Abstract
Objectives
Carpal tunnel syndrome (CTS) is a common entrapment neuropathy caused by median nerve compression. This pilot clinical trial was designed to compare the safety and effectiveness of the lidocaine patch 5% to that of naproxen 500 mg twice daily for the treatment of neuropathic pain associated with CTS.
Methods
In this 6-week, randomized, parallel-group, open-label, multicenter study, participants from 2 practice sites, aged 18 to 75 years with clinical/electrodiagnostic evidence of CTS, were randomized to receive up to 3 lidocaine 5% patches every 24 hours or naproxen 500 mg twice daily for 6 weeks. Outcome assessments included mean changes between baseline and Week 6 average pain intensity (Brief Pain Inventory [BPI]: Question 5, Average Pain Intensity [API]), an Investigator Clinical Global Impression of Improvement (CGI-I) over the course of the treatment period and a comparison of patient satisfaction (Clinical Global Assessment of Treatment [CGAT]).
Results
One hundred patients were randomized in this study, 52 in the lidocaine patch 5% group and 48 in the naproxen 500 mg twice daily group. Significant reductions in API scores were observed between baseline and Week 6 for both lidocaine patch 5% (P < .0001) and naproxen 500 mg twice daily (P = .0004); however, there were no statistically significant differences between treatments (P = .083). There was a significant (P = .016) difference in the CGI-I for lidocaine patch 5% (51.1%) compared with naproxen 500 mg twice daily (24.3%). Whereas 71.8% of the lidocaine patch 5% patients reported being “satisfied” to “very satisfied” with the treatment, only 63.2% of naproxen 500 mg twice daily patients reported likewise, although the difference was not statistically significant. Both treatments were well tolerated. Two patients reported treatment-related adverse events in the lidocaine patch 5% group and 6 in the naproxen 500 mg twice daily group, all of which were considered mild or moderate in severity.
Conclusions
This study demonstrates that the lidocaine patch 5% is effective in significantly relieving the pain associated with CTS and is well tolerated. The patch may offer patients an effective, nonsystemic, noninvasive treatment for the management of their symptoms. Further controlled studies are warranted.
Readers are encouraged to respond to George Lundberg, MD, Editor of MedGenMed, for the editor's eye only or for possible publication via email: glundberg@medscape.net
Introduction
Carpal tunnel syndrome (CTS) is a common entrapment neuropathy of the median nerve at the wrist. The lifetime incidence of CTS has been estimated to range from 2.7% to 16.0%[1,2] and is more common in women.[3] It is associated with substantial economic impact, including costs of treatment and lost revenue for employers and employees,[4] and has been estimated to cost the patient alone approximately $30,000 over the course of the syndrome.[5]
Entrapment of the median nerve is thought to be the result of noninflammatory synovial fibrosis of the flexor tendons producing the constrictive neuropathy that may be the basis of idiopathic CTS.[6,7] Both physical and physiologic alterations in the median nerve may underlie the resultant neuropathy. Patient-reported symptoms include nocturnal hand paresthesias, swelling, weakness or clumsiness (leading to a loss of functionality), and a significant number of patients experience pain. Indeed, large clinical studies (500 to 1123 hands from 379 to 740 patients) have shown that from 55% to as many as 75% of patients with mild to moderate CTS also experience pain.[8–10] This pain may be the result of sodium channel alterations that have been reported following damage or insult to peripheral nerves as well as uninjured fibers in close proximity. The change in neuronal excitability (spontaneous and ectopic discharge) contributes to and maintains the associated pain state.[11–13] Given that the analgesia produced by the lidocaine patch 5% is thought to be a result of sodium membrane stabilization,[14] this peripherally acting, topical analgesic may be a safe and appropriate treatment alternative for those patients who have pain and are diagnosed with mild-to-moderate CTS.
The lidocaine patch 5% is currently approved for the relief of pain associated with postherpetic neuralgia (PHN)[15] and is unique in that it is a peripherally acting topical analgesic that is noninvasive, with minimal systemic absorption resulting in a reduced risk of drug-drug interactions; it has an excellent safety profile.[16] The effectiveness of the lidocaine patch 5% has been demonstrated for a number of different peripheral neuropathies,[17,18] chronic low back pain,[19–21] myofascial pain,[22,23] osteoarthritis,[24–28] leg ulceration,[29] and erythromelalgia.[30,31]
For mild-to-moderate cases of CTS, treatment options include splinting of the wrist, administration of corticosteroids (oral or via local injection), oral administration of nonsteroidal anti-inflammatory drugs (NSAIDs), and local injections of anesthetics; more severe cases require surgical release.[32–34] Given the paucity of conservative, nonsurgical treatment options for CTS,[35,36] we recently studied the effect of the lidocaine patch 5% compared with corticosteroid injection for the treatment of pain seen with mild-to-moderate CTS.[37] The results of that 4-week, randomized, parallel-group, open-label pilot study suggested that the lidocaine patch 5% was well tolerated and effective in treating the pain associated with CTS. Comparable improvement in symptoms was produced following local injection of corticosteroid, without the risks associated with injections.[32,38–41] To further elucidate the effectiveness of the lidocaine patch 5% for the relief of pain and impairment of normal functioning as a result of CTS, we conducted a 6-week, randomized pilot trial comparing the safety and effectiveness of daily application of the lidocaine patch 5% to an orally administered NSAID, naproxen (500 mg twice daily).
Materials and Methods
This study was a 6-week, randomized, parallel-group, multicenter study conducted in the United States. The Ethical Review Committee, Inc. (Kansas City, Missouri) reviewed and approved the study. Patients aged 18 to 75 years with clinical and electrodiagnostic evidence of CTS were recruited. Criteria for electrodiagnostic evidence of CTS were based on the practice guidelines issued by the American Association of Electrodiagnostic Medicine, the American Academy of Neurology, and the American Academy of Physical Medicine and Rehabilitation, which consisted of a median motor nerve distal latency of more than 4.20 msec or a difference of more than 0.6 msec between the median and ulnar sensory latencies when recorded from the fourth finger.[42] In addition, patients also were required to have persistent or recurrent pain, paresthesias, or positive Phalen's or Tinel's signs. Patients signed a written informed consent form (ICF) and HIPAA statement prior to enrollment. They were not given incentives to participate in the study other than treatment at no cost. Patients were excluded from the study if they presented with another peripheral neuropathy in the involved limb, had concomitant use of the lidocaine patch 5% for any other condition, had received an injection into the carpal tunnel of the study limb within the previous 8 weeks, had surgical release of the study limb within the previous 6 months, had participated in a clinical trial within the previous 30 days, or if they were pregnant or breastfeeding. In addition, those patients with a history of peptic ulcer disease, gastrointestinal bleed, or renal failure deemed medically significant by the investigator were also excluded.
Interventions
Patients were randomly assigned at baseline to receive the lidocaine patch 5% (up to a maximum of 3 patches - 420 cm2) or oral doses of naproxen 500 mg twice daily for 6 weeks. Patients in the patch group were instructed to cover as much of the volar surface of the wrist as possible for 24 hours per day using a maximum of 3 patches per day, changing the patches on a daily basis. Treatment was allocated to each patient in a strict consecutive order using a predefined randomization sequence that was stratified on the study site. Three visits to the physician's office at 1, 3, and 6 weeks after the initial visit were scheduled. Patients assigned to the lidocaine patch 5% group were instructed to cover the volar aspect of the wrist and as much of the painful area as possible for 24 hours per day (see above) with the patches changed daily. Patients were allowed to cut the lidocaine patch 5% to shape.
The use of any other analgesic for the pain associated with CTS was not allowed, although medication to treat secondary illnesses was allowed at a stable dose. Patients were questioned at each office visit as to any concomitant medication use as well as previous analgesic use for CTS. The initial administration of the lidocaine patch 5% or naproxen 500 mg twice daily was performed at the first office visit.
Compliance was assessed at each subsequent office visit by counting the number of patches or pills recorded by the patient used since the prior visit.
Outcome Assessments
Patient assessments were conducted at the first office visit to establish baseline and after 1, 3, and 6 weeks of treatment (Visits 2, 3, and 4, respectively). At each visit, each patient completed the Brief Pain Inventory (BPI), and the investigator completed the Clinical Global Impression of Severity (CGI-S) and assessed motor function and negative sensory symptoms (numbness). The primary efficacy measure was the mean change in average daily pain (BPI, Question 5) from baseline to Week 6 (Visit 4). Secondary endpoints included an Investigator Global Impression of Improvement in pain over the course of the treatment period and a comparison of patient satisfaction (CGAT).
Safety
Safety analysis was based on patient-reported symptoms and physician observations and included all patients who received study medications. Adverse events were classified according to the Medical Dictionary for Regulatory Activities (MedDRA), and the incidence of treatment-related events was summarized.
Statistical Analysis
The primary analysis population consisted of a modified intent-to-treat population with all randomized subjects having at least 1 postrandomization observation.
Within the 7-item interference subscale on the BPI, a “completed subscale” was one that had no more than 1 missing response; an incomplete subscale had more than 1 missing response. Missing values were replaced with the average of the nonmissing completed items in order to compute a sum score. If a patient failed to complete a form (for a total score, subscale total, or individual item) after randomization (all days or visits), that patient was considered as not having any postrandomization observations for that form and was excluded from the modified intent-to-treat population for the analysis of that assessment form.
The null hypothesis to be tested in this pilot study was that there is no difference between treatments for CTS. Statistical significance was defined as P < .05, two-sided. Paired t-tests were used to analyze change from baseline efficacy endpoints within subjects while treatment comparisons on continuous variables were tested by analysis of covariance (ANCOVA) with treatment group as the between subjects factor and baseline as the covariable. For continuous variables (ordinal variables with 5 or more values) measured across visits, a repeated measures ANCOVA was performed with treatment as the between-subjects factor and the visit X treatment interaction as the within-subjects factor. Ordinal variables with less than 5 values were analyzed using the Wilcoxon rank sum test.
Results
The disposition of all of the enrolled patients is shown in Figure 1. One hundred patients were enrolled in the study and randomly assigned to receive either daily applications of the lidocaine patch 5% (n = 52) or naproxen 500 mg twice daily (n = 48). The baseline characteristics of the patients were similar between groups (Table 1). The mean age of the lidocaine patch 5% group was 55.7 years, and patients were predominantly white (86.5%) and female (65.4%), whereas the mean age of the naproxen 500 mg twice daily group was 51.5 years and patients were 75% white and 85.4% female. The majority of patients in both treatment groups had mild or moderate CTS at baseline as determined by the Clinical Global Impression of Severity of CTS, with similar baseline average daily pain scores (lidocaine patch 5%: 4.5 ± 2.5 vs naproxen 500 mg twice daily: 4.9 ± 2.6).
Figure 1.

Patient disposition of all enrolled patients.
Table 1.
Patient Demographics and Baseline Characteristics
| Parameter | Lidocaine Patch 5% (n = 52) | Naproxen 500 mg Twice Daily (n = 48) |
|---|---|---|
| Mean age ± SD, yrs | 55.7 ± 16.0 | 51.5 ± 11.8 |
| Gender, n (%) | ||
| Male | 18 (34.6) | 7 (14.6) |
| Female | 34 (65.4) | 41 (85.4) |
| Race/Ethnicity, n (%) | ||
| White | 45 (86.5) | 36 (75.0) |
| Black | 5 (9.6) | 11 (22.9) |
| Hispanic | 2 (3.9) | 1 (2.1) |
| Presence of Select Concomitant Illnesses, n (%) | ||
| Allergy/Asthma | 23 (44.2) | 19 (39.6) |
| Cardiovascular | 15 (28.9) | 11 (22.9) |
| Dermatologic | 3 (5.8) | 0 |
| Diabetes | 5 (9.6) | 6 (12.5) |
| Gastrointestinal | 15 (28.9) | 9 (18.8) |
| Hepatobiliary | 2 (3.85) | 1 (2.1) |
| Musculoskeletal | 10 (19.2) | 11 (22.9) |
| Renal/Urologic | 3 (5.8) | 2 (4.2) |
| Other | 18 (34.6) | 16 (33.3) |
| Clinical Global Impression of Severity (mean ± SD) | 2.25 ± 0.59 | 2.29 ± 0.65 |
| Mild, n (%) | 4 (7.7) | 5 (10.4) |
| Moderate, n (%) | 31 (59.6) | 24 (50.0) |
| Severe, n (%) | 17 (32.7) | 19 (39.6) |
| Average pain intensity (mean ± SD) | 4.5 ± 2.5 | 4.9 ± 2.6 |
Of the 23 patients who did not complete the study, 11 patients (5 in the patch group and 6 in the naproxen 500 mg twice daily group), had at least 1 observation after randomization and were included in the intent-to-treat population for subsequent analysis. Three patients in the lidocaine patch 5% and 2 patients in the group taking naproxen 500 mg twice daily withdrew due to adverse events.
The mean changes from baseline to Week 6 of the BPI pain intensity scores are illustrated in Figure 2. The primary endpoint of average daily pain was significantly reduced in the lidocaine patch 5% group (P < .0001) and the naproxen 500 mg twice daily group (P = .0004) at Week 6 from baseline. In addition, although there was a statistically significant drop in the other pain parameters (worst pain, least pain, and pain right now) at Week 6 in the lidocaine patch 5% group (P < .0001) and the naproxen 500 mg twice daily group (P < .004) from baseline, there was no significant difference between treatments. The least square means (LSM) change from baseline of pain relief experienced by both treatment groups was estimated at 25.5% (P < .0001, data not shown). Furthermore, the LSM change from baseline of an overall BPI interference from pain was estimated as −10.7 and −10.2 for the lidocaine patch 5% and naproxen 500 mg twice daily groups, respectively (P < .0001; data not shown). As shown in Figure 3, patients in the lidocaine patch 5% group were more likely (51.1%) to be judged by the investigator as improved compared with the naproxen 500 mg twice daily group (24.3%), according to an Investigator Clinical Global Impressions of Improvement (CGI-I) (P = .016). Assessment of the patient's level of treatment satisfaction revealed that 71.8% of those treated with the lidocaine patch 5% were “satisfied” or “very satisfied” with their treatment compared with 63.2% in the naproxen 500 mg twice daily group (Figure 4). However, statistical analysis of overall patient satisfaction did not reveal any significant difference (P = .495). Neither motor function nor negative sensory symptoms changed over the course of the study.
Figure 2.

Mean change from baseline to Week 6 of BPI pain intensity subscores.
Figure 3.

Investigator Clinical Global Impression of Improvement.
Figure 4.
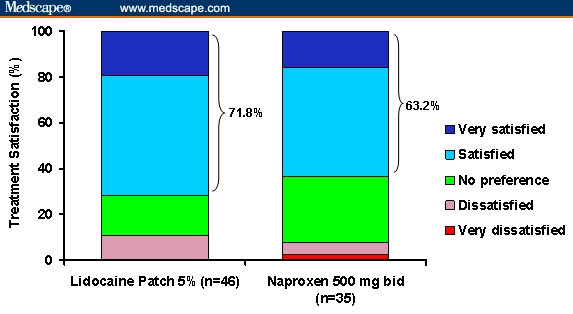
Overall patient satisfaction with treatment.
All of the adverse events (AEs) that were determined to be treatment related were mild-to-moderate in severity and are shown in Table 2. Overall, 2 patients (3.8%) experienced treatment-related adverse events with lidocaine patch 5% administration compared with 6 patients (12.5%) in the naproxen 500 mg twice daily group. Application site rash (n = 1; 1.9%) and gastrointestinal disorders, such as dyspepsia (n = 1; 1.9%) and reflux disease (n = 1; 1.9%), were reported in the lidocaine patch 5% group. In the naproxen 500 mg twice daily group, gastrointestinal disorders, such as nausea (3; 6.3%), dyspepsia (3; 6.3%), and frequent bowel movements (1; 2.1%), were reported along with nervous system disorders, such as dysgeusia (1; 2.1%) and loss of appetite (1; 2.1%).
Table 2.
Number (%) of Patients With Treatment-Related Adverse Events
| Lidocaine Patch 5% (n = 52), n (%) | Naproxen 500 mg Twice Daily (n = 48), n (%) | |
|---|---|---|
| Patients reporting AEs, n (%) | 13 (25.0) | 13 (27.1) |
| Patient withdrawals due to AEs | 3 (33.3) | 2 (14.3) |
| Patients reporting at least 1 TRAE (%) | 2 (3.8) | 7 (14.6) |
| TRAEs categorized by body system* | ||
| Nervous system disorders | 0 | 2 (4.2) |
| Dysgeusia | 0 | 1 |
| Loss of appetite | 0 | 1 |
| Gastrointestinal disorders | 1 (1.9) | 7 (14.6) |
| Nausea | 0 | 3 |
| Dyspepsia | 1 | 3 |
| Gastroesophageal reflux disease | 1 | 0 |
| Frequent bowel movements | 0 | 1 |
| Skin disorders/application site | 1 (1.9) | 0 |
| Rash | 1 | 0 |
Body system totals are not necessarily the sum of the individual adverse events because a patient may have reported 2 or more different adverse events in the same body system.
TRAE = treatment-related adverse event
Discussion
Use of the lidocaine patch 5%, a noninvasive, peripherally acting, topical analgesic for the treatment of pain associated with CTS, was comparable in efficacy to oral administration of naproxen 500 mg twice daily, without the NSAID-associated gastrointestinal AEs. Of interest was the statistically significant difference in the CGI-I between lidocaine patch 5% compared to naproxen 500 mg twice daily. Given the obvious difference in the ability of the investigator to identify those in the patch group, an unintended bias cannot be ruled out. Finally, the lidocaine patch 5% was well tolerated with no reported systemic AEs.
Although these data were generated in an open-label study, the results suggested that the lidocaine patch 5% may be an effective and safe pharmacotherapy for the treatment of mild-to-moderate CTS. Similar positive results were generated in a recent pilot study comparing the effects of the lidocaine patch 5% with local injection of corticosteroids.[37] Lidocaine patch 5% provided a comparable level of pain relief while avoiding the invasive nature of the corticosteroid injection with its associated risks. Together, these pilot studies are suggestive of the effectiveness and safety of the lidocaine patch 5% for the treatment of the pain and disability of CTS, and further controlled study is warranted. The lidocaine patch 5% targets localized damaged or dysfunctional nociceptors to block the pain signal by stabilizing abnormal sodium channels without causing an anesthetic effect, and reduces the risk of drug-drug interactions and systemic side effects due to its limited systemic absorption.
Conclusions
The lidocaine patch 5% is an effective pharmacotherapy for the treatment of mild-to-moderate pain associated with CTS and provides relief of similar magnitude to that produced following the commonly prescribed treatment with NSAIDs. The potential utility of the lidocaine patch 5% is that it may provide physicians with a noninvasive, peripherally acting, topical analgesic, thereby avoiding risk factors associated with systemic NSAID therapy. Further randomized, controlled trials are necessary to fully characterize the efficacy and safety of the lidocaine patch 5% in this patient population.
Funding Information
This study was supported by an unrestricted research grant from Endo Pharmaceuticals, Inc, Chadds Ford, Pennsylvania.
Contributor Information
Srinivas Nalamachu, Kansas City University of Medicine and Biosciences, Kansas City, Missouri; Mid America Physiatrists Overland Park, Kansas; Email: nalamachu@sbcglobal.net.
R.S. Crockett, United Biosource Corporation, Kansas City, Missouri.
Arnold R. Gammaitoni, Medical Affairs, Endo Pharmaceuticals, Inc., Chadds Ford, Pennsylvania.
Errol M. Gould, Medical Affairs, Endo Pharmaceuticals, Inc., Chadds Ford, Pennsylvania.
References
- 1.Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosen I. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282:153–158. doi: 10.1001/jama.282.2.153. [DOI] [PubMed] [Google Scholar]
- 2.Ferry S, Pritchard T, Keenan J, Croft P, Silman AJ. Estimating the prevalence of delayed median nerve conduction in the general population. Br J Rheumatol. 1998;37:630–635. doi: 10.1093/rheumatology/37.6.630. [DOI] [PubMed] [Google Scholar]
- 3.Stolp-Smith KA, Pascoe MK, Ogburn PL., Jr Carpal tunnel syndrome in pregnancy: frequency, severity, and prognosis. Arch Phys Med Rehabil. 1998;79:1285–1287. doi: 10.1016/s0003-9993(98)90276-3. [DOI] [PubMed] [Google Scholar]
- 4.Osterman AL, Whitman M, Porta LD. Nonoperative carpal tunnel syndrome treatment. Hand Clin. 2002;18:279–289. doi: 10.1016/s0749-0712(02)00023-9. [DOI] [PubMed] [Google Scholar]
- 5.Medical Condition News. Study to investigate carpal tunnel syndrome. Available at: http://www.news-medical.net/?id=3555 Accessed July 17, 2006.
- 6.Almquist EE. Painful conditions of the forearm, wrist, and hand. Bonica's Management of Pain. In: Loeser JD, Butler SH, Chapman CR, Turk DC, editors. Philadelphia, Pa: Lippincott, Williams & Wilkins; 2001. pp. 1084–1109. [Google Scholar]
- 7.Ettema AM, Amadio PC, Zhao C, Wold LE, An KN. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg Am. 2004;86-A:1458–1466. doi: 10.2106/00004623-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Padua L, Padua R, Aprile R, Tonali P. Italian multicentre study of carpal tunnel syndrome. Differences in the clinical and neurophysiological features between male and female patients. J Hand Surg [Br] 1999;24:579–582. doi: 10.1054/jhsb.1999.0255. [DOI] [PubMed] [Google Scholar]
- 9.Padua L, Padua R, Lo Monaco M, Aprile I, Tonali P. Multiperspective assessment of carpal tunnel syndrome. Neurology. 1999;53:1654–1659. doi: 10.1212/wnl.53.8.1654. [DOI] [PubMed] [Google Scholar]
- 10.Padua L, Lo Monaco M, Gregori B, Valente E, Padua R, Tonali P. Neurophysiological classification and sensitivity in 500 carpal tunnel syndrome hands. Acta Neurol Scand. 1997;96:211–217. doi: 10.1111/j.1600-0404.1997.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 11.Devor M, Govrin-Lippmann R, Angelides K. Na+ channel immunolocalization in peripheral mammalian axons and changes following nerve injury and neuroma formation. J Neurosci. 1993;13:1976–1992. doi: 10.1523/JNEUROSCI.13-05-01976.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold MS. Sodium channels and pain therapy. Curr Opin Anaesthesiol. 2000;13:565–572. doi: 10.1097/00001503-200010000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Gold MS, Weinreich D, Kim CS, et al. Redistribution of NaV1.8 in uninjured axons enables neuropathic pain. J Neurosci. 2003;23:158–166. doi: 10.1523/JNEUROSCI.23-01-00158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galer BS, Sheldon E, Patel N, Codding C, Burch F, Gammaitoni AR. Topical lidocaine patch 5% may target a novel underlying pain mechanism in osteoarthritis. Curr Med Res Opin. 2004;20:1455–1458. doi: 10.1185/030079904X2754. [DOI] [PubMed] [Google Scholar]
- 15.Endo Pharmaceuticals I. Lidoderm (lidocaine patch 5%) 2004 [Google Scholar]
- 16.Gammaitoni AR, Alvarez NA, Galer BS. Safety and tolerability of the lidocaine patch 5%, a targeted peripheral analgesic: a review of the literature. J Clin Pharmacol. 2003;43:111–117. doi: 10.1177/0091270002239817. [DOI] [PubMed] [Google Scholar]
- 17.Barbano RL, Herrmann DN, Hart-Gouleau S, Pennella-Vaughan J, Lodewick PA, Dworkin RH. Effectiveness, tolerability, and impact on quality of life of the 5% lidocaine patch in diabetic polyneuropathy. Arch Neurol. 2004;61:914–918. doi: 10.1001/archneur.61.6.914. [DOI] [PubMed] [Google Scholar]
- 18.Meier T, Wasner G, Faust M, et al. Efficacy of lidocaine patch 5% in the treatment of focal peripheral neuropathic pain syndromes: a randomized, double-blind, placebo-controlled study. Pain. 2003;106:151–158. doi: 10.1016/s0304-3959(03)00317-8. [DOI] [PubMed] [Google Scholar]
- 19.Hines R, Keaney D, Moskowitz MH, Prakken S. Use of lidocaine patch 5% for chronic low back pain: a report of four cases. Pain Med. 2002;3:361–365. doi: 10.1046/j.1526-4637.2002.02051.x. [DOI] [PubMed] [Google Scholar]
- 20.Gimbel J, Hale M, Linn R, Galer B, Kurkimills E, Gammaitoni A. Lidocaine patch 5% in patients with acute/subacute and chronic low back pain: impact on pain intensity, pain relief, and pain interference with quality of life [abstract] J Pain. 2003;4(2 suppl 1):71. [Google Scholar]
- 21.Galer B, Oleka N, Gammaitoni A. Lidocaine patch 5% improves outcomes for low-back pain and osteoarthritis patients receiving COX-2 selective or traditional NSAID therapy for pain relief [abstract] J Pain. 2005;6(3 suppl 1):S50. [Google Scholar]
- 22.Dalpiaz AS, Dodds TA. Myofascial pain response to topical lidocaine patch therapy: case report. J Pain Palliat Care Pharmacother. 2002;16:99–104. [PubMed] [Google Scholar]
- 23.Lipman A, Dalpiaz A, Lordon S. Topical lidocaine patch therapy for myofascial pain [abstract] J Pain. 2002;3(2 suppl 1):46. [PubMed] [Google Scholar]
- 24.Burch F, Codding C, Patel N, Sheldon E. Lidocaine patch 5% improves pain, stiffness, and physical function in osteoarthritis pain patients. A prospective, multicenter, open-label effectiveness trial. Osteoarthritis Cartilage. 2004;12:253–255. doi: 10.1016/j.joca.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Huppert AS. NSAID reduction through alternative pain-management programs. Med Care. 2001;39:1352–1353. doi: 10.1097/00005650-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Galer BS. A randomized, open-label study comparing the efficacy and safety of lidocaine patch 5% with celecoxib 200 mg in patients with pain from osteoarthritis of the knee [abstract] J Pain. 2005;6(3 suppl) [Google Scholar]
- 27.Gammaitoni A, Galer B, Burch F. Effectiveness and safety of the lidocaine patch 5% as monotherapy in patients with pain from osteoarthritis of the knee [abstract] J Pain. 2003;4(2 suppl 1):78. [Google Scholar]
- 28.Galer B, Kivitz A, Fairfax M, Sheldon E, Oleka N, Gammaitoni A. A randomized open-label study comparing the efficacy and safety of lidocaine patch 5% with celecoxib 200 mg in patients with pain from osteoarthritis of the knee [abstract] J Pain. 2005;6(3 suppl 1):S51. [Google Scholar]
- 29.Davis MDP. Lidocaine patch helpful in managing the chronic pain of leg ulceration. J Am Acad Dermatol. 2003;49:964. doi: 10.1016/s0190-9622(03)00770-9. [DOI] [PubMed] [Google Scholar]
- 30.Davis MDP, Sandroni P. Lidocaine patch for pain of erythromelalgia. Arch Dermatol. 2002;138:17–19. doi: 10.1001/archderm.138.1.17. [DOI] [PubMed] [Google Scholar]
- 31.Davis MDP, Sandroni P. Lidocaine patch for pain of erythromelalgia: follow-up of 34 patients. Arch Dermatol. 2005;141:1320–1321. doi: 10.1001/archderm.141.10.1320. [DOI] [PubMed] [Google Scholar]
- 32.Viera AJ. Management of carpal tunnel syndrome. Am Fam Physician. 2003;68:265–272. [PubMed] [Google Scholar]
- 33.Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2002;CD001554 doi: 10.1002/14651858.CD001554. [DOI] [PubMed] [Google Scholar]
- 34.Ly-Pen D, Andreu JL, de Blas G, Sanchez-Olaso A, Millan I. Surgical decompression versus local steroid injection in carpal tunnel syndrome: a one-year, prospective, randomized, open, controlled clinical trial. Arth Rheum. 2005;52:612–619. doi: 10.1002/art.20767. [DOI] [PubMed] [Google Scholar]
- 35.Goodyear-Smith F, Arroll B. What can family physicians offer patients with carpal tunnel syndrome other than surgery? A systematic review of nonsurgical management. Ann Fam Med. 2004;2:267–273. doi: 10.1370/afm.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bland JDP. Carpal tunnel syndrome. Curr Opin Neurol. 2005;18:581–585. doi: 10.1097/01.wco.0000173142.58068.5a. [DOI] [PubMed] [Google Scholar]
- 37.Nalamachu S, Crockett RS, Mathur D. Evaluation of the Efficacy and Safety of the Lidocaine Patch 5% Compared With Corticosteroid Injection in Improving Pain Associated With Carpal Tunnel Syndrome: a 4-Week, Randomized, Parallel-Group, Open-Label Pilot Study. J Fam Pract. 2006;55:209–214. [PubMed] [Google Scholar]
- 38.Kasten SJ, Louis DS. Carpal tunnel syndrome: a case of median nerve injection injury and a safe and effective method for injecting the carpal tunnel. J Fam Pract. 1996;43:79–82. [PubMed] [Google Scholar]
- 39.Frederick HA, Carter PR, Littler JW. Injection injuries to the median and ulnar nerves at the wrist. J Hand Surg [Am] 1992;17:645–647. doi: 10.1016/0363-5023(92)90309-d. [DOI] [PubMed] [Google Scholar]
- 40.Tavares SP, Giddins GE. Nerve injury following steroid injection for carpal tunnel syndrome. A report of two cases. J Hand Surg [Br ] 1996;21:208–209. doi: 10.1016/s0266-7681(96)80099-4. [DOI] [PubMed] [Google Scholar]
- 41.American Society of Plastic Surgeons Guidelines Committee. Arlington, Va: American Society of Plastic and Reconstructive Surgeons; 1998. Carpal tunnel syndrome: clinical practice guideline. [Google Scholar]
- 42.American Association of Electrodiagnostic Medicine. Practice parameter for electrodiagnostic studies in carpal tunnel syndrome: summary statement. Muscle Nerve. 2002;25:918–922. doi: 10.1002/mus.10185. [DOI] [PubMed] [Google Scholar]


