Abstract
The belief that breathing exercises may provide health benefits has been shared by many cultures for centuries. A case study illustrates one such FDA-approved intervention, its performance over time, and the day-to-day home blood pressure (BP) variation in response to treatment. The device used by this patient (which interactively entrains slowed and deep breathing) has been studied in 7 clinical trials. Routine use of the device (RESPeRATE, InterCure Inc., Fort Lee, New Jersey; http://www.resperate.com/MD) significantly lowered home and office BPs without adverse effects, when used alone, with lifestyle modifications, or adjunctively to antihypertensive drugs. The proposed physiological mechanism(s), the technology that guides slowed breathing, pooled clinical research outcomes, and recommendations regarding this modality in clinical practice are also reviewed. Device-guided paced breathing may offer an effective, simple, and new nonpharmacologic option for treating high BP without additional side effects, but like all lifestyle modifications, must be practiced consistently to provide benefits.
Introduction
Elevated BP is a major risk factor for adverse cardiovascular events, including myocardial infarction, stroke, heart failure, and death.[1] Antihypertensive drug therapy reduces the risk of all these endpoints,[1] but many drugs cause side effects (eg, gout, asthma, cough, pedal edema with diuretics, beta-blockers, angiotensin converting-enzyme inhibitors, and dihydropyridine calcium antagonists, respectively) and can be costly. Reduction of high BP by nonpharmacologic means (ie, lifestyle modifications) is widely recommended, either in primary prevention, or as therapy with or without antihypertensive drugs.[1,2] Unfortunately, diet and exercise alone have rarely been effective in long-term control of high BP.[3–5] In spite of the many treatment options, 50% to 80% of treated hypertensives in economically developed countries still have inadequately controlled BP.[6] Therefore, new nonpharmacologic treatment modalities for reducing high BP are of great interest.
Slow and deep breathing (“paced breathing”) has been traditionally associated with meditation and healing for hundreds of years.[7] Paced breathing plays a prominent role in behavioral methods of treating hypertension that have reported some short-term successes.[8–10] However, asking patients to perform paced breathing sessions on their own may be impractical. Apart from the prolonged training, practicing, skill, and motivation needed, effortlessly performed paced breathing involves individualized breathing patterns that typically require personal coaching. The physiological origin of the hypotensive effects of paced breathing has traditionally been attributed to the “relaxation response.”[8] While relaxation is a widely accepted, beneficial outcome of slow breathing, the antihypertensive effect of paced breathing may have a more direct physiological origin.
Case Report
A 67-year-old white woman with an 11-year history of chronic obstructive pulmonary disease and migraine headaches presented with uncontrolled hypertension for 6 months. Hypertension was initially diagnosed at age 40, and had been treated with an increasing number and doses of antihypertensive drugs. At presentation, she took hydrochlorothiazide 25 mg/day, doxazosin 1 mg/day, metoprolol succinate 50 mg/day, and felodipine 10 mg/day. In addition, aspirin 81 mg/d and sumatriptan 100 mg as needed, were prescribed. She is a nondrinker, nonsmoker, performs strenuous physical exercise twice weekly, and was otherwise healthy and without complaints. She credits her exercise program and healthy eating habits to helping her maintain her weight at 175 lb over the last 3 years, which was 25 lb less than her peak weight.
After informed consent was obtained, she was enrolled in an 8-week study that evaluated the RESPeRATE device.[11] Her initial office BP was 147/91 mm Hg (average of 3 readings, right arm, seated position, using the adult-sized cuff); heart rate of 68 and a body-mass index of 30.5 kg/m2. She was asked to use the device for one 15-minute session every afternoon or evening, and to take 3 consecutive readings of her BP at home, every morning, using a previously-validated BP monitor with a digital readout (Omron model HEM-747 IC, Omron Healthcare, Vernon Hills, Illinois).
After 2 months, her office BPs declined from 147/91 mm Hg to 130/77 mm Hg (a difference of 17/14 mm Hg). Four weeks after the end of the study, the patient's data were uploaded from both the device and home BP monitor into a specialized Web-based reporting system designed for this study. Figure 1 shows typical minute-by-minute changes of the patient's breathing pattern during a single session (#20). The figure demonstrates a reduction in her breathing rate from a normal value of 16 breaths/min (bpm) down to 6 bpm, while doubling the ratio of exhalation to inhalation time. Because she enjoyed the breathing exercise, she continued to use the device after the end of the study.
Figure 1.
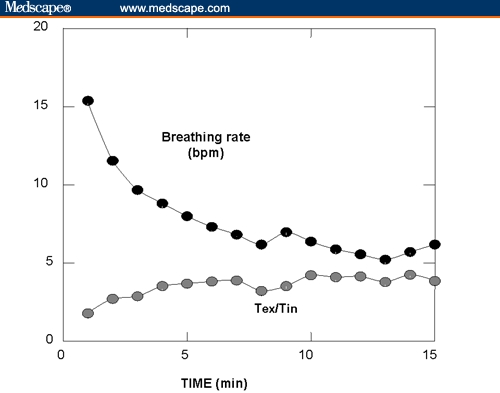
Typical changes in breathing rate and the ratio between exhale time (Tex) to inhale time (Tin) during a 15-minute session (#20) calculated from data stored in the patient's device, and uploaded to a Web-based reporting system used in research.
Figure 2 shows a different Web-based report from the same patient displaying day-by-day variations in BP over the first 3 months of use. The adherence of the patient to the treatment recommendation (of 15 minutes/day) is expressed by the time spent breathing fewer than 10 bpm during the preceding 7 days (“weekly effective time”). The mean home BP during the first 10 days of use was 161/99 mm Hg; 157/96 mm Hg between days 20-29; 151/89 mm Hg between days 50-59 and 146/85 mm Hg between days 80-89. The net BP reduction from baseline was 10/10 mm Hg after 2 months and 15/14 mm Hg after 3 months of treatment. These values showed a significant trend for BP reduction (P < .05/P < .001). The heart rate (68 ± 5 beats/min, mean ±standard deviation) did not show any significant change. The corresponding systolic load (ie, the percentage of systolic BP readings >140 mm Hg), was 95%, 95%, 70%, and 65%, respectively over the same time periods cited above. Figure 2 also shows the “weekly effective time” to be above the recommended level (45 minutes/week), demonstrating the patient's adherence to the instructions for use. During the observation period she had no change in medication, lifestyle modifications, or body-mass index, and no complaints about the device or its use.
Figure 2.
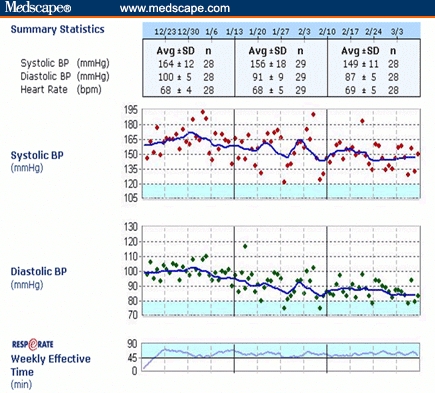
A Web-based plot of data from the case reported, including 3-month follow-up of home BP and adherence to the recommendation for using the device, using data logged automatically by the devices. The points mark daily averages of BP readings and the patient's concordance with the treatment (by “weekly effective time”). The goals for BP (<120/80 mm Hg) and concordance (> 45 min/week) are colored. The table at the top shows monthly averages of BP and heart rate.
In this patient, the lowering of home BP measurements began about 2 weeks after she initiated device-guided breathing. As in other clinical trials,[12,13] her home BPs continued to drop after the 8-week study period, as she continued to use the device.
Device Description
The device (RESPeRATE,http://www.resperate.com/MD) consists of a control box containing a microprocessor, a belt-type respiration sensor, and headphones, which provide feedback to the patient (Figure 3). During a session of device-guided breathing, the device analyzes the breathing rate and pattern and creates a personalized melody composed of 2 distinct tones – one tone for inhalation and one for exhalation. As the patient synchronizes breathing with the tones, the device gradually prolongs the exhalation tone (primarily) and slows the breathing rate to < 10 breaths/minute (“slow breathing”), as depicted in Figures 1 and 3. A record of the patient's use of the device is stored in the microprocessor for quantification of total time of device use and adherence to the regimen.
Figure 3.
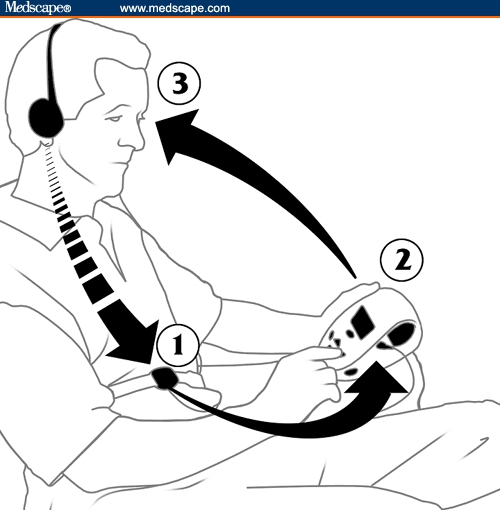
Principles of device-guided paced breathing: (1) monitoring breathing movements, (2) composing breathing-guiding tones, and (3) synchronizing breathing movements with the guiding tones.
Figure 3. Principles of device-guided paced breathing: (1) monitoring breathing movements, (2) composing breathing-guiding tones, and (3) synchronizing breathing movements with the guiding tones.
How Does RESPeRATE Work?
click here for a flash demo.
Clinical Studies
Seven separate studies[11–17] have examined the changes in office BP for subjects who used this device for 15 minutes/day for 8 weeks, compared with “control” interventions (listening to relaxing music,[12] home BP monitoring,[13,16] or both).[14] Four studies were double-blinded and randomized,[12–14] one was controlled and randomized,[16] and 2 were open-label experiences.[11,16]
A total of 286 individuals participated in these studies: 55% were men; with an average age of 57 ±11 years; body-mass index of 28 ± 4 kg/m2; and initial office BP of 150 ±13/90 ± 9 mm Hg (9% prehypertensive; 25% Stage 2). Antihypertensive drugs were taken by 78% of the subjects. Figure 4 shows a “box and whiskers plot” of the changes in office systolic BP observed in each of the 7 independent studies of patients who used the device appropriately (as assessed either by achieving ≥ 50% of the recommended 45 minutes of slow breathing per week,[13] or by an independent “help desk” in all other studies).[11,12,14–16] This figure shows that, despite some variability (note that only 14 of 18 [78%] of the subjects in the study of Grossman and colleagues[14] had a change in office systolic BP < 0 mm Hg), the mean change from baseline across all the studies was 14 mm Hg, and was fairly homogeneous across studies (P = .61 by analysis of variance).
Figure 4.
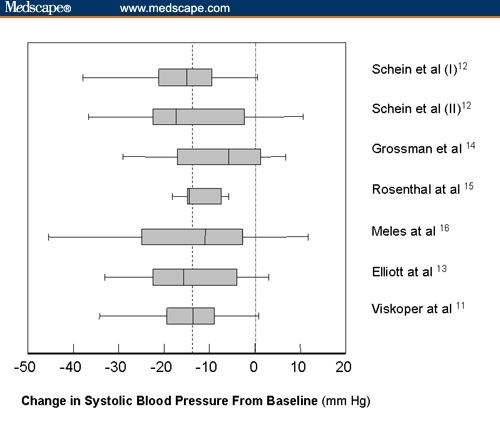
“Box and whiskers plot” of the age-adjusted changes in office systolic blood pressures seen in each of the 7 trials of the device to guide slow breathing (presented from top to bottom in chronological order of publication). The dashed vertical line represents the overall mean change in systolic blood pressure. The vertical line within each box represents the median value, while the ends of the boxes represent the 25% and 75% values for the population in each trial. The “whiskers” correspond to the extremes of the range. There was no significant heterogeneity across studies (P = .61 by analysis of variance). The first 2 studies were pooled when originally reported.[12]
Overall, the average decrease in office BP after >8 weeks of device-guided breathing among those with uncontrolled hypertension at baseline was 14/8 mm Hg, compared with control treatment of 9/4 mm Hg (P = .008 and P = .002, respectively for systolic and diastolic BPs). The difference was independent of gender and medication status. Control of BP (< 140/90 mm Hg) was seen more commonly in the group that used the device: 26% vs 4% of those with initial Stage 2 hypertension (≥ 160/100 mm Hg, P < .005); and 48% vs 34% for those with initial Stage 1 hypertension (140-159/90-99 mm Hg, P < .05). The drop in office BP was directly related to the duration of slow breathing during the 8 weeks of treatment. Those who used the device and achieved slow breathing more than 15 minutes/day had the greatest lowering of office BPs. The patient's response was almost exactly what would have been expected, based on her age, initial office BP, and weekly time of slow breathing. As with this patient, 3 to 5 weeks' use of the device was typically necessary for patients in these trials to achieve a sustained reduction in home BP.
Larger decreases in office BPs were seen in older individuals and those with higher baseline BPs, whether taking antihypertensive medications or not. BP lowering has also been seen using home BP measurements (for up to 6 months of use in one study)[16] and ambulatory BP monitoring.[15]
Potential Mechanism of Action
Inappropriately high sympathetic nervous outflow from the central nervous system is believed to be an important component in the pathophysiology of acute and chronic hypertension, which increases cardiac output and peripheral resistance. Elevated sympathetic activity is often associated with desensitization of arterial and cardiopulmonary baroreceptors, which leads to increased BP fluctuations and sustained elevations in resting BPs.
Slow breathing (< 10 bpm), especially with prolonged exhalation, appears to reduce sympathetic nerve traffic and thus causes arteriolar dilatation. The process is believed to be initiated by activated pulmonary mechanoreceptors, which respond to the increased tidal volume that accompanies slow breathing, and act in concert with cardiac mechanoreceptors to inhibit sympathetic outflow.[17,18]
Indications and Limitations
The device is indicated by the US Food and Drug Administration (FDA) for the reduction of stress and as an adjunctive therapy in hypertension. It can be combined with standard antihypertensive drugs and other nonpharmacologic interventions. It has similarly been approved and indicated in Europe, Canada, Australia, Korea, Thailand, and China. Based on the available literature,[11–16] device-guided breathing appears to be useful for: (1) prehypertensives and white-coat or labile hypertensives who might benefit from reducing stress and sympathetic activity; (2) patients with isolated systolic hypertension; and (3) patients with resistant hypertension (uncontrolled BP despite use of a diuretic and at least 2 other medications at maximum approved doses). Patients should be instructed to use the device routinely in daily 15-minute sessions, aiming to accumulate at least 45 minutes of slow breathing per week; the device can display the cumulative duration of slow breathing to assist in achieving this goal.
There are no known contraindications to the use of the device to guide slow breathing, and no adverse events have so far been reported. As with all nonpharmacologic therapies for hypertension, implementation of the regimen must be continued (typically for a few weeks) before BP lowering is seen; without continued use, any potential benefit would likely be diminished. In clinical trials, patients who did not spend the requisite 15 minutes/day in device-guided breathing did not see a significant reduction in BP. Many patients prefer to spend less than 15 seconds/day to swallow a pill, rather than spend 60 times that long performing breathing exercises. The $300 retail cost of the device (not typically reimbursed by insurance, including Medicare) is higher than many can afford, but it does come with a “money-back guarantee” (should the individual be unable to use it or should BP not be reduced). Lastly, both physicians and patients should be reminded that nonpharmacologic therapies (including device-guided breathing) should not be used in place of therapies (eg, antihypertensive drugs) that have been proven in 37 randomized clinical trials to prevent stroke (by 29 ± 2%), myocardial infarction (by 21 ± 2%), heart failure (by 29 ± 3%), and cardiovascular death (by 14 ± 2%), compared with placebo or no treatment.
Summary
Routine use of a device to guide slow breathing significantly lowers office BP without adverse effects. This modality may be a useful adjunct to current antihypertensive medications and to nonpharmacologic interventions in achieving better BP control.
Acknowledgments
The authors thank Benjamin Gavish, PhD, and Ariela Alter, PhD, for their assistance in the compilation of data, figures, and the flash video clip for this manuscript.
Contributor Information
William J. Elliott, Rush Medical College, Chicago, Illinois.
Joseph L. Izzo, Jr, Department of Medicine, State University of Buffalo, Buffalo, New York.
References
- 1.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. http://www.medscape.com/medline/abstract/12748199. [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, He J, Appel LJ, et al. Primary prevention of hypertension: Clinical and public health advisory from the National High Blood Pressure Education Program. JAMA. 2002;288:1882–1888. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- 3.Whelton PK, Appel LJ, Espeland MA, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: A randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. 1998;279:839–846. doi: 10.1001/jama.279.11.839. [DOI] [PubMed] [Google Scholar]
- 4.Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: Results of the Trials of Hypertension Prevention, Phase II. Ann Intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 5.Julius S, Nesbitt SD, Egan B, et al. Feasibility of treating prehypertension with an angiotensin receptor blocker. N Engl J Med. 2006;354:1685–1697. doi: 10.1056/NEJMoa060838. [DOI] [PubMed] [Google Scholar]
- 6.Kearny PM, Whelton M, Reynolds K, et al. World prevalence of hypertension: A systematic review. J Hypertension. 2004;22:11–19. doi: 10.1097/00004872-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Bernardi L, Sleight P, Bandinelli G, et al. Effect of rosary prayer and yoga mantras on autonomic cardiovascular rhythms: Comparative study. BMJ. 2001;323:1446–1449. doi: 10.1136/bmj.323.7327.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benson H, Rosner BA, Marzetta BR, Klemchuk HM. Decreased blood pressure in pharmacologically treated hypertensive patients who regularly elicited the relaxation response. Lancet. 1974;(i):289–291. doi: 10.1016/s0140-6736(74)92596-3. [DOI] [PubMed] [Google Scholar]
- 9.Patel C. 12-month follow-up of yoga and biofeedback in the management of hypertension. Lancet. 1975;(i):62–64. [Google Scholar]
- 10.Irvine J, Johnson DW, Jenner D, Marie GV. Relaxation and stress management in the treatment of essential hypertension. J Psychosom Res. 1986;30:437–450. doi: 10.1016/0022-3999(86)90083-8. [DOI] [PubMed] [Google Scholar]
- 11.Viskoper R, Shapira, Priluck I, et al. Non-pharmacological treatment of resistant hypertensives by device-guided slow breathing exercises. Am J Hypertens. 2003;16:484–487. doi: 10.1016/s0895-7061(03)00571-5. [DOI] [PubMed] [Google Scholar]
- 12.Schein M, Gavish B, Herz M, et al. Treating hypertension with a device that slows and regularizes breathing: A randomized double-blind controlled study. J Human Hypertens. 2001;15:271–278. doi: 10.1038/sj.jhh.1001148. [DOI] [PubMed] [Google Scholar]
- 13.Elliott WJ, Izzo JL, Jr, White WB, et al. Graded blood pressure reduction in hypertensive outpatients associated with use of a device to assist with slow breathing. J Clin Hypertens. 2004;6:553–559. doi: 10.1111/j.1524-6175.2004.03553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grossman E, Grossman A, Schein MH, Zimlichman R, Gavish B. Breathing control lowers blood pressure. J Human Hypertens. 2001;15:263–269. doi: 10.1038/sj.jhh.1001147. [DOI] [PubMed] [Google Scholar]
- 15.Rosenthal T, Alter A, Peleg E, Gavish B. Device-guided breathing exercises reduce blood pressure – ambulatory and home measurements. Am J Hypertens. 2001;14:74–76. doi: 10.1016/s0895-7061(00)01235-8. [DOI] [PubMed] [Google Scholar]
- 16.Meles E, Giannattasio C, Failla M, et al. Nonpharmacologic treatment of hypertension by respiratory exercise in the home setting. Am J Hypertens. 2004;17:370–374. doi: 10.1016/j.amjhyper.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Parati G, Izzo JL, Jr, Gavish B. Respiration and blood pressure. In: Izzo JL Jr, Black HR, editors. Hypertension Primer. 3rd ed. Baltimore, Md: Lippincott, Williams, and Wilkins; 2003. pp. 117–120. [Google Scholar]
- 18.Joseph CN, Porta C, Casucci G, et al. Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension. 2005;46:714–718. doi: 10.1161/01.HYP.0000179581.68566.7d. [DOI] [PubMed] [Google Scholar]
- 19.Elliott WJ, Black HR. The evidence-base for treatment of hypertension. In: McInnes GT, editor. Handbook of Hypertension, vol 24. New York, NY: Elsevier; 2006. In press. [Google Scholar]


