Case Presentation
The patient is a 36-year-old man who has been in the intensive care unit (ICU) for 10 days and now has upper gastrointestinal bleeding.
The patient had been in a prison facility and had no prior serious medical problems. While incarcerated, he experienced trauma to his left knee. Over a period of several days the knee became swollen and painful. He developed high fever, lethargy, and somnolence. He was transferred to our hospital. Initial evaluation revealed high fever, hypotension, tachycardia, and mental confusion. The knee and left leg were red, hot, swollen and tender. Complete blood count was normal, but bicarbonate was 14 meq/L and creatinine level was 6.5 mg/dL. Liver chemistries were mildly elevated and the coagulation profile was normal. He was admitted to the medical ICU for presumed systemic sepsis and treated with vigorous volume resuscitation, vasopressors, and antibiotics (vancomycin and cefepime). Blood cultures were positive for Streptococcus. Over 24 hours the left thigh and inguinal region developed intense swelling. Surgical consult diagnosed necrotizing fasciitis and recommended debridement. After the operation, the patient was transferred to the surgical ICU. Mechanical ventilation was necessary for 2 days following surgery. Renal failure progressed and he was started on hemodialysis for acute tubular necrosis. International normalized ratio (INR) was 1.2. Famotidine (20 mg intravenously twice daily) was ordered. He was started on nasogastric tube feedings, but this was associated with abdominal distension and large gastric residual volume. Vasopressors were stopped on hospital day 7 and hemodynamics were normal. During the first 9 days of hospitalization, serum hemoglobin declined from 15 g/dL to 10 g/dL without signs of gastrointestinal blood loss. The fall in hemoglobin level was attributed to acute illness, renal failure, and changes in intravascular volume. On the 10th hospital day, bright red blood appeared in the nasogastric tube and melena developed. He was transfused with 2 units of blood and was started on intravenous pantoprazole (80 mg rapid infusion and 8 mg/hour constant infusion). A gastroenterology consultation was requested.
Prior medical history was negative for serious illnesses, hospitalizations, or operations. The patient had a long history of heavy smoking and alcohol use.
Physical examination revealed the following vital signs: blood pressure, 128/70; heart rate, 126 beats per minute, lying; respiratory rate, 16 breaths per minute; and temperature, 98.6°F. He was awake, alert, but unable to answer questions appropriately. Bright red blood was in the nasogastric tube and it did not clear with 500 cc lavage. The skin was normal but the left lower extremity was extensively wrapped. There was no jaundice or stigmata of chronic liver disease. The lungs demonstrated diffuse rhonchi bilaterally. The cardiac examination showed only tachycardia and a hyperdynamic circulation. There was no abdominal tenderness, mass, or organomegaly. Digital rectal examination revealed no mass or tenderness, but melenic stool coated the exam glove.
Routine laboratory studies were normal except for the following: hemoglobin, 8 gm/dL; platelets, 185 × 109/L; blood urea nitrogen, 106 mg/dL; creatinine, 8 mg/dL; albumin, 1.9 g/dL; total bilirubin, 3 mg/dL; direct bilirubin, 2 mg/dL; alkaline phosphatase, 156 U/L; alanine aminotransferase, 90 U/L; aspartate aminotransferase, 172 U/L; and INR, 1.7.
Readers are encouraged to respond to George Lundberg, MD, Editor of MedGenMed, for the editor's eye only or for possible publication via email: glundberg@medscape.net
Diagnostic Questions
-
The surgeons' notes variously indicate that they suspected stress-related mucosal disease, stress-related gastric bleeding, stress erosions, stress gastritis, and stress ulcers. What do you suspect?
You should be in agreement with the surgeons, although you may not use precisely the same terms. Although these terms are often used interchangeably, they point to a distinct clinical entity recognized for many years.[1,2] The terms are actually used to differentiate the bleeding gastric lesions suspected in this patient from those bleeding gastric lesions that occur in an outpatient setting. When patients are admitted to the hospital because of clinically significant bleeding from the stomach, the source is usually deep, solitary or few ulcerative lesions found in the gastric antrum or incisura.[3] Such gastric ulcers are usually caused by aspirin, other nonsteroidal anti-inflammatory drugs (NSAIDs), or Helicobacter pylori infection. However, when gastric bleeding occurs in critically ill ICU patients, days to weeks after admission for other problems, the gastric lesions are usually multiple superficial lesions in the proximal stomach.[1,3] These lesions can progress to become multiple, shallow gastrointestinal ulcers, 0.5-2 cm in diameter, always in the acid-secreting part of the stomach (corpus and fundus). These lesions are seen in patients admitted with severe trauma, sepsis, head injury (Cushing's ulcers), burns (Curling's ulcers), or multiorgan system failure. The pathogenesis of these stress-related ulcers relates to gastric mucosal injury mediated by splanchnic hypoperfusion due to hypovolemia, depressed cardiac output, poor oxygenation, and increased vasoconstriction.[4] The initial injury is perpetuated by gastric acid, without which most injury would be self-limited.[5] Stress-related mucosal lesions are accompanied by little inflammatory reaction, making stress “gastritis” a misnomer.
-
Is stress-related mucosal disease common in patients such as the one presented?
This is a trick question on several levels. Stress-related mucosal disease is common, or uncommon, depending on specific clinical definitions and defined groups of patients.
Upper Gastrointestinal Bleeding in the Critically Ill Patient
Injury vs Clinically Significant Bleeding
In the case of critically ill patients admitted to the ICU, some form of stress-related mucosal disease occurs in most individuals.[6–8] However, the lesions are initially asymptomatic. With correction of the hemodynamic deficits, ventilatory insufficiency, and systemic response to tissue injury, the lesions remain asymptomatic and resolve. But in 5% to 25% of patients, occult or overt bleeding may occur.[9] In their classic studies, Cook and colleagues[9] differentiated occult or overt bleeding (positive nasogastric blood, mild fall in hemoglobin over several days, melena) from clinically significant bleeding (overt bleeding followed [within 24 hours] by a decrease in systolic blood pressure, rise in pulse rate, a 2-g/dL fall in hemoglobin, or a definite transfusion requirement of 2 units of blood).[9] Clinically significant bleeding (occurred in 1.5% of ICU patients in the study by Cook and colleagues) is much less common than occult or overt bleeding alone (5% to 25% of patients).[5,9,10] In a survey of 500 intensivists, Daley and colleagues[11] found that the perceived incidence of clinically significant stress-related bleeding among ICU admissions was 2%.
Low-Risk vs High-Risk ICU Patients
The frequency of clinically significant stress-related mucosal bleeding in critically ill patients is related to certain risk factors. In 1994, Cook and colleagues[9] reported that respiratory failure and coagulopathy (INR > 1.5 or platelet count < 50 per 109/L) are independent risk factors for clinically important bleeding. Bleeding was encountered in 3.7% of 847 patients who had one or both of these risk factors, vs in 0.1% of those who had neither complication. The same investigators later studied ventilated patients and found the following additional risk factors: high creatinine, not receiving enteral nutrition, and not receiving stress ulcer prophylaxis with ranitidine.[12] Recent algorithms have included other risk factors for which the evidence is weaker: severe burns (> 35% of body surface), neurologic trauma, multiple trauma, liver failure with associated coagulopathy, multiple organ failure, posttransplant (de novo), septicemia, and active gastrointestinal disease.[13]
The 1999 study by Cook and colleagues[12] found that enteral nutrition was associated with significantly lower bleeding rates, presumably due to its effect on raising intragastric pH. However, the subjects in this study were not randomized. Patients who had an inherently lower risk of bleeding could have been more likely to tolerate tube feeds. Modern ICU care emphasizes the beneficial effects of enteral feeding, but the effect of enteral nutrition on the risk of bleeding is not clear. The survey by Daley and colleagues[11] showed that current intensivists often stop stress bleeding prophylaxis when enteral feeding is initiated. In fact, enteral access for nutrition and medication is not possible in many ICU patients with bleeding, obstruction, ileus, or mesenteric ischemia (vasopressors). Intolerance to enteral feeding occurs in 15% to 50% of ICU patients, probably due to splanchnic hypoperfusion and poor motility.[4] Enteral feeding is not recommended as the sole agent for prophylaxis.[14]
The influence of H pylori on the development of stress-related injury is uncertain, but it is unlikely to have the same major etiologic role as in peptic ulcer disease.[15–18]
Four Decades Ago vs Present Time
The frequency of stress-ulcer and clinically significant ICU bleeding appears to have declined over the past 4 decades.[19] Major gastrointestinal bleeding used to occur in 30% of all severely burned patients.[6] Allen and colleagues[19] have documented that since the writing and wide acceptance of the major guidelines advocating stress-ulcer prophylaxis, most studies have found 0% to 2.8% bleeding rates in the critical care setting. In an observational study aimed at improving the appropriateness of stress-bleeding prophylaxis, Coursol and Sanzari[13] noted only a 1% bleeding rate among both patients with and without prophylaxis. Faisy and colleagues[20] collected evidence from the literature and personal observations about the low incidence of stress-related bleeding and the lack of impact of prophylaxis. No study in the new millennium has compared stress-related bleeding with placebo treatment. Faisy and colleagues[20] performed a retrospective observational study in a University Hospital ICU. They looked at 2 periods (phases) in the same ICU: 15 months with standard prophylaxis (736 patients) followed by 15 months without prophylaxis (737 patients).They recorded acute blood loss, the number of upper endoscopies, and overall mortality. Overt gastrointestinal bleeding and clinically significant gastrointestinal bleeding were defined according to the criteria used by Cook and colleagues[9] in their studies. Comparing phases 1 and 2, rates of overt gastrointestinal bleeding (14/736 and 12/737, respectively), and clinically significant gastrointestinal bleeding (10/736 and 8/737, respectively), did not differ significantly. The frequency of clinically significant bleeding was less than 1.5% throughout the 2 phases. These investigators believe that improvements in ICU care – such as oxygenation, fluid resuscitation, nutrition, antibiotics, and cardiac care – have led to improved microcirculation and tissue oxygenation in critically ill patients. This may in turn have led to the declining incidence of clinically significant gastrointestinal bleeding.
-
3.
Given the clinical features of this case, what is the significance and likely outcome of this bleeding event?
The patient is at high risk for continued morbidity and mortality. He has had clinically significant bleeding, defined as overt gastrointestinal bleeding with one of the following criteria: (a) blood pressure drop of 20 mm Hg; or (b) blood pressure drop of 10 mm Hg and heart rate increase of 20 beats/minute upon change in the orthostatic position; or (c) decrease in hemoglobin level of 20 g/dL and transfusion of 2 units of blood. Cook and colleagues[9] found that the mortality rate was 49% with clinically significant bleeding, but only 9% without such bleeding. Expert opinion still concurs that such a bleeding event carries a poor prognosis.[5,10,20] In the observations by Faisy and colleagues,[20] although total ICU mortality did not change with stress-bleeding prophylaxis, those patients with clinically significant bleeding had a mortality rate of 90% in phase 1 and 75% in phase 2. These mortality rates were 6 and 7 times the mortality rate observed for those patients without clinically significant bleeding. Ulcers associated with stress-related mucosal disease are usually multiple and superficial and are usually not amenable to therapeutic endoscopy.[4] Patients with clinically significant bleeding often do not die due to the bleeding itself, but most commonly die as a result of multiorgan failure.[20,21]
Clinical Course
The patient underwent upper gastrointestinal endoscopy. The esophagus was normal. A 2-cm ulcer oozing blood (Figures 1 and 2) was seen 2 cm beyond the gastroesophageal junction along the lesser curve of the stomach. There were 6 other ulcers scattered throughout the corpus of the stomach (Figures 3 and 4). The antrum and duodenum were normal.
Figure 1.

The ulcer was seen just beyond the gastroesophageal junction in the proximal stomach.
Figure 2.

The gastroscope is looking back upon itself to show the proximal stomach from where the bleeding is coming.
Figure 3.

Multiple other ulcers were seen in other parts of the body of the stomach.
Figure 4.
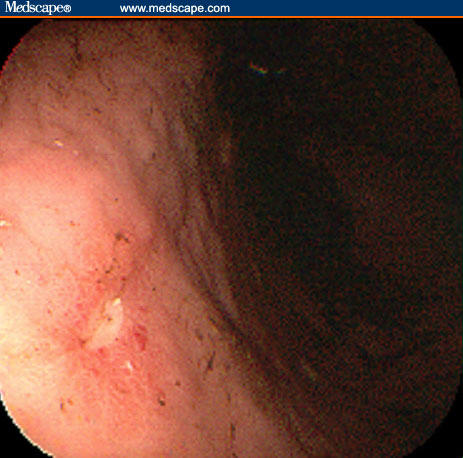
The ulcers that are not actively bleeding are smaller and more superficial.
The bleeding ulcer was treated with epinephrine injection and BICAP (bipolar circumactive probe) cautery (Figure 5).
Figure 5.

An injection catheter was used to inject epinephrine into the ulcer base to achieve hemostasis.
Hemostasis was achieved. The lesions were thought to be classic for stress-related gastric ulcerations (described previously). A continuous proton-pump inhibitor (PPI) infusion was maintained for 4 days and then an oral PPI was given twice daily. He had no further episodes of overt gastrointestinal blood loss, but did require 2 additional units of blood. The patient was discharged from the surgical ICU after 10 more days and was then discharged back to prison after 1 month. Renal function returned to normal and wound healing was achieved.
Management Questions
-
4.
Could this episode of stress-related bleeding have been prevented?
The 1996 meta-analysis by Cook and colleagues[22] supported the use of ranitidine to prevent stress-related bleeding. The largest, and best designed study of stress-ulcer prophylaxis showed that both ranitidine and sucralfate were superior to placebo in preventing bleeding.[23] In 1999, there were specific recommendations for stress-ulcer prophylaxis[24] based on treating high-risk patients with either sucralfate or ranitidine. The most recent meta-analysis could not convincingly demonstrate the efficacy of ranitidine or sucralfate for reducing clinically significant bleeding.[25] The study by Faisy and colleagues[20] suggests that prophylaxis does not affect bleeding rates. These authors believe that their data support 2 earlier reports. Ben-Menachem and colleagues[26] showed that the cost of prophylaxis was high for patients at low risk for gastrointestinal bleeding and that prophylaxis did not reduce bleeding in patients with risk factors. Erstad and colleagues[27] conducted a prospective study involving 543 patients and reported that clinically significant gastrointestinal bleeding rates were similar for patients with risk factor(s) for bleeding receiving inappropriate stress-ulcer prophylaxis and those receiving appropriate preventive therapy.
-
5.
Should this patient have received prophylaxis for stress-related bleeding?
Indications for prophylaxis for stress-related gastrointestinal hemorrhage can be controversial. Coursol and Sanzari[13] implemented a program whereby clinicians were instructed when to start and stop prophylaxis according to accepted guidelines. Their indications for stress-related gastric bleeding were based on findings reported by Cook and colleagues[9]: coagulopathy for 24 hours and mechanical ventilation for 48 hours. But they, like others, accepted a broader range of indications that are supported by weaker evidence: severe burns (> 35% of body surface), neurologic trauma, multiple trauma, liver failure with associated coagulopathy, multiple organ failure (at least 3 organs), posttransplant, septicemia, and active gastrointestinal disease. Prophylaxis was to be given to any patient with at least 1 of these factors. The patient presented here – extubated 2 days after surgery, only 2 organs failed, INR < .5 until day 10 – technically achieved the criteria for prophylaxis by the criteria of septicemia.
-
6.
On day 8 of the hospitalization, the patient was recovering from severe sepsis, on parenteral antibiotics, in renal failure (on hemodialysis), had an INR = 1.5, off vasopressors, able to take clear liquids, and had oxygen saturation of 93% on 20% O2 by nasal cannula, with large wounds in his left leg. What drug, route, and dose would you have used on day 8 of the hospitalization to prevent stress-related bleeding?
A possible therapeutic approach would be intermittent intravenous PPI, with 2 doses administered on the first day, 6 hours apart, and then 1 dose daily thereafter. This would be followed by a switch to omeprazole oral solution 40 mg daily when the patient began to tolerate oral intake. However, there is controversy regarding all aspects of stress-related bleeding prophylaxis (ie, drug, route, and dose).
Click on Next Page for an in-depth discussion of this topic.
Therapeutic Intervention
Drug
The only US Food and Drug Administration (FDA) approved intravenous medication for stress-related bleeding is cimetidine, and the most commonly recommended intravenous medication is a histamine-2 receptor antagonist (H2RA).[19] A survey of 500 intensivists was performed to assess current practice regarding prophylaxis of stress-related bleeding.[11] Of the 500 respondents, 64% used H2RAs by intermittent infusion, 23% primarily used PPIs, and 12% used sucralfate. The trend toward abandoning sucralfate and using oral and intravenous PPIs seems irreversible.
Intravenous PPIs have not been studied using clinically significant bleeding as an endpoint. Omeprazole immediate-release suspension is the only PPI drug or formulation with US FDA approval for stress-related mucosal bleeding prophylaxis. But PPIs are becoming preferred over H2RAs because they provide more potent acid suppression, maintain gastric pH ≥ 4 for prolonged periods, have a favorable adverse effect profile, have multiple administration routes, are not associated with tolerance, and are less likely to cause drug interactions mediated by cytochrome P450 enzymes.[28]
Both enteral and intravenous PPIs are potent acid suppressive agents in the ICU setting.[29] They are effective in preventing stress-related mucosal disease.[30–32] Direct comparison with ranitidine in stress-ulcer prophylaxis showed omeprazole was superior – but the groups were not of equal risk.[33] Studies suggesting increased efficacy and decreased cost with PPI use are nonrandomized and observational.[34] Recent reviews support the rationale for using PPIs in this setting, but urge that more studies be performed.[35,36] These studies will probably not be conducted because of the low rate of bleeding and the large numbers of subjects required to show a difference between ranitidine and PPI therapy.
A recent 2-part article by Devlin and colleagues[28,37] addressed the efficacy, dose, and administration route permutations that have been brought about by the availability of multiple intravenous, oral, and other PPI formulations. They also identified the numerous unanswered questions regarding the use of PPIs for prophylaxis: What is the role for intravenous PPIs relative to intravenous H2RAs? What is the appropriate intravenous PPI dose for this indication? What is the role for newer enteral PPI formulations, such as the lansoprazole oral disintegrating tablet in water, vs the sodium bicarbonate-based suspensions? When should intravenous vs enteral therapy be employed? What is the role of intravenous-to-oral switch therapy?
Route
Oral
Recently, the number of commercially available PPI formulations has greatly expanded to include several oral formulations that may be administered enterally to patients unable to swallow a tablet or capsule (eg, immediate-release omeprazole with sodium bicarbonate packaged powder for suspension; lansoprazole tablet that can be dissolved in water; esomeprazole pellets administered in water).[28] Perhaps the best study of oral PPIs for the prevention of upper gastrointestinal bleeding in critically ill patients was published in 2005.[38] In this placebo-controlled study, 359 mechanically ventilated patients were randomized to receive either omeprazole immediate-release suspension 40 mg twice on day 1 (6-8 hours apart) through a nasogastric tube followed by 40 mg daily thereafter, or intravenous cimetidine 300-mg bolus and then 50 mg/hour thereafter. Gastric aspirates were frequently sampled for blood and pH monitoring. Clinically significant bleeding occurred in 3.9% of the omeprazole-treated patients and in 5.5% of the cimetidine-treated patients. When the more liberally defined outcome of any gastrointestinal bleeding was compared between groups, fewer omeprazole-treated patients experienced any bleeding (19.1% vs 32.0% with cimetidine; P < .005). The incidence of nosocomial pneumonia was similar between groups (7.9% vs 6.1%, respectively; P > .05). Significantly more cimetidine-treated (58%) than omeprazole-treated (18%) patients had inadequate pH control (P < .001). On the basis of these data, the US FDA recently approved immediate-release omeprazole 40 mg for the prevention of stress-related mucosal bleeding. Clearly, patients who are likely to absorb a medication should receive it orally or enterally if possible.
Intravenous
Enteral medication administration is not possible in many ICU patients with bleeding, obstruction, ileus, or mesenteric ischemia (vasopressors). One study evaluated intravenous PPIs compared with H2RAs for stress ulcer prophylaxis.[39] In this study, 210 mechanically ventilated patients were randomized to receive either cimetidine (300-mg bolus, then 50 mg/hour) or 1 of 4 pantoprazole intravenous dosing regimens: 40 mg daily, 40 mg every 12 hours, 80 mg once daily, 80 mg every 12 hours, and 80 mg every 8 hours. Clinically significant gastrointestinal bleeding did not occur in any of the patients.
Dose
All of the dosing permutations for oral and parenteral PPIs are provided in the reviews by Devlin and colleagues reviews.[28,37] The strategy of administering 2 doses of PPI 6 hours apart on the first day has been used to try to inactivate more proton pumps at the beginning of therapy because with daily dosing, several days are required to obtain maximal effect.[30,38]
Discussion
Despite more than 20 years of careful study, numerous analyses, meta-analyses, and algorithms, the frequency, significance, and prevention of stress-related gastrointestinal bleeding remains mired in controversy. Comprehensive summaries of the data and issues are available.[4,5,20,28,37] Published algorithms can be used to standardize therapy.[13] As discussed previously, some experts suggest that current recommendations for H2RAs and sucralfate do not prevent the low frequency of clinically significant bleeding. Others suggest that even in the absence of clinical trials, more potent (and more costly) PPIs should be substituted for the less effective drugs so that events such as the one seen in our patient, do not occur. Unfortunately, most prophylactic regimens are used for too long and are maintained after the high-risk period.[40,41] Inappropriate use of PPIs in hospitalized patients is common and costly.[42–44]
The patient that we presented here demonstrates a classic case of stress-related gastric mucosal disease; it further represents a classic case of incomplete knowledge and incomplete application of knowledge in medicine.
Contributor Information
Aaron Woofter, Baylor College of Medicine, Houston, Texas.
Richard Goodgame, Department of Medicine, Gastroenterology Section, Baylor College of Medicine, Houston, Texas.
References
- 1.Lev R, Molot MD, McNamara J, Stremple JF. “Stress” ulcers following war wounds in Vietnam. A morphologic and histochemical study. Lab Invest. 1971;25:491–502. [PubMed] [Google Scholar]
- 2.Silen W. The clinical problem of stress ulcers. Clin Invest Med. 1987;10:270. [PubMed] [Google Scholar]
- 3.Sugawa C, Lucas CE, Rosenberg BF, et al. Differential topography of acute erosive gastritis due to trauma or sepsis, ethanol and aspirin. Gastrointest Endosc. 1973;19:127–130. doi: 10.1016/s0016-5107(73)73979-1. [DOI] [PubMed] [Google Scholar]
- 4.Martindale RG. Contemporary strategies for the prevention of stress-related mucosal bleeding. Am J Health-Syst Pharm. 2005;62(suppl 2):S11–S17. doi: 10.1093/ajhp/62.10_Supplement_2.S11. [DOI] [PubMed] [Google Scholar]
- 5.Fennerty MB. Pathophysiology of the upper gastrointestinal tract in the critically ill patient: Rationale for the therapeutic benefits of acid suppression. Crit Care Med. 2002;30(suppl):S351–S355. doi: 10.1097/00003246-200206001-00002. [DOI] [PubMed] [Google Scholar]
- 6.Czaja AJ, McAlhany JC, Pruitt BA., Jr Acute gastroduodenal disease after thermal injury. An endoscopic evaluation of incidence and natural history. N Engl J Med. 1974;291:925–929. doi: 10.1056/NEJM197410312911801. [DOI] [PubMed] [Google Scholar]
- 7.Peura DA, Johnson LF. Cimetidine for prevention and treatment of gastroduodenal mucosal lesions in patients in an intensive care unit. Ann Intern Med. 1985;103:173–177. doi: 10.7326/0003-4819-103-2-173. [DOI] [PubMed] [Google Scholar]
- 8.Lucas CE, Sugawa C, Riddle J, et al. Natural history and surgical dilemma of “stress” gastric bleeding. Arch Surg. 1971;102:266–273. doi: 10.1001/archsurg.1971.01350040028006. [DOI] [PubMed] [Google Scholar]
- 9.Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. N Engl J Med. 1994;330:377–381. doi: 10.1056/NEJM199402103300601. [DOI] [PubMed] [Google Scholar]
- 10.Devlin JW. Treatment options and formulary considerations in the management of acid suppression in critically ill patients. Am J Health-Syst Pharm. 2005;62(suppl 2):S2–S3. doi: 10.1093/ajhp/62.10_Supplement_2.S2. [DOI] [PubMed] [Google Scholar]
- 11.Daley RJ, Rebuck JA, Welage LS, Rogers FB. Prevention of stress ulceration: Current trends in critical care. Crit Care Med. 2004;32:2008–2014. doi: 10.1097/01.ccm.0000142398.73762.20. [DOI] [PubMed] [Google Scholar]
- 12.Cook D, Heyland D, Griffith L, et al. Risk factors for clinically important upper gastrointestinal bleeding in patients requiring mechanical ventilation. Crit Care Med. 1999;27:2812–2817. doi: 10.1097/00003246-199912000-00034. [DOI] [PubMed] [Google Scholar]
- 13.Coursol CJ, Sanzari SE. Impact of stress ulcer prophylaxis algorithm study. Ann Pharmacother. 2005;39:810–816. doi: 10.1345/aph.1D129. [DOI] [PubMed] [Google Scholar]
- 14.MacLaren R, Jarvis CL, Fish DN. Use of enteral nutrition for stress ulcer prophylaxis. Ann Pharmacother. 2001;35:1614–1623. doi: 10.1345/aph.1A083. [DOI] [PubMed] [Google Scholar]
- 15.Van der Voort PH, van der Hulst RW, Zandstra DF, et al. Prevalence of Helicobacter pylori infection in stress-induced gastric mucosal injury. Intensive Care Med. 2001;27:68–73. doi: 10.1007/s001340000773. [DOI] [PubMed] [Google Scholar]
- 16.Robertson MS, Cade JF, Clancy RL. Helicobacter pylori infection in intensive care: increased prevalence and a new nosocomial infection. Crit Care Med. 1999;27:1276–1280. doi: 10.1097/00003246-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Halm U, Halm F, Thein D, et al. Helicobacter pylori infection: a risk factor for upper gastrointestinal bleeding after cardiac surgery? Crit Care Med. 2000;28:110–113. doi: 10.1097/00003246-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Schilling D, Haisch G, Sloot N, et al. Low seroprevalence of Helicobacter pylori infection in patients with stress ulcer bleeding. Intensive Care Med. 2000;26:1832–1836. doi: 10.1007/s001340000724. [DOI] [PubMed] [Google Scholar]
- 19.Allen ME, Kopp BJ, Erstad BL. Stress ulcer prophylaxis in the postoperative period. Am J Health-Syst Pharm. 2004;61:588–596. doi: 10.1093/ajhp/61.6.588. [DOI] [PubMed] [Google Scholar]
- 20.Faisy C, Guerot E, Diehl J-L, Iftimovici E, Fagon J-Y. Clinically significant gastrointestinal bleeding in critically ill patients with and without stress-ulcer prophylaxis. Intensive Care Med. 2003;29:1306–1313. doi: 10.1007/s00134-003-1863-3. [DOI] [PubMed] [Google Scholar]
- 21.Lewis JD, Shin EJ, Metz DC. Characterization of gastrointestinal bleeding in severely ill hospitalized patients. Crit Care Med. 2000;28:46–50. doi: 10.1097/00003246-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Cook DJ, Reeve BK, Guyatt GH, et al. Stress ulcer prophylaxis in critically ill patients: resolving discordant meta-analyses. JAMA. 1996;275:308–314. [PubMed] [Google Scholar]
- 23.Cook D, Guyatt G, Marshall J, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. N Engl J Med. 1998;338:791–797. doi: 10.1056/NEJM199803193381203. [DOI] [PubMed] [Google Scholar]
- 24.American Society of Health-System Pharmacists. ASHP therapeutic guidelines on stress ulcer prophylaxis. Am J Health-Syst Pharm. 1999;56:347–379. doi: 10.1093/ajhp/56.4.347. [DOI] [PubMed] [Google Scholar]
- 25.Messori A, Trippoli S, Vaiani M, et al. Bleeding and pneumonia in intensive care patients given ranitidine and sucralfate for prevention of stress ulcer: metaanalysis of randomized controlled trials. BMJ. 2000;321:1–7. doi: 10.1136/bmj.321.7269.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Menachem T, McCarthy BD, Fogel R, et al. Prophylaxis for stress-related gastrointestinal hemorrhage: a cost effectiveness analysis. Crit Care Med. 1996;24:338–345. doi: 10.1097/00003246-199602000-00026. [DOI] [PubMed] [Google Scholar]
- 27.Erstad BL, Camano JM, Miller MJ, Webber AM, Fortune J. Impacting cost and appropriateness of stress ulcer prophylaxis at a university medical center. Crit Care Med. 1997;25:1678–1684. doi: 10.1097/00003246-199710000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Devlin JW, Welage LS, Olsen KM. Proton Pump Inhibitor Formulary Considerations in the Acutely Ill Part 1: Pharmacology, Pharmacodynamics, and Available Formulations. Ann Pharmacother. 2005;39:1667–1677. doi: 10.1345/aph.1G126. [DOI] [PubMed] [Google Scholar]
- 29.Roberts RW, Pitcher WD, Balaban DH, Duckworth CW, Peura DA. Nasogastric omeprazole: Effects on gastric pH in critically ill patients. Am J Gastroenterol. 1997;92:79–83. [PubMed] [Google Scholar]
- 30.Lasky MR, Metzler MH, Phillips JO. A prospective study of omeprazole suspension to prevent clinically significant gastrointestinal bleeding from stress ulcers in mechanically ventilated trauma patients. J Trauma. 1998;44:527–533. doi: 10.1097/00005373-199803000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Phillips JO, Metzler MH, Palmieri TL, et al. A prospective study of simplified omeprazole suspension for the prophylaxis of stress-related mucosal damage. Crit Care Med. 1996;24:1793–1800. doi: 10.1097/00003246-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Phillips JO, Olsen KM, Rebuck JA, et al. A randomized, pharmacokinetic and pharmacodynamic, cross-over study of duodenal or jejunal administration compared to nasogastric administration of omeprazole suspension in patients at risk for stress ulcers. Am J Gastroenterol. 2001;96:367–372. doi: 10.1111/j.1572-0241.2001.03522.x. [DOI] [PubMed] [Google Scholar]
- 33.Levy MJ, Seelig CB, Robinson NJ, et al. Comparison of omeprazole and ranitidine for stress ulcer prophylaxis. Dig Dis Sci. 1997;42:1255–1259. doi: 10.1023/a:1018810325370. [DOI] [PubMed] [Google Scholar]
- 34.Schupp KN, Schrand LM, Mutnick AH. A cost-effectiveness analysis of stress ulcer prophylaxis. Ann Pharmacother. 2003;37:631–635. doi: 10.1345/aph.1C377. [DOI] [PubMed] [Google Scholar]
- 35.Steinberg KP. Stress-related mucosal disease in the critically ill patient: Risk factors and strategies to prevent stress-related bleeding in the intensive care unit. Crit Care Med. 2002;30(suppl):S362–S364. doi: 10.1097/00003246-200206001-00005. [DOI] [PubMed] [Google Scholar]
- 36.Cash BD. Evidence-based medicine as it applies to acid suppression in the hospitalized patient. Crit Care Med. 2002;30(suppl):S373–S378. doi: 10.1097/00003246-200206001-00008. [DOI] [PubMed] [Google Scholar]
- 37.Devlin JW, Welage LS, Olsen KM. Proton pump inhibitor formulary considerations in the acutely ill Part 2: Clinical efficacy, safety, and economics. Ann Pharmacother. 2005;39:1844–1851. doi: 10.1345/aph.1G176. [DOI] [PubMed] [Google Scholar]
- 38.Conrad SA, Gabrielli A, Margolis B, et al. Randomized, double-blind comparison of immediate-release omeprazole oral suspension versus intravenous cimetidine for the prevention of upper gastrointestinal bleeding in critically ill patients. Crit Care Med. 2005;33:760–765. doi: 10.1097/01.ccm.0000157751.92249.32. [DOI] [PubMed] [Google Scholar]
- 39.Somberg L, Karlstadt R, Blatcher D. Intermittent intravenous pantoprazole maintains control of gastric pH in intensive care unit patients (abstract) Am J Gastroenterol. 2002;97(suppl):S47. [Google Scholar]
- 40.Barletta JF, Erstad BL, Fortune JB. Stress ulcer prophylaxis in trauma patients. Crit Care. 2002;6:526–530. doi: 10.1186/cc1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lam NP, Le PD, Crawford SY, et al. National survey of stress ulcer prophylaxis. Crit Care Med. 1999;27:98–103. doi: 10.1097/00003246-199901000-00034. [DOI] [PubMed] [Google Scholar]
- 42.Cornish P, Papastergiou J, Saibil F. Audit of IV pantoprazole: patterns of use and compliance with guidelines. Can J Hosp Pharm. 2002;55:20–26. [Google Scholar]
- 43.Kaplan GG, Bates D, McDonald D. Inappropriate use of intravenous proton pump inhibitors: problem extent and cost implications (abstract) Am J Gastroenterol. 2002;97:S236. [Google Scholar]
- 44.Enns R, Andrews CN, Fishman M, et al. Description of prescribing practices in patients with upper gastrointestinal bleeding receiving intravenous proton pump inhibitors: a multicenter study. Can J Gastroenterol. 2004;18:561–571. doi: 10.1155/2004/204968. [DOI] [PubMed] [Google Scholar]


