Abstract
The present study was designed to analyze the level of B-cell clonal diversity in patients with rheumatoid arthritis by using HCDR3 (third complementarity determining region of the rearranged heavy chain variable region gene) length as a marker. A modified immunoglobulin VH gene fingerprinting method using either genomic DNA or complementary (c)DNA derived from B cells of the peripheral blood, synovial fluid, and tissues of several rheumatoid arthritis patients was employed. These assays permitted the detection and distinction of numerically expanded B-cell clones from activated but not numerically expanded B-cell clones. The present data suggest that B-cell clonal expansion is a common and characteristic feature of rheumatoid arthritis and that it occurs with increasing frequency from the blood to the synovial compartments, resulting in a narrowing of the clonal repertoire at the synovial level. These clonal expansions can involve resting, apparently memory B cells, as well as activated B cells. Furthermore, some of these individual expansions can persist over extended periods of time. These findings support the hypothesis that a chronic ongoing (auto)immune reaction is operative in rheumatoid arthritis and that this reaction, at least at the B-cell level, may be unique to each individual joint. A determination of the targets of these autoimmune reactions may provide valuable clues to help understand the immunopathogenesis of this disease.
Keywords: B-lymphocyte repertoire, complementarity determining region, immunoglobulin variable region gene, rheumatoid arthritis, synovial tissue
Introduction
Rheumatoid arthritis is a chronic debilitating autoimmune disease of unknown etiology. Although the disease is characterized by synovitis of the joints, tendon sheaths, and bursae, manifestations that do not involve the synovium are not infrequent [1]. These articular and systemic manifestations appear to be mediated by immunologic processes [2]. The hallmarks of the synovial abnormalities in rheumatoid arthritis are synovial lining cell proliferation, neoangiogenesis, and inflammatory cell infiltration involving the myeloid, macrophage, and lymphoid lineages [1,2]. There has been considerable controversy regarding the relative importance of the types of cells and their products involved in the inflammatory processes of rheumatoid arthritis [3]. Nevertheless, it seems likely that all of these cell types participate to some degree in disease pathogenesis.
Evidence in support of T-cell involvement in rheumatoid arthritis involves the description of restricted subsets of T cells in the blood and synovial tissue that either express or lack certain surface membrane proteins or that express a limited set of antigen receptors. For example, clonal amplifications of CD8+ CD57+ T cells are frequently found in the T-cell repertoire of rheumatoid arthritis patients [4]. Furthermore, expanded clones of CD4+ CD28- T cells exist in the blood and synovial compartments of such patients [5] and these T cells appear to be autoreactive [6]. Finally, the T-cell receptors for antigen expressed by these and other T-cell subsets frequently display a bias in favor of receptors utilizing certain Vβ genes [5,7,8,9,10,11,12].
In contrast to the extensive studies of the clonal distribution of T cells in rheumatoid arthritis, much less is known about the level of B-cell diversity in this disease. Previous studies, however, are consistent with the interpretation that the B-cell repertoire is also restricted. For example, flow cytometric analyses of circulating B cells [13] suggested that oligoclonality exists, and cell culture experiments [14,15,16,17] demonstrated that synovial tissue explants spontaneously secrete immunoglobulins of restricted heterogeneity as defined by immunoglobulin (Ig)G subclass, isoelectric focusing, and idiotype expression. More recent molecular analyses of the immunoglobulin genes expressed by B cells in the synovial tissue of rheumatoid arthritis patients support these notions [18,19,20,21,22].
These findings are important because they suggest that, at the B- and T-cell levels, an ongoing immune reaction is occurring that is directed at restricted sets of (auto)antigens. The present study was designed to analyze further the level of clonal diversity in rheumatoid arthritis B cells by using the length of the third complementarity determining region (CDR3) of the rearranged heavy (H) chain variable region (V) gene as a marker (herein referred to as HCDR3). A modification of the immunoglobulin VH gene fingerprinting method [23] that has been used to analyze the diversity of B cells and T cells in several clinical settings [4,24,25,26] was used to address this issue. The present data suggest that B-cell clonal expansion is a common and characteristic feature of rheumatoid arthritis, that it involves both resting and activated cells, and that it can persist over extended periods of time. These findings support the idea that a chronic (auto)immune reaction is operative in rheumatoid arthritis.
Materials and methods
Patients and patient samples
Heparinized venous blood, synovial fluid, and synovial tissue were obtained from patients who fulfilled the American College of Rheumatology criteria for the diagnosis of rheumatoid arthritis [27]. Synovial tissue removed at the time of either joint replacement or therapeutic synovectomy was digested with collagenase, DNAse, and hyaluronidase to obtain single-cell suspensions. Mononuclear cells (MNCs) were isolated from cell suspensions from blood, synovial fluid, and synovial tissue by density gradient centrifugation (Ficoll-Paque; Pharmacia LKB Biotechnology, Piscataway, NJ, USA).
Isolation of DNA and RNA, and preparation of complementary DNA
Genomic DNA was isolated from MNCs using the Puregene DNA Isolation Kit (Gentra Systems, Mineapolis, MN, USA) and total RNA was isolated using Ultraspec RNA (Biotech Laboratories, Houston, TX, USA). Both of these reagents were used according to the manufacturer's instructions. One microgram of RNA was reverse transcribed to complementary (c)DNA using 200U Moloney murine leukemia virua (M-MLV) reverse transcriptase (GIBCO BRL Life Technologies, Grand Island, NY, USA), 1U RNAse inhibitor (5 Prime 3 Prime, Boulder, CO, USA) and 20 pmol oligo dT primer in a total volume of 20 μl. These ingredients were incubated at 42°C for 1 h, heated to 65°C for 10 min to stop the reactions, and then diluted to a final volume of 100 μl.
Polymerase chain reaction conditions for immunoglobulin VH gene fingerprinting assay
The original immunoglobulin VH gene fingerprinting assay [23] was modified into two stages, starting with either genomic DNA or cDNA as templates (Fig. 1). The sequences of the primers used in these reactions were published previously [28].
Figure 1.
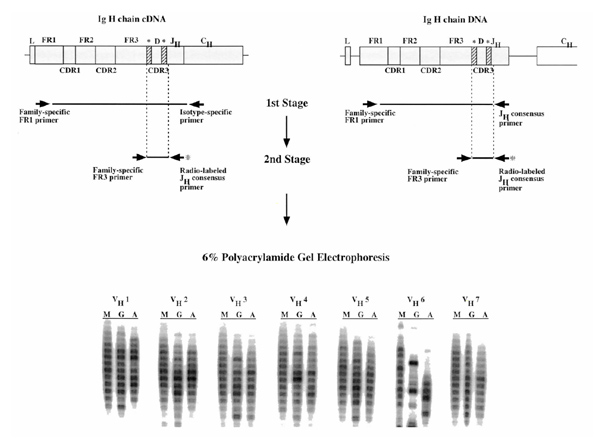
Schematic representation of the two-stage VH fingerprinting assay using either genomic DNA or complementary (c)DNA as templates. In stage I for the genomic DNA-based assay, genomic DNA was amplified using a sense VH family-specific FR1 primer in conjunction with an antisense JH consensus primer. For the cDNA-based assay, cDNA was amplified using a sense VH family-specific FR1 primer in conjunction with the appropriate antisense CH primer. In stage II, polymerase chain reaction (PCR) products generated from either genomic DNA or cDNA were amplified using a nested sense VH family-specific FR3 primer and a radiolabeled antisense nested JH consensus primer that had been end-labeled with γ32-P. The radiolabeled PCR products were electrophoresed through a 6% denaturing acrylamide sequencing gel, and then exposed to photographic film overnight. See text (Materials and methods) for further details. *Areas of coding end processing at the D-JH and VH-DJH junctions. The VH fingerprinting results displayed were derived with the cDNA-based assay.
Stage I
Genomic DNA (100ng) was amplified using a sense VH family-specific framework region (FR)1 primer in conjunction with an antisense JH consensus primer. These reactions were carried out in 50 μ l using 5 pmol of each primer, and were cycled with a 9600 GeneAmp System (Perkin Elmer, Emeryville, CA, USA) as follows: denaturation at 94°C for 40 s; annealing at 65°C for 45 s; and extension at 72°C for 40 s. After 35 cycles, extension was continued at 72°C for an additional 10 min.
cDNA (2μl) was amplified using a sense VH family-specific FR1 primer in conjunction with the appropriate antisense CH primer. The reactions were carried out in 50 μl using 5 pmol of each primer and cycled as follows: denaturation at 94°C for 45 s; annealing at 65°C for 45 s; and extension at 72°C for 45 s. After 35 cycles, extension was continued at 72°C for an additional 10 min.
Stage II
Polymerase chain reaction (PCR) products (2 μ l) generated from either genomic DNA or cDNA were amplified using 5 pmol of nested sense VH family-specific FR3 primer and radiolabeled antisense nested JH consensus primer that had been end-labeled with γ32-P (New England Nuclear, Beverly, MA, USA) using T4 polynucleotide kinase (Promega, Madison, WI, USA). The reactions were carried out in 25 μ l and cycled as follows: denaturation at 94°C for 30 s; annealing at 52°C for 45 s; and extension at 72°C for 30 s. After 15 cycles, extension was continued at 72°C for an additional 10 min. The radiolabeled PCR products that reflected the HCDR3 lengths of various B-cell clones in the cell suspension were electrophoresed through a 6% denaturing acrylamide sequencing gel for approximately 1.5 h. The gel was then dried and exposed to film overnight.
DNA cloning and sequencing
DNA sequences were determined by reamplifying the original genomic DNA using the appropriate family-specific VH leader and JH consensus primers under the following PCR conditions: denaturation at 94°C for 45s; annealing at 62°C for 30s; and extension at 72°C for 45s. After 35 cycles, extension was continued at 72°C for an additional 10min. PCR products were then cloned into TA vector (Invitrogen, San Diego, CA, USA), processed using Wizard minipreps (Promega), and sequenced using M13 forward and reverse primers, a DNA Sequencing Kit (Perkin Elmer) and an automated sequenator (Applied Biosystems, Foster City, CA, USA).
Results and discussion
Identification of B-cell clonal expansions using a modified immunoglobulin VH gene fingerprinting assay
During normal B cell development, the processes of gene segment recombination and coding end processing yield nucleotide HCDR3 lengths that are characteristic and virtually invariant for an individual B-cell clone. Therefore, these lengths can be used as signatures to identify members of a B cell clone. The immunoglobulin VH gene fingerprinting approach [23] takes advantage of the wide range of HCDR3 lengths that can occur in human B cells (approximately 5-35 amino acids) to provide an estimate of clonal diversity in polyclonal populations. When polyclonal B lymphocytes from adults are analyzed using this assay, they display a Gaussian HCDR3 length distribution around a mean of approximately 15 amino acids. The presence of an individual dominant length that differs from this Gaussian distribution can be used as an indication of a specific B-cell clonal expansion.
Figure 1 illustrates schematically the two-stage immunoglobulin VH gene fingerprinting approach that we utilized. Note that when cDNA prepared from normal peripheral blood B cells is used as a template for these VH family-specific and CH-specific assays, ladders of HCDR3 lengths that differ by three nucleotides are identified. These individual HCDR3 lengths are signatures of the various individual B-cell clones contained within the polyclonal population. The intensities of the bands in virtually all of the ladders illustrated in Figure 1 are relatively uniformly distributed around the mean. This indicates that there are no dominant HCDR3 lengths that skew the Gaussian distribution, and therefore that there are no significant clonal expansions among the B cells that express most of these VH–CH combinations. Similar results are obtained using the genomic DNA-based assay, although these results cannot be interpreted in a CH-specific manner (data not shown).
In the VH6-IgG combination (Fig. 1), however, a non-Gaussian distribution is noted, even in this normal individual. This could be a reflection of the numbers of VH genes present in the VH family being analyzed (it is more likely to see a non-Gaussian distribution in families with small numbers of individual genes) or of the state of activation of a specific clone (because activated B cells contain much higher levels of V gene messenger RNA than resting B cells). We believe that in the instance illustrated in Figure 1 the latter possibility is more likely, because the small VH2 and VH5 families (only two gene members per family) do not exhibit the same degree of oligoclonality as that observed with the only somewhat smaller VH6 family (one gene member).
Distinction between clonal expansion and clonal activation using the modified immunoglobulin VH gene fingerprinting assay
Because B-cell activation and differentiation result in dramatic increases in immunoglobulin V gene messenger RNA, these fingerprinting assays cannot readily distinguish between clonal expansion and activation when cDNA is used as a starting template. Because DNA levels are not appreciably altered by cellular activation, however, the use of genomic DNA as well as cDNA from the same sample of B cells helps to distinguish these two processes.
Thus, in the setting of specific B-cell clonal expansion without concomitant cellular activation, the DNA-based fingerprinting assay will indicate a dominant HCDR3 length, whereas the cDNA-based assay may not (data not shown). Conversely, in the setting of specific B-cell clonal activation without concomitant clonal expansion, the cDNA-based assay will indicate a dominant HCDR3 length, whereas the DNA-based assay may not. Finally, in the setting of specific B-cell clonal activation with concomitant clonal expansion, both the cDNA- and the DNA-based assays will indicate a dominant HCDR3 length.
These distinctions were very reproducible in the following studies. There were no situations in which evidence for cellular activation (either selective or accompanied by clonal expansion) was present in one set of analyses and not in a subsequent set using the same starting materials.
B cells in the blood, synovial fluid, and synovial tissue of rheumatoid arthritis patients exhibit clonal expansions of activated and resting B cells
We analyzed the peripheral blood, synovial fluid, and synovial tissue B cells of rheumatoid arthritis patients (n = 20, 10, and 5, respectively) using the genomic DNA- and cDNA-based fingerprinting assays to develop an understanding of the diversity of the B cells in these compartments. Figures 2 and 3 are illustrations of representative patients for whom concomitant blood and synovial fluid or blood and synovial tissue samples were available. In order to simplify the Figures, only the results for two large VH families (VH1 and VH3) and two small VH families (VH5 and VH6) are provided, although assays for each VH family and each major CH family (μ,γ, and α) were performed and revealed similar findings.
Figure 2.
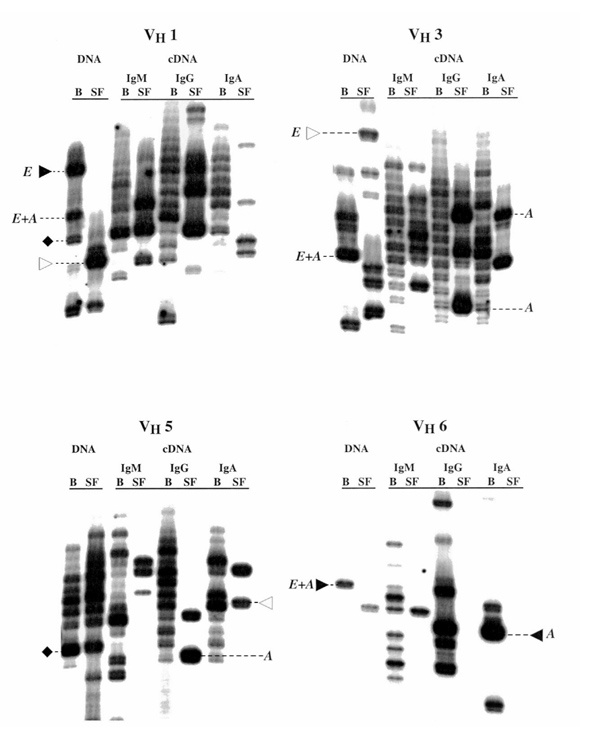
Analyses of paired samples of blood (B) and synovial fluid (SF) B cells from the same rheumatoid arthritis patient. Results using both the genomic DNA-based assay and the complementary (c)DNA-based assay for the three major immunoglobulin isotypes (M, G, and A) are shown. Note that certain clones, as represented by individual HCDR3 lengths, are restricted to the blood (▸), others to the joint (▹), and others are common to both compartments (♦). E, expanded clone; A, activated clone; E+A, same clone that is both expanded and activated.
Figure 3.
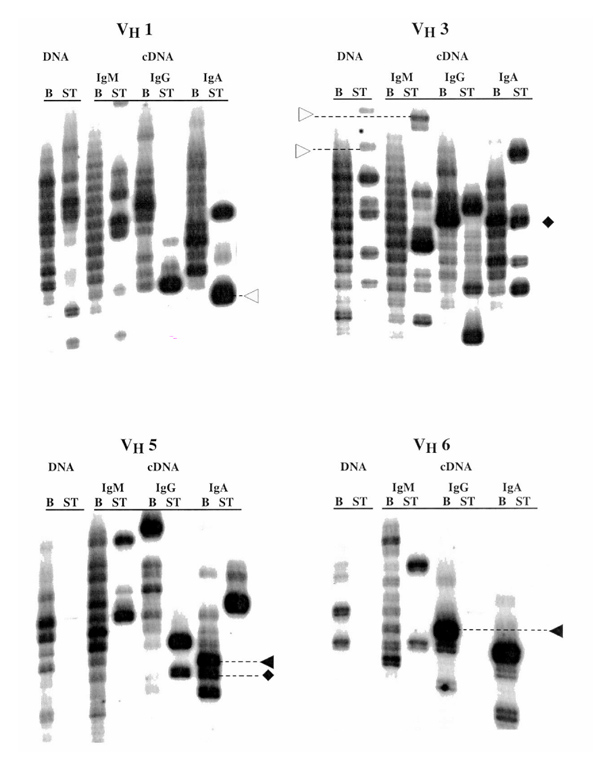
Analyses of paired samples of blood (B) and synovial tissue (ST) B cells from the same rheumatoid arthritis patient. Results using both the genomic DNA-based assay and the complementary (c)DNA-based assay for the three major immunoglobulin isotypes (M, G, and A) are shown. Certain clones are restricted to the blood (▸), others to the joint (▹), and others are common to both compartments (♦).
The genomic DNA-based assays in both patients indicated that clonal expansions are common in the blood of rheumatoid arthritis patients. This type of result was obtained with all individuals tested. It was most convincingly demonstrated by the results in Figure 3 obtained from B cells expressing genes of the VH1 and VH3 families. Because these VH families contain the largest numbers of VH genes, they would be more likely to display a polyclonal pattern.
An even more striking level of B-cell clonal dominance and expansion was seen when the genomic DNA-based assay was used to analyze B cells from the synovial fluid or synovial tissue (Figs 2 and 3). In these analyses, virtually all VH families demonstrated extensive B-cell oligoclonality. It should be pointed out that when an individual HCDR3 length comprises more than 50% of the radioactive counts of a VH–CH ladder, clonality, based on DNA sequencing, is very likely; when an individual length comprises more than 70% of the radioactivity, clonality is virtually assured (data not shown).
Collectively, these data indicate that the B-cell repertoire of rheumatoid arthritis patients is skewed away from the typical, apparently random representation of normal individuals. The reason for this discrepancy is not clear, although one possibility is that restricted antigenic exposure alters the composition of the repertoire in favor of B cells reactive with the putative antigen(s). If this is so, the progressive narrowing of the repertoire from the blood to the synovial tissue is consistent with the ideas that the antigenic exposures are originating at these sites and that the synovial compartment is supporting clonal amplification. Because these are true clonal expansions (ie increased numbers of B cells per specific clone), it is likely that the antigenic exposures are chronic and therefore are increasing the numbers of memory B cells reactive with these determinants.
In order to confirm that these clonally expanded B cells were receiving ongoing antigenic stimulation and not limited solely to the memory compartment, we employed the cDNA-based assay to distinguish clonal expansions of activated B cells from resting (memory) cells. As illustrated in Figure 2, activated B-cell clones (identified by the letter 'A' in Fig. 2) expressing each of the immunoglobulin heavy-chain isotypes were easily identified in all the VH families studied. In some instances, these activated clones were also expanded numerically (as defined by the genomic DNA-based assays, and identified by the letter 'E' in Fig. 2). In other cases, these activated clones did not appear to be numerically expanded. Similar examples can be found in Figures 3 and 4, but they are not identified by letters in order to simplify the Figures.
Figure 4.

Analyses of B cells from paired samples of blood (B) and synovial tissue from both the right (R) and left (L) hips of the same rheumatoid arthritis patient. Results using both the genomic DNA-based assay and the complementary (c)DNA-based assay for the three major immunoglobulin isotypes (M, G, and A) are shown. R, clones that are restricted to the right hip joint; L, clones that are restricted to the left hip joint; R+L, clones that are common to both the right and left hip joints.
Thus, it appears that many discrete B-cell clones exist in the synovial compartment of rheumatoid arthritis patients, and that these are increased in number, consistent with a response to a restricted antigenic challenge(s). Furthermore, these B-cell clones appear to be of both the resting (memory) and the activated types, suggesting that these antigenic challenges are chronic and ongoing. Thus, these data support and extend previous findings [18,19,20,21,22] by indicating that specific B-cell clonal amplifications can occur both in previously stimulated B cells and in currently activated B cells. The presence of activated B cells that are not increased in number is consistent either with recent in situ synovium-specific stimulation of these B-cell clones, or with the influx of activated B cells that were stimulated by antigens outside of and not necessarily relevant to the synovial compartment.
B-cell clonal expansions in the blood and synovial compartments can be restricted to one or another compartment, or be common to the two
Because the preceding data indicated that the B-cell repertoire of rheumatoid arthritis patients contains expanded clones of B cells that can be resting or activated, we investigated whether the same clones could be identified in both the blood and synovial compartments. Figures 2 and 3 illustrate data that suggest that there are expanded clones that are blood restricted, joint restricted, or are common to both compartments. Examples of blood-restricted clones are highlighted on Figures 2 and 3 with the ▸ symbol, those that are joint-restricted with the ▹ symbol, and those that are common to the two compartments with the ♦ symbol. These findings suggest that there may be a degree of cellular trafficking between the blood and the synovial tissues.
In order to address this issue, we studied the B cells of the blood and two synovial sites (right and left hip) that were obtained from the same patient within 3 h of each other (Fig. 4). In this patient, the DNA-based assay provided examples of clonal expansions that were present in only one joint (eg the VH3–JH and VH5–JH combinations in Figure 4), and the companion cDNA-based assays indicated that in some instances these expansions were either activated or resting. In only rare instances, however, did the data suggest that a similar clone was present in two different synovial tissues. DNA sequence analyses confirmed the rarity of this event (data not shown). Thus it appears that in most instances the clonal amplifications occur in situ and are not the result of trafficking from one anatomic site to another. If so, this would suggest that the antigenic challenges driving these clonal expansions may not be common to all synovial tissues, but may be generated independently at each site, possibly by ongoing tissue breakdown.
Clonal persistence in the synovial fluid compartment
If the clonal expansions identified in the joints of rheumatoid arthritis patients are due to an ongoing response to antigen, then one would predict that at least some of the clones would persist over time. To test this, we studied the synovial fluid B cells from the same joints of three patients on two occasions spanning several months. Most of the clonal expansions detected on the initial samples were not present in the subsequent samples. In a few instances, however, B-cell clonal persistence was found.
Figure 5 illustrates the best example of this phenomenon in a patient who was studied over a 4-month interval. The DNA-based assay using VH4 family-specific primers indicated the presence of two similar clones on days 0 and 120, whereas the other VH4-expressing clones detected at the first analysis were no longer present at the time of the second analysis. DNA sequence analyses of one of these two clones confirmed their identity, because each displayed the same rearranged VHDJH gene with identical VH mutations and identical HCDR3 sequences (data not shown). Therefore, certain clones can persist locally over time, suggesting that a common and persistent antigenic stimulation was operable in the joint of this rheumatoid arthritis patient.
Figure 5.
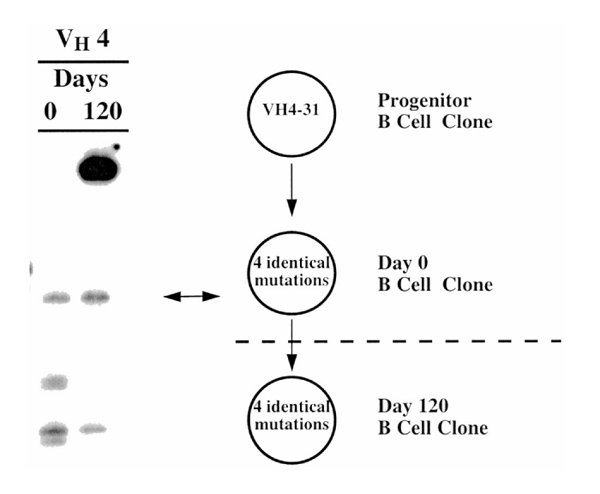
Persistence of a synovial fluid B cell clone in the same joint for 4 months. Arthrocentesis was performed on the same joint of the same patient on days 0 and 120. One of the B-cell clones identified in each of these samples was identical in both HCDR3 length and in VHDJH gene DNA sequence.
The lack of persistence of the other B-cell clones suggests two possibilities. First, the initial set of B-cell clones might have been replaced by others that recognized and responded to different antigenic epitopes on the same original immunogenic protein. This type of clonal evolution to the recognition of different epitopes on the same immunogenic moiety is common in experimental situations in which repetitive immunizations with a defined antigen are delivered [29,30,31]. The other possibility is that the B cells that disappeared over time were not reactive with tissue antigens. These could have been stimulated by irrelevant antigens in the periphery and therefore, after entering the synovial compartment, could not be restimulated and hence could not enter the memory pool and take up residence in the synovial tissue.
Conclusion
The present data indicate that clonal expansion is a common occurrence in the B-cell repertoire of rheumatoid arthritis patients. These expansions involve both resting memory B cells and activated B cells, some of which are derived from the memory B-cell compartment. Because the extent of these clonal expansions increases from the blood to the synovial compartment, this progressive narrowing in diversity implies that antigens located in the synovia are responsible for these antigen-receptor biases. In support of this hypothesis are the observations that some of these clonal expansions are joint specific. Because identical clones are rarely found in two different joints, however, these immune reactions are probably unique to each individual joint. Furthermore, because it is unlikely that each joint would harbor a different foreign antigen, these B cells are most likely reacting with autoantigens generated locally, possibly by local tissue breakdown.
Recent studies [20,32] have demonstrated that the synovial tissue of rheumatoid arthritis patients can develop lymphoid aggregates that have the cellular components of an ectopic germinal center and that can sustain B-cell clonal expansion and diversification. It is likely that the B cells that mature in these 'pseudogerminal centers' and those that we have identified in the present studies are responding to specific (auto)antigens. Therefore, the identification of the antigenic reactivities of these B cells, and in particular those B cells within the memory compartment that have presumably traversed the pseudogerminal centers and undergone (auto)antigen and T cell selection and rescue, may provide important clues to the role of B lymphocytes and their immunoglobulin molecules in the immunopathogenesis of rheumatoid arthritis.
References
- Harris ED., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990;322:1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- Firestein GS, Zvaifler NJ. How important are T cells in chronic rheumatoid synovitis? Arthritis Rheum. 1990;33:768–773. doi: 10.1002/art.1780330602. [DOI] [PubMed] [Google Scholar]
- Hingorani R, Choi IH, Akolkar P, et al. Clonal predominance of T cell receptors within the CD8+ CD45RO+ subset in normal human subjects. J Immunol. 1993;151:5762–5769. [PubMed] [Google Scholar]
- Goronzy JJ, Bartz-Bazzanella P, Hu W, et al. Dominant clonotypes in the repertoire of peripheral CD4+ T cells in rheumatoid arthritis. J Clin Invest. 1994;94:2068–2076. doi: 10.1172/JCI117561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7- CD28- T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996;97:2027–2037. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenkovic I, Stegagno M, Wright KA, et al. Clonal dominance among T-lymphocyte infiltrates in arthritis. Proc Natl Acad Sci USA. 1988;85:1179–1183. doi: 10.1073/pnas.85.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keystone EC, Minden M, Klock R, et al. Structure of T cell antigen receptor beta chain in synovial fluid cells from patients with rheumatoid arthritis. Arthritis Rheum. 1988;31:1555–1557. doi: 10.1002/art.1780311213. [DOI] [PubMed] [Google Scholar]
- Duby AD, Sinclair AK, Osborne-Lawrence SL, et al. Clonal heterogeneity of synovial fluid T lymphocytes from patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 1989;86:6206–6210. doi: 10.1073/pnas.86.16.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliard X, West SG, Lafferty JA, et al. Evidence for the effects of a superantigen in rheumatoid arthritis. Science. 1991;253:325–329. doi: 10.1126/science.1857971. [DOI] [PubMed] [Google Scholar]
- Li Y, Sun GR, Tumang JR, Crow MK, Friedman SM. CDR3 sequence motifs shared by oligoclonal rheumatoid arthritis synovial T cells. Evidence for an antigen-driven response. J Clin Invest. 1994;94:2525–2531. doi: 10.1172/JCI117624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani R, Monteiro J, Furie R, et al. Oligoclonality of V beta 3 TCR chains in the CD8+ T cell population of rheumatoid arthritis patients. J Immunol. 1996;156:852–858. [PubMed] [Google Scholar]
- Fox DA, Smith BR. Evidence for oligoclonal B cell expansion in the peripheral blood of patients with rheumatoid arthritis. Ann Rheum Dis. 1986;45:991–995. doi: 10.1136/ard.45.12.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WL, Goldberg MS, Smiley JD. Immunoglobulin G3 subclass production by rheumatoid synovial tissue cultures. J Clin Invest. 1982;69:136–144. doi: 10.1172/JCI110424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WL, Jump AA, Smiley JD. Synthesis of specific IgG idiotypes by rheumatoid synovium. Arthritis Rheum. 1990;33:1196–1204. doi: 10.1002/art.1780330821. [DOI] [PubMed] [Google Scholar]
- Smiley JD, Hoffman WL, Moore SE, Paradies LH. The humoral immune response of the rheumatoid synovium. Semin Arthritis Rheum. 1985;14:151–162. doi: 10.1016/0049-0172(85)90034-4. [DOI] [PubMed] [Google Scholar]
- Wernick RM, Lipsky PE, Marban-Arcos E, et al. IgG and IgM rheumatoid factor synthesis in rheumatoid synovial membrane cell cultures. Arthritis Rheum. 1985;28:742–752. doi: 10.1002/art.1780280704. [DOI] [PubMed] [Google Scholar]
- Bridges SL, Jr, Lee SK, Koopman WJ, Schroeder HW., Jr Analysis of immunoglobulin gamma heavy chain expression in synovial tissue of a patient with rheumatoid arthritis. Arthritis Rheum. 1993;36:631–641. [PubMed] [Google Scholar]
- Bridges SL, Jr, Clausen BE, Lavelle JC, et al. Analysis of immunoglobulin gamma heavy chains from rheumatoid arthritis synovium. Evidence of antigen-driven selection. Ann N Y Acad Sci. 1995;764:450–452. doi: 10.1111/j.1749-6632.1995.tb55862.x. [DOI] [PubMed] [Google Scholar]
- Schroder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 1996;93:221–225. doi: 10.1073/pnas.93.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges SL, Jr, Lavelle JC, Lee SK, Byer S, Schroeder HW., Jr CDR3 fingerprinting of immunoglobulin kappa light chains expressed in rheumatoid arthritis. Evidence of antigenic selection or dysregulation of gene rearrangement in B cells. Ann N Y Acad Sci. 1997;815:423–426. doi: 10.1111/j.1749-6632.1997.tb52093.x. [DOI] [PubMed] [Google Scholar]
- Berek C, Kim HJ. B-cell activation and development within chronically inflamed synovium in rheumatoid and reactive arthritis. Semin Immunol. 1997;9:261–268. doi: 10.1006/smim.1997.0076. [DOI] [PubMed] [Google Scholar]
- Deane M, Norton JD. Immunoglobulin gene 'fingerprinting': an approach to analysis of B lymphoid clonality in lymphoproliferative disorders. Br J Haematol. 1991;77:274–281. doi: 10.1111/j.1365-2141.1991.tb08570.x. [DOI] [PubMed] [Google Scholar]
- Deane M, Norton JD. Immunoglobulin heavy chain variable region family usage is independent of tumor cell phenotype in human B lineage leukemias. Eur J Immunol. 1990;20:2209–2217. doi: 10.1002/eji.1830201009. [DOI] [PubMed] [Google Scholar]
- Dono M, Hashimoto S, Fais F, et al. Evidence for progenitors of chronic lymphocytic leukemia B cells that undergo intraclonal differentiation and diversification. Blood. 1996;87:1586–1594. [PubMed] [Google Scholar]
- Fais F, Sellars B, Ghiotto F, et al. Examples of in vivo isotype class switching in IgM+ chronic lymphocytic leukemia B cells. . J Clin Invest. 1996;98:1659–1666. doi: 10.1172/JCI118961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Fais F, Ghiotto F, Hashimoto S, et al. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J Clin Invest. 1998;102:1515–1525. doi: 10.1172/JCI3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollogly JR, Cathou RE. Sequential appearance of three different anti-fluorescein combining sites in hyperimmunized rabbits: characterization by circular dichroism and binding studies. J Immunol. 1974;113:1457–1467. [PubMed] [Google Scholar]
- Berek C, Griffiths GM, Milstein C. Molecular events during maturation of the immune response to oxazolone. Nature. 1985;316:412–418. doi: 10.1038/316412a0. [DOI] [PubMed] [Google Scholar]
- Gaya A, Nieto A, Moreno C, Vives J. Affinity maturation in the arsonate system: lack of dominance of high-affinity antibody subpopulations. Immunology. 1986;58:541–544. [PMC free article] [PubMed] [Google Scholar]
- Randen I, Mellbye OJ, Forre O, Natvig JB. The identification of germinal centres and follicular dendritic cell networks in rheumatoid synovial tissue. Scand J Immunol. 1995;41:481–486. doi: 10.1111/j.1365-3083.1995.tb03596.x. [DOI] [PubMed] [Google Scholar]


