Abstract
We present here an improved RNA purification method using fast performance liquid chromatography (FPLC) size-exclusion chromatography in place of denaturing polyacrylamide gel electrophoresis (PAGE). The method allows preparation of milligram quantities of pure RNA in a single day. As RNA oligonucleotides behave differently from globular proteins in the size-exclusion column, we present standard curves for RNA oligonucleotides of different lengths on both the Superdex 75 column and the Superdex 200 size-exclusion column. Using this approach, we can separate monomer from multimeric RNA species, purify the desired RNA product from hammerhead ribozyme reactions, and isolate refolded RNA that has aggregated after long-term storage. This methodology allows simple and rapid purification of RNA oligonucleotides for structural and biophysical studies.
Keywords: RNA, FPLC, purification, NMR, X-ray crystallography
INTRODUCTION
RNA oligonucleotides are typically prepared by in vitro transcription from a DNA template with T7 RNA polymerase (Milligan et al. 1987; Puglisi and Wyatt 1995). After transcription, denaturing polyacrylamide gel electrophoresis (PAGE) is the most commonly used method to separate the desired RNA product from transcription byproducts (Wyatt et al. 1991). While this approach has been successful, it has several disadvantages (Cheong et al. 2004; Kieft and Batey 2004; Lukavsky and Puglisi 2004). First, this lengthy protocol requires multiple days to purify a single RNA sample. According to this method, after denaturing PAGE, the desired RNA is eluted from a gel matrix, concentrated, equilibrated in the desired buffer, and refolded. Water-soluble acrylamide oligomers, which are contaminants from PAGE, are difficult to remove from RNA samples (Lukavsky and Puglisi 2004). Moreover, since RNA is precipitated after the transcription reaction and purified under denaturing conditions, larger RNAs irreversibly aggregate and subsequently up to 90% of the RNA can be lost (Lukavsky and Puglisi 2004).
To overcome these disadvantages in PAGE purification, we previously reported a method using size-exclusion chromatography without requiring the denaturation of RNA (Lukavsky and Puglisi 2004). This approach produced acrylamide-free RNA samples that are suitable for structural studies. However, the protocol was not ideal. First, we found that concentration after phenol/chloroform extraction is not only a time-consuming step, but can also result in RNA aggregation and sample loss. Second, the open column used in the previous method is time consuming and low resolution. Here, we use size-exclusion chromatography columns in the fast performance liquid chromatography (FPLC) system, which allows rapid purification of homogeneous RNA samples. Milligram quantities of pure RNA are isolated from transcription and ready for structural studies in <1 d. Another advantage of size-exclusion columns in the FPLC system is their high resolution. The previous size-exclusion method employed open-column size-exclusion chromatography in which the matrix was chosen to separate between plasmid DNA and all other RNA species (Lukavsky and Puglisi 2004). By choosing appropriate size-exclusion columns, we demonstrate high-resolution separation of RNA oligonucleotides of variable sizes. The approach outlined should have broad application in the structural and biophysical investigations of systems involving RNA components.
RESULTS AND DISCUSSION
High-throughput RNA sample preparation
We describe a simple and rapid RNA purification scheme using an FPLC system. Milligram quantities of pure RNA were prepared from transcription in <1 d. As a template for in vitro transcription, we employed plasmid DNA derived from pUC119, which is prepared using the QIAGEN Plasmid Maxi kit (QIAGEN, Inc.). After restriction enzyme (BstZ17I or BsaI) treatment, the linearized plasmid template is used for in vitro transcription using T7 polymerase under standard conditions reported previously (Lukavsky and Puglisi 2004). After 2–3 h of incubation at 37°C, pyrophosphate precipitate is pelleted (3000g×10 min) and the reaction quenched by adding ethylenediaminetetraacetic acid (EDTA). An equal volume of phenol/chloroform is added to the supernatant, mixed, and centrifuged (3000g×10 min). In this process, the restriction enzyme, which is carried over with the linearized plasmid DNA, and the T7 RNA polymerase are removed from the reaction mixture, as only the aqueous phase is retained.
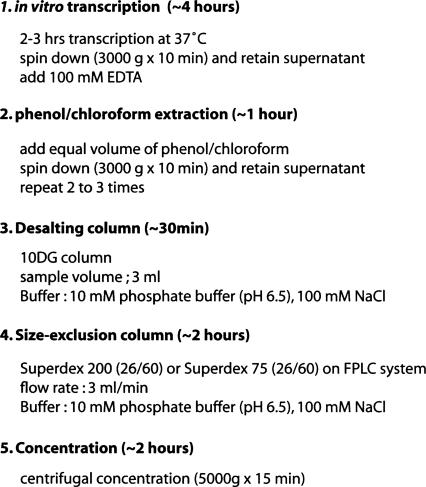
After three phenol/chloroform extractions, the aqueous phase (upper) is directly loaded on a desalting column (10DG column, Bio-Rad) to remove phenol and a significant amount of remaining NTPs. The desalting column is equilibrated with 20 mL of 10 mM phosphate buffer (pH 6.5) and 100 mM NaCl. After application to a desalting column, the eluted transcription reaction is directly loaded on a size-exclusion column without requiring any other treatments (i.e., precipitation or concentration). A summary of the methodology is presented in Figure 1.
FIGURE 1.
Schematic outline of RNA sample preparation. Estimated time for each step is shown in parentheses. Desalting and size-exclusion columns are equilibrated before use.
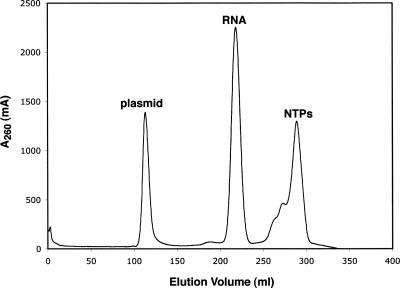
An example application of this method is shown for a 60 nucleotide (nt) RNA molecule (HIV TAR). A 5 mL transcription reaction was performed, and the phenol/chloroform-extracted RNA was passed over a desalting column. The desalted RNA fraction was collected and loaded on a Superdex 200 (26/60) column directly. Since we observed that concentration using centrifugation devices accelerates aggregation, we avoid concentrating samples at this stage. The Superdex 200 size-exclusion column was equilibrated with 10 mM phosphate buffer (pH 6.5) and 100 mM NaCl. Chromatography was performed at 3 mL/min, collecting 5 mL fractions. A typical elution profile shows three major peaks: the first contains the plasmid DNA, the second contains the desired RNA, and the third peak contains NTPs and small compounds in the transcription reaction that were not completely excluded by the desalting column (Fig. 2). The fractions containing the desired RNA were combined and concentrated using a Vivaspin 15R concentration device (Vivascience, Inc.). The molecular cutoffs of the centrifugal devices are based on globular proteins, and we observed that the molecular cutoff of RNA, which often adopts elongated conformations, is smaller than that recommended by the vendor.
FIGURE 2.
Purification of HIV TAR RNA (60 nt) by size-exclusion chromatography. Elution profile obtained from the size-exclusion chromatography step on the Superdex 200 (26/60) column. FPLC was performed at 3 mL/min with 10 mM phosphate buffer (pH 6.5) and 100 mM NaCl.
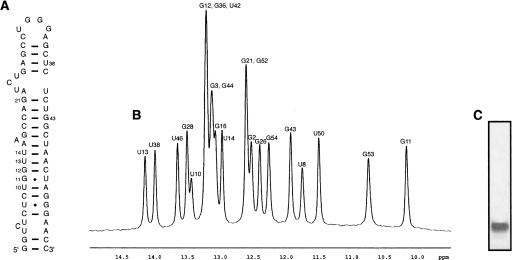
In order to highlight the usefulness of the method for structural biology studies, the imino proton region of a one-dimensional (1D) NMR spectrum of the HIV TAR RNA purified by this method is shown in Figure 3. The new purification protocol yields highly pure RNA without any contamination.
FIGURE 3.
RNA sample purity. (A) The secondary structure of the TAR RNA is shown. (B) Imino proton region of 1D 1H NMR spectra of HIV TAR RNA purified by size-exclusion chromatography. Assignments are indicated numerically above the peaks. (C) Denaturing 10% TBE gel of the NMR sample used in B, stained with toluidine blue.
FPLC size-exclusion column selection (Superdex 200 or Superdex 75)
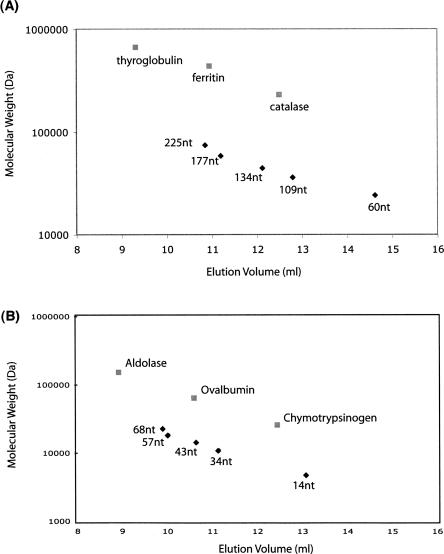
For globular proteins, the Superdex 200 is used to separate proteins of molecular weight between 10 and 600 kDa and the Superdex 75 is effective for proteins between 3 and 70 kDa. However, as shown in Figure 2, HIV TAR RNA (19.8 kDa) is eluted at 220 mL in Superdex 200 (26/60), which corresponds to a molecular weight of 111 kDa for a globular protein. Roughly a sixfold difference in apparent molecular weight between RNA and protein was observed. We therefore determined standard elution curves for RNA oligonucleotides of various lengths on the Superdex 200 and the Superdex 75 size-exclusion columns (Fig. 4). For the Superdex 200 (10/30), 225-, 177-, 134-, 109-, and 60-nt RNAs, which correspond to the molecular weights of 74.3, 58.4, 44.2, 35.9, and 19.8 kDa, respectively, were used for calibration. These RNAs are the derivatives of HCV IRES, interferon-γ mRNA, and HIV TAR (Ben-Asouli et al. 2002; Lukavsky et al. 2003; Kim et al. 2006). For the protein standards, thyroglobulin, ferritin, and catalase, which correspond to the molecular weights of 670, 440, and 240 kDa, respectively, were used for calibration. The Superdex 200 (10/30) column was equilibrated with 10 mM phosphate buffer (pH 6.5) and 100 mM NaCl. Chromatography was performed at 0.4 mL/min. The template plasmid is eluted in the void volume of the column at 8.0 mL. The 225-nt RNA (74.3 kDa) is eluted at 10.9 mL. The ferritin protein (440 kDa) also eluted at 10.9 mL. Likewise, the 177-, the 134-, the 109-, and 60-nt RNA are eluted at 11.20, 12.12, 12.79, and 14.62 mL, respectively. This result implies that RNAs behave approximately six times larger than equivalent globular proteins. NTPs and other small compounds are eluted at 17.5 mL.
FIGURE 4.
Standard curve of double-stranded RNAs on (A) Superdex 200 (10/30) and (B) Superdex 75 (10/30) columns. FPLC was performed at 0.4 mL/min in 10 mM phosphate buffer (pH 6.5) and 100 mM NaCl.
For the Superdex 75 (10/30) column, 68-, 57-, 43-, 34-, and 14-nt RNA oligonucleotides were used for calibration, which correspond to molecular weights of 22.4, 18.8, 14.1, 11.2, and 4.6 kDa, respectively. These are also derivatives of HCV IRES, interferon-γ mRNA, and HIV TAR RNA. For the protein standards, aldolase, ovalbumin, and chymotrypsinogen were used for calibration, which correspond to the molecular weights of 158, 43, and 25 kDa, respectively. As shown in Figure 4, RNAs behave about four to five times larger than globular proteins by size-exclusion chromatography.
Taken together, these observations demonstrate that the behavior of RNA greatly differs from that of proteins under native conditions on size-exclusion columns, as RNAs are often not globular but form more extended structures.
Separation of desired RNA product from cis-acting ribozyme
T7 RNA polymerase tends to add extra nucleotides to the 3′ end of synthesized RNA oligonucleotides (Milligan et al. 1987; Draper et al. 1988; Pleiss et al. 1998), often yielding RNA oligonucleotides with heterogeneous ends. As a single RNA species is typically required for high-resolution structural studies of RNAs or RNA•protein complexes, this behavior is problematic. By using cis-acting ribozyme at the 3′ ends of the RNA, this problem is readily overcome (Price et al. 1995; Ferre-D'Amare and Doudna 1996). In addition, proper usage of cis-acting ribozyme allows for efficient segmental labeling of RNA oligonucleotides, as we have demonstrated before (Kim et al. 2002). However, the ribozyme fragment must be removed during purification.
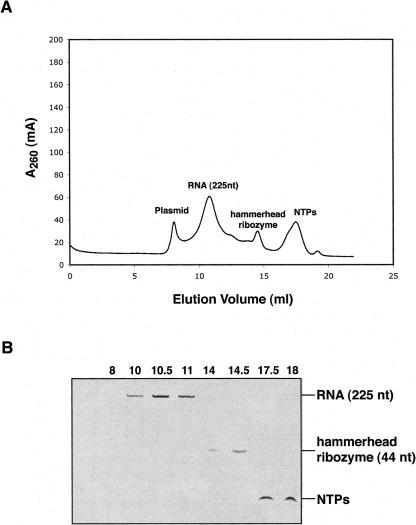
In order to test the purification of a desired RNA product from transcript and ribozyme treatment, we constructed a plasmid containing the T7 promoter, a template sequence of HCV IRES fragment (225 nt) and a 5′-cis-acting hammerhead ribozyme (44 nt) (Kim et al. 2002). During the transcription reaction, hammerhead cleavage occurs simultaneously and is completed after 2–4 h of incubation at 37°C. After T7 transcription and ribozyme reaction, the pyrophosphate precipitate is pelleted (3000g×10 min) and the reaction quenched by adding EDTA to chelate Mg2+. Subsequently, the reaction is treated as discussed previously, with phenol/chloroform extraction, application to a desalting column, and size-exclusion chromatography. The size-exclusion elution profile contains four major peaks, the first one containing the plasmid DNA followed by (in order) the desired RNA product, the hammerhead ribozyme, and the NTPs and small compounds in the transcription reaction that were not completely excluded from the desalting column (Fig. 5). The fractions containing the desired RNA are combined and concentrated as described previously. To purify the desired RNA product from the transcript and ribozyme, the RNA product should differ from transcript and ribozyme in length such that efficient separation of the RNAs by size-exclusion chromatography can be achieved.
FIGURE 5.
Purification of the desired RNA product from hammerhead ribozyme by Superdex 200 (10/30) size-exclusion chromatography. (A) Elution profile obtained from the size-exclusion chromatography is shown. FPLC was performed at 0.5 mL/min with 10 mM phosphate buffer (pH 6.5) and 50 mM NaCl. (B) The purity of the eluted sample is shown by denaturing PAGE on 5% TBE gels. The elution volume is indicated at the top of each lane.
Separation of monomeric from multimeric RNA
Many RNAs can multimerize by intermolecular formation of either Watson–Crick or noncanonical base pairs (Brion and Westhof 1997; Brunel et al. 2002). After transcription, we have observed that RNAs are often mixtures of monomer and multimers (dimer, trimer, etc.), with these equilibria depending on the RNA sequence (data not shown). In the current methodology, the ability to purify monomer away from all other RNA species is achieved through the use of FPLC in combination with large-scale size-exclusion columns. For high-resolution structural studies, it is often imperative to isolate monomeric RNA, as the inclusion of higher-order RNA complexes can lead to significant confusion in the interpretation of experimental results. Unfortunately, the RNA purification method by denaturing PAGE will not distinguish monomeric from multimeric oligonucleotides.
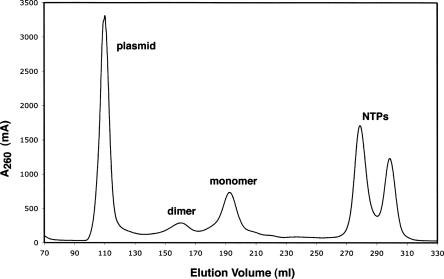
An example application is shown for CpGAIT, a 29-nt RNA molecule (Sampath et al. 2003) that exists in a monomer/dimer equilibrium after T7 transcription. After phenol/chloroform extraction, the RNA-containing species were separated into distinct peaks using the Superdex 75 (26/60) size-exclusion column (Fig. 6), and were characterized based on both denaturing and native gels (data not shown). The standard fractions corresponding to plasmid and NTPs were observed, as well as two peaks corresponding to monomeric and dimerized CpGAIT. Exclusively monomeric RNA can be purified away from all other components in a rapid and large-scale fashion. Furthermore, the purified dimer RNA can be subjected to a refolding process if desired and converted into monomer CpGAIT. Therefore, this methodology represents a significant improvement with respect to the identification and removal of higher-order complexes.
FIGURE 6.
Purification of CpGAIT (34 nt) with Superdex 75 (26/60). Elution profile obtained from the size-exclusion chromatography step using the Superdex 75 (26/60) column. FPLC was performed at 3 mL/min with 10 mM phosphate buffer (pH 6.5) and 100 mM NaCl.
Formation of RNA aggregates during storage
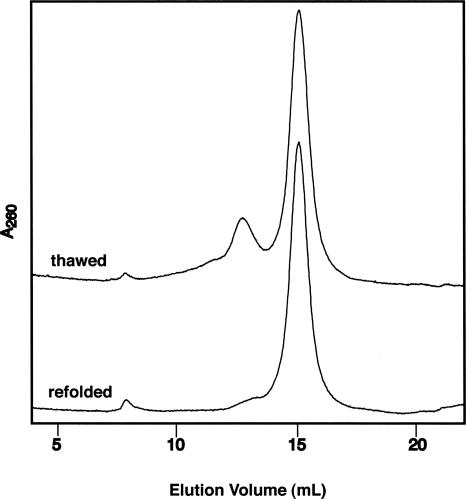
After synthesis and purification, RNA samples are often stored in aqueous buffers at either 4°C or −20°C. Many experimenters use these stored RNAs without concern for their molecular state. This is a particular concern for longer biological RNAs that may adopt multiple unimolecular and higher-order conformations. In Figure 7, we show an FPLC trace for a 69-mer RNA derived from the HIV genome. NMR studies have indicated a flexible ensemble of conformers for this RNA in solution. Upon storage at −20°C for >1 d, higher-order RNA structures are observed using FPLC. These include dimeric and higher-order species. Heating and rapid cooling of the RNA leads to recovery of the monomeric species. More severe aggregation problems were observed with RNAs >100 nt (data not shown). Thus, gel filtration is an essential step for preparation of conformationally homogeneous RNAs after storage.
FIGURE 7.
Renaturation of oligomerized RNA after storage. Superdex 200 (10/30) size-exclusion chromatography performed at 0.5 mL/min with 10 mM phosphate buffer (pH 6.5) and 50 mM NaCl. Chromatogram shows a 69mer RNA stored in −20°C freezer and thawed, without additional heating (top). Upon 90°C heating for 5 min and rapid cooling on ice, most of aggregation and dimerization is eliminated, as shown in chromatogram (bottom).
Conclusion
Purification of RNA samples is essential for biochemical investigation. The common method using PAGE is time consuming and contaminates samples with water-soluble acrylamide oligomers (Cheong et al. 2004; Kieft and Batey 2004; Lukavsky and Puglisi 2004). In this article, using the Superdex 75 or Superdex 200 column in the FPLC system, we have demonstrated fast performance and high-resolution purification of RNA. Milligram quantities of pure RNA can be isolated from transcription in <1 d, and monomeric and higher-order conformations of RNA are readily separated. The speed and relative ease of this approach are advantages over prior methods and should greatly aid biochemical and biophysical investigations of RNA.
MATERIAL AND METHODS
Large-scale plasmid preparation
The plasmid DNA pUC119 is used as a template for in vitro transcription of RNA. A template DNA fragment was designed containing a T7 promoter, a desired nucleotide sequence, and the restriction site (BstZ17I or BsaI) to run off the transcription reaction. A template DNA fragment is subcloned into pUC119 between the HindIII and EcoRI sites and transformed to Escherichia coli DH5α. A 500 mL LB culture usually yields 1 mg of DNA plasmid using QIAfilter plasmid Maxi Kits (QIAGEN, Inc.).
Transcription
After restriction enzyme (BstZ17I or BsaI) treatment, the linearized plasmid template is used for in vitro transcription using T7 polymerase. After 2 or 3 h of incubation at 37°C, pyrophosphate precipitate is pelleted (3000g × 10 min) and the reaction quenched by adding EDTA to chelate Mg2+. An equal volume of phenol/chloroform is added to the supernatant and centrifuged (3000g × 10 min). In this process, the restriction enzyme, which is carried over with the linearized plasmid DNA, and the T7 RNA polymerase are removed from the reaction mixture. After 2 or 3 phenol/chloroform extractions, the upper aqueous phase is directly loaded on the desalting column (10DG column, Bio-Rad), which is equilibrated with 20 mL of 10 mM phosphate buffer (pH 6.5) and 100 mM NaCl. A 3 mL sample is loaded to the desalting column. After the entire sample is allowed to enter the column the first 2 mL of effluent are discarded. Five milliliters of buffer is then used to elute the RNA transcript. After application to the desalting column, a 3 mL transcription reaction ends up being diluted to 5 mL. The column can be recycled and reused after a wash with 20 mL of the same buffer.
Separation of desired RNA product from cis-acting ribozyme
A complete description of the protocol has been presented elsewhere (Kim et al. 2002; Lukavsky et al. 2003). In summary, a plasmid containing the T7 promoter, a 5′-cis-acting hammerhead ribozyme (5′-GGGAGACAGGGGAGCTGATGAGTCCGTGAGGACGAAAGACTTCGGTCTA-3′), a template sequence of HCV IRES fragment (5′-CTCCCCTGTGA----AGTGTT-3′), and a 3′ BsaI restriction site was employed. The base-pairing between the ribozyme and HCV IRES fragment is highlighted in bold. Plasmid was linearized with BsaI restriction digestion prior to transcription. Hammerhead ribozyme cleavage at the 5′ end occurs simultaneously with transcription.
FPLC size-exclusion chromatography (Superdex 200 or Superdex 75)
The desalted RNA fraction is collected and loaded on the Superdex 200 (26/60) or Superdex 75 (26/60) column directly. For best resolution of the RNA peak from plasmid and NTP contaminants, a maximum load volume of 15 mL is suggested, although up to 30 mL can typically be loaded without significant difficulty. The column is equilibrated with 10 mM phosphate buffer (pH 6.5) and 100 mM NaCl. Chromatography is performed at 3 mL/min, collecting 5 mL fractions. All experiments were performed at 4°C. The fractions containing the desired RNA are combined and concentrated using Vivaspin 15R (Vivascience, Inc.).
Denaturation and refolding of aggregated RNA
Aggregated samples were diluted to low concentrations (<1 μM), heated to 90°C for 5 min, and transferred directly to ice for 15 min to rapidly drop solution temperature. The RNA solution was then transferred to room temperature (25°C) for a period of 4 h (or greater) in order to allow the temperature to re-equilibrate slowly.
NMR experiments
All NMR spectra were collected on Varian INOVA 800 NMR spectrometers at Stanford Magnetic Resonance Laboratory (SMRL). The HIV TAR RNA sample was prepared in 10 mM phosphate buffer (pH 6.5) and 100 mM NaCl. The 1D imino proton spectrum was collected in 90% H2O/10% 2H2O at 298 K.
ACKNOWLEDGMENTS
We thank M. Margaris for assistance and other members of the Puglisi laboratory for their help and advice. This research was supported by NIH AI47365 and GM69314. S.A.M. is supported by the Canadian Institutes of Health Research and the Alberta Heritage Foundation for Medical Research.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.342607.
REFERENCES
- Ben-Asouli, Y., Banai, Y., Pel-Or, Y., Shir, A., Kaempfer, R. Human interferon-γ mRNA autoregulates its translation through a pseudoknot that activates the interferon-inducible protein kinase PKR. Cell. 2002;108:221–232. doi: 10.1016/s0092-8674(02)00616-5. [DOI] [PubMed] [Google Scholar]
- Brion, P., Westhof, E. Hierarchy and dynamics of RNA folding. Annu. Rev. Biophys. Biomol. Struct. 1997;26:113–137. doi: 10.1146/annurev.biophys.26.1.113. [DOI] [PubMed] [Google Scholar]
- Brunel, C., Marquet, R., Romby, P., Ehresmann, C. RNA loop–loop interactions as dynamic functional motifs. Biochimie. 2002;84:925–944. doi: 10.1016/s0300-9084(02)01401-3. [DOI] [PubMed] [Google Scholar]
- Cheong, H.K., Hwang, E., Lee, C., Choi, B.S., Cheong, C. Rapid preparation of RNA samples for NMR spectroscopy and X-ray crystallography. Nucleic Acids Res. 2004;32:e84. doi: 10.1093/nar/gnh081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper, D.E., White, S.A., Kean, J.M. Preparation of specific ribosomal RNA fragments. Methods Enzymol. 1988;164:221–237. doi: 10.1016/s0076-6879(88)64045-6. [DOI] [PubMed] [Google Scholar]
- Ferre-D'Amare, A.R., Doudna, J.A. Use of cis- and trans-ribozymes to remove 5′ and 3′ heterogeneities from milligrams of in vitro transcribed RNA. Nucleic Acids Res. 1996;24:977–978. doi: 10.1093/nar/24.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieft, J.S., Batey, R.T. A general method for rapid and nondenaturing purification of RNAs. RNA. 2004;10:988–995. doi: 10.1261/rna.7040604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, I., Liu, C.W., Puglisi, J.D. Specific recognition of HIV TAR RNA by the dsRNA binding domains (dsRBD1-dsRBD2) of PKR. J. Mol. Biol. 2006;358:430–442. doi: 10.1016/j.jmb.2006.01.099. [DOI] [PubMed] [Google Scholar]
- Kim, I., Lukavsky, P.J., Puglisi, J.D. NMR study of 100 kDa HCV IRES RNA using segmental isotope labeling. J. Am. Chem. Soc. 2002;124:9338–9339. doi: 10.1021/ja026647w. [DOI] [PubMed] [Google Scholar]
- Lukavsky, P.J., Puglisi, J.D. Large-scale preparation and purification of polyacrylamide-free RNA oligonucleotides. RNA. 2004;10:889–893. doi: 10.1261/rna.5264804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukavsky, P.J., Kim, I., Otto, G.A., Puglisi, J.D. Structure of HCV IRES domain II determined by NMR. Nat. Struct. Biol. 2003;10:1033–1038. doi: 10.1038/nsb1004. [DOI] [PubMed] [Google Scholar]
- Milligan, J.F., Groebe, D.R., Witherell, G.W., Uhlenbeck, O.C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987;15:8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleiss, J.A., Derrick, M.L., Uhlenbeck, O.C. T7 RNA polymerase produces 5′ end heterogeneity during in vitro transcription from certain templates. RNA. 1998;4:1313–1317. doi: 10.1017/s135583829800106x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, S.R., Ito, N., Oubridge, C., Avis, J.M., Nagai, K. Crystallization of RNA–protein complexes. I. Methods for the large-scale preparation of RNA suitable for crystallographic studies. J. Mol. Biol. 1995;249:398–408. doi: 10.1006/jmbi.1995.0305. [DOI] [PubMed] [Google Scholar]
- Puglisi, J.D., Wyatt, J.R. Biochemical and NMR studies of RNA conformation with an emphasis on RNA pseudoknots. Methods Enzymol. 1995;261:323–350. doi: 10.1016/s0076-6879(95)61016-2. [DOI] [PubMed] [Google Scholar]
- Sampath, P., Mazumder, B., Seshadri, V., Fox, P.L. Transcript-selective translational silencing by γ interferon is directed by a novel structural element in the ceruloplasmin mRNA 3′ untranslated region. Mol. Cell. Biol. 2003;23:1509–1519. doi: 10.1128/MCB.23.5.1509-1519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt, J.R., Chastain, M., Puglisi, J.D. Synthesis and purification of large amounts of RNA oligonucleotides. Biotechniques. 1991;11:764–769. [PubMed] [Google Scholar]