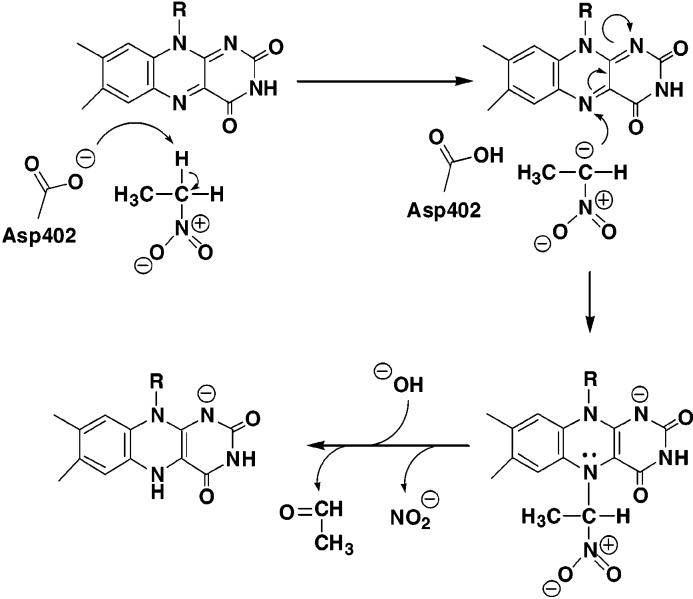
Nitroalkane oxidase (NAO) catalyzes the oxidation of nitroalkanes to the respective aldehydes or ketones with consumption of molecular oxygen and release of nitrite and hydrogen peroxide.1 This flavoenzyme is unique in its ability to oxidize neutral nitroalkanes. In the mechanism proposed for NAO (Scheme 1),2 catalysis is initiated by an active site base that deprotonates the neutral nitroalkane substrate to yield a carbanion that can attack the N-5 position of the oxidized flavin. In the absence of a crystal structure, the identity of the catalytic base has been inferred to be Asp402 by comparison of the primary structure of NAO with the homologous acyl-CoA dehydrogenase (ACAD) family of flavoenzymes.3 When this aspartate is mutated to glutamate, the rate of CH bond cleavage decreases 15–30-fold, consistent with the proposed role of Asp402.4 The objective of the present study is to more definitively assign the role of Asp402 as the catalytic base.
Although NAO has evolved to turnover neutral nitroalkanes, the nitroalkane anion is a necessary catalytic intermediate. For most enzymatic reactions, rapid equilibration or nonphysiological pK values preclude using both the protonated and the deprotonated forms of a substrate over a range of pH values. However, unlike typical carbon acids that form unstable carbanions, nitroalkanes can delocalize the negative charge into the nitro group to form a stable nitronate.5 This results in significantly lower pK values (e.g., the pK of nitroethane is 8.5) and very slow rates of proton transfer.6 Thus, within the time required to measure the initial rates of enzymatic turnover under steady-state conditions, both protonated and deprotonated nitroalkanes can be characterized as substrates. This provides a unique opportunity to probe the role of a putative catalytic base by using the preformed anion to rescue a mutant enzyme in which that active site residue has been altered.
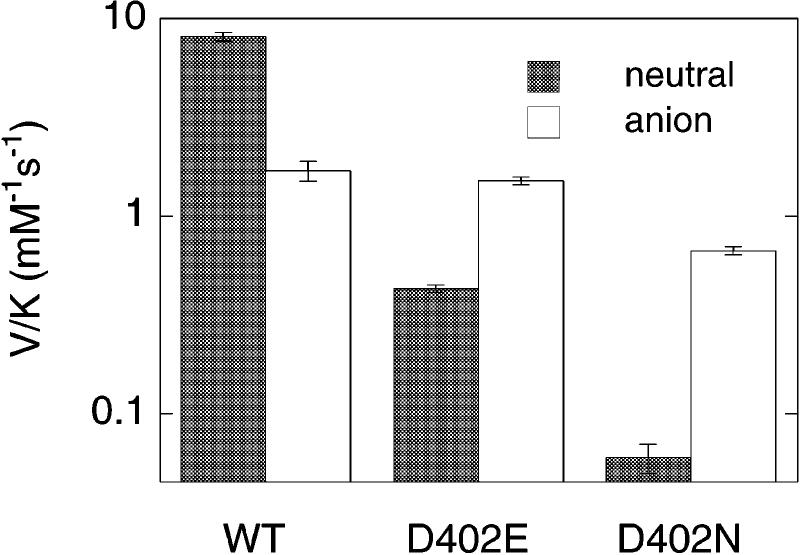
Accordingly, to determine if Asp402 is the active site base, this residue was replaced with Asn or Ala by site-directed mutagenesis, and the mutant enzymes were expressed and purified in amounts similar to those used for the wild-type enzyme.3,7 In addition, the effects of replacing Asp402 with glutamate have recently been characterized.4 CH bond cleavage is rate-limiting for the reduction of NAO by nitroethane, as was demonstrated by the observed deuterium isotope effect on the V/K value for this substrate being equal to the intrinsic isotope effect.4,8 Consequently, the ability of the mutant enzymes to abstract a proton from substrate was evaluated by comparing their V/K values to that of the wild-type enzyme (Figure 1).9 At pH 8 in 500 mM HEPES, the addition of an extra methylene group in the D402E enzyme decreases the V/K value about 20-fold with the neutral substrate. Replacing the carboxylate with an amide in the D402N enzyme has a more dramatic effect, decreasing the V/K value for neutral nitroethane at least 140-fold.10 Taking advantage of the stability of deprotonated nitroalkanes, we also examined the nitroethane anion as a substrate for the wild-type and mutant enzymes. The V/K value of the wild-type enzyme with the nitroethane anion is about 5-fold lower than the V/K value with neutral nitroethane (Figure 1).11 In contrast, the catalytic activity of the mutant enzymes is substantially greater with the deprotonated substrate than with neutral nitroethane. Thus, in enzymes in which Asp402 has been mutated, significant activity is recovered if the substrate anion is preformed in solution, consistent with the role of this residue being the formation of the anion in the active site.12
Figure 1.
The V/K values of the wild-type, D402E, and D402N enzymes with neutral nitroethane or the nitroethane anion as substrate in 500 mM HEPES, pH 8.0 at 30 °C.
Scheme 1.
Mechanism of Nitroalkane Oxidase
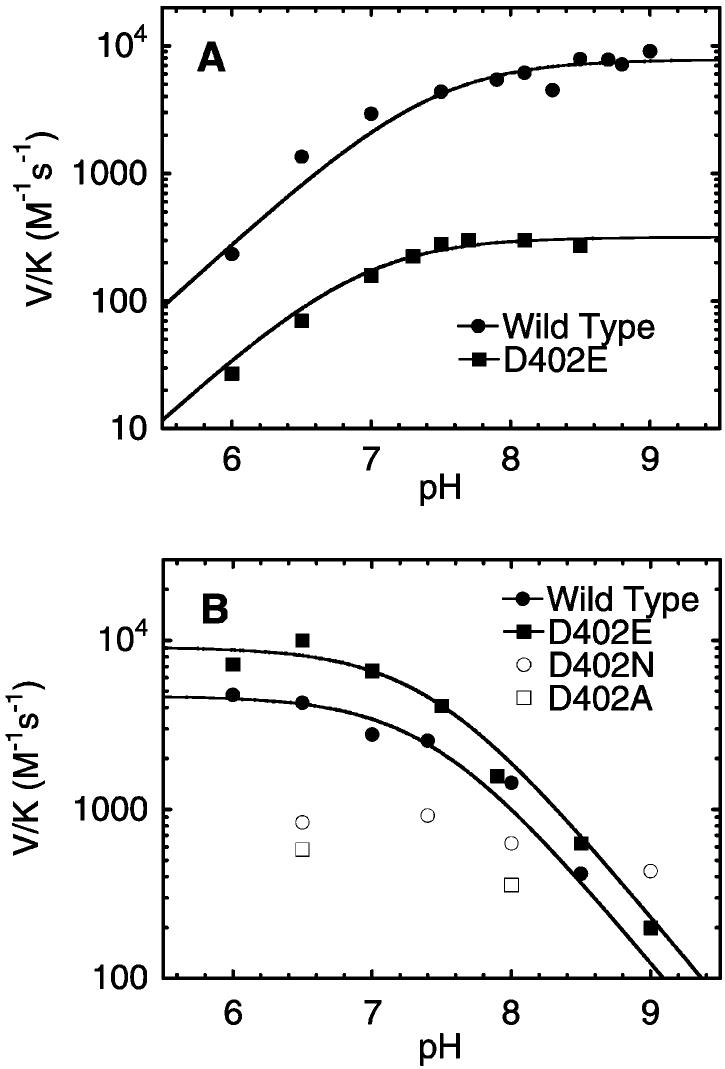
The V/K values of the wild-type and mutant enzymes with each substrate were also measured as a function of pH. With neutral nitroethane as substrate, the V/K values of the wild-type enzyme increase as the pH increases with a pK value of 7.4 ± 0.2 (Figure 2A).13 This pH dependence is reversed with the nitroethane anion as substrate, such that the V/K values increase as the pH decreases, yet the same pK value is observed (7.4 ± 0.1) (Figure 2B). The V/K values of the D402E enzyme exhibit a similar pH dependence, with a plateau at high pH for neutral nitroethane and a plateau at low pH for the nitroethane anion. The pK values for the D402E enzyme are 6.9 ± 0.1 and 7.4 ± 0.2 for the protonated and deprotonated substrates, respectively, analogous to those of wild-type NAO. The fact that the pH dependence of the V/K values for each substrate is reversed, along with the presence of only a single plateau in each case, is consistent with a carboxylate residue in the active site that must be charged to abstract a proton from the neutral substrate or neutral to bind the anionic substrate without charge–charge repulsion. In contrast, in both the D402N and the D402A enzymes, where the carboxylate at 402 is absent, the V/K values with the nitroethane anion are pH independent. Therefore, the pK value of ∼7 observed in this and other studies of NAO can be assigned to Asp402. Furthermore, although the D402E enzyme is 20-fold less active toward neutral nitroethane at high pH, there is only a 2-fold difference in the V/K values of the wild-type and D402E enzymes toward the nitroethane anion at low pH. Consequently, the D402E mutation is only inhibitory when proton abstraction is required; when this step is circumvented by using the nitroethane anion, the Asp to Glu mutation has almost no effect.
Figure 2.
pH profiles of the V/K values of the wild-type, D402E, D402N, and D402A enzymes with either (A) neutral nitroethane or the (B) nitroethane anion at 30 °C.
In conclusion, the results of this study are consistent with a primary role for Asp402 as the catalytic base in the mechanism of nitroalkane oxidase. The rare ability to generate a stable catalytic intermediate by preforming the nitroethane anion has allowed us to demonstrate that deprotonation of the neutral substrate is (i) an early, and likely the initial, step in catalysis, and (ii) the main function of Asp402 because bypassing this step rescues inactive enzymes in which this residue has been mutated.
Acknowledgment
This research was supported in part by grants to P.F.F. from the NIH (GM58698) and the Robert A. Welch Foundation (A-1245).
References
- 1.Kido T, Hashizume K, Soda K. J. Bacteriol. 1978;133:53–58. doi: 10.1128/jb.133.1.53-58.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gadda G, Edmondson RD, Russell DH, Fitzpatrick PF. J. Biol. Chem. 1997;272:5563–5570. doi: 10.1074/jbc.272.9.5563. [DOI] [PubMed] [Google Scholar]
- 3.Daubner SC, Gadda G, Valley MP, Fitzpatrick PF. Proc. Natl. Acad. Sci. U.S.A. 2002;99:2702–2707. doi: 10.1073/pnas.052527799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valley MP, Fitzpatrick PF. Biochemistry. 2003;42:5850–5856. doi: 10.1021/bi034061w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feuer H. The Chemistry of the Nitro and Nitroso Groups, Part 1. Interscience; New York: 1969. Chapter 7. [Google Scholar]
- 6.Bernasconi CF. Acc. Chem. Res. 1992;25:9–16. [Google Scholar]
- 7. Less than one-half of the active sites of the D402A enzyme are purified with flavin bound, and the enzyme is less stable than other mutant enzymes. Because most of the characteristics of the D402A enzyme were similar to those of the D402N enzyme, a minimal amount of data was collected for this less stable variant.
- 8.Gadda G, Fitzpatrick PF. Biochemistry. 2000;39:1406–1410. doi: 10.1021/bi992255z. [DOI] [PubMed] [Google Scholar]
- 9. All assays were conducted and all data were fit as previously described.4,8.
- 10. A V/K value for the D402A enzyme was not determined under these conditions, but at pH 8 in 50 mM HEPES, the V/K values for the D402A and D402N enzymes with the neutral substrate are identical (0.020 ± 0.002 and 0.017 ± 0.003 mM−1 s−1, respectively).
- 11. The nitroethane anion was generated by mixing concentrated neutral nitroethane with a 2-fold excess of potassium hydroxide and incubating overnight.
- 12. It is possible that the D402N and D402A enzymes are much less active with the neutral substrate than indicated in that the measured activity may actually be due to the oxidation of a small amount of the nitroethane anion generated in situ. The measured rates of these enzymes with neutral nitroethane are comparable to the buffer-catalyzed nonenzymatic rate of nitroethane deprotonation in 500 mM HEPES at pH 8 (data not shown), and there is a decrease in the V/K value of the D402N enzyme with neutral nitroethane upon decreasing the buffer concentration from 500 to 50 mM HEPES (0.06 ± 0.01 to 0.020 ± 0.002 mM−1 s−1). No changes in the V/K values of the wild type and D402E enzymes are observed in this concentration range.
- 13. [N-Morpholino]-ethanesulfonic acid (MES) was used for pH 6–6.5, N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid] (HEPES) for pH 7–8.1, and N-tris[hydroxymethyl]methyl-3-aminopropanesulfonic acid (TAPS) for pH 7.9–9. In all cases, the concentration of the buffer was 500 mM to neutralize the hydroxide used to form the anion.