Short abstract
CD8+ T cells dominate the lymphocyte population in synovial fluid in chronic inflammatory arthritis. It is known that these CD8+ T cells are often clonally or oligoclonally expanded, but their specificity and their relevance to the pathogenesis of joint disease has remained unclear. We found that as many as 15.5% of synovial CD8+ T cells may be specific for a single epitope from an Epstein-Barr virus lytic cycle protein. The virus-specific T cells within the joint showed increased expression of markers of activation and differentiation compared with those in the periphery, and retained their functional capacity to secrete proinflammatory cytokines on stimulation. These activated, virus-specific CD8+ T cells could therefore interact with synoviocytes, either by cell-cell contact or by a cytokine network, and play a 'bystander' role in the maintenance of inflammation in patients with arthritis.
Keywords: CD8+ T cell, Epstein-Barr virus lytic cycle, human leucocyte antigen peptide tetrameric complex, rheumatoid arthritis, viral immunity
Abstract
Introduction:
Epstein-Barr virus (EBV) is transmitted orally, replicates in the oropharynx and establishes life-long latency in human B lymphocytes. T-cell responses to latent and lytic/replicative cycle proteins are readily detectable in peripheral blood from healthy EBV-seropositive individuals. EBV has also been detected within synovial tissue, and T-cell responses to EBV lytic proteins have been reported in synovial fluid from a patient with rheumatoid arthritis (RA). This raises the question regarding whether T cells specific for certain viruses might be present at high frequencies within synovial fluid and whether such T cells might be activated or able to secrete cytokines. If so, they might play a 'bystander' role in the pathogenesis of inflammatory joint disease.
Objectives:
To quantify and characterize T cells that are specific for epitopes from EBV, cytomegalovirus (CMV) and influenza in peripheral blood and synovial fluid from patients with arthritis.
Methods:
Peripheral blood mononuclear cells (PBMCs) and synovial fluid mononuclear cells (SFMCs) were obtained from patients with inflammatory arthritis (including those with RA, osteoarthritis, psoriatic arthritis and reactive arthritis). Samples from human leucocyte antigen (HLA)-A2-positive donors were stained with fluorescent-labelled tetramers of HLA-A2 complexed with the GLCTLVAML peptide epitope from the EBV lytic cycle protein BMLF1, the GILGFVFTL peptide epitope from the influenza A matrix protein, or the NLVPMVATV epitope from the CMV pp65 protein. Samples from HLA-B8-positive donors were stained with fluorescent-labelled tetramers of HLA-B8 complexed with the RAKFKQLL peptide epitope from the EBV lytic protein BZLF1 or the FLRGRAYGL peptide epitope from the EBV latent protein EBNA3A. All samples were costained with an antibody specific for CD8. CD4+ T cells were not analyzed. Selected samples were costained with antibodies specific for cell-surface glycoproteins, in order to determine the phenotype of the T cells within the joint and the periphery. Functional assays to detect release of IFN-γ or tumour necrosis factor (TNF)-α were also performed on some samples.
Results:
The first group of 15 patients included 10 patients with RA, one patient with reactive arthritis, one patient with psoriatic arthritis and three patients with osteoarthritis. Of these, 11 were HLA-A2 positive and five were HLA-B8 positive. We used HLA-peptide tetrameric complexes to analyze the frequency of EBV-specific T cells in PBMCs and SFMCs (Figs 1 and 2). Clear enrichment of CD8+ T cells specific for epitopes from the EBV lytic cycle proteins was seen within synovial fluid from almost all donors studied, including patients with psoriatic arthritis and osteoarthritis and those with RA. In donor RhA6, 9.5% of CD8+ SFMCs were specific for the HLA-A2 restricted GLCTLVAML epitope, compared with 0.5% of CD8+ PBMCs. Likewise in a donor with osteoarthritis (NR4), 15.5% of CD8+ SFMCs were specific for the HLA-B8-restricted RAKFKQLL epitope, compared with 0.4% of CD8+ PBMCs. In contrast, we did not find enrichment of T cells specific for the HLA-B8-restricted FLRGRAYGL epitope (from the latent protein EBNA3A) within SFMCs compared with PBMCs in any donors. In selected individuals we performed ELISpot assays to detect IFN-γ secreted by SFMCs and PBMCs after a short incubation in vitro with peptide epitopes from EBV lytic proteins. These assays confirmed enrichment of T cells specific for epitopes from EBV lytic proteins within synovial fluid and showed that subpopulations of these cells were able to secrete proinflammatory cytokines after short-term stimulation.
We used a HLA-A2/GILGFVFTL tetramer to stain PBMCs and SFMCs from six HLA-A2-positive patients. The proportion of T cells specific for this influenza epitope was low (<0.2%) in all donors studied, and we did not find any enrichment within SFMCs.
We had access to SFMCs only from a second group of four HLA-A2-positive patients with RA. A tetramer of HLA-A2 complexed to the NLVPMVATV epitope from the CMV pp65 protein reacted with subpopulations of CD8+ SFMCs in all four donors, with frequencies of 0.2, 0.5, 2.3 and 13.9%. SFMCs from all four donors secreted TNF after short-term incubation with COS cells transfected with HLA-A2 and pp65 complementary DNA. We analyzed the phenotype of virus-specific cells within PBMCs and SFMCs in three donors. The SFMC virus-specific T cells were more highly activated than those in PBMCs, as evidenced by expression of high levels of CD69 and HLA-DR. A greater proportion of SFMCs were CD38+, CD62L low, CD45RO bright, CD45RA dim, CD57+ and CD28- when compared with PBMCs.
Discussion:
This work shows that T cells specific for certain epitopes from viral proteins are present at very high frequencies (up to 15.5% of CD8+ T cells) within SFMCs taken from patients with inflammatory joint disease. This enrichment does not reflect a generalized enrichment for the 'memory pool' of T cells; we did not find enrichment of T cells specific for the GILGFVFTL epitope from influenza A or for the FLRGRAYGL epitope from the EBV latent protein EBNA3A, whereas we found clear enrichment of T cells specific for the GLCTLVAML epitope from the EBV lytic protein BMLF1 and for the RAKFKQLL epitope from the EBV lytic protein BZLF1.
The enrichment might reflect preferential recruitment of subpopulations of virus-specific T cells, perhaps based on expression of selectins, chemokine receptors or integrins. Alternatively, T cells specific for certain viral epitopes may be stimulated to proliferate within the joint, by viral antigens themselves or by cross-reactive self-antigens. Finally, it is theoretically possible that subpopulations of T cells within the joint are preferentially protected from apoptotic cell death. Whatever the explanation, the virus-specific T cells are present at high frequency, are activated and are able to secrete proinflammatory cytokines. They could potentially interact with synoviocytes and contribute to the maintenance of inflammation within joints in many different forms of inflammatory arthritis.
Introduction
Epstein-Barr virus (EBV) is a common gammaherpesvirus that infects over 90% of individuals worldwide [1]. The virus is transmitted orally, replicates in the oropharynx and subsequently establishes life-long latent infection of B lymphocytes [2]. Replicative infection is associated with the expression of approximately 70EBV 'lytic' proteins, whereas latent infection is associated with the expression of up to nine 'latent' proteins [3,4]. The cellular immune response plays a crucial role in controlling EBV infection. T-cell responses to EBV latent proteins have been well studied and are easily detectable in healthy EBV-seropositive individuals [5,6,7]. More recently, responses to some of the EBV lytic cycle proteins have also been described [7,8,9,10]; reactivities to these antigens are frequently found to be more abundant than those directed against the EBV latent antigens in healthy EBV carriers [7,9,10].
An association between EBV and rheumatoid arthritis (RA) was first proposed on the basis of high titres of EBV-specific antibodies found in some patients with RA [11,12]. The observation that some of the anti-EBV antibodies were cross-reactive with autoantigens such as collagen was put forward as a further argument in support of the link [13]. The importance of these findings in the pathophysiology of rheumatoid arthritis has never been established, however. More recently the cloning of T cells that are specific for EBV antigens from synovial fluid taken from a patient with RA [14] has reopened the debate regarding the importance of this virus in the aetiology of arthritis. David-Ameline et al [15] showed that a large proportion of T-cell clones derived from synovial fluid taken from one individual with RA, under polyclonal activation conditions, recognized EBV-transformed lymphoblastoid cell lines in an human leucocyte antigen (HLA)-restricted manner. Subsequent work revealed that these T-cell clones recognized epitopes from EBV lytic cycle proteins [14]. Analysis of the T-cell receptor use of the EBV-specific T cell clones and of the T-cell receptor repertoire of synovial fluid lymphocytes [14,15] suggested that the EBV-specific T cells were clonally expanded within the synovial fluid from this donor. In some other donors, short-term T-cell lines derived from synovial fluid lymphocytes, but not those derived from peripheral blood lymphocytes, secreted low levels of tumour necrosis factor (TNF) in response to stimulation with an EBV antigen [14,16]. These results suggested that EBV-specific T cells might form a component of joint infiltrating lymphocytes in patients with RA.
In the present study we used tetrameric HLA-peptide complexes [17] to investigate the T-cell response to EBV, cytomegalovirus (CMV) and influenza in individuals with inflammatory arthritis. We made tetramers of HLA molecules complexed to peptide epitopes from EBV latent and lytic proteins [7,10], to an epitope from a CMV structural protein [18] and to an epitope from influenza A matrix protein [19]. We used these tetramers to quantify and characterize virus-specific CD8+ T cells within samples of peripheral blood and synovial fluid taken from patients with inflammatory arthritis. The experiments show that CD8+ T cells specific for certain viral antigens are enriched within synovial fluid. The enrichment could reflect recruitment into, stimulation within or preferential survival within inflamed joints. The virus-specific T cells are activated and subpopulations are able to secrete proinflammatory cytokines. These T cells could therefore interact with synoviocytes and play a role in the maintenance of inflammation in chronic arthritis.
Materials and methods
Patient samples
Samples of synovial fluid and peripheral venous blood were initially obtained from a group of 15 EBV-seropositive patients, 13 of whom were HLA-A2 positive and/or HLA-B8 positive. Ten patients (RhA1-RhA10) suffered from RA, one from reactive arthritis (NR1), one from psoriatic arthritis (NR2) and three from osteoarthritis (NR3-NR5). Details of these patients are given in Table 1. Peripheral blood mononuclear cells (PBMCs) and synovial fluid mononuclear cells (SFMCs) were isolated using Lymphoprep (Nycomed Pharma, Oslo, Norway) gradient centrifugation and cryopreserved in 10% dimethyl sulphoxide, 90% fetal calf syndrome at -70°C. Only synovial lymphocytes were available for analysis from a smaller second group of HLA-A2-positive RA patients (RA3, RA11, RA14 and RA15), whose clinical status has been described elsewhere [16].
Table 1.
Patient details
| Patients | HLA-A | HLA-B | Diagnosis | |
| Group 1 | RhA1 | 2, 29 | 44, 49 | Rheumatoid arthritis |
| RhA2 | 2, 29 | 39, 44 | Rheumatoid arthritis | |
| RhA3 | 2, 3 | 7, 62 | Rheumatoid arthritis | |
| RhA4 | 2, 31 | 7, 8 | Rheumatoid arthritis | |
| RhA5 | 1, 2 | 8, 62 | Rheumatoid arthritis | |
| RhA6 | 2 | - | Rheumatoid arthritis | |
| RhA7 | 1, 29 | 8, 44 | Rheumatoid arthritis | |
| RhA8 | 2, 30 | 44, 52 | Rheumatoid arthritis | |
| RhA9 | 2, 3 | 7, 35 | Rheumatoid arthritis | |
| RhA10 | 29, 32 | 40, 44 | Rheumatoid arthritis | |
| NR1 | 1,2 | 27,49 | Reactive arthritis | |
| NR2 | 2, 36 | 8, 60 | Psoriatic arthritis | |
| NR3 | 1, 2 | 7, 62 | Osteoarthritis | |
| NR4 | 1, 31 | 40, 52 | Osteoarthritis | |
| NR5 | - | 8 | Osteoarthritis | |
| Group 2 | RA3 | 1, 2 | 8, 44 | Rheumatoid arthritis |
| RA11 | 2 | 27, 44 | Rheumatoid arthritis | |
| RA14 | 2 | 15, 44 | Rheumatoid arthritis | |
| RA15 | 2, 11 | 8, 27 | Rheumatoid arthritis |
The HLA-B type of patient RhA6 and the HLA-A type of patient NR5 Were not determined.
Human leucocyte antigen typing
Genomic DNA was extracted from whole blood using a Puregene kit (Gentra systems, Minneapolis, MN, USA) according to the manufacturer's instructions. Each patient was tissue typed using polymerase chain reaction, as previously described [20]. HLA-A and HLA-B types are summarized in Table 1.
Tetrameric class I human leucocyte antigen-peptide complexes.
HLA-peptide tetramers were produced as previously described [7,10]. Briefly, recombinant class I heavy chain or β2-microglobulin was produced in Escherichia coli cells transformed with the relevant expression plasmids. Expression of the heavy chain was limited to the extracellular domain and the sequence of this domain was modified by the addition of a substrate sequence for BirA biotinylation at the carboxyl terminus. The HLA-peptide complexes were folded in vitro using 30mg heavy chain, 25mg β2-microglobulin and 10mg peptide, then biotinylated using purified BirA enzyme (Avidity, Denvery, CO, USA). The biotinylated complexes were recovered by fast performance liquid chromatography (FPLC) purification and ion exchange chromatography. Tetramers were made by mixing biotinylated protein complex with streptavidin-phycoerythrin (PE) at a molar ratio of 4:1. The three HLA-A2 tetrameric complexes synthesized for this study contained either the influenza A virus matrix peptide GILGFVFTL [19] (A2/FluM tetramer), the EBV BMLF1 peptide GLCTLVAML [9,14] (A2/GLC tetramer), or the CMV pp65 peptide NLVPMVATV [18] (A2/NLV tetramer). Two HLA-B8 tetramers were also made, with the EBV EBNA3A peptide FLRGRAYGL [21] (B8/FLR tetramer), or the EBV BZLF1 peptide RAKFKQLL [8,9] (B8/RAK tetramer).
ELISpot assay for IFN-γ release by stimulated T cells
This assay was used to detect IFN-γ production by T cells in fresh samples of PBMCs and SFMCs on antigen stimulation. Ninety-six-well polyvinylidene difluoride backed plates (Millipore, Watford, UK) were precoated with 15 μ g/ml of anti-IFN-γ monoclonal antibody 1-DIK(Mabtech, Stockholm, Sweden). PBMCs were added in duplicate wells at 2.5×105, 1.25×105 and 6.25×104 cells/well and incubated overnight at 37°C, 5% carbon dioxide with the exact 8-mer or 9-mer peptide antigen(2 μ mol/l final concentration). The cells were discarded the following day and the second biotinylated anti-IFN-γ mono-clonal antibody, 7-B6-1 biotin (Mabtech, Stockholm, Sweden), was added at 1 μ g/ml and left for 3h at room temperature, followed by streptavidin-conjugated alkaline phosphatase (Mabtech, Stockholm, Sweden) for a further 2h. Individual cytokine-producing cells were detected as dark spots after a 30-min reaction with 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium using a pre-mixed alkaline phosphatase conjugate substrate kit (Bio-Rad, Richmond, CA, USA). The spots were counted under a dissection microscope. The number of specific responders was calculated after subtracting negative control values.
COS transfections and T cell stimulation assay
COS cells were transfected with HLA-A2 and pp65 complementary DNA as previously described [16]. CD8+ SFMCs (104 and 105) were incubated with the transfected COS cells for 6h. The culture supernatant was harvested and tested for TNF-α content by measuring culture supernatant cytotoxicity against Wehi 164 clone 13 in a colorimetric assay as previously described [16].
Cell staining and flow cytometry
PBMCs were incubated for 30min in phosphate-buffered saline with 0.16% bovine serum albumin and 0.1% sodium azide, containing 0.5mg/ml phycoerythrin-labelled tetrameric complex, washed and then stained on ice with saturating amounts of an anti-CD8 monoclonal antibody directly conjugated to Tricolor (Caltag Laboratories, San Francisco, CA, USA). For phenotypic analysis, selected samples were also incubated with one of a panel of mono-clonal antibodies directed against cell-surface markers. This panel consisted of anti-CD28 fluorescein isothiocyanate (FITC) (Immunotech, Marseilles, France), anti-CD45RA FITC (Immunotech, Marseilles, France), anti-CD45RO FITC (DAKO, Glostrup, Denmark), anti-CD57 FITC (Becton-Dickinson, Mountain View, CA, USA), anti-CD62L FITC (Pharmingen, San Diego, CA, USA), anti-CD69 FITC (DAKO) and anti-HLA DR FITC (DAKO).
All samples were fixed in 2% formaldehyde and analyzed using a fluorescent antibody cell sorter using CELLQuest software (Becton-Dickinson). For two-colour analysis 50000 live cells were analyzed. For three-colour analysis 200000 live cells were analyzed. In each experiment the lymphocyte pool was identified using forward and side scatter analysis and markers were set to analyze the CD8hi subset of CD3+ T cells. CD4+ T-cell responses were not analyzed in the present study.
Results
Presence of T cells specific for Epstein-Barr virus in synovial fluid
In the first group of patients we used HLA-peptide tetrameric complexes to analyze the frequency of CD8+ T cells specific for two EBV lytic protein epitopes, the HLA-A2 restricted epitope (GLCTLVAML) from BMLF1 [9,14] and the HLA-B8 restricted epitope (RAKFKQLL) from BZLF1 [8,9], and for an EBV latent protein epitope, the HLA-B8 restricted epitope (FLRGRAYGL) from EBNA3A [21].
We initially studied 11 HLA-A2+ patients with inflammatory arthritis. In patient RhA6, 9.5% of CD8+ T cells within synovial fluid were specific for the GLCTLVAML epitope from BMLF1, whereas only 0.5% CD8+ T cells within peripheral blood were specific for this epitope (Figs 1a and 2a). Likewise in patient RhA5, T cells that were specific for the GLCTLVAML epitope were clearly enriched within synovial fluid (4.5% of CD8+ T cells) when compared with peripheral blood (1.0%; Figs 1b and 2a). In four of the six other HLA-A2+ individuals with RA (RhA2, RhA3, RhA8, RhA9), we also found higher frequencies of T cells specific for GLCTLVAML in synovial fluid than in peripheral blood (Fig. 2a). This finding was not restricted to patients with RA; we found enrichment of GLCTLVAML-specific T cells in synovial fluid taken from patients with psoriatic arthritis (NR2) and osteoarthritis (NR3; Fig. 2a).
Figure 1.

Staining of paired samples of peripheral blood and synovial fluid with human leucocyte antigen (HLA)-peptide tetrameric complexes. Paired samples of peripheral blood mononuclear cells (PBMCs; left column) and synovial fluid mononuclear cells (SFMCs; right column) from donors (a) RhA6, (b, c) RhA5 and (d) RhA7 were stained with a monoclonal antibody that was specific for CD8 and with (a, b) the A2/GLCTLVAML tetramer or (c, d) the B8/RAKFKQLL tetramer. The percentage frequency of CD8+ T cells that reacted with the tetrameric complexes is shown; 50 000 live cells were included in the analysis.
Figure 2.

T cells specific for the Epstein-Barr virus (EBV) lytic protein epitopes GLCTLVAML and RAKFKQLL are enriched within synovial fluid. Paired samples of peripheral blood mononuclear cells (PBMCs) and synovial fluid mononuclear cells (SFMCs) were stained with (a) the A2/GLCTLVAML tetramer or (b) the B8/RAKFKQLL tetramer together with a monoclonal antibodies specific for CD8. The percentage of CD8+ T cells that react with the relevant tetrameric complex is shown. L, left knee; R, right knee.
We identified five HLA-B8+ patients, three of whom suffered from RA (RhA4, RhA5, RhA7), one of whom had psoriatic arthritis (NR2) and one of whom had osteoarthritis (NR5). In patient RhA5, 13.1% CD8+ T cells within synovial fluid reacted with the B8-RAK tetrameric complex compared with 4.3% within peripheral blood (Figs 1c and 2b). Similarly in patient RhA7, we found that 7.3% of CD8+ T cells were specific for the RAKFKQLL epitope in synovial fluid compared with 0.9% CD8+ T cells within the periphery (Figs 1d and 2b). In the third RA patient, as well as in patients with psoriatic arthritis and osteoarthritis, the frequencies of T cells specific for the RAKFKQLL epitope were also much higher in synovial fluid than in peripheral blood (Fig. 2b).
T cells that were specific for the HLA-B8 restricted epitope (FLRGRAYGL) from the EBV latent protein EBNA3A were either undetectable or present at only very low frequencies (<0.1% of CD8+ T cells) in three of the five HLA-B8+ patients studied. In donor RhA7 we found FLRGRAYGL-specific T cells at a frequency of 0.5% CD8+ T cells in peripheral blood and 0.4% CD8+ T cells in synovial fluid. In a patient with osteoarthritis (NR4) we found FLRGRAYGL-specific T cells at a frequency of 0.4% in peripheral blood and 0.2% in synovial fluid. Thus, using this technique we did not find evidence for enrichment of T cells specific for this EBV latent epitope within synovial fluid of patients with inflammatory arthritis.
In general the frequencies of EBV-reactive T cells within peripheral blood of the patients studied were similar to those previously reported in healthy individuals [7] using this technique, despite the use of immunosuppressive drugs.
In selected individuals we performed ELISpot assays to detect IFN-γ secreted by SFMCs and PBMCs after a short incubation in vitro with peptide epitopes from EBV lytic proteins (Fig. 3). We calculated that the estimates of frequency of virus-specific T cells obtained with an ELISpot assay for IFN-γ secretion were a mean of 19.9-fold and 20.1-fold lower than those obtained with tetramer staining in peripheral blood and synovial fluid, respectively. We have previously shown that in healthy control individuals estimates of frequency of EBV-specific T cells obtained with the ELISpot assay are a mean of 4.4-fold lower than those obtained by staining with tetrameric complexes [7]. Thus, the ability of the virus-specific T cells to secrete IFN-γ appeared to be impaired both in peripheral blood and synovial fluid in the present patient group. The correlation between the numbers of T cells that were detected using an ELISpot assay versus tetramer staining was relatively poor in this group of patients, suggesting interindividual and intersite differences in the functional capacity of the antigen-specific T cells. Despite these limitations, the results of the assays showed enrichment for T cells that were specific for epitopes from EBV lytic proteins within synovial fluid, and showed that subpopulations of these cells are able to secrete proinflammatory cytokines after short-term stimulation.
Figure 3.

Frequency of IFN-γ secreting antigen-specific T cells detected using an ELISpot assay. ELISpot assays to detect IFN-γ secreted by T cells following short-term incubation with (a) the GLCTLVAML peptide or (b) the RAKFKQLL peptide were performed on paired samples of peripheral blood mononuclear cells (PBMCs) and synovial fluid mononuclear cells (SFMCs). The number of spots detected per 106 mononuclear cells is shown.
T cells specific for cytomegalovirus may also be present at high frequencies within synovial fluid
In light of recent results that suggest increased responses of synovial lymphocytes from some RA patients to CMV antigens derived from pp65 and IE1 proteins [16], we also assessed the number of SFMCs specific for a CMV epitope in a second group of four patients. HLA-A2 tetramers carrying a CMV-derived epitope (pp65 NLVPMVATV) were made and used to stain CD8+ synovial fluid lymphocytes from four HLA-A2+ RA patients (RA3, RA11, RA14 and RA15). Paired samples of peripheral blood from these donors were not available for comparative analysis. The frequency of A2/NLV-reactive cells varied greatly from one patient to another, but reached up to 13.9% in one patient (RA15; Table 2). Consistent with these findings, CD8+ SFMCs from these donors secreted TNF-α after short-term incubation with COS cells transfected with HLA-A2 and pp65 complementary DNA (Table 2) [16].
Table 2.
Frequency of, and tumour necrosis factor secretion by, Synovial T cells specific for cytomegalovirus
| TNF response (pg/ml) | |||
| Patient | HLA-A2-NLV+/CD8+ T cells (%) | 104 | 105 |
| RA3 | 0.2 | 3 | 45 |
| RA11 | 2.3 | 21 | 102 |
| RA14 | 0.5 | 10 | 61 |
| RA15 | 13.9 | 60 | 70 |
Tumour necrosis factor (TNF) response was estimated by titrating the mononuclear cells after short-term incubation with COS cells transfected with human leucocyte antigen (HLA)-A2 and pp65 complementary DNA, as described by Scotet et al [16].
T cells specific for influenza A are not present at high frequencies within synovial fluid
We used a tetramer of HLA-A2 complexed with the GILGFVFTL epitope from influenza A matrix protein to stain paired samples of peripheral blood and synovial fluid from six HLA-A2 patients, four of whom had RA, one of whom had osteoarthritis and one of whom had reactive arthritis. Influenza-specific CD8+ T cells were undetectable in both PBMC and SFMC preparations in four donors. The frequencies of the influenza-specific CD8+ T cells in the other two donors were very low (<0.2%) in peripheral blood and showed no enrichment within synovial fluid.
Phenotype of Epstein-Barr virus-specific T lymphocytes within peripheral blood and synovial fluid
We analyzed expression of cell surface markers of activation and differentiation by EBV antigen-specific cells in paired samples of peripheral blood and synovial fluid in donor RhA7 (Figs 4 and 5). CD69 and HLA-DR are often upregulated by activated CD8+ T cells; these molecules were found to be expressed at higher levels on RAKFKQLL-specific T cells within synovial fluid than in those found in peripheral blood (Fig. 4). Coexpression of CD69 and HLA-DR represents an unusual phenotype, but one that has been described previously within the joint [22]. CD38 expression has also been used to identify activated cells; this molecule was expressed on 56% of RAKFKQLL-specific T cells within the joint, compared with on 15% within the periphery (Fig. 5a).
Figure 4.
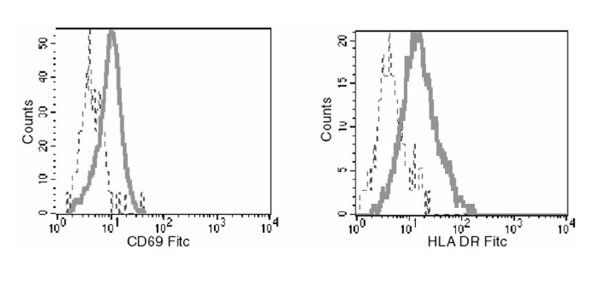
Expression of CD69 and human leucocyte antigen (HLA) DR by B8-RAKFKQLL-specific T cells in peripheral blood and synovial fluid from patient RhA7. Samples of peripheral blood mononuclear cells (dashed lines) and synovial fluid mononuclear cells (continuous lines) were stained with the B8/RAKFKQLL tetramer, with anti-CD8 and with monoclonal antibodies specific for CD69 or HLA-DR; 200 000 live cells were analyzed, and expression of CD69 and HLA-DR by the populations of CD8+ B8/RAKFKQLL tetramer reactive cells is shown. FITC, fluorescein isothiocyanate
Figure 5.
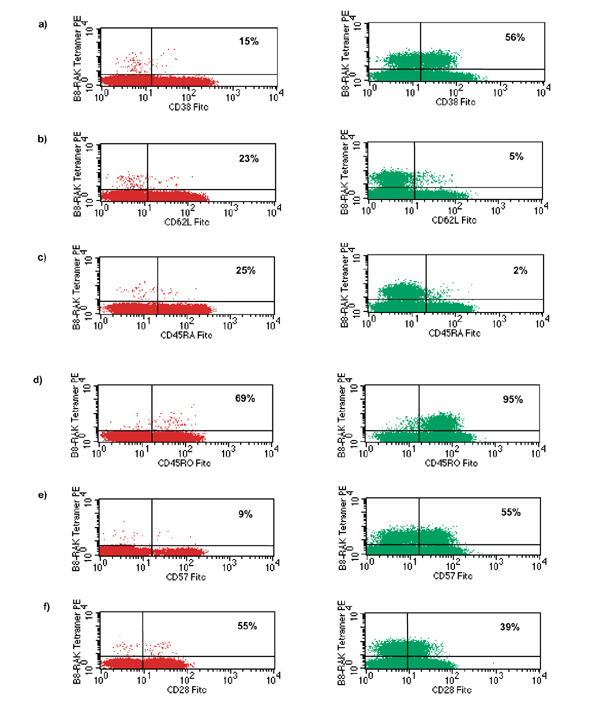
The phenotype of RAKFKQLL-specific T cells in peripheral blood and synovial fluid from patient RhA7. Samples of peripheral blood mononuclear cells (PBMCs; left column) and synovial fluid mononuclear cells (SFMCs; right column) were stained with anti-CD8, the B8/RAKFKQLL tetramer and with monocloncal antibodies specific for (a) CD38, (b) CD62L, (c) CD45RA, (d) CD45RO, (e) CD57 and (f) CD28. The percentage frequency of CD8+ tetramer reactive cells that stain with the phenotypic markers are shown; 200000 live cells were included in the analysis. FITC, fluorescein isothiocyanate.
L-selectin (CD62L) is downregulated after antigen stimulation, with expression often being regained in the stable memory state [23]. This molecule was expressed on only 5% of the RAKFKQLL-specific synovial fluid lymphocytes, compared with on 23% of the cells within peripheral blood (Fig. 5b).
CD45RA was not expressed at high levels by significant numbers of RAKFKQLL-specific cells within synovial fluid, but was expressed by 25% of those within peripheral blood (Fig. 5c). The vast majority of RAKFKQLL-specific cells within synovial fluid were CD45RO bright; 69% of these antigen-specific cells within the periphery expressed CD45RO [24] (Fig. 5d).
Expression of CD57, a glycoprotein of unknown function, is thought to occur on CD8+ T cells in a late differentiation compartment [25]. This molecule was expressed on 9% of RAKFKQLL-specific T cells within peripheral blood and on 55% of these cells within synovial fluid (Fig. 5e).
CD28 is a costimulatory molecule that binds B7; down-regulation of CD28 expression is associated with a diminished proliferative capacity and may reflect a state of late-terminal differentiation [26]. This molecule was expressed on 55% of RAKFKQLL-specific T cells within peripheral blood and on 39% of T cells in synovial fluid (Fig. 5f).
Analysis of GLCTLVAML-specific T cells within peripheral blood and synovial fluid from two further donors, with RA and osteoarthritis, respectively, revealed the same pattern, with EBV-specific T cells within the synovial fluid showing an increase in markers of activation and late differentiation as compared with those in peripheral blood (data not shown).
Discussion
The present study shows that virus-specific CD8+ T cells are enriched in synovial fluid from individuals with inflammatory arthritis. In particular, CD8+ T cells specific for two epitopes from EBV lytic cycle antigens (GLCTLVAML from BMLF1 and RAKFKQLL from BZLF1) may be present at very high frequency within the joints of EBV-seropositive patients. In donor RhA5, staining with tetrameric HLA-peptide complexes showed that T cells specific for these two epitopes accounted for 17.6% of all CD8+ T cells within synovial fluid (over 106 cells in a single joint aspirate). The findings described are not specific for RA and we obtained similar results in a patient with psoriatic arthritis (NR2) and in patients with osteoarthritis (NR3 and NR4). We also found very large numbers of T cells specific for an epitope (NLVPMVATV) from the CMV tegument protein pp65 in one patient with RA, and clearly detectable populations of T cells specific for this epitope in three other patients with RA. We did not find high frequencies of T cells specific for an epitope (FLRGRAYGL) from the EBV latent protein EBNA3A or of T cells specific for an epitope (GILGFVFTL) from the influenza A matrix protein within SFMCs in the patients studied. Thus, the synovial T-cell population is not simply a 'concentrated' pool of peripheral memory T cells. Many factors may contribute to the enrichment of T cells specific for certain viral antigens within the joint; these include preferential migration of subsets of T cells, local stimulation within the joint and protection from apoptotic cell death within the joint.
T-cell migration to sites of inflammation is a carefully controlled process [27,28,29] and T cells with an activated and memory phenotype may be preferentially recruited to a site of inflammation. The experiments described here show that the antigen-specific T cells within PBMCs are relatively more common in the CD62Llo, CD45RO+ and CD45RA- compartments than in the CD62Lhi, CD45RO- and CD45RA+ compartments. Thus, selection for activated/memory T cells is likely to account at least partly for the observed enrichment of virus-specific T cells within the joints. Current experiments are aimed at investigating the importance of expression of integrins and chemokine receptors by T cells in the recruitment of virus-specific T cells into joints [27,30,31].
It is also possible that T cells are stimulated to proliferate within the joints. Preliminary experiments (unpublished data) suggest that a small proportion of CD8+ T cells (usually <5%), including some EBV-specific T cells, are in cell cycle within synovial fluid. the stimulus to proliferation might be the relevant viral antigen itself. CMV dna has been detected in rheumatoid synovium [32]. Bcells as well as T cells are recruited into inflamed joints and, in EBV-seropositive individuals, a subpopulation of these Bcells will be latently infected with EBV. One might therefore expect to find this virus within inflamed joints. Although some early studies [33] found no evidence of EBV infection within joints, other reports [34,35] described detection of EBV DNA within the joints of patients with RA and one study [36] described the use of in situ hybridization to detect EBV-encoded small RNA1 and LMP1 transcripts in synovial lining cells from RA patients. Furthermore Koide et al [37] derived a fibroblastoid cell line that expressed EBV proteins from the synovium of a patient with RA. Very recently, Edinger et al [38] reported detection of EBV DNA within synovia from 10 out of 11 patients with RA. That study also provides evidence of transcription of EBV EBER1 and BZLF1 in samples of synovia from patients with RA and osteoarthritis. Thus, expression of BZLF1 within the joint may be stimulating T cells specific for the HLA-B8 restricted RAKFKQLL epitope from BZLF1. Transcription of BMLF1 has, however, not been detected within synovial tissue, suggesting that alternative mechanisms may be responsible for driving the proliferation of CD8+ T cells specific for the HLA-A2 restricted epitope (GLCTLVAML) from BMLF1. One possible alternative is that antigen-presenting cells such as dendritic cells may take up EBV antigens and subsequently be recruited to joints where they present epitopes from the EBV antigens by 'cross-presentation' [39]. A second possibility is that the virus-specific T cells are being stimulated by cross-reactive self-antigens expressed within the joint. In favour of this is the fact that a subpopulation of T cells specific for the HLA-B8 restricted RAKFKQLL peptide epitope is able to cross-react with a self peptide from a serine-threonine kinase [40].
Relative resistance to apoptotic cell death is a further theoretical factor that could influence the frequency of the virus-specific T cells within the joint. Although there is evidence that T cells within the joint are protected from apoptosis by type 1 IFN [41] there is no obvious reason to believe that the T cells specific for the RAKFKQLL and GLCTLVAML epitopes should survive more efficiently than other CD8+ T cells.
The results of the ELISpot assays for IFN-γ release after incubation of SFMCs with peptide epitopes from EBV and of the assays for TNF-α release after incubation of SFMCs with COS cells transfected with HLA-A2 and CMV pp65 suggest that the T cells within the synovial fluid retain their capacity to secrete proinflammatory cytokines. Within the joint, the secretion of such cytokines could lead to activation of synoviocytes and hence to the maintenance of inflammation [42]. Cell-cell contact between the activated virus-specific T cells within the joint and the synoviocyte population is a second mechanism whereby the virus-specific T cells might interact with the indigenous cells within the joint and contribute to the pathogenesis of inflammatory joint disease [43].
Importantly, these experiments show that large numbers of T cells within the joint are specific for epitopes from certain viral proteins. Many previous studies analyzed T-cell receptor use of T cells within the joint and found evidence of clonality, and concluded that the T cells are being driven by a specific self-antigen. Paliard et al [44] found that clonality was particularly marked within Vβ 14+ T cells within synovial fluid and that Vβ 14+ T cells were not well represented within peripheral blood of patients with RA. Those authors suggested that a superantigen might have caused activation of Vβ 14+ T cells, with recruitment of selected Vβ 14+ T cell clones into the joint followed by deletion of Vβ 14+ T cells in the periphery.
It will be interesting to analyze Vβ usage of our HLA-viral peptide tetramer-reactive CD8+ T cells. From the work we have described, it seems likely that at least some of the clonally expanded populations of CD8+ T cells found in synovial fluid are specific for viral antigens. The reasons for the presence of large numbers of synovial T cells specific for certain viral epitopes and not others remain unclear, and the role that these virus-specific 'bystander' T cells may play in the maintenance of inflammation needs further investigation.
References
- Rickinson AB, Kieff E. Epstein-Barr virus. Virology, 3rd ed. Edited by Fields BN, Knipe DM, Howley PM. Philadelphia: Lippincott-Raven, 1996. pp. 2397–2446.
- Massuci MG, Ernberg I. Epstein-Barr virus: adaptation to a life within the immune system. Trends Microbiol. 1994;2:125–130. doi: 10.1016/0966-842x(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Sixbey JW, Nedrud JG, Raab-Traub N, Hanes RA, Pagano JS. Epstein-Barr virus replication in oropharyngeal epithelial cells. . N Engl J Med. 1984;310:1225–1230. doi: 10.1056/NEJM198405103101905. [DOI] [PubMed] [Google Scholar]
- Tierney RJ, Steven N, Young LS, Rickinson AB. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J Virol. 1994;68:7374–7385. doi: 10.1128/jvi.68.11.7374-7385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RJ, Kurilla MG, Brooks JM, et al. Identification of target antigens for the human cytotoxic T cell response to Epstein-Barr virus (EBV): implications for the immune control of EBV-positive malignancies. J Exp Med. 1992;176:157–168. doi: 10.1084/jem.176.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R, Burrows SR, Kurilla MG, et al. Localisation of Epstein-Barr virus cytotoxic T cell epitopes using recombinant vaccinia: implications for vaccine development. J Exp Med. 1992;176:169–176. doi: 10.1084/jem.176.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LC, Gudgeon N, Annels NE, et al. T cells specific for EBV in healthy virus carriers. J Immunol. 1999;162:1827–1835. [PubMed] [Google Scholar]
- Bogedain C, Wolf H, Modrow S, Stuber G, Jilg W. Specific cytotoxic T lymphocytes recognise the immediate-early transactivator Zta of Epstein-Barr virus. J Virol. 1995;69:4872–4879. doi: 10.1128/jvi.69.8.4872-4879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven N, Annels NE, Kumar A, et al. Immediate early and early lytic cycle proteins are frequent targets of the Epstein-Barr virus-induced cytotoxic T cell response. J Exp Med. 1997;185:1605–1617. doi: 10.1084/jem.185.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan MFC, Tan L, Annels N, et al. Direct visualisation of antigen-specific CD8+ T cells during the primary immune response to Epstein Barr virus in vivo. . J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano MA, Carson DA, Slovin SF, Richman DD, Vaughan JH. Antibodies to Epstein-Barr virus-determined antigens in normal subjects and in patients with seropositive rheumatoid arthritis. . Proc Natl Acad Sci USA. 1979;76:5825–5828. doi: 10.1073/pnas.76.11.5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao QY, Rickinson AB, Gaston JS, Epstein MA. Disturbance of the Epstein-Barr virus-host balance in rheumatoid arthritis patients: a quantitative study. Clin Exp Immunol. 1986;64:302–310. [PMC free article] [PubMed] [Google Scholar]
- Baboonian C, Venables PJ, Williams DG, Williams RO, Maini RN. Cross reaction of antibodies to a glycine/alanine repeat sequence of Epstein-Barr virus nuclear antigen-1 with collagen, cytokeratin, and actin. . Ann Rheum Dis. 1991;50:772–775. doi: 10.1136/ard.50.11.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotet E, David-Ameline J, Peyrat M-A, et al. T cell response to Epstein-Barr virus transactivators in chronic rheumatoid arthritis. J Exp Med. 1996;184:1791–1800. doi: 10.1084/jem.184.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Ameline J, Lim A, Davodeau F, et al. Selection of T cells reactive against autologous B lymphoblastoid cells during chronic rheumatoid arthritis. J Immunol. 1996;157:4697–4706. [PubMed] [Google Scholar]
- Scotet E, Peyrat M-A, Saulquin X, et al. Frequent enrichment for CD8 T cells reactive against common herpes viruses in chronic inflammatory lesions: towards a reassessment of the physiopathological significance of T cell clonal expansions found in autoimmune inflammatory processes. Eur J Immunol. 1999;29:973–985. doi: 10.1002/(SICI)1521-4141(199903)29:03<973::AID-IMMU973>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Altman JD, Moss PAH, Goulder PR, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- Wills MR, Carmichael AJ, Mynard K, et al. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J, Elvin J, Latron F, et al. Identification of the nonamer peptide from influenza A matrix protein and the role of pockets of HLA A2 in its recognition by cytotoxic T lymphocytes. Eur J Immunol. 1992;22:903–907. doi: 10.1002/eji.1830220404. [DOI] [PubMed] [Google Scholar]
- Bunce M, O'Neil CM, Barnardo MC, et al. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 and DQB1 by PCR with 144 primer mixes utilising sequence-specific primers (PCR-SSP). . Tissue Antigens. 1995;46:355–367. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- Burrows SR, Rodda SJ, Suhrbier A, Geysen HM, Moss DJ. The specificity of recognition of a cytotoxic T lymphocyte epitope. Eur J Immunol. 1992;22:191–195. doi: 10.1002/eji.1830220128. [DOI] [PubMed] [Google Scholar]
- Iannone F, Corrigall VM, Kingsley GH, Panayi GS. Evidence for the continuous recruitment and activation of T cells into the joints of patients with rheumatoid arthritis. Eur J Immunol. 1994;24:2706–2713. doi: 10.1002/eji.1830241120. [DOI] [PubMed] [Google Scholar]
- Spertini O, Luscinskas FW, Kansas GS, et al. Leukocyte adhesion molecule (LAM-1, L selectin) interacts with an inducible endothelial cell ligand to support leucocyte adhesion. J Immunol . 1991;147:2565–2573. [PubMed] [Google Scholar]
- Akbar AN, Terry L, Timms A, Beverley PC, Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. . J Immunol. 1988;140:2171–2178. [PubMed] [Google Scholar]
- D'Angeac AD, Monier S, Pilling D, et al. CD57+ T lymphocytes are derived from CD57- precursors by differentiation occurring in late immune responses. Eur J Immunol . 1994;24:1503–1511. doi: 10.1002/eji.1830240707. [DOI] [PubMed] [Google Scholar]
- Azuma M, Phillips JH, Lanier LL. CD28- T lymphocytes. Antigenic and functional properties. J Immunol. 1993;150:1147–1159. [PubMed] [Google Scholar]
- Qin S, Rottman JB, Myers P, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest . 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med . 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz T. Lymphocyte homing to sites of inflammation. Curr Opin Immunol. 1992;4:287–293. doi: 10.1016/0952-7915(92)90078-s. [DOI] [PubMed] [Google Scholar]
- Yokota A, Murata N, Saiki O, et al. High avidity state of leukocyte function-associated antigen-1 on rheumatoid synovial fluid T lymphocytes. J Immunol. 1995;155:4118–4124. [PubMed] [Google Scholar]
- Dustin ML, Springer TA. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988;107:321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einsele H, Steidle M, Muller CA, et al. Demonstration of cytomegalovirus (CMV) DNA and anti-CMV response in the synovial membranes and serum of patients with rheumatoid arthritis. J Rheumatol. 1992;19:677–681. [PubMed] [Google Scholar]
- Brousset P, Caulier M, Cantagrel A, et al. Absence of Epstein-Barr virus carrying cells in synovial membranes and subcutaneous nodules of patients with rheumatoid arthritis. Ann Rheum Dis. 1993;52:608–609. doi: 10.1136/ard.52.8.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Nikkari S, Skurnik M, et al. Detection of herpesviruses by polymerase chain reaction in lymphocytes from patients with rheumatoid arthritis. Arthritis Rheum. 1993;36:1080–1086. doi: 10.1002/art.1780360808. [DOI] [PubMed] [Google Scholar]
- Mousavi-Jazi M, Bostrom L, Lovmark C, et al. Infrequent detection of cytomegalovirus and Epstein-Barr virus DNA in synovial membrance of patients with rheumatoid arthritis. J Rheumatol . 1998;25:623–628. [PubMed] [Google Scholar]
- Takei M, Mitamura K, Fujiwara S, et al. Detection of Epstein-Barr virus-encoded small RNA 1 and latent membrane protein 1 in synovial lining cells from rheumatoid arthritis patients. Int Immunol. 1997;9:739–743. doi: 10.1093/intimm/9.5.739. [DOI] [PubMed] [Google Scholar]
- Koide J, Takada K, Sugiura M, et al. Spontaneous establishment of an Epstein-Barr virus-infected fibroblast line from the synovial tissue of a rheumatoid arthritis patient. J Virol. 1997;71:2478–2481. doi: 10.1128/jvi.71.3.2478-2481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger JW, Bonneville M, Scotet E, et al. EBV gene expression not altered in rheumatoid synovia despite the presence of EBV antigen-specific T cell clones. J Immunol . 1999;162:3694–3701. [PubMed] [Google Scholar]
- Albert M, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Misko IS, Cross SM, Khanna R, et al. Cross reactive recognition of viral, self and bacterial peptide ligands by human class I-restricted cytotoxic T lymphocyte clonotypes: implications for molecular mimicry in autoimmune disease. Proc Natl Acad Sci USA. 1999;96:2279–2284. doi: 10.1073/pnas.96.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling D, Akbar AN, Girdlestone J, et al. Interferon-γ mediates stromal rescue of T cells from apoptosis. . Eur J Immmunol. 1999;29:1041–1050. doi: 10.1002/(SICI)1521-4141(199903)29:03<1041::AID-IMMU1041>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Klimiuk PA, Yang H, Goronzy JJ, Weyand CM. Production of cytokines and metalloproteinases in rheumatoid synovitis is T cell dependent. Clin Immunol. 1999;90:65–78. doi: 10.1006/clim.1998.4618. [DOI] [PubMed] [Google Scholar]
- Sebbag M, Parry SL, Brennan FL, Feldmann M. Cytokine stimulation of T lymphocytes regulates their capacity to induce monocyte production of tumor necrosis factor-alpha, but not interleukin-10: possible relevance to pathophysiology of rheumatoid arthritis. Eur J Immunol. 1997;27:624–632. doi: 10.1002/eji.1830270308. [DOI] [PubMed] [Google Scholar]
- Paliard X, West SG, Lafferty JA, et al. Evidence for the effects of a superantigen in rheumatoid arthritis. Science . 1991;253:325–329. doi: 10.1126/science.1857971. [DOI] [PubMed] [Google Scholar]


