Case Presentation
Presenting Complaint
A 58-year-old man is referred to Johns Hopkins Hospital, Baltimore, Maryland, with worsening headache and cognition.
The patient was in his usual state of health until 4 months earlier, when he began to develop bitemporal headaches. Mild and intermittent at first, the headaches became progressively more severe and constant. His primary physician prescribed several over-the-counter medications, but these had little effect. Two months later, he was admitted to a local community hospital for persistent symptoms, which now also included mild neck stiffness, occasional nausea, and a burning sensation in his scalp. At this time, the patient noted that his headaches worsened upon coughing, sneezing, or bending over. He also felt that his thinking was “slower” and he complained of memory difficulties. In addition, he was sleeping up to 18 hours a day, which was a marked change from his usual 7 to 8 hours of nightly sleep. Magnetic resonance imaging (MRI) of the brain was read as normal. He was discharged with a prescription for hydromorphone, which provided only modest benefit for his headaches.
His symptoms continued to worsen during the next 2 months, at which point he was referred to Johns Hopkins Hospital.
Past Medical History
The patient has hypertension, chronic sinusitis, and a remote history of bleeding gastric ulcers.
Social History
He lives with his wife and children. He is a professional videographer, but unable to work currently because of his condition. He denies any tobacco, alcohol, or illicit drug use.
Family History
Multiple family members are known to have coronary artery disease and hypertension, but no family history of headaches, unexplained encephalopathy, or other neurologic conditions is known.
Medications
Currently takes clopidogrel, hydrochlorothiazide, lisinopril, hydromorphone, and prochlorperazine.
Allergies
The patient has no known drug allergies.
Review of Systems
The patient complains of a dry cough but denies any fever, chills, night sweats, or weight loss. He reports no contacts with other sick individuals and no recent travel outside his hometown. His one pet, a dog, passed away of “old age” several months earlier.
- In this patient, features of the headache that are worrisome for a serious underlying cause include all of the following except:
- New-onset headache in patient older than 50 years of age
- Worsening of headache with coughing
- Worsening cognition and memory
- Bilateral character of headaches
- Accompanying nausea
- Accompanying neck stiffness
Headache “Red Flags”
“Red flags” for the presence of serious underlying disorders as a cause of acute or subacute headache can be remembered by using the mnemonic SNOOP:
Systemic symptoms or illness (including fever, persistent or progressive vomiting, stiff neck, pregnancy, cancer, immunocompromised state, anticoagulated);
Neurologic signs or symptoms (including altered mental status, focal neurologic symptoms or signs, seizures, or papilledema);
Onset is new (especially in those age 40 years or older) or sudden;
Other associated conditions (eg, headache is subsequent to head trauma, awakens patient from sleep, or is worsened by Valsalva maneuvers);
Prior headache history that is different (eg, headaches now are of different pattern or are rapidly progressive in severity or frequency).
When such red flags are present, neuroimaging (computed tomography [CT] or MRI) is indicated to investigate secondary causes of headache.
Physical Exam
The patient is a tall, well-appearing gentleman who seems comfortable in bed. He is afebrile, with stable vital signs. Pertinent findings on general examination include normal temporal artery pulsations bilaterally, absence of oral ulcerations, no neck stiffness, no lymphadenopathy, and no rashes or splinter hemorrhages. Fundoscopic exam is unremarkable.
His neurologic exam is notable for his abnormal mental status. He is somnolent though quite easily arousable, and oriented to person and date but not to the location. Although he can register 3 objects, he does not recall any of them after several minutes despite prompting. His long-term memory appears to be relatively spared, but he cannot recall most of the events over the past few months, including the death of his dog. He follows multistep commands, albeit slowly; his speech, too, is slow but not dysarthric. The rest of his neurologic exam is unremarkable except for some mild bradykinesia throughout. He demonstrates no cogwheeling, tremor, or postural instability.
Imaging Studies
The patient was transferred to us with films of a repeat MRI of the brain obtained on the day before transfer. The following is a T1 sequence obtained after gadolinium administration.
-
2.All of the following should be included in the radiological differential diagnosis except:
- Carcinomatous meningitis
- Giant cell arteritis (aka temporal arteritis)
- Multiple sclerosis
- Central nervous system (CNS) Lyme disease
- Spontaneous intracranial hypotension
Pachymeningeal Enhancement
The meninges are composed of 3 layers which, from outermost to innermost, are known as the dura mater, the arachnoid, and the pia mater. The dura mater, also known as the “pachymeninx,” is called such because it is the thickest of the layers. The differential diagnosis of pachymeningeal enhancement is broad, but includes:
Infectious diseases (including tuberculosis, fungal infections, Lyme disease, syphilis, HTLV-1, and cysticercosis);
Autoimmune disorders (including sarcoidosis, Sjögren's syndrome, Beh¸et's disease, Wegener's disease, rheumatoid arthritis, and giant cell arteritis);
Neoplastic processes (including lymphoma, carcinomatous meningitis, skull-based metastases, and meningioma);
Intracranial hypotension;
Rare entities such as idiopathic hypertrophic pachymeningitis.
The following fluid-attenuated inversion-recovery (FLAIR) and T1 sequence of this patient are without gadolinium administration:
Laboratory Investigations
Complete blood count, electrolytes, and hepatic panel were normal. TSH, B12, and ammonia level were unremarkable. A Westergren erythrocyte sedimentation rate was normal, and the serum rapid plasma reagin (RPR) was negative, as were HIV, hepatitis, and Lyme serologies. Autoimmune investigations including antinuclear antibodies (ANA), anti-neutrophil cytoplasm antibodies (ANCA), anti-Ro/La, C3, C4, serum protein electrophoresis (SPEP), and urine protein electrophoresis (UPEP) were normal or negative.
Lumbar puncture in the lateral decubitus position demonstrated an opening pressure of 9 cm H2O. Cerebrospinal fluid (CSF) analysis revealed 3 white blood cells with 95% mononuclear cells, 0 red blood cells, glucose of 65 mg/dL, and a protein of 85 mg/dL. Gram stain and bacterial culture were negative, as were fungal and mycobacterial cultures. Viral polymerase chain reactions of the CSF were negative. CSF VDRL test and Lyme titer were negative. No oligoclonal bands were detected and the IgG index was normal. Cytopathology and flow cytometry were unremarkable.
-
3.What is the most likely diagnosis?
- Dandy-Walker malformation
- Subdural empyema
- Spontaneous intracranial hypotension
- Neurosarcoidosis
- Cerebral venous sinus thrombosis
Discussion
Intracranial hypotension was first described by Schultenbrand in 1938. He recognized that “aliquorrhea,” a deficiency in cerebrospinal fluid, could result in headaches predominantly when upright. He considered that the deficiency in CSF could result from decreased production, increased absorption, or loss of CSF through leakage.
Pathophysiology of Intracranial Hypotension
It has since been recognized that intracranial hypotension results, in the vast majority of cases, from CSF leakage. The resulting CSF hypovolemia leads to a reduction in the buoyant force on the brain, causing the brain to sag downward, particularly when the patient is upright. This downward descent of the brain, in turn, leads to traction on vascular structures, causing orthostatic headaches. Traction on cranial nerves can cause a variety of cranial neuropathies. In addition, the Monroe-Kellie doctrine, a longstanding and oft-quoted tenet of clinical neurology, dictates that the total volume within the cranial vault must remain constant. Therefore, when CSF volume is reduced, a compensatory elevation in the cerebral blood volume occurs, often in the venous channels and lakes of the dura. This leads to pachymeningeal enhancement and the accumulation of subdural fluid collections, which are also a volume compensatory mechanism.
The most common setting in which CSF hypovolemia and hypotension is encountered is subsequent to lumbar puncture, after which up to 25% of patients may develop orthostatic headaches. Use of an atraumatic spinal needle and a smaller needle size can reduce the incidence of post-lumbar puncture headaches, presumably by lowering the incidence of symptomatic CSF leakage. Other secondary causes of CSF hypovolemia include trauma, CSF leakage after cranial surgery, and CSF over-drainage via a ventriculoperitoneal or ventriculoatrial shunt.
Intracranial hypotension is termed “spontaneous” in the absence of the above secondary conditions. We have limited understanding of the pathophysiology of CSF leak formation in spontaneous intracranial hypotension. CSF leaks tend to occur in the thoracic region of the spinal cord, and the presence of connective tissue disorders such as Marfan syndrome and Ehlers-Danlos type II may predispose to their formation.
Clinical Features
The most common clinical feature of intracranial hypotension is the presence of orthostatic headaches (ie, headaches exacerbated in the upright position and relieved upon recumbency). However, the headaches associated with this condition can assume almost any form, including chronic daily headaches, exertional headaches, or acute thunderclap-like headaches. A minority of patients, predominantly the elderly, will not complain of significant headaches.
Other clinical manifestations may include neck stiffness, nausea, a number of cranial neuropathies (particularly involving nerves III, IV, VI, or VII), and dizziness. A variety of visual manifestations can occur, including photophobia, blurring of vision, and occasionally visual field cuts. Some patients complain of altered hearing, describing it as muffled, or as if they are listening through a seashell. Rarely, alterations in mental status can occur, and can range from a mild encephalopathy to stupor or even coma.
Diagnostic Evaluation
CSF findings are highly variable in spontaneous intracranial hypotension (SIH), but are quite useful for excluding infectious, neoplastic, or autoimmune causes that may mimic the clinical and radiologic presentation of SIH. Of importance, despite the name “intracranial hypotension,” the opening pressure may not be decreased. Indeed, the CSF opening pressure is greater than 9 cm H20 in up to one third of patients. Because a normal opening pressure does not exclude the diagnosis of SIH, it has been proposed that “CSF hypovolemia” may be a more apt name for this condition. Of interest, the CSF white blood cell (WBC) count may be abnormally elevated (> 5 cells/mm3) in over one half of patients, for reasons that are unclear. Regardless, an abnormally elevated CSF WBC count should prompt a thorough search for infectious, inflammatory, or neoplastic disorders. The most common CSF abnormality, found in the majority of patients with SIH, is an elevated protein level.
MRI scanning has revolutionized the diagnosis of SIH. Diffuse pachymeningeal enhancement is the most common finding, present in over 80% of cases. Some authors have described that this diffuse enhancement appears “wave-like,” particularly in the frontal lobes, in contrast to the nodular enhancement that is seen in infectious, neoplastic, or autoimmune processes. Other common imaging findings include subdural fluid collections, cerebellar tonsillar descent, crowding of the posterior fossa, and engorgement of the pituitary gland.
-
4.What is the most appropriate next step for this patient?
- Ventriculoperitoneal shunt to control intracranial pressure
- Cisternogram to identify area of CSF leak
- CT myelogram to identify area of CSF leak
- Strict bed rest to speed resolution of CSF leak
- Injection of fibrin sealant to stop CSF leak
Treatment of SIH
Initial therapeutic maneuvers are conservative and should focus on symptomatic treatment of the patient. If symptomatic treatment is unsuccessful, then the site of CSF leakage may need to be identified before more aggressive therapies are undertaken.
Conservative Measures
Initial treatment is similar to that of a post-lumbar puncture headache. Combinations of strict bed rest, ample hydration, and oral or intravenous caffeine are often employed, with variable results. If the patient does not improve over the course of several days, then an epidural blood patch (EBP) in the lumbar region is the treatment of choice. Effects of lumbar EBP are attributed to volume replacement by compression of the dura as well as a latent effect of blood tracking rostrally to seal the CSF leak. The success rate with EBP for patients with SIH is approximately 30%, far lower than the impressive success rates in post-lumbar puncture headache. This is likely because, in SIH, the site of CSF leakage is distant from the site of the blood patch; furthermore, the anatomy of the leak may be more complex than a simple hole or tear in the dura produced by a spinal needle. Lumbar EBP may be repeated several times. If the patient is still without benefit, then the site of CSF leakage is identified before more invasive therapeutic measures.
Identification of the Site of CSF Leakage
The site of the CSF leak in patients with SIH is often the thoracic region. Several imaging techniques may be used to identify the exact area of leakage. Because the majority of CSF leaks produce only a small volume of fluid and are only intermittently active, radionuclide cisternography can be very useful in detecting and localizing a CSF leak. A radioactive tracer, Indium-111, is introduced into the subarachnoid space via lumbar puncture, and its course is followed for 24-48 hours. Findings that are consistent with SIH include absence of tracer in the cerebral convexities and early presence of tracer in the kidneys and bladder. Less frequently, the actual site of parathecal CSF leakage is identified. Rarely, a dural tear at the skull base may cause SIH; this should be suspected in individuals who present with symptoms of intracranial hypotension and who have CSF rhinorrhea. Detection of radioactivity in nasal pledgets during radionuclide cisternography can confirm a skull base CSF leak.
If the site of CSF leakage is not identified by cisternography, then CT myelography and/or gadolinium-enhanced MRI scan may be of benefit for identifying the area of leakage.
Further Treatment Measures
Once the site of CSF leakage is identified, several additional therapeutic options should be considered. Initial treatment often consists of a directed epidural blood patch in the region of the leak, which in some instances is performed under the guidance of CT. For patients who remain symptomatic after directed EBP, surgical repair of the CSF leak should be considered. In well-selected patients in whom the site of CSF leakage has been properly identified, surgical repair can be effective. Recent reports suggest that epidural patching with fibrin glue may be a promising alternative to treatment of SIH, but its efficacy has not yet been widely proven.
Outcome
Our patient did not improve with bed rest and hydration. We performed 2 lumbar EBPs, the second of which resulted in substantial improvement of symptoms. Within 1-2 weeks, his cognitive status had returned to normal and he resumed work.
A month later, his symptoms returned after hitting golf balls at the driving range. A cisternogram and CT myelogram revealed evidence of a thoracic CSF leak at the T8 level. An EBP directed at the T8 level was performed, and his symptoms resolved. He has remained symptom-free since then, and has resumed his normal activities, including golfing. We did not identify any predisposing factors thought to be associated with SIH, including dural sac abnormalities or connective tissue disorders, in our patient.
Figure 1.

T1 sequence after gadolinium administration reveals diffuse pachymeningeal enhancement (arrows).
Figure 2.
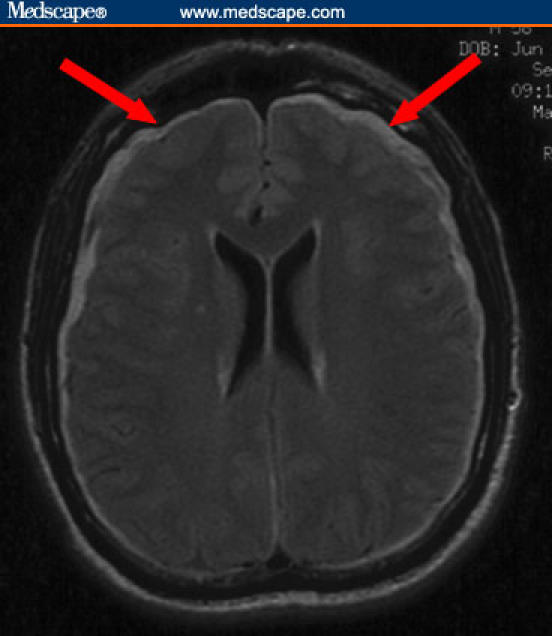
FLAIR sequence shows bilateral subdural fluid collections (arrows).
Figure 3.
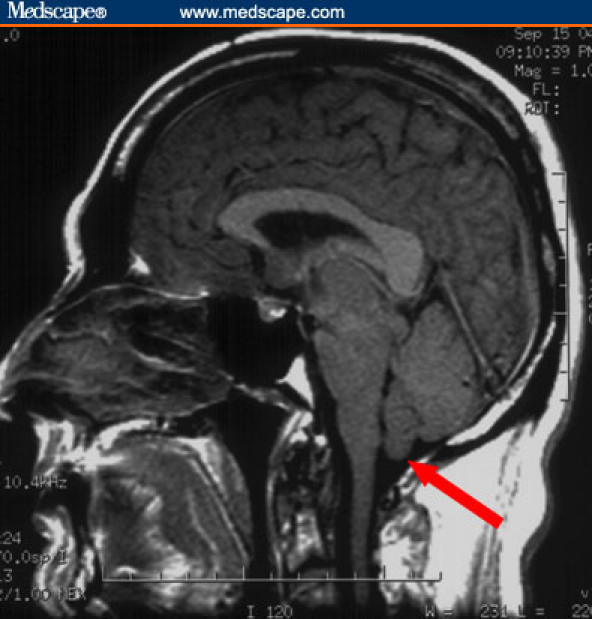
Sagittal T1 sequence demonstrates crowding of the posterior fossa and cerebellar tonsillar descent (arrow).
Contributor Information
Arun Venkatesan, Department of Neurology, The Johns Hopkins University, Baltimore, Maryland.
Michael A. Williams, Department of Neurology, The Johns Hopkins University, Baltimore, Maryland.
Suggested Reading
- Dodick D. Headache as a symptom of ominous disease. What are the warning signals? Postgrad Med. 1997;101:46–50. 55–56, 62–64. doi: 10.3810/pgm.1997.05.217. [DOI] [PubMed] [Google Scholar]
- Joubert J. Diagnosing headache. Aust Fam Physician. 2005;34:621–625. [PubMed] [Google Scholar]
- Kupersmith MJ, Martin V, Heller G, Shah A, Mitnick HJ. Idiopathic hypertrophic pachymeningitis. Neurology. 2004;62:686–694. doi: 10.1212/01.wnl.0000113748.53023.b7. [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Shiga Y, Hasegawa T, et al. CSF hypovolemia vs intracranial hypotension in “spontaneous intracranial hypotension syndrome.”. Neurology. 2003;60:941–947. doi: 10.1212/01.wnl.0000049933.51044.81. [DOI] [PubMed] [Google Scholar]
- Mokri B. Headaches caused by decreased intracranial pressure: diagnosis and management. Curr Opin Neurol. 2003;16:319–326. doi: 10.1097/01.wco.0000073933.19076.c0. [DOI] [PubMed] [Google Scholar]
- Schievink WI, Maya MM, Moser FM. Treatment of spontaneous intracranial hypotension with percutaneous placement of a fibrin sealant. Report of four cases. J Neurosurg. 2004;100:1098–1100. doi: 10.3171/jns.2004.100.6.1098. [DOI] [PubMed] [Google Scholar]
- Tosaka M, Sato N, Fujimaki H, Takahashi A, Saito N. Wave-like appearance of diffuse pachymeningeal enhancement associated with intracranial hypotension. Neuroradiology. 2005;47:362–367. doi: 10.1007/s00234-005-1366-8. [DOI] [PubMed] [Google Scholar]


