabilized with 0.3% saponin (Sigma-Aldrich) in FACS buffer. Allophycocyanin- or PE-conjugated anti-IFN-γ were added, and the cells were stained for 20 min on ice. For BrdU staining, cells were permeabilized with 1% paraformaldehyde/0.05% Tween 20 for 30 min at room temperature (RT), followed by 30 min at 4°C. Cells were pelleted and incubated in 1 ml of DNase solution (0.15 M NaCl, 4.2 mM MgCl2, and 150 KU/ml DNase I) for 30 min at RT. Cells were pelleted, and 20 μl of anti-BrdU (BD Pharmingen) was added and cells were incubated for 30 min at RT. Cells were washed with FACS buffer and analyzed using a FACSCalibur flow cytometer (BD Biosciences). Results were analyzed using FlowJo software (Tree Star).
Statistics
Results represent the mean ± SD unless otherwise stated. Statistical significance was determined by Student’s t test or F test when significance was determined over several experiments. In addition, ranked data were analyzed using a Mann-Whitney U test. A p value of <0.05 was considered significant.
Results
ICOS is expressed on CD4+/IL-4+ T cells during T. muris infection
In mice resistant to T. muris, infection induces a Th2 response, and studies have shown that protective immunity is dependent on CD4+ T cells secreting IL-4 (43). To assay the expression of ICOS during this infection, resistant BALB/c bicistronic IL-4 reporter mice, where GFP expression is a marker of IL-4 gene expression (4get mice) (44), were challenged with T. muris, and mLN cells were harvested. Following infection, the frequency of CD4+/GFP+ T cells increased 3- to 10-fold (Fig. 1, a and b). Analysis of the CD4+/GFP- and the CD4+/GFP+ T cells for ICOS expression revealed that the GFP- cells had a modest shift in ICOS expression over isotype control levels (Fig. 1c, lower panel); however, allCD4+/IL-4-expressing T cells expressed ICOS, and the level of expression increased following infection (mean fluorescent intensity: naive = 126 ± 21; day 18 = 210 ± 16) (Fig. 1c, upper panel). Thus, protective immunity to T. muris is associated with an expansion of Th2 cells, and this population expresses high levels of ICOS.
FIGURE 1.
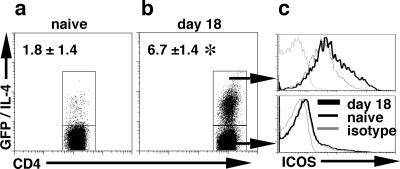
ICOS expression on CD4+ Th2 effector cells. BALB/c 4get mice were infected with 200 T. muris eggs, and after 18 days of infection, mLN cells were recovered from naïve (n = 8) (a) and infected mice (n = 16) (b) and incubated with Abs to CD4 and ICOS. Representative ICOS expression on GFP/IL-4+ (c, upper panel) and GFP/IL-4- (c, lower panel) from naive and infected mice is presented. The numbers in each plot are the percentage (±SD) of live CD4+ T cells that are GFP/IL-4+ from 8 naive and 16 infected mice over four individual experiments. Significance is p < 0.001 and is illustrated by *.
Defective Th2 cytokine production and increased susceptibility to T. muris in ICOS-/- mice
To test the significance of B7RP-1-ICOS interactions in protective type 2 responses, BALB/c WT and ICOS -/- mice were infected with T. muris and immune responses, and the infection outcome was assessed after 18 days. Analysis of T. muris-specific T cell responses revealed that restimulated mLN cells from infected BALB/c mice made a dominant Th2 response with high levels of IL-4 (Fig. 2a), IL-13 (Fig. 2b), and low levels of IFN-γ (Fig. 2c). Although Ab is not required for clearance of T. muris, it provides a surrogate indicator of the polarity of the antiparasite T cell response. As previously reported, BALB/c mice infected with T. muris expressed high levels of parasite-specific IgG1 (Fig. 2d) and total IgE (Fig. 2e) both indicators of a Th2 response. This dominant type 2 mediated immunity led to the expulsion of worms by day 18 (Fig. 2f). In contrast, ICOS-/- mice were deficient in IgG1 and IgE levels (Fig. 2, d and e) with no corresponding increase in titers of T. muris-specific IgG2a (data not shown). This generalized defect in B cell responses is consistent with the important role ICOS plays in B cell function (45, 46). In addition, mLN cells from infected ICOS-/- mice restimulated with worm Ag produced significantly less IL-4 and IL-13 (Fig. 2, a and b), but there was a significant increase in parasite-specific IFN-γ (Fig. 2c; p < 0.001). Consistent with these suboptimal type 2 responses, ICOS-/- mice harbored a substantial worm burden at day 18 (Fig. 2f).
FIGURE 2.
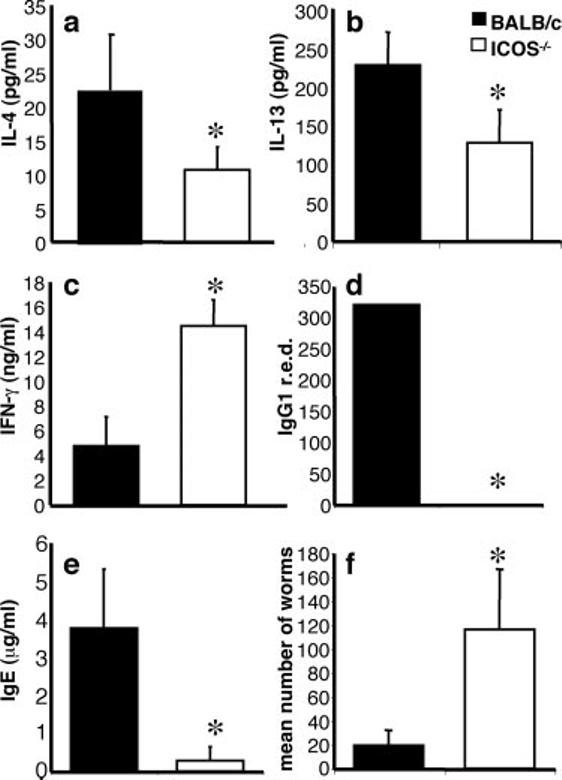
Defective cytokine and Ab responses in T. muris-infected ICOS-/- mice. BALB/c and ICOS-/- mice were infected with T. muris eggs, and after 18 days of infection serum, mLN and guts were harvested. mLN were restimulated with parasite Ag, and supernatants were harvested and measured for the production of IL-4 (a), IL-13 (b), and IFN-γ (c). T. muris-specific IgG1 reciprocal end-point dilution (r.e.d.) (d) and total IgE (e) levels were measured by ELISA. Worm burdens (f) were counted from the large intestine as described in Materials and Methods. Data shown are means of at least three mice and are representative of at least three individual experiments. Significance within experiments (b-f) and across experiments (a) where p < 0.05 is illustrated by *.
Neutralization of IFN-γ reveals an ICOS-independent pathway for Th2 cell generation
Previous studies demonstrated that blockade of the B7/CD28 pathway resulted in a failure to expel T. muris, but resistance could be restored by blocking IFN-γ (47). To test whether ICOS-/- mice would generate a protective Th2 response if IFN-γ was blocked, mice were infected with T. muris and treated with anti-IFN-γ at days 4, 8, 12, and 16. Although no increase was noted in the IL-4 response (data not shown), there was a trend toward increased T. muris-specific IL-5 (Fig. 3a), and IL-13 levels in recall assays were significantly recovered (Fig. 3b). As before, ICOS-/- mice had low levels of parasite-specific IgG1 and IgE responses; however, mice that were given anti-IFN-γ treatment displayed a significant increase in parasite-specific IgG1 (Fig. 3c) and complete recovery of total IgE levels compared with untreated ICOS-/- mice (Fig. 3d).
FIGURE 3.
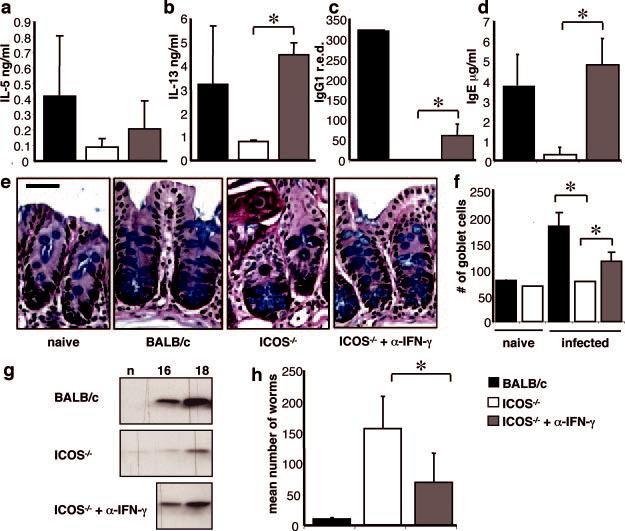
Anti-IFN-γ-treated ICOS-/- mice are resistant to T. muris. BALB/c and ICOS-/- mice were infected with T. muris and treated with control or anti-IFN-γ mAb and sacrificed at day 18 postinfection. mLN were restimulated with parasite Ag, and supernatants were harvested and measured for the production of IL-5 (a) or IL-13 (b). T. muris-specific IgG1 (c) and total IgE (d) were measured from serum by ELISA. Cecal sections were harvested and stained for goblet cells (e), and the numbers of goblet cells were counted (f), as described in Materials and Methods. Levels of RELMβ protein were analyzed by Western blot following digestion of stool samples from naive (n) and day 16- and 18-infected mice (g). Worm burdens (h) were counted from sections of the large intestine as described in Materials and Methods. Data shown are averages of at least three mice and are representative of at least three individual experiments. Significance where p < 0.05 is illustrated by *.
Previous studies have shown that the ability to expel T. muris is associated with goblet cell hyperplasia and secretion of RELMβ in the gut, a goblet cell-specific protein (36). Thus, at day 18 postinfection, BALB/c mice exhibit pronounced increases in crypt elongation, goblet cell numbers (Fig. 3, e and f), and significant secretion of RELMβ (Fig. 3g), whereas all of these were defective in ICOS-/- mice (Fig. 3, e-g). However, the recovery of type 2 responses in anti-IFN-γ-treated ICOS-/- mice was also reflected in enhanced goblet cell responses and secretion of RELM β (Fig. 3, e and f). Accordingly, recovery of Th2 cell cytokine production and gut inflammation in ICOS-/- mice treated with anti-IFN-γ resulted in a significant decrease in worm burden compared with untreated ICOS-/- mice (Fig. 3h). Taken together, these results indicate that, in the absence of IFN-γ, ICOS is not required for the generation of a protective CD4+ Th2 cell response.
ICOS-/- mice exhibit delayed but intact type 2 responses and immunity to T. muris
The above data imply the presence of an ICOS-independent pathway for the development of T. muris-specific Th2 cells, yet at day 18 postinfection, in ICOS-/- mice there was a pronounced defect in protective type 2 immune responses. To determine whether the Th2 cell defect persists beyond day 18 postinfection, infected BALB/c and ICOS-/- mice were analyzed at day 35 postinfection. Restimulation of BALB/c mLN results in undetectable levels of IL-13 and IL-5 (Fig. 4, a and b), which is consistent with contraction of T cell responses in the absence of parasites. In addition, goblet cell hyperplasia is less pronounced (Fig. 4, c and d), and RELMβ levels peak at day 18 and are down-regulated by day 35 (Fig. 4e). However, at this later time point, ICOS-/- mice produced increased amounts of IL-13 and IL-5 compared with BALB/c mice (Fig. 4, a and b) and exhibited significant goblet cell hyperplasia (Fig. 4, c and d). Furthermore, at this late time point, there was substantial production of RELMβ in ICOS-/- mice (Fig. 4e). Lastly, this delay in the expansion of type 2 responses in ICOS-/- mice can be observed in the kinetics of the worm burden. As before, although infection levels are equivalent between BALB/c and ICOS-/- mice at day 12, by day 18 BALB/c mice exhibit worm expulsion, whereas ICOS-/- mice still have a significant worm burden. However, by day 35, coinciding with the development of Th2 cell responses and the onset of goblet cell hyperplasia, the worm burden in ICOS-/- mice is reduced dramatically and by day 54 is almost absent (Fig. 4f).
FIGURE 4.
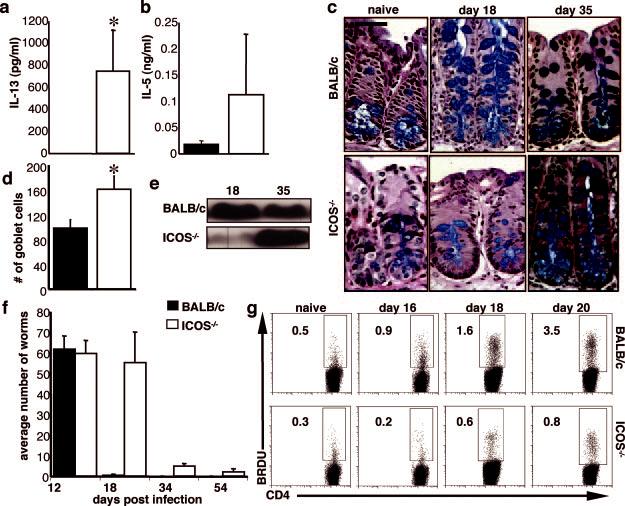
Delay in the Th2 response in the absence of ICOS. BALB/c and ICOS-/- mice were infected with T. muris and sacrificed at day 18 and 35 postinfection. mLN were restimulated with parasite Ag, and supernatants were harvested and measured for the production of IL-13 (a) or IL-5 (b). Cecal sections were harvested, stained, and counted for goblet cells as described in Materials and Methods (c and d). Levels of RELMβ protein were analyzed by Western blot following digestion of stool samples from day 18- and day 35-infected mice (e). Worm burdens (f) were counted from sections of the large intestine as described in Materials and Methods at days 12, 18, 34, and 54 postinfection. g, Mice were injected i.p. 1 day before sacrifice with 200 μg of BrdU/mouse, and following sacrifice at days 0, 16, 18, and 20 postinfection, mLN cells were incubated with Abs to CD4 and BrdU as described in Materials and Methods. Numbers represent the frequency of live CD4+ cells that are BrdU+. Data shown are averages of at least three mice and are representative of at least two individual experiments. Significance where p < 0.05 is illustrated by *.
Despite initial results suggesting that ICOS is required for the generation of a Th2 response during infection with T. muris, it is clear that by day 35 the ICOS-/- mice develop protective type 2 responses. This suggests that the defects noted in the type 2 response in ICOS-/- mice at day 18 postinfection may be due to the poor expansion of CD4+ Th2 cells (17, 48) rather than a failure to differentiate. To test this possibility, infected BALB/c and ICOS-/- mice were injected with BrdU 1 day before sacrifice, and the mLN CD4+ T cells were analyzed for their ability to incorporate this thymidine analog, an indicator of proliferation, at various time points postinfection. Although naive BALB/c and ICOS-/- mice had similar background levels of BrdU incorporation, following infection at days 16, 18, and 20, ICOS-/- mice had significantly fewer proliferating CD4+ T cells with on average a 4-fold decrease in the percentage of BrdU+CD4+ T cells (Fig. 4g). The delay in the CD4+ T cell response and the kinetics of the worm burden imply that ICOS is not essential for the differentiation of Th2 cell subsets, but rather is required for optimal expansion of these cells.
ICOS is required for optimal activation and expansion of CD4+ T cells during T. gondii infection
Previous reports from this laboratory and others have implicated ICOS in the generation of Th1 cell responses (48 -52). To determine whether the importance of ICOS during type 2 responses for the expansion of Th2 cells also applies during type 1 responses, ICOS-/- mice were infected with T. gondii. Protection from this intracellular parasite requires the production of IL-12 and IFN-γ to control parasite replication. Before infection, there are no differences in total splenic numbers or composition from BALB/c and ICOS-/- spleens (data not shown); however, following 7 days of infection with T. gondii, there is a significant (p < 0.05) defect in the number of splenic cells and PECs recovered from ICOS-/- mice compared with control mice (Fig. 5a). This defect, although less severe, is also apparent at day 14 postinfection. Analysis of the cellular composition revealed that while the percentage of CD8+ T cells remained the same, there was a significant (p < 0.05) reduction in the percentage of CD4+ T cells in the spleen and the PECs of ICOS-/- mice following infection (Fig. 5b). In addition, although the numbers of activated CD8+ T cells were comparable (data not shown), the proportion of CD4+ T cells in the spleen that were activated (CD44high, CD62Llow) was significantly less in ICOS-/- mice compared with wild-type controls (Fig. 5c, top panel).
FIGURE 5.
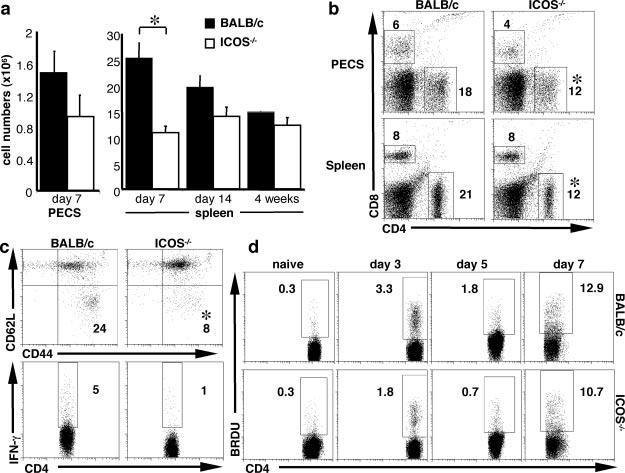
CD4+ T cell defect in ICOS-/- mice following T. gondii infection. BALB/c and ICOS-/- mice were infected with 20 cysts of T. gondii. At 7 days postinfection, mice were sacrificed, and PEC and splenic cells were counted (a) and incubated with Abs to CD4 and CD8 (b) and the activation markers CD44 and CD62L (c, upper panel). Following 48 h of restimulation with parasite Ag (sTAg), cells were stimulated with PMA and ionomycin for 4h in the presence of brefeldin A and stained for intracellular IFN-γ (c, lower panel) as described in Materials and Methods. d, Mice were injected i.p. 1 day before sacrifice with 200 μg of BrdU/mouse, and following sacrifice at days 0, 3, 5, and 7 postinfection, splenic cells were incubated with Abs to CD4 and BrdU as described in Materials and Methods. Numbers represent the frequency of live CD4+ T cells that are BrdU+. Data shown are averages of at least three mice and are representative of at least three individual experiments. Significance where p < 0.05 is illustrated by *.
As with the expansion of Th2 cells during T. muris infection, analysis of BrdU incorporation in splenic cells isolated from ICOS-/- mice following infection with T. gondii showed a delayed expansion of CD4+ T cells compared with wild-type mice (Fig. 5d). Examination of the ability of T cells to make IFN-γ in response to parasite Ag revealed that the percentage of CD4+ T cells producing IFN-γ was reduced in ICOS-/- mice (Fig. 5c, lower panel). However, there was equivalent IFN-γ production by CD8+ T cells, and overall levels of IL-12 and IFN-γ protein, although diminished, were not significantly defective in ICOS-/- cultures (data not shown). Despite the decrease in CD4+ T cell numbers and activation status in infected ICOS-/- mice, there is sufficient production of IFN-γ that these mice did not succumb to infection at this acute time point. Nevertheless, consistent with the studies described above, these data reveal that in addition to being required for the expansion of CD4+ Th2 cells, ICOS is also necessary for the optimal expansion of CD4+ Th1 cells during acute toxoplasmosis.
Reduced inflammation during chronic T. gondii infection
Unlike T. muris, the presence of a protective T cell response to T. gondii does not lead to sterile immunity, and the parasite will form cysts that persist for the lifetime of the host (53). To determine whether the defect in CD4+ T cell expansion seen during the acute phase of infection is also a function of the chronic stage of infection, T cell responses were analyzed 4-6 wk postinfection. At this point, chronically infected mice develop toxoplasmic encephalitis associated with the presence of parasites in the brain. Therefore, as well as investigating the splenic T cell response, BMNC were examined for T cell composition and cytokine production. At this time point, wild-type and ICOS-/- mice have similar numbers of splenic CD4+ T cells (Figs. 5a and 6a); however, in the CNS, the defect in CD4+ T cells persists, with a significant decrease in the total number of cells (Fig. 6b) and the percentage of CD4+ T cells harvested from the brain (Fig. 6a). Despite this difference in numbers, these CD4+ and CD8+ T cells isolated from the CNS of BALB/c and ICOS-/- mice are equally capable of producing IFN-γ in response to parasite Ag (Fig. 6c); however, the defect in the numbers of CD4+ T cells likely accounts for the significant reduction in secreted IFN-γ (Fig. 6d). Associated with the reduced CD4+ Th1 cell response there is significantly less inflammation in the brains of chronically infected resistant ICOS-/- mice (BALB/c 2 ± 0.3, ICOS-/- 1 ± 0.4, p = 0.005) (Fig. 6e). Thus, ICOS-/- mice can generate a protective type 1 response; however, the decrease in CD4+ Th1 expansion leads to less inflammation in the brain.
FIGURE 6.
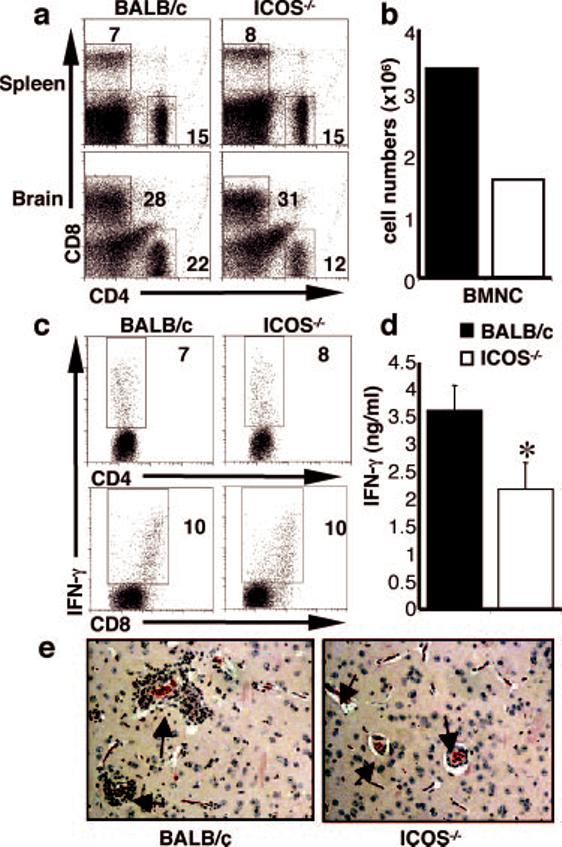
ICOS-/- mice have less inflammation at the chronic stage of T. gondii infection. BALB/c and ICOS-/- mice were infected with T. gondii, and after 4 wk of infection, BMNC numbers were counted (b), and these and splenic cells were incubated with Abs to CD4 and CD8 (a). BMNC were cultured for 48 h with parasite Ag (sTAg) and stained for intracellular IFN-γ (c) as described in Materials and Methods. Live and cell-specific gates were applied. Supernatants were harvested and measured for IFN-γ (d) by ELISA. Paraffin sections of brains from naive and infected BALB/c and ICOS-/- mice were stained with H+E (e); arrows point to blood vessels to highlight the difference in inflammation, in particular in the perivascular infiltrate. Data shown are averages of at least three mice and are representative of at least three individual experiments. Significance where p < 0.05 is illustrated by *.
Discussion
Since the original description of the B7RP-1/ICOS interaction, a consensus has emerged that this pathway has an important role in the ability of B cells to undergo class switching. In contrast, its contribution to the development of polarized helper T cell subsets has been less clear. There are conflicting reports that place ICOS solely in the differentiation of Th2 cells (5, 6, 54): those that suggest that it is important in Th1 cells (15, 51) and studies that give ICOS a degree of significance in Th1 and Th2 cell generation (4, 48, 50, 52). It has been unclear whether these apparently contradictory findings were simply a reflection of the individual experimental systems being used, or if ICOS was a key receptor involved in dictating polarity of Th cell responses. In the experimental systems examined in this report, ICOS is not required for the ability of a protozoan or helminth parasite to generate Th1 or Th2 cells, respectively. Rather, the decreased Th responses noted during these infections appear to be a secondary consequence of the reduced expansion of these subsets. Thus, challenge of ICOS-/- mice with T. muris resulted in a significant defect in the development of protective CD4+ Th2 cells associated with decreased infection-induced incorporation of BrdU by CD4+ T cells and a marked delay in worm expulsion. Since resistance to T. muris is independent of Ab production, (Refs. 55 and 56; D. Artis, unpublished observations), the decreased resistance is unlikely to be related to the role of ICOS in B cell functions (1, 4, 22-24). Nevertheless, these studies revealed the development of an ICOS independent protective Th2 response that was observed in the absence of IFN-γ or at later (day 35) time points. Similarly, the absence of ICOS during toxoplasmosis resulted in a reduction in the numbers of activated CD4+ T cells associated with lower levels of BrdU incorporation, but those CD4+ T cells that became activated were capable of producing equivalent levels of IFN-γ. Similarly, using a noninfectious model, Garside and colleagues (48) showed that, when TCR transgenic T cells specific for OVA were differentiated under Th1 or Th2 conditions in vitro and then transferred into naive recipients, both subsets required ICOS for subsequent expansion.
In many respects, the finding that ICOS is necessary for optimal type 2 responses to T. muris is similar to the initial finding that this molecule was required for the development of inflammatory responses in the lungs mediated by Th2 cells (5). However, the emergence of protective immunity in the absence of IFN-γ or at later time points concurs with reports that ICOS was not required for type 2 responses that develop after infection with Nippostrongylus or Brugia malayi (13, 16, 18, 54). These apparent differences may be explained by the type of antigenic stimulation used in these experimental systems. In the model originally reported by Coyle and colleagues (5), OVA was used as the T cell-specific Ag that generated lung inflammation, and this represents a transient stimulus with no inherent ability to induce Th2 responses. In contrast, challenge with N. brasiliensis results in active larval migration through multiple tissues before establishing in the gut. This pathogen is generally regarded as one of the most potent inducers of type 2 responses (57), and it has been shown previously that these infection-induced Th2 cells develop independently of costimulatory molecules and other canonical signals, such as IL-4 and IL-13, associated with the development of Th2 responses (58-61). Similarly, the implantation of B. malayi in the peritoneum and the long-term survival of these metabolically active adults provides a sustained Ag load and a powerful stimulus for Th2 responses. When compared with these models, infection with T. muris is relatively quiescent for the first week as the eggs hatch and produce individual larvae that are confined to the gut and which only become apparent after the first week. Thus, during the early phase of this infection, there is a low Ag load associated with limited inflammation, whereas by day 16 -18 there is considerable Ag load and damage caused to the intestinal epithelium. In the absence of ICOS, there were defective Trichuris-specific Th2 responses during the early phase of infection, but with the development and continued presence of adults, there is the emergence of protective type 2 CD4+ T cells. These kinetics are consistent with a model in which this costimulatory molecule promotes the development of Th2 cells when the stimulus is weak, whereas with a more powerful insult, these responses are ICOS independent.
One of the main properties attributed to ICOS has been to provide second signals to previously activated T cells for the production of several cytokines (IL-4 and IFN-γ), as well as IL-10. While IL-10 is a growth factor for B cells, it is also a potent inhibitor of Th1 and Th2 responses, and in its absence, mice infected with T. muris or T. gondii develop a lethal T cell-mediated inflammatory response (53, 62, 63). With the emergence of additional reports that associated ICOS with IL-10 production (4, 8) and the differentiation of regulatory T cells, a prominent source of IL-10 (19, 21, 64), one possible outcome of these experiments was that in the absence of ICOS-mediated activation of regulatory T cells, infected mice might develop immunopathology. However, in these studies, infection of ICOS-/- mice did not lead to the development of any obvious T cell-mediated disease. These findings may indicate that either ICOS is not involved in regulatory T cell generation in these particular models or that regulatory T cells have a minor role in controlling inflammation during either of these infections. Indeed, in ICOS-/- BALB/c mice infected with T. gondii, the reduced CD4+ T cell response was associated with less severe CNS inflammation. As BALB/c mice are relatively resistant to toxoplasmic encephalitis, the long-term consequences of these observations are hard to gauge. However, the recent availability of ICOS-/- mice on a genetic background susceptible to chronic toxoplasmosis (C57BL/6) has allowed preliminary experiments, which indicates that the reduced pathology observed on a BALB/c background translates into decreased mortality rates during the later phase of infection in C57BL/6 mice (E. H. Wilson and C. A. Hunter, unpublished observations). A similar outcome was previously noted with CD28-/- mice during the chronic phase of toxoplasmosis (65), and these phenotypes highlight the role of costimulation in the generation of protective immunity and its pathological consequences.
With the original development of the two signal hypothesis for B cells and its application to T cells, there has been an acceptance of the critical role of costimulation in the regulation of lymphocyte responses. Although costimulatory properties have been ascribed to many molecules (for review, see Refs. 3 and 66), probably the best understood interaction is provided by signaling through CD28, which, when combined with MHC/TCR interactions, leads to increased production of the T cell growth factor IL-2 and expression of the IL-2R. In contrast, ICOS does not regulate the production of IL-2, and while there is a role for this costimulator in T cell expansion, the molecular basis for these effects are unclear. Studies that have compared the signaling events downstream of CD28 and ICOS have shown that both can bind PI3K, leading to Akt activation, but that stimulation through ICOS results in a more robust activation of this pathway. In addition, there is a different pattern of MAPK activation, with stimulation through ICOS activation leading to decreased phosphorylation of JNK compared with CD28 (67). Whether the differential signaling of ICOS leads to prosurvival or expansion signals that are unique from those provided by CD28 remains to be determined. It is also important to acknowledge that these second signals are likely to occur in environments that contain cytokines (IL-12 and IL-4) with defined roles to induce polarization but which also provide proliferative signals (68, 69), and it is unclear whether there is additional cross-talk between these pathways. It is to be expected that the contribution of individual costimulatory pathways during an immune response will be a function of many factors from the type of Ag or stimulus to the strength of polarizing signals that are present. Nevertheless, the studies presented here clarify the requirement for ICOS during infection-induced Th1 and Th2 responses and supports the idea of a more general role for this pathway in the regulation of CD4+ T cell expansion rather than dictating their polarity.
Footnotes
- sTAg
- soluble toxoplasma Ag
- BMNC
- brain mononuclear cell
- mLN
- mesenteric LN
- PEC
- peritoneal exudates cell
- CD62L
- Lselectin
- RT
- room temperature
- RELM
- resistin-like molecule.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This work was supported by the State of Pennsylvania (to C.A.H.); National Institutes of Health Grants AI41158 and AI42334 (to C.A.H.), and AI61570 (to D.A.); Molecular Biology Core, Morphology Core and Pilot Feasibility Program of the National Institute of Diabetes and Digestive and Kidney Diseases Center Grant DK50306 (to D.A.); the Crohn’s and Colitis Foundation of America’s William and Shelby Modell Family Foundation Research Award (to D.A.); and the Irvington Institute for Immunological Research Postdoctoral Fellowship (to C.Z.).
References
- 1.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T cell costimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 2.Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, et al. T cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 3.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu. Rev. Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 4.McAdam AJ, Chang TT, Lumelsky AE, Greenfield EA, Boussiotis VA, Duke-Cohan JS, Chernova T, Malenkovich N, Jabs C, Kuchroo VK, et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J. Immunol. 2000;165:5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- 5.Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, Nguyen T, Burwell T, Schneider H, Gonzalo JA, et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- 6.Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, Boucher LM, Bouchard D, Chan VS, Duncan G, et al. ICOS is essential for effective T helper cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 7.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat. Rev. Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 8.Lohning M, Hutloff A, Kallinich T, Mages HW, Bonhagen K, Radbruch A, Hamelmann E, Kroczek RA. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J. Exp. Med. 2003;197:181–193. doi: 10.1084/jem.20020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nurieva RI, Treuting P, Duong J, Flavell RA, Dong C. Inducible costimulator is essential for collagen-induced arthritis. J. Clin. Invest. 2003;111:701–706. doi: 10.1172/JCI17321. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Watanabe M, Watanabe S, Hara Y, Harada Y, Kubo M, Tanabe K, Toma H, Abe R. ICOS-mediated costimulation on Th2 differentiation is achieved by the enhancement of IL-4 receptor-mediated signaling. J. Immunol. 2005;174:1989–1996. doi: 10.4049/jimmunol.174.4.1989. [DOI] [PubMed] [Google Scholar]
- 11.Rutitzky LI, Ozkaynak E, Rottman JB, Stadecker MJ. Disruption of the ICOS-B7RP-1 costimulatory pathway leads to enhanced hepatic immunopathology and increased γ interferon production by CD4 T cells in murine schistosomiasis. Infect. Immun. 2003;71:4040–4044. doi: 10.1128/IAI.71.7.4040-4044.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stolberg VR, Chiu BC, Komuniecki E, Freeman CM, Chensue SW. Analysis of inducible costimulatory molecule participation during the induction and elicitation of granulomatous responses to mycobacterial and schistosomal antigens. Cell. Immunol. 2005;237:45–54. doi: 10.1016/j.cellimm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Scales HE, Ierna MX, Gutierrez-Ramos JC, Coyle AJ, Garside P, Lawrence CE. Effect of inducible costimulator blockade on the pathological and protective immune responses induced by the gastrointestinal helminth Trichinella spiralis. Eur. J. Immunol. 2004;34:2854–2862. doi: 10.1002/eji.200324364. [DOI] [PubMed] [Google Scholar]
- 14.Gajewska BU, Tafuri A, Swirski FK, Walker T, Johnson JR, Shea T, Shahinian A, Goncharova S, Mak TW, Stampfli MR, Jordana M. B7RP-1 is not required for the generation of Th2 responses in a model of allergic airway inflammation but is essential for the induction of inhalation tolerance. J. Immunol. 2005;174:3000–3005. doi: 10.4049/jimmunol.174.5.3000. [DOI] [PubMed] [Google Scholar]
- 15.Hubbard VM, Eng JM, Ramirez-Montagut T, Tjoe KH, Muriglan SJ, Kochman AA, Terwey TH, Willis LM, Schiro R, Heller G, et al. Absence of inducible costimulator on alloreactive T cells reduces graft-versushost disease and induces Th2 deviation. Blood. 2005;106:3285–3292. doi: 10.1182/blood-2005-01-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loke P, Zang X, Hsuan L, Waitz R, Locksley RM, Allen JE, Allison JP. Inducible costimulator is required for type 2 antibody isotype switching but not T helper cell type 2 responses in chronic nematode infection. Proc. Natl. Acad. Sci. USA. 2005;102:9872–9877. doi: 10.1073/pnas.0503961102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Berkel ME, Schrijver EH, Hofhuis FM, Sharpe AH, Coyle AJ, Broeren CP, Tesselaar K, Oosterwegel MA. ICOS contributes to T cell expansion in CTLA-4 deficient mice. J. Immunol. 2005;175:182–188. doi: 10.4049/jimmunol.175.1.182. [DOI] [PubMed] [Google Scholar]
- 18.Kopf M, Coyle AJ, Schmitz N, Barner M, Oxenius A, Gallimore A, Gutierrez-Ramos JC, Bachmann MF. Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J. Exp. Med. 2000;192:53–61. doi: 10.1084/jem.192.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 20.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J. Exp. Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vermeiren J, Ceuppens JL, Van Ghelue M, Witters P, Bullens D, Mages HW, Kroczek RA, Van Gool SW. Human T cell activation by costimulatory signal-deficient allogeneic cells induces inducible costimulator-expressing anergic T cells with regulatory cell activity. J. Immunol. 2004;172:5371–5378. doi: 10.4049/jimmunol.172.9.5371. [DOI] [PubMed] [Google Scholar]
- 22.McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, Freeman GJ, Sharpe AH. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 23.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, Chen L. B7-H3: a costimulatory molecule for T cell activation and IFN-γ production. Nat. Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 24.Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Drager R, Eibel H, Fischer B, Schaffer AA, Mages HW, et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat. Immunol. 2003;4:261–268. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- 25.Deng ZB, Zhu W, Lu CM, Shi Q, Ju SG, Ma HB, Xu Y, Zhang XG. An agonist human ICOS monoclonal antibody that induces T cell activation and inhibits proliferation of a myeloma cell line. Hybrid Hybridomics. 2004;23:176–182. doi: 10.1089/1536859041224299. [DOI] [PubMed] [Google Scholar]
- 26.Lorentzen AR, Celius EG, Ekstrom PO, Wiencke K, Lie BA, Myhr KM, Ling V, Thorsby E, Vartdal F, Spurkland A, Harbo HF. Lack of association with the CD28/CTLA4/ICOS gene region among Nor-wegian multiple sclerosis patients. J. Neuroimmunol. 2005;166:197–201. doi: 10.1016/j.jneuroim.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Shilling RA, Pinto JM, Decker DC, Schneider DH, Bandukwala HS, Schneider JR, Camoretti-Mercado B, Ober C, Sperling AI. Cutting edge: polymorphisms in the ICOS promoter region are associated with allergic sensitization and Th2 cytokine production. J. Immunol. 2005;175:2061–2065. doi: 10.4049/jimmunol.175.4.2061. [DOI] [PubMed] [Google Scholar]
- 28.Yang JH, Zhang J, Cai Q, Zhao DB, Wang J, Guo PE, Liu L, Han XH, Shen Q. Expression and function of inducible costimulator on peripheral blood T cells in patients with systemic lupus erythematosus. Rheumatology. 2005;44:1245–1254. doi: 10.1093/rheumatology/keh724. [DOI] [PubMed] [Google Scholar]
- 29.Bradley JE, Jackson JA. Immunity, immunoregulation and the ecology of trichuriasis and ascariasis. Parasite Immunol. 2004;26:429–441. doi: 10.1111/j.0141-9838.2004.00730.x. [DOI] [PubMed] [Google Scholar]
- 30.Cliffe LJ, Grencis RK. The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Adv. Parasitol. 2004;57:255–307. doi: 10.1016/S0065-308X(04)57004-5. [DOI] [PubMed] [Google Scholar]
- 31.Aliberti J. Host persistence: exploitation of anti-inflammatory pathways by Toxoplasma gondii. Nat. Rev. Immunol. 2005;5:162–170. doi: 10.1038/nri1547. [DOI] [PubMed] [Google Scholar]
- 32.Lieberman LA, Hunter CA. The role of cytokines and their signaling pathways in the regulation of immunity to Toxoplasma gondii. Int. Rev. Immunol. 2002;21:373–403. doi: 10.1080/08830180213281. [DOI] [PubMed] [Google Scholar]
- 33.Denkers EY, Gazzinelli RT. Regulation and function of T cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 1998;11:569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohrs M, Blankespoor CM, Wang ZE, Loots GG, Afzal V, Hadeiba H, Shinkai K, Rubin EM, Locksley RM. Deletion of a coordinate regulator of type 2 cytokine expression in mice. Nat. Immunol. 2001;2:842–847. doi: 10.1038/ni0901-842. [DOI] [PubMed] [Google Scholar]
- 35.Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23:419–429. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, et al. RELMβ/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc. Natl. Acad. Sci. USA. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villarino AV, Stumhofer JS, Saris CJ, Kastelein RA, de Sauvage FJ, Hunter CA. IL-27 limits IL-2 production during Th1 differentiation. J. Immunol. 2006;176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 38.Cai G, Kastelein R, Hunter CA. Interleukin-18 (IL-18) enhances innate IL-12-mediated resistance to Toxoplasma gondii. Infect. Immun. 2000;68:6932–6938. doi: 10.1128/iai.68.12.6932-6938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunter CA, Chizzonite R, Remington JS. IL-1β is required for IL-12 to induce production of IFN-γ by NK cells: a role for IL-1β in the T cell-independent mechanism of resistance against intracellular pathogens. J. Immunol. 1995;155:4347–4354. [PubMed] [Google Scholar]
- 40.Blewett DA, Miller JK, Harding J. Simple technique for the direct isolation of toxoplasma tissue cysts from fetal ovine brain. Vet. Rec. 1983;112:98–100. doi: 10.1136/vr.112.5.98. [DOI] [PubMed] [Google Scholar]
- 41.Sharma SD, Mullenax J, Araujo FG, Erlich HA, Remington JS. Western blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J. Immunol. 1983;131:977–983. [PubMed] [Google Scholar]
- 42.Villegas EN, Wille U, Craig L, Linsley PS, Rennick DM, Peach R, Hunter CA. Blockade of costimulation prevents infection-induced immunopathology in interleukin-10-deficient mice. Infect. Immun. 2000;68:2837–2844. doi: 10.1128/iai.68.5.2837-2844.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grencis RK. Cytokine-mediated regulation of intestinal helminth infections: the Trichuris muris model. Ann. Trop. Med. Parasitol. 1993;87:643–647. doi: 10.1080/00034983.1993.11812823. [DOI] [PubMed] [Google Scholar]
- 44.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 45.Mak TW, Shahinian A, Yoshinaga SK, Wakeham A, Boucher LM, Pintilie M, Duncan G, Gajewska BU, Gronski M, Eriksson U, et al. Costimulation through the inducible costimulator ligand is essential for both T helper and B cell functions in T cell-dependent B cell responses. Nat. Immunol. 2003;4:765–772. doi: 10.1038/ni947. [DOI] [PubMed] [Google Scholar]
- 46.Wong SC, Oh E, Ng CH, Lam KP. Impaired germinal center formation and recall T cell-dependent immune responses in mice lacking the costimulatory ligand B7-H2. Blood. 2003;102:1381–1388. doi: 10.1182/blood-2002-08-2416. [DOI] [PubMed] [Google Scholar]
- 47.Urban J, Fang H, Liu Q, Ekkens MJ, Chen SJ, Nguyen D, Mitro V, Donaldson DD, Byrd C, Peach R, et al. IL-13-mediated worm expulsion is B7 independent and IFN-γ sensitive. J. Immunol. 2000;164:4250–4256. doi: 10.4049/jimmunol.164.8.4250. [DOI] [PubMed] [Google Scholar]
- 48.Smith KM, Brewer JM, Webb P, Coyle AJ, Gutierrez-Ramos C, Garside P. Inducible costimulatory molecule-B7-related protein 1 interactions are important for the clonal expansion and B cell helper functions of naive, Th1, and Th2 T cells. J. Immunol. 2003;170:2310–2315. doi: 10.4049/jimmunol.170.5.2310. [DOI] [PubMed] [Google Scholar]
- 49.Rottman JB, Smith T, Tonra JR, Ganley K, Bloom T, Silva R, Pierce B, Gutierrez-Ramos JC, Ozkaynak E, Coyle AJ. The costimulatory molecule ICOS plays an important role in the immunopathogenesis of EAE. Nat. Immunol. 2001;2:605–611. doi: 10.1038/89750. [DOI] [PubMed] [Google Scholar]
- 50.Greenwald RJ, McAdam AJ, Van der Woude D, Satoskar AR, Sharpe AH. Cutting edge: inducible costimulator protein regulates both Th1 and Th2 responses to cutaneous leishmaniasis. J. Immunol. 2002;168:991–995. doi: 10.4049/jimmunol.168.3.991. [DOI] [PubMed] [Google Scholar]
- 51.Villegas EN, Lieberman LA, Mason N, Blass SL, Zediak VP, Peach R, Horan T, Yoshinaga S, Hunter CA. A role for inducible costimulator protein in the CD28-independent mechanism of resistance to Toxoplasma gondii. J. Immunol. 2002;169:937–943. doi: 10.4049/jimmunol.169.2.937. [DOI] [PubMed] [Google Scholar]
- 52.Wassink L, Vieira PL, Smits HH, Kingsbury GA, Coyle AJ, Kapsenberg ML, Wierenga EA. ICOS expression by activated human Th cells is enhanced by IL-12 and IL-23: increased ICOS expression enhances the effector function of both Th1 and Th2 cells. J. Immunol. 2004;173:1779–1786. doi: 10.4049/jimmunol.173.3.1779. [DOI] [PubMed] [Google Scholar]
- 53.Wilson EH, Wille-Reece U, Dzierszinski F, Hunter CA. A critical role for IL-10 in limiting inflammation during toxoplasmic encephalitis. J. Neuroimmunol. 2005;165:63–74. doi: 10.1016/j.jneuroim.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 54.Miyahira Y, Akiba H, Ogawa SH, Ishi T, Watanabe S, Kobayashi S, Takeuchi T, Aoki T, Tezuka K, Abe R, et al. Involvement of ICOS-B7RP-1 costimulatory pathway in the regulation of immune responses to Leishmania major and Nippostrongylus brasiliensis infections. Immunol. Lett. 2003;89:193–199. doi: 10.1016/s0165-2478(03)00137-8. [DOI] [PubMed] [Google Scholar]
- 55.Else KJ, Grencis RK. Antibody-independent effector mechanisms in resistance to the intestinal nematode parasite Trichuris muris. Infect. Immun. 1996;64:2950–2954. doi: 10.1128/iai.64.8.2950-2954.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Betts J, deSchoolmeester ML, Else KJ. Trichuris muris: CD4+ T cell-mediated protection in reconstituted SCID mice. Parasitology. 2000;121(Pt 6):631–637. [PubMed] [Google Scholar]
- 57.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites: masters of regulation. Immunol. Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 58.Lawrence RA, Gray CA, Osborne J, Maizels RM. Nippostrongylus brasiliensis: cytokine responses and nematode expulsion in normal and IL-4-deficient mice. Exp. Parasitol. 1996;84:65–73. doi: 10.1006/expr.1996.0090. [DOI] [PubMed] [Google Scholar]
- 59.Fallon PG, Jolin HE, Smith P, Emson CL, Townsend MJ, Fallon R, McKenzie AN. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity. 2002;17:7–17. doi: 10.1016/s1074-7613(02)00332-1. [DOI] [PubMed] [Google Scholar]
- 60.Liu Z, Liu Q, Pesce J, Whitmire J, Ekkens MJ, Foster A, VanNoy J, Sharpe AH, Urban JF, Jr., Gause WC. Nippostrongylus brasiliensis can induce B7-independent antigen-specific development of IL-4-producing T cells from naive CD4 T cells in vivo. J. Immunol. 2002;169:6959–6968. doi: 10.4049/jimmunol.169.12.6959. [DOI] [PubMed] [Google Scholar]
- 61.Holland MJ, Harcus YM, Balic A, Maizels RM. Th2 induction by Nippostrongylus secreted antigens in mice deficient in B cells, eosinophils or MHC class I-related receptors. Immunol. Lett. 2005;96:93–101. doi: 10.1016/j.imlet.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 62.Schopf LR, Hoffmann KF, Cheever AW, Urban JF, Jr., Wynn TA. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J. Immunol. 2002;168:2383–2392. doi: 10.4049/jimmunol.168.5.2383. [DOI] [PubMed] [Google Scholar]
- 63.Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ, and TNF-α. J. Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 64.de Jong YP, Rietdijk ST, Faubion WA, Abadia-Molina AC, Clarke K, Mizoguchi E, Tian J, Delaney T, Manning S, Gutierrez-Ramos JC, et al. Blocking inducible co-stimulator in the absence of CD28 impairs Th1 and CD25+ regulatory T cells in murine colitis. Int. Immunol. 2004;16:205–213. doi: 10.1093/intimm/dxh019. [DOI] [PubMed] [Google Scholar]
- 65.Reichmann G, Villegas EN, Craig L, Peach R, Hunter CA. The CD28/B7 interaction is not required for resistance to Toxoplasma gondii in the brain but contributes to the development of immunopathology. J. Immunol. 1999;163:3354–3362. [PubMed] [Google Scholar]
- 66.Lebedeva T, Dustin ML, Sykulev Y. ICAM-1 co-stimulates target cells to facilitate antigen presentation. Curr. Opin. Immunol. 2005;17:251–258. doi: 10.1016/j.coi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 67.Arimura Y, Kato H, Dianzani U, Okamoto T, Kamekura S, Buonfiglio D, Miyoshi-Akiyama T, Uchiyama T, Yagi J. A co-stimulatory molecule on activated T cells, H4/ICOS, delivers specific signals in Th cells and regulates their responses. Int. Immunol. 2002;14:555–566. doi: 10.1093/intimm/dxf022. [DOI] [PubMed] [Google Scholar]
- 68.Hu-Li J, Shevach EM, Mizuguchi J, Ohara J, Mosmann T, Paul WE. B cell stimulatory factor 1 (interleukin 4) is a potent costimulant for normal resting T lymphocytes. J. Exp. Med. 1987;165:157–172. doi: 10.1084/jem.165.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kubin M, Kamoun M, Trinchieri G. Interleukin 12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J. Exp. Med. 1994;180:211–222. doi: 10.1084/jem.180.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]


