Keratoacanthomas associated with sorafenib therapy
Sorafenib (BAY 43-9006; Nexavar®) is one of the new generation of oral small-molecule anti-neoplastic agents targeting multiple kinases and is approved for use in the treatment of metastatic renal cell carcinoma. There are currently greater than 50 NIH-sponsored clinical trials investigating sorafenib for the treatment of other solid cancers.1
Sorafenib inhibits several tyrosine and serine/threonine kinases (vascular endothelial growth factor (VEGF) receptor 2 and 3, platelet derived growth factor (PDGF) receptor B-Raf, Raf-1, Flt3, c-kit, β, and RET).2,3 Common sorafenib toxicities include diarrhea, hypertension, fatigue, and cutaneous lesions (facial and scalp erythema, focal acral erythema/acral erythrodysesthesia/hand-foot syndrome, alopecia, and splinter hemorrhages).
Herein we report three patients without a history of skin cancer or previous exposure to radiation, who developed keratoacanthomas (KA) during treatment with sorafenib. All three patients had Fitzpatrick type II skin and a history of significant photodamage.
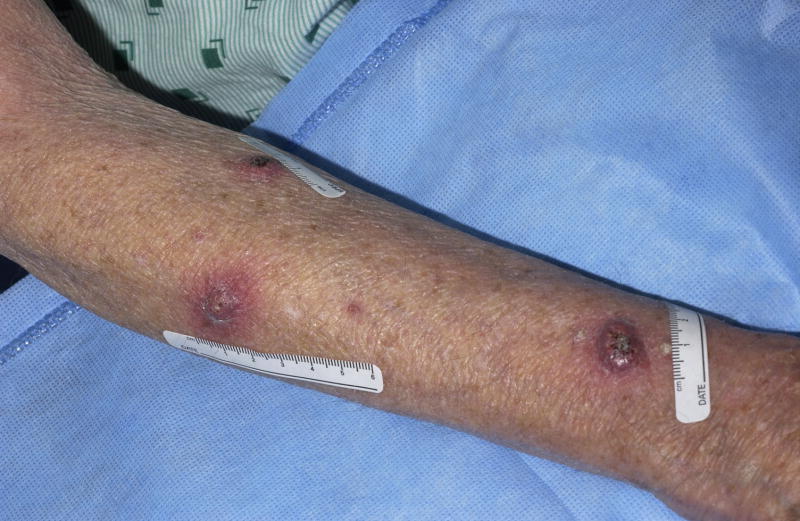
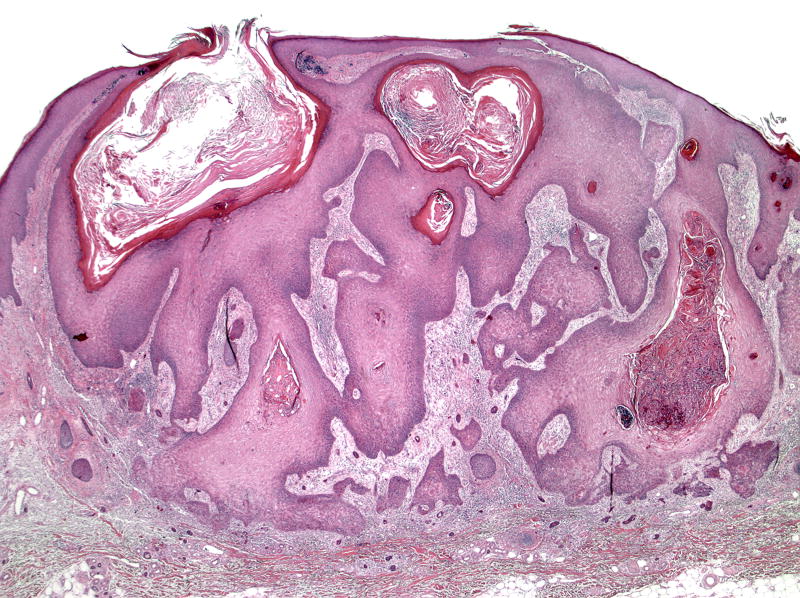
A 74-year-old white male with metastatic lung cancer developed nine rapidly growing dome-shaped tender nodules with central keratotic cores on his upper extremities and right neck after three months of sorafenib (400mg BID) (Figure 1). Excisional biopsy confirmed the clinical diagnosis of KA (Figure 2). Although the KAs decreased in size during a 2-week drug holiday, the KAs enlarged again upon restarting sorafenib. Additional KAs later developed, which eventually led to discontinuation of sorafenib. All KAs were surgically excised.
Figure 1.
Figure 2.
The second patient was a 65-year-old white male treated with sorafenib, 200 mg BID, for metastatic prostate cancer. Sorafenib was decreased to 200mg QD due to worsening neuropathy and fatigue. Biopsy of a small papule on his right nasal sidewall revealed an early KA. Within the next two months, he developed multiple rapidly growing papules on his left cheek, trunk, and bilateral upper and lower extremities. Histology of six papules was consistent with KA. No new lesions developed with two additional months of sorafenib.
The third patient was a 63-year-old white female with metastatic leiomyosarcoma treated with sorafenib 200 mg BID for 7 months, then dose-reduced to 200 mg QD. After 14 months of sorafenib, a 6 x 8 mm papule with central keratotic core on the right forearm developed over three weeks. Excisional biopsy verified the diagnosis of KA. No other KAs developed following three additional months of therapy, when sorafenib was discontinued for disease progression.
The development of KAs has not been previously described in association with sorafenib. This finding has occurred in fewer than 3 % of patients treated with sorafenib at our institution. Putative causes of keratoacanthomas include immunosuppression,4 photodamage, viral infection (HPV), genetic predisposition, and chemical carcinogen exposure. Sorafenib is not a classic immunosuppressive agent and has not been associated with increased risk of opportunistic infections.
Because it may be difficult clinically to differentiate KAs from squamous cell carcinoma, histologic examination following complete excision is recommended. Although KAs have been reported to regress spontaneously, the clinical course of KAs in this setting is unknown. If patients continue to develop multiple large KAs that require repeated excisions (as in Case 1), discontinuation of sorafenib should be considered. However, the morbidity of these lesions must be weighed against the benefit this novel drug may be exerting against a patient’s primary or metastatic disease.
Footnotes
Conflict of Interest Disclosure: None declared
Funding source: None
References
- 1. [Accessed August 8, 2006.]; www.clinicaltrials.gov.
- 2.Strumberg D, Seeber S. Raf kinase inhibitors in oncology. Onkologie. 2005;28:101–7. doi: 10.1159/000083373. [DOI] [PubMed] [Google Scholar]
- 3.Carlomagno F, Anaganti S, Guida T, et al. BAY 43-9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst. 2006;98:326–34. doi: 10.1093/jnci/djj069. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan JJ, Colditz GA. Keratoacanthoma in a sub-tropical climate. Australas J Dermatol. 1979;20:34–40. doi: 10.1111/j.1440-0960.1979.tb00122.x. [DOI] [PubMed] [Google Scholar]