Abstract
Objective
Previous studies of subjects with dementia of the Alzheimer type (DAT) have reported correlations between increases in activity of the hypothalamic-pituitary-adrenal (HPA) axis and hippocampal degeneration. In this study, we sought to determine whether increases in plasma cortisol, a marker of HPA activity, were associated with clinical and cognitive measures of the rate of disease progression in DAT subjects.
Method
Thirty-three subjects with very mild and mild DAT and 21 nondemented comparison subjects were assessed annually for up to 4 years using the Clinical Dementia Rating scale and a battery of neuropsychological tests. Plasma was obtained at 8AM on a single day and assayed for cortisol. Rates of change over time in the clinical and cognitive measures were derived from growth curve models.
Results
In the DAT subjects, but not in the nondemented comparison subjects, higher plasma cortisol levels were correlated with more rapidly increasing symptoms of dementia and more rapidly decreasing performance on neuropsychological tests associated with temporal lobe function. Similar correlations were not observed between higher plasma cortisol levels and clinical and cognitive assessments obtained at the single assessment closest in time to the plasma collection.
Conclusions
Higher HPA activity, as reflected by increased plasma cortisol levels, is associated with more rapid disease progression in DAT subjects.
Introduction
Chronic psychosocial stressors trigger increases in the levels of glucocorticoid (GC) stress hormones that, in turn, have deleterious effects on the structure and function of central nervous system (CNS) structures, especially the hippocampus (1, 2). For example, in rodents, atrophy of neuronal dendrites within the cornu ammonis (CA) of the hippocampus (3) and deficits in spatial memory (4) develop after administration of chronic behavioral stress or the GC hormone, corticosterone. In humans with chronic depression, a psychiatric disorder frequently associated with increased activity of the hypothalamic-pituitary-adrenal (HPA) axis (5), decreases in the volume of the hippocampus have been correlated with the duration of illness (6, 7). The cellular mechanisms underlying the potentially neurotoxic effects of GC hormones are under intense investigation (5). In animals, behavioral stressors and corticosterone administration increase excitatory amino acid release (8, 9), as well as the expression of N-methyl-D-aspartate (NMDA) -type glutamate receptors (10). Thus, GC-triggered increases in excitotoxicity have been proposed as one mechanism of GC-induced neuronal injury (5). Also, because the hippocampus plays a central role in inhibiting the activity of the HPA axis (11), hippocampal damage could produce a repetitive cycle of increasing HPA dysregulation and ongoing hippocampal injury (1).
GC-related neuronal injury has also been proposed as a mechanism by which the CNS might be affected by “healthy” aging (1, 12). Age-related increases in corticosterone levels were first reported in rodents more than 25 years ago (13), and have been correlated with spatial memory deficits (14). More recently, correlations have been reported between increased plasma cortisol levels, memory impairments, and smaller hippocampal volumes in non-demented elderly subjects (15, 16). However, caution should be used in interpreting such findings since a portion of such subjects could have preclinical forms of Alzheimer’s disease (AD) (17). In another study, Lupien, et al (18), suggested that elderly subjects could be clustered into subgroups characterized by increases, decreases, and no change in plasma cortisol levels over time. Again, a possible explanation for such findings is that the subjects in such subgroups may differ with regard to the frequency of preclinical forms of AD.
Increases in HPA axis activity have also been directly associated with AD. Increases in plasma cortisol levels have been reported in individuals with probable AD (19, 20, 21, 22, 23), but have been generally interpreted as evidence that the disease process of AD (i.e., AD-induced hippocampal degeneration) leads to dysinhibition of the HPA axis. In line with this hypothesis, correlations have been reported between increases in HPA axis activity and dementia severity (24) or hippocampal volume loss (25, 26) in individuals with probable AD. Finally, decreases in cortical concentrations of corticotropin-releasing hormone (CRH) and increases in CRH receptors have been reported in post-mortem studies of AD subjects (27, 28). However, as with the correlations between plasma cortisol and hippocampal volumes, these findings have been interpreted as evidence of the effect of the AD disease process on CRH expression.
The purpose of the current study was to assess the relationship between plasma cortisol levels and clinical and cognitive measures of the rate of disease progression in subjects with very mild or mild dementia of the Alzheimer type (DAT). Our hypothesis was that higher levels of HPA axis activity, as reflected by higher plasma cortisol levels, would be correlated with more rapid progression of clinical and cognitive deficits in DAT subjects. Among cognitive measures, we were most interested in the relationship between cortisol levels and measures of memory performance, because of previous reports linking increased HPA axis activity to hippocampal volume loss and dysfunction in elderly individuals (5). We also studied non-demented subjects matched to the DAT subjects with respect to age and gender so that normative relationships, if any, between cortisol levels and cognition could be assessed.
Methods
All subjects in this study were community dwelling and enrolled in longitudinal studies of aging and dementia at the Alzheimer’s Disease Research Center (ADRC) at Washington University School of Medicine. Written informed consent was obtained from each subject after the nature and risks of the study were explained. Each subject was assessed on an annual basis using a standard protocol (29) that included semi-structured interviews with the subject and a collateral source (generally a spouse or an adult child) who was knowledgeable about the subject. The Clinical Dementia Rating scale (CDR) was used as the primary method of recording the presence and severity of dementia (30). The CDR rates the presence or absence of cognitive impairment on a 5 point scale (0, 0.5, 1, 2, 3 - from none to severe) in 6 domains or “boxes”: 1) memory, 2) orientation, 3) judgment and problem solving, 4) function in the community, 5) function at home and hobbies, and 6) personal care. An overall CDR score is then derived using the individual ratings in the 6 domains in accordance with standard scoring rules (30), such that a CDR score of 0 indicates no dementia and CDR scores of 0.5, 1, 2, and 3 indicate very mild, mild, moderate and severe dementia, respectively. Also, the individual box scores can be totaled to yield a sum-of-boxes total score (29) that ranges from 0 (0x6 when there is no impairment in any domain) to 18 (3x6 when there is maximal impairment in all domains). Inter-rater reliability for the CDR is high (31, 32). Individuals at our ADRC rated as CDR 0.5 progress in a predictable manner to greater stages of dementia severity, and at autopsy, the large majority of such individuals have neuropathological AD (33). Similarly impaired individuals have been considered by other investigators as having “mild cognitive impairment” (34).
The subjects recruited for this study had CDR scores of 1 (mild dementia), 0.5 (very mild dementia), or 0 (no dementia) at the time of their assessment. To measure the rate of change of dementia severity in conjunction with the date of plasma sampling in each subject, all available annual CDR assessments were used for a time period of up to two years before and two years after the date of plasma sampling. The average number of assessments available in the subject groups was similar (i.e., mean (SD) - 3.2 (0.4) for CDR 1 subjects, 3.2 (0.7) for CDR 0.5 subjects, and 2.7 (0.8) for CDR 0 subjects). CDR sum-of-boxes total scores were used as the clinical measure of dementia severity. Also, because of suggested links between hypercortisolemia and depression in the elderly (5), symptoms of depression were assessed at the time of the annual assessments using the Geriatric Depression Scale (GDS) (35).
To measure the rate of change in cognitive function, the subjects’ performance was assessed using a comprehensive neuropsychological battery obtained in conjunction with the annual assessments, but independently of the protocol that yielded the CDR. Clinicians were unaware of the results of neuropsychological test battery and the psychometricians were unaware of the results of the CDR evaluation. The neuropsychological battery included measures of episodic memory, semantic memory, speeded psychomotor performance, visuospatial ability, and attention (36). It was previously used to describe the pattern of cognitive deficits in 407 individuals with very mild and mild DAT (i.e., CDR 0.5 and 1) (37). In that study, a factor analysis revealed three factors that accounted for ~70% of the variance in cognitive performance. In a subset of these subjects who later came to autopsy (n = 41), scores for the three factors were correlated with the frequency of β-amyloid plaques in three general regions of the brain (i.e., temporal lobe, parietal lobe and frontal lobe). Thus, the three factor scores were named according to these brain regions (i.e., Temporal Factor, Parietal Factor, Frontal Factor). In the present study, these three factor scores were calculated for each of the subjects using weightings derived from the prior factor analyses on the subjects with very mild and mild DAT (37).
Apolipoprotein E (apoE) gene allele status was also determined in all of the subjects; 3 (5.6%) subjects had two apoE4 alleles, 18 (33.3%) subjects had one apoE4 allele, and 33 (61.1%) subjects had no apoE4 alleles.
Cortisol levels were determined using plasma samples collected in conjunction with a study of cerebrospinal fluid biomarkers of AD conducted by two of the authors (AMF and DMH). Blood samples were collected onto ice by venipuncture between 7:45 and 8:00 AM after an overnight fast, plasma was prepared, and then stored at −80° C until the time of assay. Cortisol concentrations were assayed in duplicate using a commercially available double-antibody radioimmunoassay (Clinical Assays, DiaSorin, Stillwater, MN). Mean values were used for all analyses. The interassay coefficient of variation (CV) of this assay is less than 15% at cortisol concentrations between 3 and 40 μg/ml and the limit of detection is 1 μg/ml.
Statistical analyses to test for an association between the rate of change of sum-of-boxes total scores and neuropsychological battery factor scores and plasma cortisol concentrations were performed using general linear mixed models. Random coefficients models were constructed (38), which assumed a linear growth for CDR total scores and the three neuropsychological battery factor scores over time for each subject and an unstructured covariance matrix between the intercept and the slope across the subjects within the three groups of subjects defined by their CDR scores (i.e., 1, 0.5 and 0). We further assumed that the slope over time was a linear function of cortisol levels, allowing us to assess the association between the longitudinal rate of change of the sum-of-boxes total scores and neuropsychological battery factor scores and plasma cortisol levels. This assumption was justified because no higher order term provided a significant effect in any of the models used in the analyses. In all T or F tests from these models, the Satterthwaite’s approximation was used to adjust the degrees of freedom for the denominator (39). All these models were implemented using PROC MIXED/SAS (40).
Results
The clinical characteristics, sum-of-boxes scores, neuropsychological battery factor scores, and plasma cortisol levels for the three groups of subjects (i.e., CDR 1, 0.5 and 0) are summarized in Table 1. One-way ANOVA suggested a trend toward a group effect for plasma cortisol levels (F=2.3, df=2,51, p=.092); post-hoc comparisons of the mean plasma cortisol levels across groups suggested that CDR 1 subjects had slightly higher plasma cortisol levels than both CDR 0 (p = .013) and CDR 0.5 (p = .046) subjects, but that CDR 0 and CDR 0.5 subjects had similar plasma cortisol levels (p = .47).
Table 1.
Clinical Characteristics and Plasma Cortisol Concentrations in Elderly Subjects With and Without Dementia of the Alzheimer Type
| Group | CDR 0 | CDR 0.5 | CDR 1 |
|---|---|---|---|
| N | 21 | 23 | 10 |
| Age (years) | 77.6 (9.7) | 74.2 (7.5) | 76.0 (4.6) |
| Sex (M/F) | 7/14 | 15/8 | 4/6 |
| Sum-of Boxes | .07 (.18) | 2.17 (1.00) | 6.50 (1.68) |
| Temporal Factor | 1.42 (.80) | .72 (.80) | −.78 (.41) |
| Frontal Factor | .49 (.71) | .34 (.66) | −.41 (.88) |
| Parietal Factor | .78 (.32) | .62 (.51) | −.17 (.62) |
| Cortisol (μg/ml) | 14.26 (4.10) | 15.08 (3.13) | 17.98 (4.29) |
Values for all variables, except for N and Sex, are group means (SD). For age, Sum-of-Boxes, Temporal Factor, Frontal Factor, and Parietal Factor, the assessments conducted closest in time to the time of plasma cortisol sampling were used to calculate the group means.
Combining the two groups of subjects with DAT (i.e., CDR 0.5 and CDR 1) together (n = 33), there was a significant positive association between higher plasma cortisol levels and a more positive rate of change (i.e., worsening) in sum-of-boxes scores (T=2.86, p=.007). Also, there was a significant negative association between higher plasma cortisol levels and a more negative rate of change (i.e., worsening) in Temporal Factor scores (T=−2.59, p=.014), but no association with the rates of change in Parietal or Frontal Factor scores. The magnitude of these associations was substantial, as reflected by estimating the impact of a physiologically-relevant change in plasma cortisol. For an increase of 1 μg/ml in plasma cortisol, the annual rate of change in sum-of-boxes scores would be increased by 0.150 (SE=0.052) or 19% of the observed rate of change in sum-of-boxes scores (0.80 (SE=0.190)), and the annual rate of change in Temporal Factor scores would be decreased by 0.025 (SE=0.010) or 16% of the observed rate of change in Temporal Factor scores (−0.16 (SE=0.040)). In contrast, there were no significant associations between plasma cortisol levels and sum-of-box scores or Temporal Factor scores obtained from the assessment closest in time to the collection of the blood cortisol sample in the combined group of DAT subjects.
When the CDR 0.5 subjects (n = 23) were evaluated separately, there again were significant associations between plasma cortisol levels and the rates of change in sum-of-boxes scores (T=2.53, p=.017) and Temporal Factor scores (T=−2.58, p=.016), but not Parietal Factor scores or Frontal Factor scores. However, when the CDR 1 subjects (n = 10) were evaluated separately, there were no significant associations between plasma cortisol levels and the rates of change in sum-of-boxes scores, Temporal Factor scores, Parietal Factor scores or Frontal Factor scores.
In the nondemented subjects (i.e., CDR 0) (n = 21), there were no significant associations between plasma cortisol levels and the rates of change in sum-of-boxes scores, Temporal Factor scores, Parietal Factor scores or Frontal Factor scores.
When apoE allelic status was taken into the consideration, the positive association between plasma cortisol levels and the rate of change in sum-of-boxes scores in DAT subjects (i.e., CDR 0.5 and CDR 1 combined) with no apoE4 alleles remained significant (T=2.76, p=0.009), but not in DAT subjects with at least 1 apoE4 allele. Also, our data suggested a trend for an association between higher plasma cortisol levels and a more negative rate of change in Temporal Factor scores in DAT subjects with at least 1 apoE4 allele (T=−1.94, p=0.063), but not in DAT subjects with no apoE4 alleles. There were no significant associations between plasma cortisol levels and sum-of-box scores or Temporal Factor scores in non-demented subjects (i.e., CDR 0) regardless of their apoE status.
There was no significant difference in mean GDS scores obtained from the assessment closest in time to the collection of the plasma cortisol sample among the three groups of subjects (i.e., CDR 1, 0.5 and 0) (F=2.27, p=0.118). There was also no significant correlation between plasma cortisol levels and GDS scores obtained during the assessment closest in time to the collection of the blood cortisol sample in the CDR 1 subjects (Pearson’s r=−0.22, p=0.598), the CDR 0.5 subjects (r=−0.11, p=0.700), or in the CDR 0 subjects (r=−0.14, p=0.587).
Discussion
These results provide preliminary support for our hypothesis that increased GC hormone levels, as reflected by morning plasma cortisol concentrations, are associated the rate of change of both clinical and cognitive measures of dementia severity in subjects with DAT. It is especially intriguing that plasma cortisol levels were associated with the rate of change in the neuropsychological Factor measure weighted towards memory function and previously found to be associated with AD neuropathologic burden in the temporal lobe (i.e., Temporal Factor scores) (37). This association is consistent with previous reports of relationships between increased HPA axis activity and age-related hippocampal volume loss (5). There have been conflicting reports about the density of GC receptor expression in the hippocampus relative to other regions of the primate brain (41, 42, 43). Nonetheless, our findings raise the intriguing possibility that increased GC levels may have a disproportionate impact on the AD disease process as it develops in the hippocampus and other structures of the medial temporal lobe (e.g., entorhinal cortex) (44).
The fact there were no significant associations between plasma cortisol levels and measures of dementia severity at the time of blood sampling suggests that increased cortisol levels were associated with more rapid rates of disease progression rather than the severity of disease. Taking this interpretation one step further, these results suggest that increased activity of the HPA axis as reflected by plasma cortisol levels may be associated with an acceleration of the disease process in AD, rather than being the product of the degenerative effects of the disease on the hippocampus and HPA dysinhibition.
We were surprised to find no associations between plasma cortisol and the rates of change in sum-of-boxes or neuropsychological Factor scores in the elderly non-demented subjects. This finding is in apparent conflict with the prior report of a correlation between plasma cortisol levels and the severity of cognitive impairment in non-demented elderly volunteers (16). One explanation for this discrepancy might be that the age range was too limited in our small group of CDR 0 subjects (see Table 1). Also, our CDR 0 subjects were rigorously screened to exclude even very mild signs of dementia. At the present time, only 3 (5.6%) of these individuals have come to autopsy, so it is not possible to determine the frequency of preclinical AD in our nondemented subjects. Also, we did not find any association between the severity of depressive symptoms and plasma cortisol levels in any of the subject groups. However, any subjects who met syndromal criteria for depression were also excluded from our study.
Our findings of a relationship between plasma cortisol levels and markers of disease progression in DAT subjects may be particularly applicable to the early stages of AD. This is suggested by the fact that significant correlations were observed between plasma cortisol concentrations and measures of disease progression in the CDR 0.5 subjects, but not in the CDR 1 subjects, when these groups were examined separately. While the correlations between plasma cortisol levels and markers of disease progression would have been more difficult to evaluate in the small group of CDR 1 subjects, the values of the correlations observed in CDR 1 subjects were not even suggestive of a trend.
At present, the mechanism by which increased HPA axis activity could accelerate the disease process of AD is unknown. Behavioral stressors and administration of GC hormones have been reported to increase excitatory amino acid release (8, 9) and the expression of NMDA-type glutamate receptors (10) in rodents. Using mice that overexpress the human form of amyloid precursor protein (APP), we recently observed that chronic stress (i.e., isolation) accelerated the deposition of amyloid plaques as well as appearance of deficits in learning and memory that usually accompany Aβ deposition (45). Also, Harris-White and Chu (46) have reported that GC hormones may decrease the clearance of Aβ, which itself can have neurotoxic effects (47). Alternatively, behavioral stressors could influence cognition by decreasing hippocampal neurogenesis (48). Thus, stress-related increases in GC levels could either directly or indirectly influence neuronal dysfunction and cognitive impairment associated with Aβ deposition.
There are some notable weaknesses to this study. First, our measure of the subjects’ stress responsivity was plasma cortisol levels at one time point on a single day. Our study was retrospective and the only available plasma samples were collected in coordination with a study of CSF AD biomarkers, which involved obtaining blood and CSF on a single day. However, a correlation has been previously reported between 8AM cortisol levels and dementia severity in DAT subjects (24). Also, our subjects may have had anticipatory anxiety on that day related to the lumbar puncture. Nonetheless, the associations found between our simple measure of HPA axis activity and the rates of clinical and neuropsychological decline were substantial. Second, the samples available for this study, and especially the sample of mildly demented subjects (i.e., CDR 1), were small. Larger numbers of subjects with a wider range of dementia severity would have made it possible to more clearly determine whether the relationship between plasma cortisol concentrations and the rate of disease progression were specific to a particular stage of AD.
The results of this study need to be confirmed and extended by examining other measures of disease progression in larger numbers of DAT subjects that have been assessed over longer periods of time and wider ranges of dementia severity. Studies of neuroanatomical markers of disease progression, such as measures of the structure of the hippocampus itself (49,50), would be particular useful. If the hypothesis that stress can increase GC levels and accelerate the progression of the disease process of AD is confirmed, it would give impetus to the development of therapeutic approaches, both pharmacological (51, 52) and non-pharmacological (53), to decrease stress and the levels of stress-related GC hormones.
Figure 1. Correlations Between Plasma Cortisol Concentrations and Measures of Disease Progression in Subjects with Very Mild to Mild DAT.
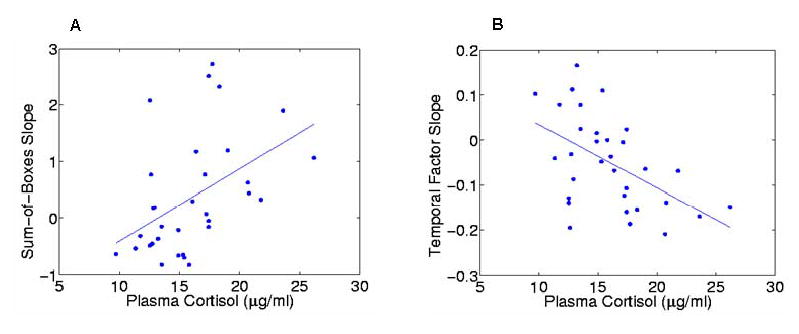
Relationships between plasma cortisol concentrations and measures of disease progression were examined in a combined group of subjects with very mild (CDR 0.5) and mild (CDR 1) DAT. Panel A shows the scatterplot for the association between cortisol concentrations and rates of change (i.e., slopes) in sum-of-box scores. Panel B shows the scatterplot for the association between 8AM cortisol concentrations and rates of change (i.e., slopes) in Temporal Factor scores. Plasma was collected for cortisol at 8AM after an overnight fast. Rates of change in sum-of-box scores were generated using general linear mixed models and up to 4 annual assessments.
Acknowledgments
This research was supported by PHS grants AG 025824, AG 03991, AG 05681, and AG 20794. The authors thank the Clinical, Psychometric, and Biostatistical Cores of the Washington University Alzheimer’s Disease Research Center (ADRC) for subject assessments and data management.
References
- 1.Sapolsky RM: Stress, the Aging Brain, and the Mechanisms of Neuron Death. MIT Press, 1992; Cambridge
- 2.Fuchs E, Flugge G. Stress, glucocorticoids and structural plasticity of the hippocampus. Neurosci Biobeh Rev. 1998;23:295–300. doi: 10.1016/s0149-7634(98)00031-1. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA2 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 4.Coburn-Litvak PS, Pothakos K, Tata DA, McCloskey DP, Anderson BJ. Chronic administration of corticosterone impairs spatial reference memory before spatial working memory in rats. Neurobiol Learning Mem. 2003;80:11–23. doi: 10.1016/s1074-7427(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 5.McEwen BS. The neurobiology of stress: From serendipity to clinical relevance. Brain Research. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 6.Sheline YI, Wang PO, Gado MH, Csernansky JG, Vannier MV. Hippocampal atrophy in recurrent major depression. Proc Nat’l Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5044. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowy MT, Gault L, Yamamoto BK. Adrenalectomy attenuates stress-induced elevations in extracellular glutamate concentrations in the hippocampus. J Neurochem. 1993;61:1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- 9.Venero C, Borrell J. Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus: A microdialysis study in freely moving rats. Eur J Neurosci. 1999;11:2465–2473. doi: 10.1046/j.1460-9568.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- 10.Weiland NG, Orchinik M, Tanapat P. Chronic corticosterone treatment induces parallel changes in N-methyl-D-aspartate receptor subunit messenger RNA levels and antagonist binding sites in the hippocampus. Neurosci. 1997;78:653–662. doi: 10.1016/s0306-4522(96)00619-7. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocrine Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 12.Hibbard C, Yau JLW, Seckl JR. Glucocorticoids and the ageing hippocampus. J Anat. 2000;197:553–562. doi: 10.1046/j.1469-7580.2000.19740553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landfield P, Waymire J, Lynch G. Hippocampal aging and adrenocorticoids: Quantitative correlations. Science. 1978;202:1098–1102. doi: 10.1126/science.715460. [DOI] [PubMed] [Google Scholar]
- 14.Issa A, Gauthier S, Meaney MJ. Hypothalamic-pituitary-adrenal activity in aged cognitively impaired and cognitively unimpaired aged rats. J Neurosci. 1990;10:3247–3254. doi: 10.1523/JNEUROSCI.10-10-03247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lupien S, Lecours AR, Lussier I, Schwartz G, Nair NPV, Meaney MJ. Basal cortisol levels and cognitive deficits in human aging. J Neurosci. 1994;14:2893–2903. doi: 10.1523/JNEUROSCI.14-05-02893.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lupien SJ, de Leon M, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 17.Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Ann Neurol. 1999;5(3):358–68. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 18.Lupien S, Lecours AR, Schwartz G, Sharma S, Hauger RL, Meaney MJ, Nair NPV. Longitudinal study of basal cortisol levels in healthy elderly subjects: Evidence for subgroups. Neurobiol Aging. 1996;17:95–105. doi: 10.1016/0197-4580(95)02005-5. [DOI] [PubMed] [Google Scholar]
- 19.Davis KL, Davis BM, Greenwald BS, Mohs RC, Mathe AA, Johns CA, Horvath TB. Cortisol and Alzheimer’s Disease, I: Basal studies. Am J Psychiatry. 1986;143:300–305. doi: 10.1176/ajp.143.3.300. [DOI] [PubMed] [Google Scholar]
- 20.Weiner MF, Vobach S, Olsson K, Svetlik D, Risser RC. Cortisol secretion and Alzheimer’s disease progression. Biol Psychiatry. 1997;42:1030–1038. doi: 10.1016/s0006-3223(97)00165-0. [DOI] [PubMed] [Google Scholar]
- 21.Swanwick GRJ, Kirby M, Bruce I, Buggy F, Coen RF, Coakley D, Lawlor BA. Hypothalamic-pituitary-adrenal axis dysfunction in Alzheimer’s Disease. Am J Psychiatry. 1998;155:286–289. doi: 10.1176/ajp.155.2.286. [DOI] [PubMed] [Google Scholar]
- 22.Umegaki H, Ikari H, Nakahata H, Endo H, Suzuki Y, Ogawa O, Nakamura A, Yamamoto T, Iguchi A. Plasma cortisol levels in elderly female subjects with Alzheimer’s disease: A cross-sectional and longitudinal study. Brain Res. 2000;881:241–243. doi: 10.1016/s0006-8993(00)02847-x. [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen S, Nasman B, Carlstrom K, Olsson T. Increased levels of adrenocortical and gonadal hormones in mild to moderate Alzheimer’s disease. Dementia Geriatr Cogn Disord. 2002;13:74–79. doi: 10.1159/000048637. [DOI] [PubMed] [Google Scholar]
- 24.Miller TP, Taylor J, Rogerson S, Mauricio M, Kennedy Q, Schatzberg A, Tinklenberg J, Yesavage J. Cognitive and noncognitive symptoms in dementia patients: relationship to cortisol and dehydroepiandrosterone. Int Psychogeriatr. 1998;10:85–96. doi: 10.1017/s1041610298005171. [DOI] [PubMed] [Google Scholar]
- 25.DeLeon MJ, McRae T, Tsai JR, George AE, Marcus DL, Freedman M, Wolf AP, McEwen B. Abnormal cortisol response in Alzheimer’s disease linked to hippocampal atrophy. Lancet. 1988;ii:391–392. doi: 10.1016/s0140-6736(88)92855-3. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien JT, Ames D, Schweitzer I, Colman P, Desmond P, Tress B. Clinical and magnetic resonance imaging correlates of hypothalamic-pituitary-adrenal axis function in depression and Alzheimer’s disease. Br J Psychiatry. 1996;168:679–687. doi: 10.1192/bjp.168.6.679. [DOI] [PubMed] [Google Scholar]
- 27.Auchus AP, Green RC, Nemeroff CB. Cortical and subcortical neuropeptides in Alzheimer’s disease. Neurobiol Aging. 1994;15:589–595. doi: 10.1016/0197-4580(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 28.Davis KL, Mohs RC, Marin DB, Purohit DP, Perl DP, Lantz M, Austin G, Haroutunian V. Neuropeptide abnormalities in patients with early Alzheimer disease. Arch Gen Psychiatry. 1999;56:981–987. doi: 10.1001/archpsyc.56.11.981. [DOI] [PubMed] [Google Scholar]
- 29.Berg L, Miller JP, Storandt M, Duchek J, Morris JC, Rubin EH, Burke WJ, Coben LA. Mild senile dementia of the Alzheimer type. 2 Longitudinal assessment Ann Neurol. 1988;23:477–484. doi: 10.1002/ana.410230509. [DOI] [PubMed] [Google Scholar]
- 30.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 31.Burke WJ, Miller JP, Rubin EH, Morris JC, Coben LA, Duchek J, Wittels IG, Berg L. Reliability of the Washington University Clinical Dementia Rating (CDR) Arch Neurol. 1988;45:31–42. doi: 10.1001/archneur.1988.00520250037015. [DOI] [PubMed] [Google Scholar]
- 32.Morris JC, Ernesto C, Schafer K, Coats M, Leon S, Sano M, Thal LJ, Woodbury P. Clinical dementia rating training and reliability in multicenter studies: The Alzheimer’s Disease Cooperative Study experience. Neurology. 1997;48:228–236. doi: 10.1212/wnl.48.6.1508. [DOI] [PubMed] [Google Scholar]
- 33.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer’s disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 34.Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack CR, Jr, Galasko DR, Doody R, Kaye J, Sano M, Mohs R, Gauthier S, Kim HT, Jin S, Schultz AN, Schafer K, Mulnard R, van Dyck CH, Mintzer J, Zamrini EY, Cahn-Weiner D, Thal J. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 35.Yesavage J, Brink T, Rose R, Lum O. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 36.Storandt M, Grant EA, Miller JP, Morris JC. Rates of progression in mild cognitive impairment and early Alzheimer’s disease. Neurology. 2002;59:1034–1041. doi: 10.1212/wnl.59.7.1034. [DOI] [PubMed] [Google Scholar]
- 37.Kanne SM, Balota DA, Storandt M, McKeel DW, Morris JC. Relating anatomy to function in Alzheimer's disease: Neuropsychological profiles predict regional neuropathology 5 years later. Neurology. 1998;50:979–985. doi: 10.1212/wnl.50.4.979. [DOI] [PubMed] [Google Scholar]
- 38.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 39.Giesbrecht FG, Burns JC. Two-stage analysis based on a mixed model: large sample asymptotic theory and small sample simulation results. Biometrics. 1985;41:477–486. [Google Scholar]
- 40.Littell R, Milliken GA, Stroup W, Wolfinger R: SAS System for Mixed Models. SAS Institute Inc. Cary NC. 1996
- 41.Patel PD, Lopez JF, Lyons DM, Burke S, Wallace M, Schatzberg AF. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J Psychiatr Res. 2000;34:383–92. doi: 10.1016/s0022-3956(00)00035-2. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci. 2000;20:4657–68. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pryce CR, Feldon J, Fuchs E, Knuesel I, Oertle T, Sengstag C, Spengler M, Weber E, Weston A, Jongen-Relo A. Postnatal ontogeny of hippocampal expression of the mineralocorticoid and glucocorticoid receptors in the common marmoset monkey. Eur J Neurosci. 2005;21:1521. doi: 10.1111/j.1460-9568.2005.04003.x. [DOI] [PubMed] [Google Scholar]
- 44.Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topological and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cerebral Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- 45.Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Effects of isolation stress on hippocampal neurogenesis, memory, and amyloid plaque deposition in APP (Tg2576) mutant mice. Neuroscience. 2004;127:601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 46.Harris-White ME, Chu T. Estrogen (E2) and glucocorticoid (Gc) effects on microglia and Aβ clearance in vitro and in vivo. Neurochem International. 2001;39:435–448. doi: 10.1016/s0197-0186(01)00051-1. [DOI] [PubMed] [Google Scholar]
- 47.Miguel-Hidalgo JJ, Alvarez XA, Cacabelos R, Quack G. Neuroprotection by memantine against neurodegeneration induced by β-amyloid (1–40) Brain Res. 2002;958:210–221. doi: 10.1016/s0006-8993(02)03731-9. [DOI] [PubMed] [Google Scholar]
- 48.Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–71. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- 49.Jack CR, Petersen RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Kokmen E. Rate of medial temporal lobe atrophy in typical aging and Alzheimer’s disease. Neurology. 1998;51:993–999. doi: 10.1212/wnl.51.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang L, Swank J, Glick IE, Gado MH, Miller MI, Morris JC, Csernansky JG. Changes in hippocampal volume and shape across time distinguish dementia of the Alzheimer type from healthy aging. NeuroImage. 2003;20:667–682. doi: 10.1016/S1053-8119(03)00361-6. [DOI] [PubMed] [Google Scholar]
- 51.Pomara N, Doraiswamy PM, Tun H, Ferris S. Mifepristone (RU 486) for Alzheimer's disease. Neurology. 2002;58(9):1436. doi: 10.1212/wnl.58.9.1436. [DOI] [PubMed] [Google Scholar]
- 52.Belanoff JK, Jurik J, Schatzberg LD, DeBattista C, Schatzberg AF. Slowing the progression of cognitive decline in Alzheimer's disease using mifepristone. Mol Neurosci. 2002;19:201–6. doi: 10.1007/s12031-002-0033-3. [DOI] [PubMed] [Google Scholar]
- 53.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–53. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]


