Abstract
OBJECTIVE
To review the clinical features of Parkinson disease (PD) and other causes of motor parkinsonism with an emphasis on diagnosis in elderly patients.
SOURCES OF INFORMATION
MEDLINE and Google Scholar were searched for original research articles describing clinical diagnosis of parkinsonism. Consensus statements and articles summarizing diagnostic criteria for parkinsonian syndromes were also reviewed. Most evidence was levels II or III.
MAIN MESSAGE
Diagnosis of PD is made clinically and can be challenging. In older patients, PD can present with general functional decline and nonspecific symptoms. Clinical criteria for diagnosing PD and the TRAP mnemonic can be helpful. A 2-week trial of levodopa-carbidopa treatment can be considered. Specific signs and a minimal response to levodopa treatment suggest other causes of parkinsonism. Clinical features of other causes of parkinsonism are reviewed in the article.
CONCLUSION
Parkinsonism and PD are common in older patients. Family physicians should consider parkinsonism in the differential diagnosis of patients who have falls and exhibit general functional decline.
Abstract
OBJECTIF
Rappeler les caractéristiques cliniques de la maladie de Parkinson (MP) et les autres causes de parkinsonisme moteur, avec une insistance particulière sur le diagnostic chez la personne âgée.
SOURCE DE L’INFORMATION
On a recherché dans MEDLINE et Google Scholar les articles de recherche décrivant le diagnostic clinique du parkinsonisme. On a également révisé des déclarations consensuelles et des articles résumant les critères diagnostiques du syndrome parkinsonien. Les preuves étaient principalement de niveaux II ou III.
PRINCIPAL MESSAGE
Le diagnostic de la MP est d’ordre clinique et il peut s’avérer difficile. Chez le patient âgé, la MP peut s’accompagner d’un déclin fonctionnel global et de symptômes non spécifiques. Les critères cliniques de diagnostic et l’aide mnémotechnique TRAP peuvent être utiles. On peut tenter un traitement de 2 semaines avec la lévodopa-carbidopa. Certains signes spécifiques et une faible réponse à la lévodopa suggèrent d’autres causes de parkinsonisme, lesquels sont passé en revue dans cet article.
CONCLUSION
Le parkinsonisme et la MP sont fréquents chez le patient âgé. Le médecin de famille devrait penser au parkinsonisme lors du diagnostic différentiel des patients qui présentent des chutes et un déclin fonctionnel général.
EDITOR’S KEY POINTS.
Parkinsonism and Parkinson disease (PD) are not infrequent causes of functional decline in elderly people, but the diagnosis is often missed early on because patients present with nonspecific symptoms.
Physicians should consider parkinsonism and PD in the differential diagnosis when older people present with recent decline. Use of the TRAP mnemonic is helpful: tremor, rigidity, akinesia, and postural instability. In addition, changes in handwriting (micrography) are often seen.
Once parkinsonism is identified, physicians should consider other causes besides PD, including medication side effects, vascular parkinsonism, and dementia with Lewy bodies. Drug-induced parkinsonism is most important to diagnose, given that it can be reversed.
A 2-week trial of levodopa-carbidopa will reveal a marked improvement in PD, but not in the other forms of parkinsonism.
POINTS DE REPÈRE DU RÉDACTEUR.
Le parkinsonisme et la maladie de Parkinson (MP) sont des causes relativement fréquentes de déclin fonctionnel chez le sujet âgé, mais le diagnostic est souvent manqué en phase précoce parce que les symptômes sont non spécifiques.
En présence d’un patient âgé qui présente un déclin fonctionnel, le médecin doit penser au parkinsonisme et à la MP lors du diagnostic différentiel. TRAP (pour tremor, rigidity, akinesia et postural instability) est une aide mnémotechnique utile. De plus, la calligraphie est souvent modifiée (micrographie).
Une fois le parkinsonisme identifié, le médecin doit envisager les causes autres que la MP, notamment les effets médicamenteux indésirables, le parkinsonisme vasculaire et la démence à corps de Lewy. Le parkinsonisme d’origine médicamenteuse est particulièrement important à identifier puisqu’il peut être corrigé.
Un traitement de 2 semaines à la lévodopa-carbidopa produira une amélioration importante dans la MP, mais non dans les autres formes de parkinsonisme.
Functional decline is common among frail older patients. The differential diagnosis of functional decline is large and includes medication side effects, congestive heart failure, unrecognized dementia or depression, and other common and uncommon illnesses.
Parkinson disease (PD) and parkinsonism sometimes present with a nonspecific history, and experienced physicians might initially miss the physical features of PD unless it is considered in the differential diagnosis. Idiopathic PD is the most common form of parkinsonism, but it is important for family physicians to be able to recognize other causes of parkinsonism. The purpose of this paper is to review the diagnostically important features of PD and to describe other causes of parkinsonism.
Case history
Mr T.G., aged 84, is brought to your office by his daughter because he has been losing weight over the last 8 months. He lives alone, and his daughter has noted that he has lost almost 5 kg and has become withdrawn. She reports that he is having difficulty with meal preparation and now requires personal assistance from home-care services for bathing and dressing. He seems to be depressed, as he has “lost his usual spark.” She also reports that he has fallen twice in the last few months when preparing food at the kitchen counter. He describes falling backward. Although he suffered no injuries, he has been less active because he fears falling again.
Sources of information
MEDLINE and Google Scholar were searched for original research articles on physical examination and clinical diagnosis of PD and related conditions. Articles describing examination maneuvers feasible in family physicians’ offices were the focus. The search aimed to identify articles on clinical assessment and criteria for diagnosing parkinsonism published subsequent to the systematic review of physical findings by Rao et al1 (ie, between 2002 and 2005). MeSH headings used were Parkinson disease/diagnosis, hypokinesia/diagnosis, neurological examination, and physical examination. Two consensus articles summarizing diagnostic criteria for multiple system atrophy and dementia with Lewy bodies (DLB) were used. Most of the evidence in research papers, articles about development of clinical criteria, and papers on methods used to develop consensus criteria was levels II or III. The authors’ clinical experiences are mentioned, where appropriate.
Main message
The prevalence of PD and parkinsonism increases with age. Researchers estimate that United Kingdom residents have a 1 in 60 chance of developing parkinsonism during their lives, and that the prevalence of PD in people older than 65 is almost 2%.2,3 In nursing homes, the prevalence is approximately 6%.4-6
Diagnosis of parkinsonism is challenging for all physicians. Studies have attempted to assess the accuracy of clinical diagnosis by performing structured clinical examinations or autopsies to confirm the diagnosis.7-10 About 15% of patients diagnosed with PD do not fulfill clinical or pathologic criteria for the disease, and the diagnosis can be easily missed. In studies of PD identification, up to 20% of PD patients had not been diagnosed by previous physicians (level II evidence).7,10
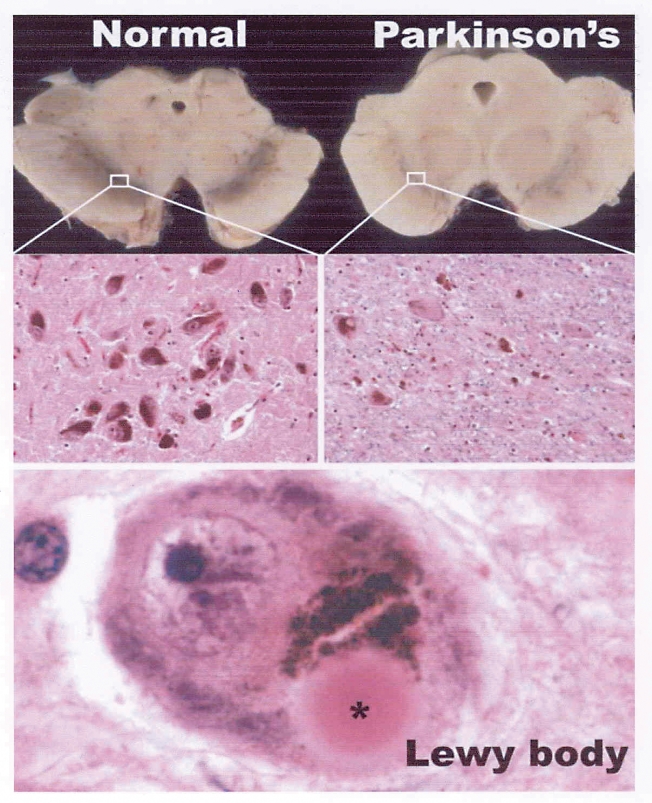
A brief review of the pathology of PD might help physicians to consider this diagnosis when seeing patients with a variety of presenting symptoms. The main pathology in PD is degeneration of pigmented neurons in the brainstem (Figure 1). Through a microscope you can typically see intracellular Lewy bodies. The neurons of the substantia nigra pars compacta are particularly affected, which results in depletion of dopamine to its primary projection area, the striatum. The overall outcome of this depletion is an overactive subthalamic nucleus, which increases activity of the major inhibitory output nuclei (globus pallidus and substantia nigra pars reticulata), which in turn results in increased thalamic inhibition and problems with motor output. The presence of Lewy bodies in the cortex as well as in deeper structures is the main feature that distinguishes PD from DLB.
Figure 1. Appearance of the substantia nigra in a normal midbrain and in the midbrain of a patient with Parkinson disease.
In the normal midbrain, the substantia nigra is darkly pigmented. In idiopathic Parkinson disease, marked pallor is due to degeneration and loss of dopaminergic neurons. Some of the surviving neurons contain characteristic eosinophilic Lewy body inclusions.
Recognition and diagnosis of PD
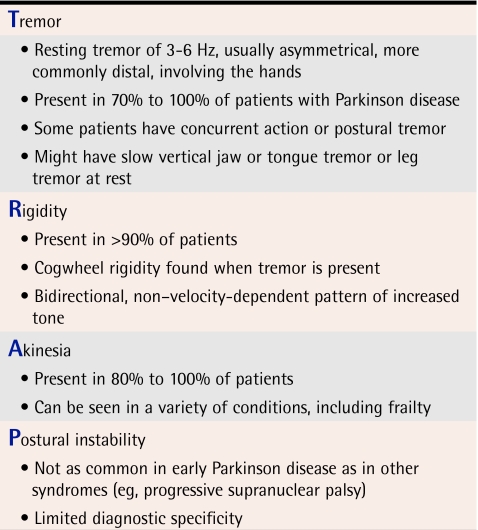
The first step to recognizing PD is to be aware of its common presenting features. One of the challenges of PD is that its symptoms and signs are often insidious. Unless physicians are actively seeking these signs and symptoms, they might consider a diagnosis of PD only when more prominent findings are noted. Most family physicians are aware of the mnemonic TRAP (Table 111,12), which describes the main clinical features of PD. Many of these findings, however, can be assessed only through some form of examination. Other features of PD become apparent purely by observing patients during visits and hence are important in family practices.
Table 1.
TRAP mnemonic for Parkinson disease
Gait
Although postural instability is a core feature, changes in gait usually start before instability is noted and often before patients start to have falls. The gait of patients with PD is somewhat similar to the so-called senile gait of aging: forward flexion of the trunk and decreased height and length of step. Patients with PD usually have noticeably less arm swing, sometimes asymmetrically. In our experience, this might be the first thing noted on casual observation of a patient that raises the possibility of parkinsonism. Observing patients when they turn might reveal the en bloc small steps (with minimal trunk rotation while turning around) and is an important part of gait assessment (level II evidence).1
Facial appearance
It is easy to miss the subtle development of the masklike face of PD (hypomimia). Recognition of hypomimia is increased when patients drool. Patients with PD and other parkinsonian diseases have difficulty swallowing saliva frequently enough and often drool. When patients are observed forever dabbing at their lips during interviews, we have found it important to consider parkinsonism, particularly if patients are taking neuroleptic drugs. Changes in the rate of blinking are sometimes observed; patients with PD often blink less frequently than most people do, but blepharospasm might also be present (level II evidence).1
Other features
Development of micrography might prompt a patient to visit a physician’s office or might be picked up by a doctor observing a patient doing a written task. Micrography tends to get progressively worse as patients write, although it can be evident in all writing (level II evidence).1
As noted in the case history, a nonspecific presentation of PD is common and includes psychiatric symptoms (level II evidence).13 Depression is the most common psychiatric feature early in the course of PD; its prevalence is estimated at 10%. Up to 50% of patients develop symptoms of depression during the course of PD.13,14 Psychotic features tend to be associated with pharmacologic treatment13 or with alternative diagnoses, such as DLB. Dementia is a frequent complication of PD that occurs after several years, but it is not common in the earlier stages of disease.
A systematic review published in 20031 evaluated the precision and accuracy of clinical examination for PD. Micrography had a positive likelihood ratio (LR) ranging from 2.8 to 5.9, the LR for shuffling gait ranged from 3.3 to 15, the LR for tremor as a symptom ranged from 1.3 to 17, and the LR for a combination of rigidity and bradykinesia was 4.5. Other symptoms mentioned included difficulty turning in bed (LR 13), opening jars (LR 6.1), and rising from a chair (LR 1.9 to 5.2). Difficulty rising from a chair can reflect frailty and proximal muscle weakness in elderly patients who do not have PD (level II evidence).1
Tremor might be a presenting complaint less frequently in older than in younger patients, but they might have more marked gait changes. Akinesia and rigidity do not appear to be influenced by age.15 Continued blinking during a series of glabellar taps has reasonable sensitivity for PD.16 Olfactory dysfunction might be noticed early on in both PD and DLB, but is not commonly assessed in family physicians’ offices17 (level II evidence).
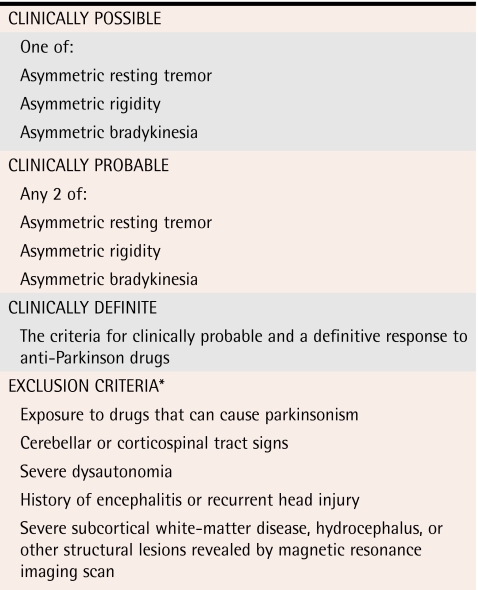
Several sets of clinical criteria for PD have been published and are summarized in Table 211,18,19 (levels II and III evidence). Specific motor maneuvers for diagnosing PD include rapidly alternating hands, tapping the thumb with the index finger, and opening and closing the hands to look for slowing and loss of amplitude. Getting patients to tap their heels on the ground by raising an entire leg at least 3 inches might reveal bradykinesia. Watching patients rise from an appropriate chair can be done easily in an examination room. A trial of levodopa-carbidopa with close follow-up for several weeks can be informative (level II evidence).20 Failure to respond to levodopa-carbidopa should lead to review of the diagnosis of PD.
Table 2.
*See Table 3 also.
Reprinted with permission from Samii et al.19
Differential diagnoses
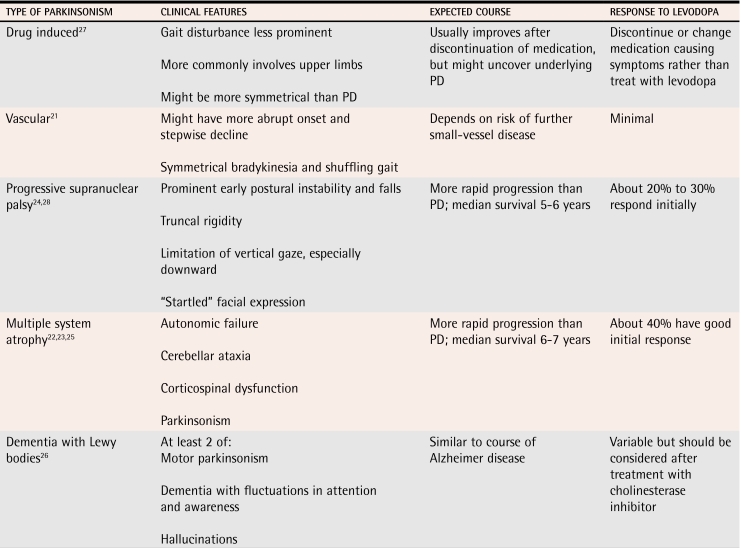
Once PD is identified, it is important to consider other conditions in the differential diagnosis. As mentioned above, diagnosis of PD is often found to be inaccurate at specialty clinics or at postmortem review. Presence of “red-flag” clinical features (Table 311,12) and lack of response to levodopa treatment should alert family physicians to the possibility of an alternative diagnosis. The main conditions to consider are drug-induced parkinsonism, vascular parkinsonism, progressive supranuclear palsy, multiple system atrophy, and DLB. Diagnostic criteria exist for most of these conditions; their main features are listed in Table 421-28 (levels II and III evidence).22-26 Although diagnosis of these conditions might seem intimidating, most of them can be identified in the office. The diagnosis is normally confirmed by a neurologist. Neuroimaging does not have a big role.
Table 3.
Table 4.
Clinical features of other causes of parkinsonism
PD—Parkinson disease.
Frailty.
Frail elderly people without PD can sometimes have features of parkinsonism. During normal aging, pigmented neurons are lost from the substantia nigra, but with a different pattern from that of PD.29 In our clinical experience, this can be particularly challenging when patients are weak or deconditioned after acute illness. A trial of levodopa can help distinguish between changes due to age and due to PD.
Medications.
The most important cause of parkinsonism to identify is drug-induced parkinsonism, because of the potential for reversal with discontinuation of the medication(s) causing symptoms. Physicians are aware of the risk of parkinsonism when using older neuroleptics, such as haloperidol. Atypical agents might still cause problems, especially in very old patients taking high doses.30 Risperidone is cited as the agent that most commonly causes parkinsonism, but all agents can cause it (level I evidence).31 Antiemetics, particularly prochlorperazine and metoclopramide, can cause parkinsonism after prolonged use, usually for at least several months.
Parkinson-plus
A variety of akinetic-rigid syndromes can cause parkinsonian features (Table 421-28). Of these, progressive supranuclear palsy is the most common and sometimes presents in patients younger than those in which PD presents. Diagnosis of progressive supranuclear palsy should be considered when patients have postural instability early on and limitation of downward gaze. Multiple system atrophy is a grouping of related conditions: Shy-Drager syndrome, in which autonomic nervous system failure predominates, olivopontocerebellar atrophy, and striatonigral degeneration. Multiple system atrophy should be considered when patients present with parkinsonism and marked autonomic dysfunction or with cerebellar findings (olivopontocerebellar atrophy). Most of these conditions have limited response to levodopa and tend to progress more rapidly than PD (level II evidence).25
Dementia with Lewy bodies
Related pathologically to PD dementia, DLB is a relatively common cause of dementia. There are diagnostic criteria, but an important feature is development of dementia temporally associated (usually within a year) with motor parkinsonism. Patients with PD usually develop dementia only after years with the disease and with severe motor symptoms. In patients with DLB, parkinsonism is usually relatively mild, and tremor is a less common finding than in PD. Treatment of psychiatric symptoms with cholinesterase inhibitors could worsen motor symptoms. Studies have not shown this to be common, but patients should be monitored (level I evidence).32
Case conclusion
You see Mr T.G. walking down the hall to the examining room and note that he has decreased arm swing and a shuffling gait. When you talk to him in the room, you notice he dabs at his mouth with a handkerchief regularly and has little facial expression. There is no tremor, but when he writes a sentence at your request, his writing shows progressive micrography. He has difficulty getting out of the chair in your examining room, and when he walks and comes back to sit down, you note en bloc turning. There is no evidence of depression.
You cautiously prescribe levodopa-carbidopa starting at half a tablet once daily and titrated up to 1 tablet at 07:00 AM, 11:00 AM, and 4:00 PM (given at least 45 minutes before meals to increase absorption). Upon return, he and his daughter report that his appetite and function have improved and that he has had no more falls. He can get up from the chair, and his gait has improved in speed and length of stride.
Conclusion
Parkinson disease and parkinsonism are common in older patients. Basic knowledge of presenting features could increase the likelihood of identifying conditions before functional decline sets in. Older patients sometimes present with nonspecific functional problems. Considering the differential diagnosis is important to identify treatable conditions and to access specialist support appropriately.
Levels of evidence.
Level I: At least one properly conducted randomized controlled trial, systematic review, or meta-analysis
Level II: Other comparison trials, non-randomized, cohort, case-control, or epidemiologic studies, and preferably more than one study
Level III: Expert opinion or consensus statements
Biographies
Dr Frank teaches in the Division of Geriatric Medicine at Queen’s University and at St Mary’s of the Lake Hospital in Kingston, Ont.
Dr Pari teaches in the Division of Neurology at Queen’s University and at the Kingston General Hospital.
Dr Rossiter teaches in the Department of Pathology and Molecular Medicine at Queen’s University and at the Kingston General Hospital.
References
- 1.Rao G, Fisch L, Srinivasan S, D’Amico F, Okada T, Eaton C, et al. Does this patient have Parkinson disease? JAMA. 2003;289(3):347–353. doi: 10.1001/jama.289.3.347. [DOI] [PubMed] [Google Scholar]
- 2.De Lau LM, Giesbergen PC, de Rijk MC, Hofman A, Koudstaal PJ, Breteler MM. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology. 2004;63(7):1240–1244. doi: 10.1212/01.wnl.0000140706.52798.be. [DOI] [PubMed] [Google Scholar]
- 3.De Rijk MC, Launer LJ, Berger K, Breteler MM, Dartigues JF, Baldereschi M, et al. Prevalence of Parkinson’s disease in Europe. A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 Suppl 5):21–23. [PubMed] [Google Scholar]
- 4.Lapane KL, Fernandez HH, Friedman JH. Prevalence, clinical characteristics, and pharmacologic treatment of Parkinson’s disease in residents in long-term care facilities. SAGE Study Group. Pharmacotherapy. 1999;19(11):1321–1327. doi: 10.1592/phco.19.16.1321.30877. [DOI] [PubMed] [Google Scholar]
- 5.Larsen JP. Parkinson’s disease as a community health problem: study in Norwegian nursing homes. The Norwegian Study Group of Parkinson’s Disease in the Elderly. BMJ. 1991;303(6805):741–743. doi: 10.1136/bmj.303.6805.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell SL, Kiely DK, Kiel DP, Lipsitz LA. The epidemiology, clinical characteristics, and natural history of older nursing home residents with a diagnosis of Parkinson’s disease. J Am Geriatr Soc. 1996;44(4):394–399. doi: 10.1111/j.1532-5415.1996.tb06408.x. [DOI] [PubMed] [Google Scholar]
- 7.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology. 1992;42(6):1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- 8.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson’s disease. Arch Neurol. 1993;50(2):140–148. doi: 10.1001/archneur.1993.00540020018011. [DOI] [PubMed] [Google Scholar]
- 9.Hughes AJ, Daniel SE, Lees AJ. The clinical features of Parkinson’s disease in 100 histologically proven cases. Adv Neurol. 1993;60:595–599. [PubMed] [Google Scholar]
- 10.Schrag A, Ben-Shlomo Y, Quinn N. How valid is the clinical diagnosis of Parkinson’s disease in the community? J Neurol Neurosurg Psychiatry. 2002;73(5):529–534. doi: 10.1136/jnnp.73.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson’s disease. Ann Neurol. 1992;32(Suppl 1):125–127. doi: 10.1002/ana.410320721. [DOI] [PubMed] [Google Scholar]
- 12.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56(1):33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 13.Nuti A, Ceravolo R, Piccinni A, Dell’Agnello G, Bellini G, Gambaccini G, et al. Psychiatric comorbidity in a population of Parkinson’s disease patients. Eur J Neurol. 2004;11(5):315–320. doi: 10.1111/j.1468-1331.2004.00781.x. [DOI] [PubMed] [Google Scholar]
- 14.Tandberg E, Larsen JP, Aarsland D, Cummings JL. The occurrence of depression in Parkinson’s disease. A community-based study. Arch Neurol. 1996;53(2):175–179. doi: 10.1001/archneur.1996.00550020087019. [DOI] [PubMed] [Google Scholar]
- 15.Nagayama H, Hamamoto M, Nito C, Takagi S, Miyazaki T, Katayama Y. Initial symptoms of Parkinson’s disease with elderly onset. Gerontology. 2000;46(3):129–132. doi: 10.1159/000022147. [DOI] [PubMed] [Google Scholar]
- 16.Brodsky H, Dat VK, Thomas M, Jankovic J. Glabellar and palmomental reflexes in Parkinsonian disorders. Neurology. 2004;63(6):1096–1098. doi: 10.1212/01.wnl.0000140249.97312.76. [DOI] [PubMed] [Google Scholar]
- 17.Katzenschlager R, Lees AJ. Olfaction and Parkinson’s syndromes: its role in differential diagnosis. Curr Opin Neurol. 2004;17(4):417–423. doi: 10.1097/01.wco.0000137531.76491.c2. [DOI] [PubMed] [Google Scholar]
- 18.Ward CD, Gibb WR. Research diagnostic criteria for Parkinson’s disease. Adv Neurol. 1990;53:245–249. [PubMed] [Google Scholar]
- 19.Samii A, Nutt JG, Ransom BR. Parkinson’s disease. Lancet. 2004;363:1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- 20.Clarke CE, Davies P. Systematic review of acute levodopa and apomorphine challenge tests in the diagnosis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2000;69(5):590–594. doi: 10.1136/jnnp.69.5.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zijlmans JC, Daniel SE, Hughes AJ, Revesz T, Lees AJ. Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov Disord. 2004;19(6):630–640. doi: 10.1002/mds.20083. [DOI] [PubMed] [Google Scholar]
- 22.Gilman S, Low PA, Quinn N, Albanese A, Ben-Shlomo Y, Fowler CJ, et al. Consensus statement on the diagnosis of multiple system atrophy. J Auton Nerv Syst. 1998;74(2-3):189–192. [PubMed] [Google Scholar]
- 23.Gilman S, Low P, Quinn N, Albanese A, Ben-Shlomo Y, Fowler C, et al. [Consensus on the diagnosis of multi-system atrophy]. Neurologia. 1999;14(9):425–428. [PubMed] [Google Scholar]
- 24.Litvan I, Agid Y, Calne D, Campbell B, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47(1):1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, McKeith I, et al. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord. 2003;18(5):467–486. doi: 10.1002/mds.10459. [DOI] [PubMed] [Google Scholar]
- 26.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson EW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 27.Hassin-Baer S, Sirota P, Korczyn AD, Treves TA, Epstein B, Shabtai H, et al. Clinical characteristics of neuroleptic-induced Parkinsonism. J Neural Transm. 2001;108(11):1299–1308. doi: 10.1007/s007020100006. [DOI] [PubMed] [Google Scholar]
- 28.Tolosa E, Valldeoriola F, Marti MJ. Clinical diagnosis and diagnostic criteria of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome). J Neural Transm Suppl. 1994;42:15–31. doi: 10.1007/978-3-7091-6641-3_2. [DOI] [PubMed] [Google Scholar]
- 29.Kish SJ, Shannak K, Rajput A, Deck JH, Homykiewicz O. Aging produces a specific pattern of striatal dopamine loss: implications for the etiology of idiopathic Parkinson’s disease. J Neurochem. 1992;58(2):642–648. doi: 10.1111/j.1471-4159.1992.tb09766.x. [DOI] [PubMed] [Google Scholar]
- 30.Alexopoulos GS, Streim J, Carpenter D, Docherty JP. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(Suppl 2):5–99. [PubMed] [Google Scholar]
- 31.Mullen J, Jibson MD, Sweitzer D. A comparison of the relative safety, efficacy, and tolerability of quetiapine and risperidone in outpatients with schizophrenia and other psychotic disorders: the quetiapine experience with safety and tolerability (QUEST) study. Clin Ther. 2001;23(11):1839–1854. doi: 10.1016/s0149-2918(00)89080-3. [DOI] [PubMed] [Google Scholar]
- 32.Wesnes KA, McKeith IG, Ferrara R, Emre M, Del Ser T, Spano PF, et al. Effects of rivastigmine on cognitive function in dementia with lewy bodies: a randomised placebo-controlled international study using the cognitive drug research computerised assessment system. Dement Geriatr Cogn Disord. 2002;13(3):183–192. doi: 10.1159/000048651. [DOI] [PubMed] [Google Scholar]