Abstract
Objective
There is a rapidly evolving debate on the indications and appropriate duration of therapy for postmenopausal hormone therapy. The objective of this meta-analysis was to examine the specific relationships of postmenopausal estrogen therapy (ET), postmenopausal combined (estrogen-progestogen) hormone therapy (CHT), and the incidence of breast cancer.
Design
We performed computerized searches of MEDLINE and CancerLit through September 2003 and reviewed reference lists of retrieved studies and meta-analyses. We included English-language studies that identified noncontraceptive postmenopausal hormone use; reported on the risks of “current use” of ET and/or CHT and breast cancer incidence; were case-control, cohort, or experimental; and reported either an odds ratio (OR), relative risk (RR), or HR with CIs. Two investigators were involved during all stages of study selection and independently extracted all data selected for inclusion in meta-analyses.
Results
Meta-analysis of 13 studies of ET and breast cancer (700,000 women) resulted in an OR of 1.16 (95% confidence limits [CL] 1.06, 1.28), with estimates for less than 5 years use 1.16 (1.02, 1.32) and more than 5 years use 1.20 (1.06, 1.37). Meta-analysis of eight studies of CHT and breast cancer (650,000 women) resulted in an OR of 1.39 (95% CL 1.12, 1.72), with estimates for less than 5 years use 1.35 (1.16, 1.57) and more than 5 years use 1.63 (1.22, 2.18).
Conclusions
Data from observational studies support the association of increased but considerably different risks for breast cancer incidence among current users of ET and CHT. These represent the first pooled estimates for ET. CHT estimates correspond to those from randomized trials.
Keywords: Hormone therapy, Breast cancer, Menopause
There is a rapidly evolving debate on the indications and appropriate duration of therapy for postmenopausal hormone therapy (HT). Initially, use focused on the treatment of perimenopausal symptoms, but the purported benefits were quickly broadened to include protectionfromcardiovasculardisease,age-related dementias, osteoporosis, and colon cancer.1 Most recently, the perceived role of HTs has shifted from one of broad indications, long-term use, and substantial optimism to narrow indications, short-term use, and great skepticism.2
Since the publication of the results of the Women’s Health Initiative (WHI),3,4 a primary prevention trial of HT and coronary heart disease, and the follow-up to the Heart and Estrogen/Progestin Replacement Study (HERS II),5 a secondary prevention trial on the same topic, concerns have focused on whether the potential risks of HT outweigh the benefits for selected patients, many patients, or even a majority of patients. HERS II5 found increased rates of venous thromboembolism and biliary tract surgery in the group assigned to receive 0.625 mg/day of conjugated estrogens and 2.5 mg/day of medroxyprogesterone acetate (MPA). Rates of cancer, however, were not elevated in treated women relative to controls. One arm of the WHI was stopped early because of a finding of increased breast cancer risk among users of combined conjugated estrogens and MPA, with an unadjusted hazard ratio (HR) of 1.26 (95% confidence limits [CL] 1.00, 1.59). A second arm of the WHI that was stopped more recently, due to the relative certainty that a beneficial cardiovascular effect would not be demonstrated with longer follow-up, found no increased risk of breast cancer among users of estrogen-alone preparations, with an HR of 0.77 (95% CL 0.59, 1.01).
The Agency for Healthcare Research and Quality (AHRQ) has also completed a report on HT.6 They found an association between the “current use” of HT and breast cancer as well as conflicting evidence of increased risk for long-term use. They concluded that there was no evidence of increased risk for combined estrogen-progestogen regimens over estrogen-alone regimens. This report, however, based many of its findings on meta-analyses that were confounded by the inclusion of premenopausal oral contraceptive use.7–9 The US Preventive Services Task Force concluded that there was fair to good evidence that HT increases the incidence of breast cancer, with best evidence for estrogen plus progestogen.10
The role of HT has been re-evaluated since the publication of the WHI, HERS II, and AHRQ reports. The US Food and Drug Administration codified its revised position on HT by requiring changes to the package inserts for all female hormonal therapies.11 Meanwhile, numerous new reports from observational studies have been published on the association between specific regimens of HT and breast cancer, providing the opportunity for a closer examination of the topic which can better incorporate duration of use into estimates of risk, examine different regimens, and provide more generalizable results than trials alone. Especially when absolute risk is small, individual studies may be ill-equipped to estimate risks precisely because of random error. Meta-analysis of such studies may help average out the “noise,” albeit not correcting for any potential systematic biases in the original data.
For these reasons, we undertook a systematic review that differs from previous work in four ways. First, it excludes contraceptive use from systematic assessment of HT and breast cancer risk. Second, it separates estimates for estrogen-alone therapy (ET) and combined estrogen-progestogen hormone therapy (CHT) regimens. Third, it looks at the duration of use, separated into less than 5 years or greater than 5 years. Fourth, it relies solely on estimates of ET/CHT “current use.”
METHODS
Data sources
Studies and review articles relating postmenopausal hormone therapy and cancer were identified in the MEDLINE (1966 through September 2003) and CancerLit (1975 through September 2003) databases. Search terms included hormone replacement therapy, estrogen replacement, cancer, and neoplasm (see Appendix 1 for the complete search strategy).
Study selection criteria
Studies had to be conducted in postmenopausal women with the title available in English to be considered for this systematic review. Data on cancer incidence, mortality, pathology, or stage had to be reported. Studies selected for inclusion met the following requirements: 1) the study was observational or interventional with a comparison group (eg, case-control, cohort, quasi-experimental, or experimental, but not case series); 2) the study incorporated longitudinal ascertainment of exposure and disease (ascertainment could be prospective or historical, but not cross-sectional); 3) the study reported data on rates of cancer in at least two groups (“never” and “current” users), or the study included a summary estimate (eg, odds ratio, relative risk) with reported CIs or a precise P value; and 4) the study had to distinguish between noncontraceptive and contraceptive estrogen use in its presentation of results. Reports selected for meta-analyses additionally had to provide estimates of risk for women using ET or CHT at study inception (current use). Estimates for “current use” of HT among women enrolling in a study, as compared with “past use” or “ever use,” have consistently found the greatest risk associations with breast cancer and are also most comparable to estimates from randomized trials such as HERS and WHI that start women on HT or placebo at study inception.12,13 Two investigators reviewed all titles and studies included in meta-analyses. The full text of the citation was retrieved for those with no abstract available. We excluded editorials, letters, and nonsystematic reviews. For datasets that were presented in multiple publications, we selected those with the most up-to-date results, longest follow-up, or most pertinent outcomes. We did not pursue unpublished data because several prior meta-analyses conducted in this area found no contribution from this added step. We conducted a separate search to identify prior meta-analyses of HT and cancer and used their reference lists to find additional studies not identified by database searches. Appendix 2 summarizes the findings of the literature search.
Data extraction
We abstracted included studies into evidence tables modeled on those of the AHRQ report.6 Pertinent data were initially abstracted by one investigator, compared with results found by the AHRQ reviewers where available, and independently abstracted by another investigator. Discrepancies were resolved by consensus.
Data synthesis
We conducted meta-analyses of studies on the “current use” of ET/CHT and its relationship to incident cases of breast cancer. We used the methods of DerSimonian and Laird14 to compute point estimates and 95% CLs with Stata software (version 7) using the “meta” command. Because no meaningful differences were found between the random effects and fixed effects analyses, only random effects results are presented. When results from observational studies and randomized trials were available on the same topic, separate meta-analyses were conducted because of different potentials for bias among studies versus trials.15 Heterogeneity was assessed using the Q test, I2 and further evaluated with exploratory meta-regression.16,17 Whenever possible, adjusted odds ratios or RRs were used as estimates of the true relation between HT and breast cancer. We present “study quality” ratings based on methods described by the US Preventive Services Task Force,18 but limit our use of these ratings because they do not take account of bias directions and so are potentially misleading.19 To assess publication bias we used the “trim and fill” method (“metatrim” in Stata).20
RESULTS
Search results
From a sample of 2,474 titles reviewed (1,669 MEDLINE, 594 CancerLit, and 211 from prior meta-analyses) we identified 10 meta-analyses, 56 reports of case-control studies, 41 reports of cohort studies, and 4 reports of randomized trials with data on the relationship between breast cancer and HT. Studies that are included in the meta-analyses are listed in Table 1. Other studies that met all the inclusion criteria but were not included in meta-analyses (because they did not provide data on “current use” of ET/CHT, or are presented in other publications of the same dataset) are listed in Appendix 3. Other than updated reports of data presented earlier, all exclusions were of studies that only provided data on HT “ever use” or “past use.” Both of these classifications of exposure are subject to considerable recall bias on type of agent and duration of use, and hence may underestimate risks compared to estimates based on “current use.” All data elements relative to the meta-analyses had two reviewers who came to consensus on all items. Studies included in prior meta-analyses but excluded in this review are listed in Appendix 4 along with reasons for their exclusion.
TABLE 1.
Studies included in the meta-analyses
| Design/Qualitya | Author | Study name or location | Setting | Country | Years enrolled | Study size | Baseline age range (mean), y |
|---|---|---|---|---|---|---|---|
| CC/G | Stanford 199521 | Washington state | Population based | United States | 1988–90 | 942 | 50–64 |
| CC/F | Henrich 199822 | Yale | Hospital based | United States | 1987–92 | 654 | 47–88 (66) |
| CC/G | Chen 200223 | Group Health Cooperative | Population based | United States | 1990–95 | 1,397 | 50–74 |
| CC/G | Newcomb 200224 | 3 State | Population based | United States | 1992–94 | 10,869 | 50–79 |
| CC/G | Weiss 200225 | CARES | Population based | United States | 1994–98 | 3,823 | 35–64 |
| Cohort/G | Mills 198926 | 7th-Day Adventist | Population based | United States | 1976–82 | 20,341 | (55.4) |
| Cohort/G | Colditz 199527 | Nurses Health | Population based | United States | 1976–86 | 69,586 | 30–55 |
| Cohort/G | Folsom 199528 | Iowa | Population based | United States | 1986–91 | 41,837 | 55–69 |
| Cohort/G | Lucas 199829 | Osteoporotic Fractures | Population based | United States | 1986–92 | 7,250 | >65 |
| Cohort/P | Sourander 199830 | Finland | Population based | Finland | 1987–95 | 6,433 | 57–65 |
| Cohort/G | Schairer 200031 | BCDDPb | Population based | United States | 1973–80 | 2,082 | (58) |
| Cohort/G | Porch 200213 | Women’s Health Study (WHS) | Population based | United States | 1993–00 | 17,835 | >45 |
| Cohort/G | Beral 200312 | Million Women | Population based | United Kingdom | 1996–01 | >1 mill | 50–64 |
| RCT/G | Hulley 20025 | HERS II | Population based | United States | 1993–00 | 2,763 | (67) |
| RCT/G | Rossouw 20024 | Women’s Health Initiative | Population based | United States | 1993–98 | 16,608 | 50–79 (63) |
CC, case-control; RCT, randomized controlled trial; G, good; F, fair; P, poor; according to rating system of Harris et al.18
Breast Cancer Detection Demonstration Project.
Postmenopausal hormone therapy formulations
There are numerous formulations of HT that vary by agent and dose. In general, women used estradiol-based regimens in European studies and conjugated estrogens in US studies. Earlier studies report higher doses of estrogen (eg, >1.25 mg/day conjugated estrogens32) than more recent studies (eg, 0.625 mg/day of conjugated estrogens5). The use of opposed estrogens (ie, in combination with a progestogen) gained widespread acceptance in the 1980s after many studies found estrogen-alone regimens were associated with high rates of endometrial cancer in women with a uterus.33 In a nationally representative cohort of US women, use of CHT increased from 7% in 1979–1983 to 37% in 1992–1995.31 Hence, studies of women on HT conducted in the 1970s or earlier are presumed to have women exposed to estrogen alone unless explicitly stated otherwise. Combined hormone therapy consists of a form of estrogen delivered either sequentially or simultaneously with a form of progestogen. Early CHT formulations delivered estrogen for most days of the cycle with a progestogen (eg, levonorgestrel or MPA) added during the final week—sequential CHT; however, most of the CHT studies identified in this review are of estrogen and progestogen delivered simultaneously—continuous CHT. If a study listed the progestogen type and dose used, which occurred infrequently, this information was abstracted. All but one study12 included in the CHT meta-analysis were conducted in the United States, where MPA is the predominant progestogen.34 The Million Women Study, conducted in Europe, had approximately 17% of progestogen users taking MPA, 39% taking norethisterone, and 44% taking norgestrel/levonorgestrel.12
Estrogen therapy and risk of breast cancer
Eight cohort and five case-control studies addressed the association of ET and breast cancer (see Table 2). Meta-analysis of these 13 studies including 701,160 women results in a pooled OR of 1.16 (95% CL 1.06, 1.28), with the Q test P = 0.003 suggesting heterogeneity and I2 = 53% (see Figure 1). Exploratory meta-regression analyses to determine potential sources of this heterogeneity did not detect a relation of the odds ratio to design (case control versus cohort), location (US versus Europe), enrollment or publication year, study size, quality rating (good versus poor/fair), years of follow-up, or adjustment for socioeconomic status.
TABLE 2.
Point estimates and 95% confidence limits for studies included in the meta-analyses
| Author/Year | Study | <5 years use | ≥5 years use | Study size | Overall |
|---|---|---|---|---|---|
| Estrogen therapy | |||||
| Mills 1989 | 7th-Day Adventist | 1.88 (1.30, 2.73) | 2.05 (1.15, 3.63) | 7,839 | 1.69 (1.12, 2.55) |
| Colditz 1995 | Nurses Health | 1.18 (1.02, 1.36) | 1.46 (1.28, 1.66) | 69,586 | 1.32 (1.14, 1.54) |
| Folsom 1995 | Iowa | 1.45 (1.03, 2.06) | 1.21 (0.92, 1.60) | 36,942 | 1.24 (0.99, 1.56) |
| Stanford 1995 | Washington | 1.00 (0.69, 1.43) | 0.77 (0.53, 1.13) | 662 | 0.90 (0.70, 1.30) |
| Henrich 1998 | Yale | – | – | 654 | 1.52 (0.77, 2.99) |
| Lucas 1998 | Osteoporotic Fractures | – | – | 5,278 | 1.33 (0.75, 2.35) |
| Sourander 1998 | Finland | – | – | 6,560 | 0.57 (0.27, 1.20) |
| Schairer 2000 | BCDDP | – | 1.10 (1.00, 1.30) | 37,084 | 1.10 (1.00, 1.30) |
| Chen 2002 | Group Health | 1.29 (0.87, 1.91) | 1.84 (1.04, 3.27) | 757 | 1.17 (0.85, 1.60) |
| Newcomb 2002 | 3 State | 1.07 (0.84, 1.37) | 1.34 (1.12, 1.59) | 9,200 | 1.25 (1.08, 1.45) |
| Porch 2002 | WHS | 0.96 (0.58, 1.58) | 0.99 (0.65, 1.53) | 12,219 | 0.96 (0.65, 1.42) |
| Weiss 2002 | CARES | 0.92 (0.72, 1.17) | 0.81 (0.63, 1.04) | 2,354 | 0.84 (0.67, 1.06) |
| Beral 2003 | Million Women | 1.05 (0.69, 1.59) | 1.34 (1.24, 1.44) | 512,025 | 1.30 (1.22, 1.38) |
| Pooled ET | 1.16 (1.02, 1.32) | 1.20 (1.06, 1.37) | 701,160 | 1.16 (1.06, 1.28) | |
| Combined estrogen-progestin therapy | |||||
| Colditz 1995 | Nurses Health | – | – | 69,586 | 1.41 (1.15, 1.74) |
| Stanford 1995 | Washington | 1.40 (0.70, 2.70) | 0.50 (0.10, 1.70) | 572 | 0.90 (0.60, 1.20) |
| Schairer 2000 | BCDDP | 1.40 (1.10, 1.90) | – | 21,323 | 1.40 (1.10, 1.90) |
| Chen 2002 | Group Health | – | – | 700 | 1.49 (1.04, 2.12) |
| Newcomb 2002 | 3 State | 1.32 (1.02, 1.70) | 1.50 (1.09, 2.06) | 8,490 | 1.39 (1.12, 1.71) |
| Porch 2002 | WHS | 1.11 (0.81, 1.52) | 1.76 (1.29, 2.39) | 12,211 | 1.37 (1.05, 1.78) |
| Weiss 2002 | CARES | 1.02 (0.69, 1.51) | 1.37 (1.06, 1.77) | 2,222 | 1.22 (0.99, 1.50) |
| Beral 2003 | Million Women | 1.63 (1.37, 1.93) | 2.21 (2.09, 2.34) | 540,455 | 2.00 (1.91, 2.09) |
| Pooled CHT | 1.35 (1.16, 1.57) | 1.63 (1.22, 2.18) | 655,559 | 1.39 (1.12, 1.72) | |
Notes:
Mills 1989 includes patients followed up until 1982, at which time combined regimen use was negligible in the United States.
Mills 1989 ET duration estimates based on age-adjusted pooled estimates for <1 and 1–5 years, and 6–10 and ≥10 years.
Colditz 1995 ET duration estimates based on pooled estimates for data for 1–23 and 24–59 months, and 60–119 and ≥120 months.
Overall CHT data from Nurses Health Study are also presented in Grodstein 1997 with RR 0.76 (0.56–1.02).
Folsom 1995 classified as ET (follow-up ended 1991, when most HT was still ET; they estimate <20% CHT).
Henrich 1998 ET estimate includes approximately 5% of women on CHT.
Schairer 2000 CHT <5 years estimate based on 3.6 years average use given for overall CHT users.
Schairer 2000 ET ≥5 years estimate based on 10.3 years average use given for overall ET users.
Chen 2002 ≥5 years ET estimate based on 60 continuous months of “recent” use in the past 5 years.
Weiss 2002 ET and CHT <5 year data based on pooled estimates for data from 0–6, 6–23, and 24–59 months.
Beral 2003 duration data based on pooled estimates for data for <1 and 1–4 years, and 5–9 and ≥10 years.
FIG. 1.

Current use of estrogen therapy and breast cancer risk. Forest plot of observational studies on the association between “current use” of estrogen therapy and the incidence of breast cancer. Each square represents the point estimate of a study, with the size of the square corresponding to the study size. Thirteen studies (see Table 2 for corresponding references) result in a pooled OR of 1.16 (95% CL 1.06, 1.28).
Nine studies provided estimates for less than 5 years of ETuse: OR 1.16 (1.02, 1.32) with the Q test P = 0.09 and I2 = 27%. Ten studies provided data for 5 or more years of use: OR 1.20 (1.06, 1.37) with the Q test P < 0.001 and I2 = 70%.
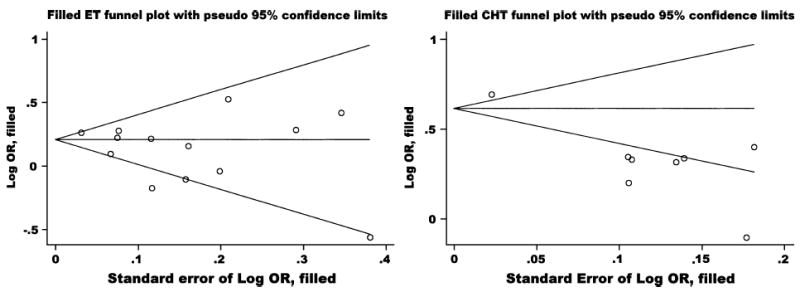
Nonparametric tests for publication bias resulted in no studies being trimmed or filled (see Appendix 5), suggesting no significant likelihood of missed studies. Furthermore, any studies not published would have to be large with highly protective findings to influence the statistical pooling in light of the over 700,000 women included in this meta-analysis.
We identified one randomized trial that met all inclusion criteria. The Women’s Health Initiative showed an unadjusted HR of 0.77 (95% CL 0.59, 1.01) and an adjusted HR of 0.77 (95% CL 0.57, 1.06), with an average of 6.8 years of follow-up.3
Combined estrogen-progestogen therapy and risk of breast cancer
We conducted a meta-analysis of observational studies of CHT and breast cancer (four cohort and four case-control studies) that excluded two randomized trials: WHI and HERS (see Table 2). Meta-analysis of these eight “good quality”studies with 655,559 women yields a pooled OR of 1.39 (95% CL 1.12, 1.72) with a heterogeneity P of < 0.001 and I2 = 87% (see Fig. 2). Exploratory meta-regression analyses to determine potential sources of this heterogeneity did not detect a relation of the OR to design (case control versus cohort), enrollment or publication year, years of follow-up, or adjustment for socioeconomic status. The only factors that relate to the OR are predominant type of progestogen (MPA versus other), study location (US versus Europe), and sample size (P < 0.001). By excluding the Million Women Study (RR 2.00, 95% CL 1.91, 2.09) the pooled OR for seven studies is 1.32 (95% CL 1.19, 1.46) with a heterogeneity P = 0.36 and I2 = 0%. For this meta-analysis, the Million Women Study is the only one conducted in Europe, it is the largest study, and the only one that had norgestrel/levonorgestrel as the predominant type of progestogen.
FIG. 2.

Current use of combined (estrogen-progestogen) hormone therapy and breast cancer risk. Forest plot of observational studies on the association between “current use” of combined therapy and the incidence of breast cancer. Each square represents the point estimate of a study, with the size of the square corresponding to the study size. Eight studies (see Table 2 for corresponding references) result in a pooled odds ratio of 1.39 (95% CL 1.12, 1.72).
Six studies provided estimates for less than 5 years of CHT use: OR 1.35 (1.16, 1.57) with the Q test P = 0.17 and I2 = 11%. Five studies provided data for 5 or more years of use: OR 1.63 (1.22, 2.18) with the Q test P < 0.001 and I2 = 74%. Nonparametric tests for publication bias resulted in no studies being trimmed or filled (see Appendix 5). If small positive studies are more likely to be reported than small negative studies, then the “trim and fill” method would compensate and “fill in” the missing studies and create an adjusted pooled estimate.20
We identified two randomized trials that met all inclusion criteria. The Heart and Estrogen/progestin Replacement Study (HERS) study5 (the fully randomized and blinded trial excluding the open-label observational cohort portion of HERS II) showed an HR of 1.30 (95% CL 0.77, 2.19) and the WHI showed an adjusted HR of 1.26 (95% CL 0.83, 1.92) and an unadjusted HR of 1.26 (95% CL 1.00, 1.59).4
What types of progestogens in CHT are associated with higher risk for breast cancer?
Available data are limited with respect to explicit comparisons of breast cancer risk by type of progestogen used. AHRQ found insufficient evidence to conclude that elevated risk is associated with certain types of progestogens relative to others. HERS II showed a trend toward increased risks associated with a regimen with 2.5 mg/day MPA. The same oral regimen was used in the WHI and showed increased risk. In cohort and case-control studies, MPA was the most common agent taken by women exposed to CHT, and most studies found increased risk for CHT regimens. Although the Million Women Study found a higher risk associated with CHT use than all the studies conducted in the United States, whether this can be attributed to the predominant type of progestogen used or another as-yet-unidentified confounder has not been determined.
DISCUSSION
In this meta-analysis of observational studies of postmenopausal hormone use, there is an increased risk of breast cancer incidence associated with the current use of ET (1.16, 95% CL 1.06, 1.28) and a considerably larger risk associated with the current use of CHT (1.39, 95% CL 1.12, 1.72). Data from randomized trials do not give a conclusive answer for breast cancer risk from postmenopausal HT (see Fig. 3). Results from HERS with approximately 4 years of follow-up and WHI with about 5 years of follow-up correspond well to the pooled results obtained from observational studies of CHT with less than 5 years duration of use. In Fig. 3, there are trends in the pooled observational results toward both a dose-response relationship (with longer durations of use corresponding to larger risks) and greater risk associated with the addition of a progestogen to the hormone therapy regimen. That the observational evidence reinforces data from two randomized trials of CHT helps give credence to the pooled estimates for ET.
FIG. 3.

Comparisons between randomized trials and observational studies of hormone therapy and breast cancer incidence by duration of hormone therapy use. Plots of point estimates and adjusted 95% CL for the association observed in individual randomized trials and pooled observational studies of estrogen therapy and combined estrogen-progestogen therapy.
However, the risk of breast cancer reported in the ET arm of the WHI appears to contradict the risk estimates we derived from a substantial sample of pooled observational data. The WHI investigators concluded “Women considering taking CEE should be counseled about an increased risk of stroke but can be reassured about no excess risk of heart disease or breast cancer for at least 6.8 years of use”3 (emphasis added). We believe the data suggest that differences exist between estrogen-alone and combined estrogen-progestogen regimens, but would recommend full disclosure to potential HT users about even minor elevations in breast cancer risk.
Prior observational studies led us to recommend HT based on biased results from “past use” and “ever use” estimates (not just “current use”), inclusion of pre-menopausal women, and mixing of exposures attributed to HT from ET and CHT. The present meta-analysis represents the least confounded and most specific one to date, including several large studies that were missed or omitted by prior meta-analyses. The authors of the AHRQ summary concluded that there was no evidence of increased risk for CHT regimens over ET regimens and conflicting evidence of increased risk for long-term use.6 They did not, however, conduct new meta-analyses on these questions. Their conclusions were based on the results of three prior meta-analyses,7–9 which did not differentiate between ET and CHT. Furthermore, these prior meta-analyses had significant limitations: 1) the Collaborative Group’s reanalysis of 51 epidemiologic studies7 (OR 1.21, P < 0.001) includes 10 studies of premenopausal women using oral contraceptives (see Appendix 4) and we identified five cohort studies12,13,29–31 and six case-control studies22–25,35,36 that were published since the time of their pooling; 2) the Sillero-Arenas meta-analysis8 (RR 1.23, 95% CL 1.12, 1.35) was based on only six studies (which they did not explicitly identify); and 3) the meta-analysis by Colditz et al9 (RR 1.40, 95% CL 1.20, 1.63) included only three studies, one of which explicitly states that it included women on oral contraceptives.37
Several studies included in this analysis were conducted during the 1980s, a time of transition from estrogen-alone regimens to estrogen plus progestogen for women who had not had a hysterectomy. Confounding (or self-selection) bias among women or their physicians might have been strongest during this period. Hence, any presumptions on type of agent relative to effect on breast cancer risk are most susceptible to bias. Furthermore, a “healthy user” bias has been well-documented among studies of HT and cardiovascular disease.38 Despite the potential for a “healthy user” bias, we still found an increased risk for breast cancer among “current users” of ETand CHT relative to “never users” of any HT. An earlier meta-analysis of HT and cardiovascular disease39 found that higher socioeconomic status (and its associated better health) accounted for most of the observed protective association from HT40,41. We found no such association with breast cancer incidence and socioeconomic status for either the ET meta-analysis (P = 0.756) or the CHT meta-analysis (P = 0.678).
Although factors such as the type of estrogen (derived from equine or “natural” sources), type of progestogen (testosterone based or not), and other factors might help provide biologic explanations for this heterogeneity,42,43 there were insufficient data in the reports included in the current analyses to further evaluate their specific roles. The sensitivity analysis that included seven of eight studies (which had MPA as the predominant agent) found that the type of progestogen explained a substantial portion of the heterogeneity observed in the CHT meta-analysis. But this might also be attributable to study size, study location, or other factors. Future research in this area should clearly differentiate premenopausal and postmenopausal estrogen and progestogen exposure, attempt to quantify lifetime estrogen (and progestogen) exposure patterns, and attempt to address specific risks by type of estrogen and progestogen.
The meta-analysis of ET in the current report represents the “first look” at such significant HT data without the inclusion of progestogen, and provides clinically meaningful estimates for patients and providers on the risks of this specific type of postmenopausal hormone therapy. The present report suggests that a woman taking ET for a few (<5) years of perimenopausal use will have considerably different risks compared to a woman needing CHT or long-term (>5 years) treatment. Women with disabling menopause symptoms might find that risk acceptable for the short term. Until this report, recommendations for HTuse did not differ for these women with clinically different risk profiles and needs.
CONCLUSIONS
In conclusion, our analysis shows that current use of ET and CHT are associated with increased but different risks of breast cancer. Our conclusions differ from prior reports because we excluded studies confounded by premenopausal oral contraceptive use, included numerous studies published since the time of the last review, and focused on estimates of ET/CHT “current use.” We believe these new findings should be thoughtfully considered as policy and clinical decision making undergo re-evaluation.
Appendix 1. MEDLINE search strategy
| #1 Search hormone replacement therapy |
| #2 Search estrogen replacement |
| #3 Search #1 OR #2 |
| #4 Search cancer |
| #5 Search neoplasm |
| #6 Search #4 OR #5 |
| #7 Search #3 AND #6 |
| #8 Search #3 AND #6 Field: All Fields, Limits: English, Human |
| #9 Search (#8) AND (randomized controlled trial [PTYP] OR drug therapy [SH] OR therapeutic use [SH:NOEXP] OR random* [WORD]) |
| #10 Search (#8) AND (cohort studies [MESH] OR risk [MESH] OR (odds [WORD] AND ratio* [WORD]) OR (relative [WORD] AND risk [WORD]) OR (case control* [WORD] OR case-control studies [MESH])) |
| #11 Search #9 OR #10 |
Strategy adapted from Haynes RB, Wilczynski N, McKibbon KA, Walker CJ, Sinclair JC. Developing optimal search strategies for detecting clinically sound studies in MEDLINE. J Am Med Inform Assoc 1994;1:447–458.
APPENDIX 2. Hormone therapy systematic review flow chart. *Presented in detail in Appendix 3, with reports of 56 case-control, 41 cohort, and 4 randomized trial datasets. †Rejected articles presented data that was updated in another article or did not provide estimates for “current use” of ET/CHT compared with “never use.”

Appendix 3.
See Appendix 3 table in Figures and Tables section.
Appendix 3. All studies that met inclusion criteria.
| Design | Author | Year | Journal | Setting | Country | Years enrolled | Study size a | Baseline age range (mean) , y |
|---|---|---|---|---|---|---|---|---|
| CC | Boston Collaborative | 1974 | N Engl J Med | Hospital based | United States | 1972 | 825 | 45–69 |
| CC | Brinton | 1981 | Cancer | Population based | United States | 1973–77 | 1,960 | 35–74 |
| CC | Brinton | 1986 | Br J Cancer | Population based | United States | 1977–80 | 4,218 | 35–74 |
| CC | Brinton | 1998 | Menopause | Population based | United States | 1990–92 | 1,950 | <55 |
| CC | Brownson | 1988 | Arch Intern Med | Population based | United States | 1979–86 | 2,149 | (63) |
| CC | Byrne | 2000 | Cancer | Population based | United States | 1976–92 | 743 | 30–55 |
| CC | Casagrande | 1976 | J Natl Cancer Inst | Population based | United States | 1969–73 | 266 | 50–64 |
| CC | Chen | 2002 | JAMA | Population based | United States | 1990–95 | 1,397 | 50–74 |
| CC | Daling | 2002 | Cancer | Population based | United States | 1994–98 | 3,602 | <65 |
| CC | Ettinger | 1996 | Obstet Gynecol | Population based | United States | 1969–73 | 454 | 55–69 |
| CC | Ewertz | 1988 | Int J Cancer | Population based | Denmark | 1983–84 | 990 | <70 |
| CC | Fioretti | 1999 | Brit J Cancer | Hospital based | Italy | 1983–94 | 1,247 | 22–80 |
| CC | Grodstein | 1997 | N Engl J Med | Population based | United States | 1976–94 | 40,000 | 30–55 |
| CC | Harris | 1992 | J Natl Cancer Inst | Hospital based | United States | 1987–89 | 1,124 | (55) |
| CC | Henrich | 1998 | J Clin Epidemiol | Hospital based | United States | 1987–92 | 654 | 47–88 (66) |
| CC | Hiatt | 1984 | Cancer | Population based | United States | 1971–79 | 239 | 37–56 |
| CC | Hoover | 1981 | J Natl Cancer Inst | Population based | United States | 1969–75 | 956 | (57.3) |
| CC | Horwitz | 1984 | Am J Med | Hospital based | United States | 1976–79 | 921 | >45 (63) |
| CC | Huang | 1999 | Int J Cancer | Hospital based | Taiwan | 1995–96 | 300 | 26–82 (49.6) |
| CC | Hulka | 1982 | Am J Obstet Gynecol | Hospital based | United States | 1977–78 | 772 | (64.8) |
| CC | Jick | 1980 | Am J Epidemiol | Hospital based | United States | 1975–78 | 236 | 45–64 |
| CC | Kaufman | 1984 | JAMA | Hospital based | United States, Canada | 1976–81 | 1,317 | <70 (51) |
| CC | Kaufman | 1991 | Am J Epi | Hospital based | United States, Canada | 1980–86 | 3,763 | 40–69 (59) |
| CC | Kelsey | 1981 | J Natl Cancer Inst | Hospital based | United States | 1977–79 | 1,173 | 45–74 |
| CC | Kirsh | 2002 | Cancer Causes Control | Population based | Canada | 1995–96 | 807 | 20–74 |
| CC | La Vecchia | 1992 | Int J Cancer | Hospital based | Italy | 1983–85 | 5,606 | <75 |
| CC | La Vecchia | 1986 | Int J Cancer | Hospital based | Italy | 1983–85 | 1,524 | <75 |
| CC | La Vecchia | 1995 | Br J Cancer | Hospital based | Italy | 1991–94 | 5,157 | 23–74 |
| CC | Levi | 1996 | Eur J Cancer Prev | Hospital based | Swiss | 1990–95 | 737 | 23–70 |
| CC | Li | 2002 | Cancer | Population based | United States | 1992–94 | 769 | 30–74 |
| CC | Li | 2000 | Cancer | Population based | United States | 1988–90 | 1,029 | 50–64 |
| CC | Lipworth | 1995 | Int J Cancer | Hospital based | Greece | 1983–94 | 2,368 | (56.4) |
| CC | Magnuson | 1999 | Int J Cancer | Population based | Sweden | 1993–95 | 5,408 | 50–74 |
| CC | McDonald | 1986 | Breast Cancer Res Treat | Population based | United States | 1977–78 | 714 | 50–74 |
| CC | Moorman | 2000 | Am J Pub Health | Population based | United States | 1993–96 | 804 | 20–74 |
| CC | Newcomb | 2002 | Cancer Epidemiol Biomarkers Prev | Population based | United States | 1992–94 | 10,869 | 50–79 |
| CC | Newcomb | 1995 | Am J Epidemiol | Population based | United States | 1989–91 | 6,828 | <75 |
| CC | Nomura | 1985 | NCI Monogr | Hospital based | United States | 1975–80 | 344 | 45–74 |
| CC | Nomura | 1986 | Int J Cancer | Hospital based | United States | 1975–80 | 344 | 45–74 |
| CC | Olsson | 2001 | Br J Cancer | Population based | Sweden | 1990–92 | 29,508 | 25–65 |
| CC | Palmer | 1991 | Am J Epidemiol | Population based | Canada | 1982–86 | 1,821 | <70 |
| CC | Persson | 1997 | Int J Cancer | Population based | Sweden | 1990–92 | 2,175 | 40–74 |
| CC | Pike | 2000 | Steroids | Population based | United States | 1987–96 | 3,534 | 55–64 |
| CC | Rohan | 1988 | Med J Aust | Population based | Australia | 1982–84 | 569 | 20–74 |
| CC | Ross | 2000 | J Natl Cancer Inst | Population based | United States | 1987–96 | 3,534 | 55–64 |
| CC | Ross | 1980 | JAMA | Population based | United States | 1971–77 | 414 | 50–74 |
| CC | Sartwell | 1977 | J Natl Cancer Inst | Hospital based | United States | 1969–72 | 651 | 20–74 |
| CC | Sherman | 1983 | Cancer | Hospital based | United States | 1974–78 | 226 | (60) |
| CC | Stanford | 1995 | JAMA | Population based | United States | 1988–90 | 942 | 50–64 |
| CC | Talamini | 1985 | Int J Epidemiol | Hospital based | Italy | 1980–83 | 741 | 27–79 |
| CC | Tavani | 1997 | Cancer Epidemiol Biomarkers Prev | Hospital based | Italy | 1981–94 | 5,984 | 22–74 |
| CC | Weinstein | 1993 | Int J Epidemiol | Population based | United States | 1984–86 | 1,697 | 20–79 |
| CC | Weiss | 2002 | Obstet Gynecol | Population based | United States | 1994–98 | 3,823 | 35–64 |
| CC | Wingo | 1987 | JAMA | Population based | United States | 1980–82 | 3,014 | 25–54 |
| CC | Wynder | 1978 | Cancer | Hospital based | United States | 1969–75 | 897 | >30 |
| CC | Yang | 1992 | Cancer Causes Control | Population based | Canada | 1988–89 | 1,384 | <75 |
| Cohort | Adami | 1989 | Int J Cancer | Population based | Sweden | 1977–87 | 23,244 | .35 (54.5) |
| Cohort | Beral | 2003 | Lancet | Population based | United Kingdom | 1996–01 | >1 mill | 50–64 |
| Cohort | Bland | 1980 | Cancer | Population based | United States | N/R | 405 | 37–84 |
| Cohort | Buring | 1987 | Am J Epidemiol | Population based | United States | 1976–80 | 33,335 | 30–55 |
| Cohort | Chen | 2002 | Ann Intern Med | Population based | United States | 1980–94 | 44,187 | 30–55 |
| Cohort | Colditz | 1995 | N Engl J Med | Population based | United States | 1976–86 | 69,586 | 30–55 |
| Cohort | Colditz | 1990 | JAMA | Population based | United States | 1976–86 | 23,607 | 30–55 |
| Cohort | Colditz | 1992 | Cancer Causes Control | Population based | United States | 1976–86 | 23,965 | 30–55 |
| Cohort | Colditz | 2000 | Am J Epidemiol | Population based | United States | 1976–86 | 58,520 | 30–55 |
| Cohort | DeLignieres | 2002 | Climacteric | Population based | France | 1975–87 | 3,175 | 20–59 |
| Cohort | Folsom | 1995 | Am J Public Health | Population based | United States | 1986–91 | 41,837 | 55–69 |
| Cohort | Gambrell | 1983 | Obstet Gynecol | Hospital based | United States | 1975–81 | 5,563 | 31–92 |
| Cohort | Gapstur | 1999 | JAMA | Population based | United States | 1986–96 | 37,105 | 55–69 |
| Cohort | Gapstur | 1995 | Cancer Epidemiol Biomarkers Prev | Population based | United States | 1986–92 | 37,105 | 55–69 |
| Cohort | Goodman | 1997 | Prev Med | Population based | Japan | 1979–81 | 22,200 | 30+ |
| Cohort | Hammond | 1979 | Am J Obstet Gynecol | Hospital based | United States | 1974 | 610 | (43) |
| Cohort | Henderson | 1991 | Arch Intern Med | Population based | United States | 1981–85 | 8,881 | (73) |
| Cohort | Hunt | 1987 | Br J Obstet Gynaecol | Menopause clinics | United Kingdom | 1978–82 | 4,544 | (50) |
| Cohort | Hunt | 1990 | Br J Obstet Gynaecol | Menopause clinics | United Kingdom | 1978–82 | 4,544 | (50) |
| Cohort | Lando | 1999 | Am J Prev Med | Population based | United States | 1971–74 | 5,761 | 25–74 (55) |
| Cohort | Lucas | 1998 | Am J Epidemiol | Population based | United States | 1986–92 | 7,250 | >65 |
| Cohort | Manjer | 2001 | Int J Cancer | Population based | Sweden | 1983–92 | 5,865 | (54.1) |
| Cohort | Miller | 1992 | Can Med Assoc J | Population based | Canada | 1980–85 | 39,405 | 50–59 |
| Cohort | Mills | 1989 | Cancer | Population based | United States | 1976–82 | 20,341 | (55.4) |
| Cohort | Olsson | 2001 | Br J Cancer | Population based | Sweden | 1990–92 | 29,508 | 25–65 |
| Cohort | Persson | 1996 | Int J Cancer | Population based | Sweden | 1977–80 | 22,579 | (54.5) |
| Cohort | Persson | 1999 | Cancer Causes Control | Population based | Sweden | 1977–80 | 23,246 | <70 |
| Cohort | Porch | 2002 | Cancer Causes Control | Population based | United States | 1993–00 | 17,835 | >45 |
| Cohort | Pukkala | 2001 | Cancer Causes Control | Population based | Finland | 1994–97 | 94,505 | All |
| Cohort | Risch | 1994 | Am J Epidemiol | Population based | Canada | 1976–90 | 32,790 | 43–49 |
| Cohort | Schairer | 1994 | Cancer Causes Control | Population based | United States | 1973–80 | 42,020 | (57.4) |
| Cohort | Schairer | 1997 | Epi | Population based | Sweden | 1977–87 | 23,246 | >35 (54.5) |
| Cohort | Schairer | 1999 | J Natl Cancer Inst | Population based | United States | 1973–80 | 2,675 | (58) |
| Cohort | Schairer | 2000 | JAMA | Population based | United States | 1973–80 | 2,082 | (58) |
| Cohort | Schuurman | 1995 | Cancer Causes Control | Population based | Netherlands | 1986 | 62,573 | 55–69 |
| Cohort | Sellers | 1997 | Ann Intern Med | Population based | United States | 1986–93 | 35,919 | 55–69 |
| Cohort | Sourander | 1998 | Lancet | Population based | Finland | 1987–95 | 6,433 | 57–65 |
| Cohort | Sturgeon | 1995 | Epidemiology | Population based | United States | 1973–80 | 49,017 | (57.4) |
| Cohort | Vakil | 1983 | Cancer Detect Prev | 20 private practices | Canada | 1960–70 | 1,483 | 32–62 |
| Cohort | Willis | 1996 | Cancer Causes Control | Population based | United States | 1982–91 | 676,526 | 59.2 |
| Cohort/CC | Bergkvist | 1989 | N Engl J Med | Population based | Sweden | 1977–84 | 23,244 | (53.7) |
| RCT | Hulley | 1998 | JAMA | Population based | United States | 1993–98 | 2,763 | (67) |
| RCT | Hulley | 2002 | JAMA | Population based | United States | 1993–00 | 2,763 | (67) |
| RCT | Nachtigal | 1992 | Obstet Gynecol | Hospital based | United States | 1965–88 | 164 | (55) |
| RCT | Rossouw | 2002 | JAMA | Population based | United States | 1993–98 | 16,608 | 50–79 |
CC, case-control; RCT, randomized controlled trial.
Study size = number of breast cancer cases.
Appendix 4. Studies included in other meta-analyses but excluded in this report
| Author, Year | Journal | Reason for rejection |
|---|---|---|
| Siskind, 1989 | Am J Epidemiol | no HT data |
| Ravnihar, 1979 | Eur J Cancer | OCPs not HT |
| Hislop, 1986 | Cancer Detect Preven | no HT data |
| Lawson, 1981 | Am J Epidemiol | no differentiation between OCPs and HT |
| Byrd, 1977 | Ann Surg | case series |
| Thomas, 1982 | J Natl Cancer Inst | case series |
| Hoover, 1976 | N Engl J Med | case series |
| Craig, 1974 | J Natl Cancer Inst | cross-sectional study |
| Burch, 1975 | Front Hormone Res | case series |
| Dupont, 1999 | Cancer | case series |
| Dupont, 1989 | Cancer | case series |
| La Vecchia, 1995 | Br J Cancer | did not differentiate HT into ET and CHT |
| Levi, 1996 | Eur J Cancer Prev | did not differentiate HT into ET and CHT |
| Included in Collaborative Group’s meta-analysis | ||
| Lee, 1987 | J Natl Cancer Inst | OCPs not HT |
| Paul, 1990 | Int J Cancer | OCPs not HT |
| Ursin, 1992 | Epidemiology | OCPs not HT |
| Rookus, 1994 | Lancet | OCPs not HT |
| White, 1994 | J Natl Cancer Inst | OCPs not HT |
| Brinton, 1995 | J Natl Cancer Inst | OCPs not HT |
| Morabia, 1993 | Prev Med | OCPs not HT |
| Vessey, 1983 | Br J Cancer | OCPs not HT |
| Ravnihar, 1988 | Neoplasia | OCPs not HT |
| WHO Collab, 1990 | Br J Cancer | OCPs not HT |
OCPs, oral contraceptives; HT, (postmenopausal) hormone therapy; ET, (postmenopausal) estrogen therapy; CHT, (postmenopausal) combined hormone therapy.
APPENDIX 5. Funnel plots for publication bias
Footnotes
The funding sources, Berlex Labs and the VA/RWJ Clinical Scholars Program, had no role in the collection, analysis, or interpretation of the data or in the decision to submit the manuscript for publication.
References
- 1.Kirschstein R. Menopausal hormone therapy: summary of a scientific workshop. Ann Intern Med. 2003;138:361–364. doi: 10.7326/0003-4819-138-4-200302180-00029. [DOI] [PubMed] [Google Scholar]
- 2.Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. N Engl J Med. 2003;348:645–650. doi: 10.1056/NEJMsb022365. [DOI] [PubMed] [Google Scholar]
- 3.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 4.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 5.Hulley S, Furberg C, Barrett-Connor E, et al. Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:58–66. doi: 10.1001/jama.288.1.58. [DOI] [PubMed] [Google Scholar]
- 6.Humphrey LL. Hormone Replacement Therapy and Breast Cancer: Systematic Evidence Review Number 14 Rockville, MD: Agency for Health Care Research and Quality, US Department of Health and Human Services, 2002. [PubMed]
- 7.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047–1059. [PubMed] [Google Scholar]
- 8.Sillero-Arenas M, Delgado-Rodriguez M, Rodigues-Canteras R, Bueno-Cavanillas A, Galvez-Vargas R. Menopausal hormone replacement therapy and breast cancer: a meta-analysis. Obstet Gynecol. 1992;79:286–294. [PubMed] [Google Scholar]
- 9.Colditz GA, Egan KM, Stampfer MJ. Hormone replacement therapy and risk of breast cancer: results from epidemiologic studies. Am J Obstet Gynecol. 1993;168:1473–1480. doi: 10.1016/s0002-9378(11)90784-4. [DOI] [PubMed] [Google Scholar]
- 10.US Preventive Services Task Force. Postmenopausal hormone replacement therapy for primary prevention of chronic conditions: recommendations and rationale. Ann Intern Med. 2002;137:834–839. doi: 10.7326/0003-4819-137-10-200211190-00013. [DOI] [PubMed] [Google Scholar]
- 11.FDA: Hormone therapy drug labels to change. 2003 vol: Reuters Health 2003.
- 12.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 13.Porch JV, Lee IM, Cook NR, Rexrode KM, Burin JE. Estrogen-progestin replacement therapy and breast cancer risk: the Women’s Health Study (United States) Cancer Causes Control. 2002;13:847–854. doi: 10.1023/a:1020617415381. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Savitz DA. Interpreting Epidemiologic Evidence: Strategies for Study Design and Analysis New York: Oxford University Press; 2003.
- 16.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20:21–35. doi: 10.1016/s0749-3797(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 19.Greenland S, O’Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics. 2001;2:463–471. doi: 10.1093/biostatistics/2.4.463. [DOI] [PubMed] [Google Scholar]
- 20.Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. JAMA. 2000;95:89–98. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 21.Stanford JL, Weiss NS, Voigt LF, Daling JR, Habel LA, Rossing MA. Combined estrogen and progestin hormone replacement therapy in relation to risk of breast cancer in middle-aged women. JAMA. 1995;274:137–142. [PubMed] [Google Scholar]
- 22.Henrich JB, Kornguth PJ, Viscoli CM, Horwitz RI. Postmenopausal estrogen use and invasive versus in situ breast cancer risk. J Clin Epidemiol. 1998;51:1277–1283. doi: 10.1016/s0895-4356(98)00116-4. [DOI] [PubMed] [Google Scholar]
- 23.Chen CL, Weiss NS, Newcomb P, Barlow W, White E. Hormone replacement therapy in relation to breast cancer. JAMA. 2002;287:734–741. doi: 10.1001/jama.287.6.734. [DOI] [PubMed] [Google Scholar]
- 24.Newcomb PA, Titus-Ernstoff L, Egan KM, et al. Postmenopausal estrogen and progestin use in relation to breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:593–600. [PubMed] [Google Scholar]
- 25.Weiss LK, Burkman RT, Cushing-Haugen KL, et al. Hormone replacement therapy regimens and breast cancer risk(1) Obstet Gynecol. 2002;100:1148–1158. doi: 10.1016/s0029-7844(02)02502-4. [DOI] [PubMed] [Google Scholar]
- 26.Mills PK, Beeson WL, Phillips RL, Fraser GE. Prospective study of exogenous hormone use and breast cancer in Seventh-day Adventists. Cancer. 1989;64:591–597. doi: 10.1002/1097-0142(19890801)64:3<591::aid-cncr2820640305>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 27.Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332:1589–1593. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- 28.Folsom AR, Mink PJ, Sellers TA, Hong CP, Zheng W, Potter JD. Hormonal replacement therapy and morbidity and mortality in a prospective study of postmenopausal women. Am J Public Health. 1995;85:1128–1132. doi: 10.2105/ajph.85.8_pt_1.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucas FL, Cauley JA, Stone RA, et al. Bone mineral density and risk of breast cancer: differences by family history of breast cancer. Study of Osteoporotic Fractures Research Group. Am J Epidemiol. 1998;148:22–29. doi: 10.1093/oxfordjournals.aje.a009554. [DOI] [PubMed] [Google Scholar]
- 30.Sourander L, Rajala T, Raiha I, Makinen J, Erkkola R, Helenius H. Cardiovascular and cancer morbidity and mortality and sudden cardiac death in postmenopausal women on oestrogen replacement therapy (ERT) Lancet. 1998;352:1965–1969. doi: 10.1016/S0140-6736(98)05066-1. [DOI] [PubMed] [Google Scholar]
- 31.Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283:485–491. doi: 10.1001/jama.283.4.485. [DOI] [PubMed] [Google Scholar]
- 32.Ross RK, Paganini-Hill A, Gerkins VR, et al. A case-control study of menopausal estrogen therapy and breast cancer. JAMA. 1980;243:1635–1639. [PubMed] [Google Scholar]
- 33.Whitehead MI, Townsend PT, Pryse-Davies J, et al. Actions of progestins on the morphology and biochemistry of the endometrium of postmenopausal women receiving low-dose estrogen therapy. Am J Obstet Gynecol. 1982;142:791–795. doi: 10.1016/s0002-9378(16)32490-5. [DOI] [PubMed] [Google Scholar]
- 34.Stahlberg C, Pederson AT, Lynge E, Ottesen B. Hormone replacement therapy and risk of breast cancer: the role of progestins. Acta Obstet Gynecol Scand. 2003;82:335–344. [PubMed] [Google Scholar]
- 35.Daling JR, Malone KE, Doody DR, et al. Relation of regimens of combined hormone replacement therapy to lobular, ductal, and other histologic types of breast carcinoma. Cancer. 2002;95:2455–2464. doi: 10.1002/cncr.10984. [DOI] [PubMed] [Google Scholar]
- 36.Kirsh V, Kreiger N. Estrogen and estrogen-progestin replacement therapy and risk of postmenopausal breast cancer in Canada. Cancer Causes Control. 2002;13:583–590. doi: 10.1023/a:1016330024268. [DOI] [PubMed] [Google Scholar]
- 37.Ravnihar B, Seigel DG, Lindtner J. An epidemiologic study of breast cancer and benign breast neoplasias in relation to the oral contraceptive and estrogen use. Eur J Cancer. 1979;15:395–405. doi: 10.1016/0014-2964(79)90074-4. [DOI] [PubMed] [Google Scholar]
- 38.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 39.Humphrey LL, Chan BK, Sox HC. Postmenopausal hormone replacement therapy and the primary prevention of cardiovascular disease. Ann Intern Med. 2002;137:273–284. doi: 10.7326/0003-4819-137-4-200208200-00012. [DOI] [PubMed] [Google Scholar]
- 40.Grodstein F, Manson JE, Stampfer MJ. Postmenopausal hormone replacement therapy [Letter] Ann Intern Med. 2003;138:688. doi: 10.7326/0003-4819-138-8-200304150-00028. [DOI] [PubMed] [Google Scholar]
- 41.Laine C. Postmenopausal hormone replacement therapy: how could we have been so wrong? Ann Intern Med. 2002;137:290. doi: 10.7326/0003-4819-137-4-200208200-00015. [DOI] [PubMed] [Google Scholar]
- 42.Meyerson SJ. Postmenopausal hormone replacement therapy [Letter] Ann Intern Med. 2003;138:687. doi: 10.7326/0003-4819-138-8-200304150-00026. [DOI] [PubMed] [Google Scholar]
- 43.Chausmer AB. Postmenopausal hormone replacement therapy [Letter] Ann Intern Med. 2003;138:687–688. doi: 10.7326/0003-4819-138-8-200304150-00027. [DOI] [PubMed] [Google Scholar]


