Abstract
Previously we reported that oxytocin (OT)-containing neurons of the hypothalamic paraventricular nucleus (PVN) project to the preBötzinger complex (preBötC) region and phrenic motoneurons innervating the diaphragm (D). The aim of these studies was to determine pathways involved in PVN stimulation-induced changes in upper airway and chest wall pumping muscle activity. In addition, we determined the role of OT-containing neurons in the PVN in mediating increased respiratory output elicited by PVN stimulation. Neuroanatomical experiments, using pseudorabies virus (PRV) as a transneuronal tracer in C8 spinalectomized animals showed that PVN neurons project to hypoglossal motoneurons innervating the genioglossus (GG) muscle. Furthermore, microinjection of the PVN with bicuculline, a GABAA receptor antagonist, significantly increased (P<0.05) peak electromyographic activity of GG (GGEMG) and of DEMG, frequency discharge, and arterial blood pressure (BP) and heart rate. Prior injection of oxytocin antagonist [d-(CH2)5, Tyr(Me)2,Orn8]-vasotocin(OVT) intracisternally or blockade of oxytocin receptors in the preBötC region with oxytocin antagonist L-368,899, diminished GGEMG and DEMG responses and blunted the increase in BP and heart rate to PVN stimulation. These data show that PVN stimulation affects central regulatory mechanisms via the preBötC region controlling both respiratory and cardiovascular functions. The parallel changes induced by PVN stimulation were mediated mainly through an OT-OT receptor signaling pathway.
Keywords: Transneuronal labeling, Pseudorabies virus, diaphragm and genioglossus electromyographic activity, arterial pressure, heart rate
1. INTRODUCTION
The PVN is a complex structure that initiates endocrine and autonomic responses to stress and behavioral changes (3, 6). It receives inputs from visceral receptors, circulating hormones and limbic circuits and transmits information to multiple CNS sites (11,53), including cell groups regulating respiratory drive (27,30,58) and sympathetic outflow (6,13,24,26,27,41,48). Chemical stimulation of the PVN by microinjection of glutamate elevated frequency and peak diaphragmatic discharge (60). Furthermore, microinjection of bicuculline, a γ-aminobutyric acid (GABA)A receptor antagonist into the PVN of conscious rats increased minute ventilation, mean arterial pressure (MAP), heart rate, and oxygen consumption (52).
The respiratory changes induced by stimulation of PVN cells could be mediated via multiple pathways including the rostral ventrolateral region of the medulla oblongata (RVLM) where inspiratory rhythm generating neurons (preBötzinger Complex; preBötC) are located (56). This region receives inputs from the PVN (28, 39) and expresses oxytocin receptors (39). Furthermore, this ventrolateral medullary area plays an important role in coordinating phrenic and hypoglossal nerve discharge or the activity of the muscles that they innervate (21, 23). Thus, the respiratory-related network within the region corresponding to the preBötC could be involved in mediating responses of the diaphragm and genioglossus muscle to stimulation of PVN neurons. However, PVN neurons may affect respiratory drive to upper airway dilating and chest wall pumping muscles via direct projections to motoneurons innervating these muscles. Furthermore, through projections to the nucleus tractus solitarius (NTS; 11, 50, 51), the PVN could affect respiratory output and sympathetic nerve activity at manifold points (35).
The physiological significance of the preBötC region in mediating parallel changes in the activity of inspiratory chest wall pumping and upper airway dilating muscles, elicited by activation of PVN neurons, is not well understood. Moreover, it is not known which specific neurotransmitter relays information from the PVN to the preBötC or other sites that could affect breathing and cardiovascular function.
Oxytocin (OT) is one among numerous neurotransmitters expressed by neurons of the PVN that project to respiratory-related sites in the brainstem and spinal cord (39). This neuropeptide, in addition to its well known hormonal action, produces neuronal effects in various regions of the central nervous system, including the RVLM (30), a site controlling respiratory drive and sympathetic nerve activity. Located within this region is the preBötC, the site thought to generate inspiratory rhythmic breathing (56). However, it is not known whether the release of OT within this region mediates changes in respiratory drive and cardiovascular function induced by PVN stimulation.
Therefore, in these studies we tested the hypothesis that the effect of PVN stimulation on diaphragm and genioglossus muscle responses and cardiovascular changes are mediated through PVN-OT containing neurons innervating the preBötC region. The data support the assumption that release of oxytocin from the PVN activates oxytocin receptors in the pre-Bötzinger neurons inducing a parallel increase in respiratory drive to the genioglossus muscle and the diaphragm and stimulating sympathetic outflow, causing an elevation in arterial pressure and heart rate.
2. Material and Methods
Experiments were performed on adult male Sprague-Dawley rats (250-350 g) housed in the Howard University College of Medicine Veterinary Services Facility. The rats were given food and water ad libitum and were exposed to a normal 12:12-h light- dark cycle. All experimental procedures were approved by the Howard University Institutional Animal Care and Use Committee and are according to NIH guidelines for the care and use of laboratory animals.
2. 1. Neuroanatomical studies: Transneuronal retrograde labeling experiments.
Previously, we have shown that OT-containing cells project to both the preBötC and phrenic motoneurons. In the present studies, we determined whether PVN neurons innervate hypoglossal motor cells. Pseudorabies virus (PRV), a transneuronal tracer, was injected into the genioglossus muscle of C8 spinalectomized animals. This model was used to avoid viral transmission via sympathetic pathways as described previously (19,20). Briefly, Sprague—Dawley rats were anesthetized with pentobarbital (40 mg/kg, i.p.). Spinalectomy at the C8 level was performed and the pudental nerves were dissected and sectioned to prevent urine retention. The genioglossus muscle of the tongue was exposed and injected unilaterally with eight to ten 100 nl injections of PRV (Bartha strain, 3.55×l07 plaque forming units/ml). The rats were carefully monitored and given sterile saline (1 ml/100 g body weight) subcutaneously every 12 h.
Five days after PRV injections, rats were anesthetized with pentobarbital and perfused intracardially with 0.9% saline, which was followed by perfusion with 4% paraformaldehyde. Brains were removed, post-fixed in 4% paraformaldehyde and transferred to a 30% sucrose solution. Brains were cut at 50 μm in the transverse plane on a freezing microtome. A one-in-five series of brainstem sections extending from 10 mm caudal to Bregma to the decussation of the pyramids, were incubated in a 1:60 000 dilution of pig anti-PRV (1:60,000) for 12-16 h, rinsed with phosphate buffer and then exposed to a 1:100 solution of biotinylated rabbit anti-pig antibody (3-4 h) and visualized by reacting the sections with diaminobenzidine using an ABC kit (Vector Laboratories, Burlingame, CA).
2.2. Physiological studies
2.2.1. General preparation.
For physiological studies, animals were anesthetized with an intraperitoneal injection of urethane (1.5 g/kg) and supplemental doses of urethane (0.1-0.3 g/kg iv) were given during surgery as required, based on nociceptive reflex responses and increases in arterial blood pressure. The rats were instrumented with catheters in the carotid artery for recording arterial pressure and monitoring arterial blood pH, PCO2 and PO2 (Radiometer ABL5, Radiometer Westlake, OH) and in the jugular vein for administration of anesthetic and fluids. The arterial catheter was attached to a calibrated pressure transducer connected to a blood pressure preamplifier (Buxco Research Systems, Wilmington, NC)
2.2.2. Electromyographic recordings.
Electromyographic recordings were obtained as described previously (39). Briefly, a midline incision was made on the ventral surface of the neck for bilateral vagotomy in the cervical region and for tracheotomy. The rats were intubated, placed in a prone position and mechanically ventilated with 100% oxygen at 10ml/kg body weight using a rodent ventilator (Harvard Apparatus, Holliston, MA). Core body temperature was monitored with a rectal probe and maintained between ∼37° and 38° C with a temperature-controlled heating pad (F S T, San Francisco, CA). Bipolar electrodes, which consisted of two teflon-coated stainless steel wires with bared tips, were inserted into the genioglossus muscle (GG) and into the right side of the costal region of the diaphragm (D) for recording electromyographic activity (GGEMG, DEMG). The rats were then placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) with blunt ear bars and the dorsal brain regions overlying the right sides of the hypothalamic PVN and the preBötzinger complex were reached through small craniotomies. The stereotaxic coordinates for the PVN were 1.6-2.1 mm caudal to Bregma, 0.3-0.4 mm lateral to the midline and 7.8 mm ventral to dura. Stereotaxic coordinates for the preBötC within the ventral respiratory group were 12.3-12.9 mm caudal to Bregma, 1.8-2.1 mm from midline, and 8.0-10.0 mm ventral to dura as previously reported (45).
As we described previously (39), DEMG and GGEMG signals were amplified, bandpass filtered, rectified, and integrated to give moving average traces. These signals along with analog outputs from the blood pressure amplifier and the gas analyzer (ADInstruments, Colorado Springs, CO), which measured end tidal CO2, were sent to a Power Lab data acquisition system (ADInstruments) and visualized on the computer with Chart software (ADInstruments). At the beginning of experiments, arterial blood pH was adjusted to ∼ 7.35- 7.45 with administration of NaHCO3 as required and PCO2 was kept within ∼ the 34- 38 mm Hg range by adjusting the rate of the ventilator.
2.3. Experimental Protocols
2.3.1. Stimulation of PVN with GABAA receptor antagonist.
In the first series of experiments (n=10), based on preliminary dose response studies, bicuculline [-]methiodide (Sigma-Aldrich, St. Louis, MO) was microinjected into the PVN unilaterally (50 nl of a 1 mM solution) with a Hamilton syringe connected to a UMP2 pressure pump with microprocessor (World Precision Instruments, Sarasota, FL). Control studies were conducted by injecting the same volume (50 nl) of physiological saline into the regions of interest. In these studies we avoided use of the excitatory amino acid glutamate because of the narrow window between its stimulating and depolarizing blockade effects on the neurons of interest (33).
2.3.2. Midcollicular Decerebration.
To determine whether the effects of bicuculline are mediated through suprapontine neural structures rather than via resorbed, circulating bicuculline, the drug was microinjected into the PVN (n=5), following midcollicular decerebration. Briefly, small parietal craniotomies were made 1-2 mm rostral to the interaural line on the left and right sides. A thin blunt spatula was inserted horizontally on each side and then swept back across the depth, preserving the large blood vessels. A 1-h stabilization period was allowed before resuming the experiment by unilateral microinjection of a dose of bicuculline that was 2-fold higher than the original concentration. Decerebration was verified by visual inspection of the brain after fixation. Only animals with complete decerebration were included in the study.
2.3.3. Intracisternal Injection of Oxytocin Receptor Antagonist.
To define, whether OT mediates the effects of PVN stimulation, OT receptors were blocked by intracisternal injection of an OT antagonist. For this, the head of the animal was fixed into a stereotaxic instrument in a nose-down position and the skin and muscle overlying the occipital bone were retracted. A small part of the occipital bone was removed to visualize the fourth ventricle via the atlantooccipital membrane. Oxytocin receptor blocker, [d-(CH2)5, Tyr(Me)2, Orn8]-vasotocin (AVT) (Sigma— Aldrich Chemical Company) was dissolved in artificial cerebrospinal fluid (aCSF) and slowly injected into the 4th ventricle (n=7 rats) in a fixed volume of 2 μl of a 0.1mM solution of the drug. We employed AVT because it is a potent peptide OT receptor blocker and is widely used in pharmacological and physiological experiments (8, 9, 44). Control experiments with intracisternal injection of 2 μl of CSF were carried out in 2 rats.
2.3.4. Microinjection of Oxytocin Receptor Antagonist in the preBötzinger region.
Since drugs administered intracisternally could affect multiple sites, we defined the degree of involvement of the preBötC network in response to activation of OT signaling pathways by microinjecting OT receptor antagonist into the right rostral ventrolateral medulla (RVLM) region that contains the preBötC. For these microinjection studies, we used a non-peptide OT antagonist, L-368,899 (Sigma-Aldrich). This compound is more selective for the oxytocin receptor and has minimal or no effect on vasopressin receptors (46). In preliminary studies, we tested the effects of prior microinjection of the OT receptor antagonist L-368,899 on respiratory and cardiovascular changes elicited by OT injected into the preBötzinger region. The results showed that L-368,899; diminished the effects of 50 nL of 1 mM OT solution microinjected into the preBötzinger region, but failed to block changes induced by microinjection of vasopressin into the same area, such as an increase in mean arterial blood pressure and diaphragm activity. This indicated to us a high level of its specificity for OT receptors. L-368,899 (0.1mM, 20 nl) was microinjected into the preBötC region and responses were recorded (n=6). After reaching a steady state condition, 4-7 min, the PVN was stimulated ipsilaterally with the same dose of bicuculline as in the absence of the OT receptor antagonist.
2.3.5. Microinjection of GABAA receptor agonist in the preBötzinger region.
We conducted complementary experiments in determining the overall role of the preBötzinger region in mediating respiratory and cardiovascular effects of PVN activation. Muscimol, a specific GABAA receptor agonist (50 nl of a 10 mM solution) was unilaterally microinjected (n=6 rats) into the right preBötzinger region, using the coordinates given above. Muscimol decreased respiratory discharge of both the D and GG muscle and decreased arterial pressure. Only muscimol injections were considered to be in the preBötzinger region when muscimol administration reduced inspiratory drive. To avoid the effects of decreased respiratory drive on responses of the studied variables to PVN stimulation, peak activity of the diaphragm was brought close to its baseline level prior to injection of muscimol. This was accomplished by reducing the rate of the ventilator, consequently, increasing arterial CO2 and respiratory drive to values comparable to prior to muscimol administration, which was ∼ 30% of the drive recorded by ventilating animals with 7% CO2 at the beginning of the experiment. We needed to increase end tidal CO2, on average of 1.2 -1.4% to reach the expected level of respiratory drive. When a steady state was achieved, ipsilateral microinjection of bicuculline into the PVN was performed, and the studied variables were recorded.
2.4. Histology.
At the end of each experiment, rats were overdosed with urethane and perfused intracardially with 0.9% saline, which was followed by 4% paraformaldehyde. Brains were removed, postfixed in 4% paraformaldehyde and transferred into a 30% sucrose solution prior to cryosectioning with Leica cryostat (Leica Microsystems, Bannockburn, IL). Sections were mounted onto glass slides to identify injection sites.
2.5. Data Analysis.
The baseline values for DEMG and GGEMG activities were analyzed from moving average signals and were quantified in arbitrary units within a 3-5 min period for 10 consecutive breaths immediately before microinjection of bicuculline into the PVN. To determine changes in tonic and phasic electrical activity of the genioglossus muscle evoked by microinjection of bicuculline into the PVN, peak response amplitudes were also determined for 10 consecutive breaths. Mean arterial blood pressure and heart rate were measured for the corresponding baseline and peak response periods. Mean peak DEMG and GGEMG activity, respiratory frequency (f), diaphragm minute activity (DEMG x f), genioglossus min activity (GGEMG x f) and inspiratory and expiratory times were quantified and calculated. Baseline and peak response amplitudes were determined similarly for midcollicular decerebration, OT blocker and muscimol experiments.
Measured and calculated cardiorespiratory variables were analyzed as appropriate with a paired student’s t-test or a one-way repeated measures ANOVA, followed by Tukey’s post hoc test for comparisons between drugs (Sigmastat, SYSTAT Software Inc., Chicago, IL). Summary data in the text and figures are expressed as means ± SE. Differences were considered statistically significant at P < 0.05.
3. Results
3. 1. Neuroanatomical studies:
Transneuronal retrograde labeling experiments. Five days after PRV injections into the genioglossus muscle of C8 spinalectomized animals, the majority of PRV infected cells was observed ipsilaterally within the ventral and ventrolateral portions of hypoglossal motoneurons at 14 mm caudal to Bregma and immediately caudal to the area postrema. Within the PVN, labeled cells were also localized mainly ipsilaterally, in the dorsal parvocellular subnucleus shown in Fig. 1 and to a lesser extent within the medial parvocellular, ventral parvocellular, and magnocellular subnuclei.
Figure 1.

A: Immunohistochemical localization of PRV-positive neurons five days after injection of PRV into the genioglossus muscle of C8 spinalectomized animals. PRV infected cells were prominent in the ventral and ventrolateral portions of the hypoglossal nucleus on the same side that PRV was injected into the tongue. B: Transneural infected cells within the PVN were observed predominantly ipsilateral in the dorsal parvocellular subnucleus (dP). C and D: Schematic drawings based on the atlas of Paxinos and Watson (1998). cc, central canal; NTS, nucleus tractus solitarius; XII, hypoglossal nucleus; NA, nucleus ambiguus; III V, third ventricle; PVN, hypothalamic paraventricular nucleus; AHA, anterior hypothalamic area. Scale bar= 100 μm.
3.2. The effect of PVN stimulation on diaphragm and genioglossus muscle activities.
In 4 of 10 animals in this study, saline vehicle was injected into the PVN prior to injection of bicuculline. Ventilatory measurements following saline administration did not change dramatically from baseline values. DEMG decreased by 1.7%, while Ti, Te, and frequency only increased slightly (P>0.05) by 3.9, 2.3 and 3.5%, respectively. GGEMG, blood pressure and heart rate insignificantly decreased (P>0.05) on average by less than 2%.
In contrast, activation of the PVN by microinjection of bicuculline increased DEMG and GGEMG peak activity frequency discharge and breathing rate. Typically, the response peaked within 3-5 minutes from the start of injection and was followed by a decline back to preinjection levels within 10-20 minutes. Microinjection sites that elicited changes in respiratory drive and arterial pressure are not shown in Fig 2. Integrated tracings for DEMG discharges are presented in Fig. 3A and average results for all studied variables are shown in Fig. 3B -F). Rats microinjected unilaterally with bicuculline showed an increase (P<0.05) in peak DEMG activity by 109 % from 0.49 ± 0.01 to 0.94 ± 0.13 units. Inspiratory time did not change significantly (P> 0.05). However, expiratory duration was shortened (P<0.05) by 22.8 ± 6.8 % from a mean of 1.13 ± 0.07 to 0.85 ± 0.07s, which led to a 28.8% increase (P<0.05) in frequency (Fig 3); thus Min DEMG changed dramatically by 172% from 22.7± 4.0 to 49.8 ± 5.0 units. Results for GGEMG were similar to the results for diaphragm activity (Fig.4). Peak GGEMG activity increased from 0.59 ± 0.12 to 1.02 ± 0.19, a 91.8% change (P<0.05); Min GGEMG also increased significantly (P<0.05) by 148.7% (Fig. 4B and C).
Figure 2.

A: Sites of injection of bicuculline into the paraventricular nucleus of the hypothalamus that caused an increase in diaphgram and genioglossal muscle activity. B: Schematic showing location of the preBötzinger complex and the sites injected with oxytocin antagonist L-368,899 that blocked respiratory response of PVN stimulation with bicuculline. Missed or control injections are not shown.
Figure 3.

A: Representative integrated electromyographic signals from the diaphragm are shown for baseline activity and at peak response following PVN stimulation by microinjection of bicuculline. B-E: Average responses for diaphragm amplitude (DEMG, Minute diaphragm (Min DEMG activity, expiratory duration (Te) and frequency of diaphragm discharge (f) are shown. Values are means ± S.E. for 10 animals. * P<0.05.
Figure 4.

A: Representative integrated electromyographic signals from the genioglossus are shown for baseline activity and at peak response following PVN stimulation with bicuculline. B. Average responses for genioglossus muscle amplitude (GGEMG) and Minute activity (Min GGEMG). Values are means ± S.E. for 10 animals. * P<0.05.
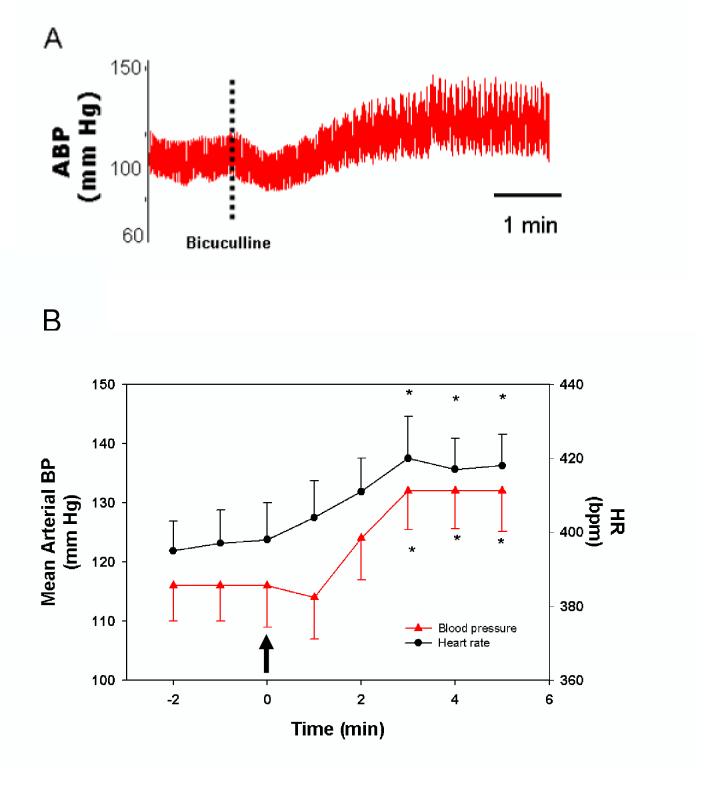
Microinjection of bicuculline caused an increase in arterial blood pressure as exemplified in Fig 5A. Arterial blood pressure and heart rate were significantly elevated within 3 minutes after PVN stimulation (Figure 5B). Mean arterial blood pressure increased from the control value of 116 ± 7 to 132 ± 8 mm Hg following treatment (P<0.05). Heart rate was elevated slightly, but significantly (P<0.05) by 5.6% from 395 ± 11 to 417 ± 12 beats/min.
Figure 5.
A: Tracing of arterial pressure response to PVN stimulation with bicuculline as indicated by the dashed vertical line. B: Stimulation of the PVN with bicuculline, indicated by the arrow, increased mean arterial blood pressure (mm Hg) and heart rate (bpm, beats per minute). Values are means ± S.E. for 10 animals. * Significantly different (P<0.05) from the zero time point.
In experiments, when injection sites were located in the nearby regions, 500-700 μm dorsal or lateral to the PVN borders, the same amount of bicuculline had no detectable effect on diaphragm and genioglossus muscle activity. These data are not shown in Fig. 2.
3.3. The effect of PVN stimulation on diaphragm and genioglossus muscle activity after midcollicular decerebration.
In decerebrated rats, PVN stimulation with bicuculline had no effect (P >0.05) on minute DEMG activity (before vs. after bicuculline: 20.5 ± 3.6 vs. 20.2 ± 3.6) minute GGEMG activity (16.5 ± 3.6 vs. 23.8 ± 11.0), arterial blood pressure (115 ± 4.0 vs. 112 ± 4.0 mmHg), or heart rate (426 ± 36 vs. 432 ± 34 beats/min). These findings indicate that changes observed in nondecerebrated rats were due to activation of PVN neurons and not due to spill over of bicuculline into the cerebrospinal fluid.
3.4. The effect of PVN stimulation on diaphragm and genioglossus muscle activities after intracisternal injection of oxytocin receptor antagonist.
Data for intracisternal injection of OT blocker AVT, in a concentration (2 μl of a 0.1mM solution of the drug); that blocks the stimulatory effects of 1mM OT, were analyzed only in rats in which anatomical studies confirmed that microinjections of bicuculline were performed in the PVN as described in sections 3.2 and 3.3. In these animals, administration of AVT elicited a slight decrease in DEMG, but a slight increase in GGEMG, arterial blood pressure and heart rate. However, AVT diminished the effects of PVN stimulation on these variables. In Table 1, baseline variables prior to intervention, the steady- state condition after administering AVT intracisternally and stimulation of the PVN with bicuculline are presented.
Table 1.
Effect of PVN stimulation on cardiorespiratory variables after intracisternal blockade of OT receptors.
| Drug | DEMG (Au) | Min DEMG | Ti (s) | Te (s) | f (breaths/min) | GGEMG (Au) | Min GGEMG | BP (mmHg) | HR (bpm) |
|---|---|---|---|---|---|---|---|---|---|
| ControlBaseline | 0.38 ± 0.05 | 18.6 ± 2.6 | 0.22 ± 0.02 | 1.01 ± 0.05 | 49 ± 2 | 0.60 ± 0.10 | 30 ± 5 | 110 ± 6 | 415 ± 10 |
| AVT | 0.37 ± 0.05 | 18.4 ± 2.8 | 0.24 ± 0.02 | 0.99 ± 0.08 | 50 ± 3 | 0.62 ± 0.13 | 31 ± 5 | 112 ± 7 | 426 ± 11 |
| Bicuculline | 0.38 ± 0.05 | 19.4 ± 2.9 | 0.22 ± 0.02 | 0.99 ± 0.07 | 51 ± 3 | 0.49 ± 0.09 | 25 ± 4 | 107 ± 5 | 430 ± 11* |
Values are means ± SE; n = 7 animals.
Differences between baseline and changes induced by AVT were not significant (P>0.05). Bicuculline administration did not significantly change (P>0.05) the values recorded post AVT. Heart rate for microinjection of bicuculline was significantly elevated above the baseline value (P<0.05).
3.5. The effect of PVN stimulation on diaphragm and genioglossus muscle activities after microinjection of oxytocin receptor antagonist in the preBötzinger region.
In this series of experiments, we microinjected L-368,899, an OT receptor antagonist, into the preBötzinger region. As shown in Table 2, blockade of OT receptors tended to decrease respiratory drive, significantly lowering minute GGEMG activity and arterial pressure (P<0.05). Furthermore, there was a decrease in DEMG and reduced frequency discharge, but the OT antagonist abolished changes (P>0.05) in DEMG activity, minute DEMG (before vs after bicuculline: (19.8 ± 3.7 vs 18.3 ± 3.7), minute GGEMG activity (20.7 ± 2.2 vs 19.7 ± 3.2, arterial blood pressure (92 ± 6.0 vs 93 ± 7.0 mmHg), or heart rate (422 ± 7.0 vs 417 ± 5.0 beats/min) elicited by PVN stimulation. These findings indicate that changes in the studied variables induced by stimulation of PVN neurons are mediated mainly by a release of OT within the preBötzinger region and activation of OT receptors.
Table 2.
Effect of PVN stimulation on cardiorespiratory variables after blockade of OT receptors in preBötC
| Drug | DEMG (Au) | Min DEMG | Ti (s) | Te (s) | f (breaths/min) | GGEMG (Au) | Min GGEMG | BP (mmHg) | HR (bpm) |
|---|---|---|---|---|---|---|---|---|---|
| ControlBaseline | 0.46 ± 0.07 | 24.0 ± 3.7 | 0.26 ± 0.02 | 0.91 ± 0.07 | 53 ± 3 | 0.49 ± 0.03 | 25.7 ± 2.1 | 106 ± 6 | 426 ± 6 |
| L-368899 | 0.39 ± 0.07 | 19.8 ± 3.7 | 0.25 ± 0.02 | 0.94 ±0.06 | 51 ± 3 | 0.40 ± 0.04 | 20.7 ± 2.2* | 92 ± 6* | 422 ± 7 |
| Bicuculline | 0.35 ± 0.07 | 18.3 ± 3.7 | 0.21 ± 0.04 | 0.97 ± 0.09 | 52 ± 3 | 0.38 ± 0.06 * | 19.7 ± 3.2* | 93 ± 7 | 417 ± 5 |
Values are means ± SE; n=6 animals.
Represents differences (P < 0.05) between baseline and after administration of L-368899 for Min GGEMG and arterial blood pressure (BP). Differences between values post L-368899 and bicuculline were not significant (P>0.05) indicating that blockade of OT receptor within the preBötC inhibited responses elicited by PVN stimulation. Represents a significant difference (P<0.05) between the treatment and baseline value.
3.6. Effect of the PVN stimulation on diaphragm and genioglossus muscle activities after microinjection of GABAA agonist in the preBötzinger region.
To define the degree of involvement of the preBötzinger region in mediating respiratory drive to the GG and D and in eliciting cardiovascular responses to PVN stimulation, muscimol was administered unilaterally into the preBötzinger region to diminish the responses of preBötC neurons to afferent inputs from the PVN. Muscimol decreased peak D and GG activity as well as arterial blood pressure, which was brought close to preinjection levels by adjusting the ventilatory rate and, consequently, increasing end tidal PCO2 on average by 1.2-1.4%. Under these conditions, PVN stimulation by microinjection of bicuculline following administration of muscimol, a GABAA R agonist, in the preBötzinger region, had no effect (P>0.05) on DEMG, GGEMG, or duration of the respiratory cycle. Furthermore, arterial pressure and heart rate only changed slightly (P>0.05) by 3.7 and 1%, respectively in response to PVN stimulation, suggesting that the preBötzinger region is involved in mediation of both respiratory and cardiovascular changes induced by PVN stimulation.
4. Discussion
The results of the present study show that an increase in inspiratory activity of the diaphragm and genioglossus muscle, elevation of arterial blood pressure and heart rate elicited by stimulation of the PVN are mediated mainly through OT projections to the preBötzinger complex region and activation of OT -receptors within this area. In addition, the data confirmed previous findings showing that activation of PVN neurons elicited an increase in respiratory drive and arterial blood pressure in anesthetized (60) and in non-sedated, normotensive rats (52).
The present findings are discussed by focusing on 1) neuroanatomical studies, 2) comparison of current results with previous findings related to the involvement of PVN OT-containing neurons in autonomic control, and 3) the functional significance of the results.
4.1. Transneuronal labeling studies.
Pseudorabies virus (PRV), as a transneuronal tracer, is used in a number of studies (16, 19, 20, 27, 29, 36, 54, 55). Following microinjection of PRV, the virus is retrogradely transported from the genioglossus muscle to first order neurons (hypoglossal motoneurons) where it replicates and passes transneuronally to second order neurons, including those projecting to hypoglossal cells that innervate the genioglossus muscle. In spinalectomized rats, five days after PRV injections into the genioglossus muscle, labeled cells were found mainly ipsilaterally at the level and immediately caudal to the obex in the ventral and ventrolateral subdivisions of the hypoglossal nucleus. A few scattered PRV positive cells were observed intermingled with unlabeled hypoglossal motoneurons in the more dorsomedial and dorsolateral subregions.
Following injection of PRV into the genioglossus muscle, second order neurons were observed within the PVN dorsal and medial ventral subregions known to project to respiratory-related sites in the medulla oblongata and spinal cord and to express OT or vasopressin traits (28, 39). To better address the question of dual function of PVN neurons in autonomic and motor control, future studies may include simultaneously injecting two different viral strains of a transynaptic tracer, each strain expressing a unique reporter gene as used in determining the sympatho-motor circuitry involved in mediating stress responses (29).
If replication time is prolonged through central polysynaptic circuits, the virus can also label higher order cells (17, 18, 34). Hence, we cannot exclude the possibility that some of these cells could be third order neurons. Furthermore, as with other viruses, some neurons do not express receptors for the virus, or PRV infection can produce neuronal death of receptive neurons, thereby reducing the number of labeled cells. Also there is the potential for non-specific extracellular spread of PRV from compromised infected neurons, leading to lateral, non-specific labeling. In the present studies, cytolytic processes of infected cells were reduced using the attenuated strain of Bartha PRV. As shown in this study, this strain expresses neurotropism for PVN neurons innervating hypoglossal neurons that project to the genioglossus muscle. Furthermore, the non-specific spread of the virus should not be considered a problem since its spread is restricted by activation of astrocytes, microglia, and reactive gliosis, isolating infected neurons from non-infected ones, thereby preventing non-specific neuronal infection (17, 18, 34).
It should be mentioned that in the C8 spinalectomized animals, as described in the previous studies (19,20), sympathetic pathways are eliminated as a possible route for the virus to reach supraspinal structures. Therefore, we believe that transneuronal labeling of the PVN neurons, following injection of tracer into the genioglossus muscle are second order neurons that innervate hypoglossal motoneurons.
4.2. Physiological studies.
PVN neurons project to a number of CNS sites (30), including brainstem and spinal cord nuclei that are known to be involved in central regulation of respiration (28,39,60), sympathetic outflow (26,27), and cholinergic output to visceral organs (19, 20, 36). Furthermore OT-containing PVN neurons innervating the brain stem—spinal cord—respiratory-related sites (39) arise from the dorsal and medial ventral PVN subnuclei that project to hypoglossal motoneurons, and vasopressor neurons of the rostral ventrolateral medulla oblongata (27).
The PVN is involved in coordination of respiratory and cardiovascular responses to a number of stimuli (39, 53), including those from peripheral chemoreceptors (41, 48). Previously, it has been shown that bilateral microinjection of glutamate into the PVN in urethane-anesthetized vagotomized and ventilated rats caused an increased in peak DEMG activity and DEMG frequency discharge. These changes were associated with elevation of arterial blood pressure (52).Subsequently, in well designed studies, Schlenker and colleagues reported that unilateral microinjection of bicuculline, a γ-aminobutyric acid (GABA)A receptor antagonist, into the PVN of conscious rats increased breathing frequency, volume of a breath, mean arterial pressure, heart rate, and oxygen consumption for up to 10 min following injection (52). In the present study we used a GABAA receptor antagonist instead of glutamate to stimulate PVN neurons. We avoided a use of glutamate since its application after a brief period of excitation time could cause a long-lasting (up to 30 min) decrease in excitability or silencing of discharge, probably due to a depolarizing block and disturbances in the ionic composition of the extracellular space (33). Previous studies have shown that local injections of oxytocin in the PVN elicit a positive feedback mechanism for the oxytocinergic activation (31), suggesting that oxytocin injections into the PVN could be used for studies aimed to examine the physiologic role of the PVN oxytocinergic pathway. We did not use oxytocin administration since previous studies provided evidence that blockade of GABAA receptors expressed by PVN neurons affects both respiratory and cardiovascular function (52). Using low concentrations of GABAA receptor antagonist bicuculline, we circumvent possible difficulties and generated the present results that extend previous data (52, 60), showing that PVN stimulation also increases genioglossus muscle activity. While the first series of experiments clearly demonstrate PVN involvement in regulation of respiratory drive to different inspiratory muscle groups and in coordination of breathing activity and cardiovascular function, they do not indicate which PVN neurons mediate these responses, nor the neurotransmitters involved. Therefore, in the second series of our studies, we focused on this issue and proved our hypothesis that under well defined and reproducible experimental conditions, OT-containing cells mediate major changes in respiratory drive and cardiovascular function.
Physiological experimental design has its own limitations,therefore the results need to be interpreted with caution. Difficulties are mainly related to drug delivery and selectivity of drugs used. For drug delivery, we used pressure ejection. We found this method to be very useful in administering drugs in a controlled volume to discrete brainstem regions. None-the-less, drug delivery by this method into the PVN may affect cells other than OT-producing neurons. Furthermore, variations in the size of the head, could lead to ejection of the drug outside of targeted regions. However, head size in rats of the same age and strain may differ only slightly. Hence, the majority of experimental rats (approx. 85%) was microinjected within the PVN and responded with an increase in respiratory drive following bicuculline administration. Missed injections, or control administrations of used drug, lateral or dorsal to PVN borders (500-700 μm), had no observable effects on recorded variables.
Difficulties could also occur due to low specificity of OT receptor antagonists. Initially, we employed AVT, a potent peptide OT receptor blocker that is widely used in pharmacological and physiological experiments (8, 9, 43). For our preBotC experiments, we used a non-peptide antagonist, L-368,899, because it is more selective for the oxytocin receptor (46). To achieve even greater specificity over pharmacological blockade, RNA interference (RNAi) technology with small interfering RNA (siRNA) can be used to inhibit target gene expression. Exogenous interfering RNAs have been delivered to cells in vivo via viral infection (59), lipofection (4) and electroporation (1). In addition to potential toxicity associated with RNAi, the time required for recovery after surgical intervention and the transient knockdown of target gene expression may not be compatible for acute experiments as performed in the present study.
Oxytocin-responsive cells and OT-receptors are found along the neural axis (13, 16, 38, 40, 47). Cell signaling generated by OT is mediated via OT receptors, which are typical members of the rhodopsin-type (class I) G protein-coupled receptor (GPCR) family (5). We found that OT receptors are present in the ventrolateral medulla where NK-1 receptor expressing neurons are located. Their activation by exogenous OT mimics respiratory and cardiovascular responses elicited by PVN stimulation (39). However, the changes caused by stimulation of PVN neurons could be mediated through projections to other brainstem sites involved in autonomic control (14, 51, 58), i.e., the nucleus tractus solitarius (8, 24). Therefore, prior blockade of these effector responses by intracisternal administration of the OT receptor antagonist but not vehicle into the 4th ventricle, does not exclude this possibility. However, microinjection of OT antagonist within the preBötzinger region similarly abolished the observed changes. Similarly, prior administration of specific GABAA receptor agonist muscimol into the preBötC, nearly abolished responses of the genioglossus muscle and diaphragm, and cardiovascular changes induced by PVN stimulation. These experiments indicate that neurons within the ventrolateral region of the medulla oblongata where inspiratory rhythm generating cells are found (56), mediate respiratory and cardiovascular effects of stimulation of PVN oxytocin-containing cells. This is accomplished mainly through propriobulbar neurons to hypoglossal motor cells regulating genioglossus muscle activity, via bulbospinal neurons to phrenic nuclei controlling diaphragm discharge, and via connectivity with the intermediolateral cell column influencing sympathetic outflow, consequently arterial pressure and heart rate, as presented in Fig.6.
Figure 6.

The PVN-brainstem-spinal cord network regulating respiratory drive and cardiovascular function. The preBötC region is an integrative site transmitting excitatory information from PVN oxytocin-containing neurons to medullary neurons projecting to hypoglossal motor cells controlling the activity of the genioglossus muscle, bulbospinal motoneurons projecting to phrenic nuclei that drive the diaphragm and to bulbospinal neurons regulating sympathetic outflow, arterial blood pressure and heart rate.
Our conceptual model as diagrammatically presented in Fig. 6, is supported by findings generated two decades ago, showing that excitatory substances administered systemically, intraventricularly, or applied topically on the ventrolateral medulla increase hypoglossal nerve activity largely through a neuronal network located beneath the ventrolateral medullary surface (21, 23). Furthermore, cooling, local anesthesia, or activation of GABAA receptors beneath the surface of ventrolateral medulla, abolishes phrenic and preferentially hypoglossal nerve activity and responses to hypercapnic loading (22). Alterations in this network could lead to parallel changes in respiration and cardiovascular function (10). Hence, the preBötC region is an integrative site for respiratory and cardiovascular function and is involved in transmitting excitatory information from the PVN to upper airway dilating and chest wall pumping muscles, as well as to bulbospinal neurons regulating sympathetic outflow. Studies elucidating anatomic connections between the preBötC region and brainstem neurons regulating sympathetic outflow could provide a definitive explanation for the present results.
The hypoglossal nucleus receives excitatory and inhibitory inputs from the brain stem and suprapontine regions to produce signals to the genioglossus muscle regulating suckling, swallowing, and speech (12, 15, 25, and 57). The findings of the present study raise the possibility that PVN innervation of hypoglossal motoneurons could be related to these functions rather then directly controlling respiratory discharge of hypoglossal neurons.
The PVN plays an important role in modulating the sympathoexcitatory component of peripheral, but not central chemoreflexes (41, 48). Stimulation of the PVN increased arterial pressure and heart rate, which were diminished by prior blockade of OT receptors, or muscimol administered into the ventrolateral medulla. The data suggest that vasopressor neurons outside of the ventrolateral pressor region do not play a critical role in mediating a PVN stimulation-induced increase in arterial pressure and heart rate. Furthermore, these results suggest that activation of PVN OT-producing neurons elicit parallel changes in respiratory and cardiovascular functions by a single mechanism that control these interlinked systems.
4.3. The functional implications.
Respiration is regulated by both involuntary (neural, endocrine, metabolic, and emotional components) and voluntary controlling mechanisms. Understandably, being a vital and continuous process, breathing is not dependent on endocrine hormones but it is modulated by a number of molecules, including PVN peptides such as OT. Previous studies suggested that OT affects respiratory drive via direct stimulation of the preBötC within the ventrolateral medulla oblongata (39). The present findings indicate that through this site, endogenously released OT regulates both respiratory and cardiovascular function.
The parvocellular OT-containing neurons are involved in the regulation of appetite. While lesions in the PVN in rat brains and OT receptor antagonism cause overeating and obesity (2, 7, 32), central administration of oxytocin and an oxytocin agonist decrease food intake in rats (37, 43), and could be involved in anorexia and the bulimia nervosa syndrome (3). A loss of PVN neurons producing OT or functional abnormalities in OT-OT receptor signaling may cause obesity and increased susceptibility to hypoventilation and obstructive sleep apnea (42). In humans, a decrease in the activity of tongue muscles during sleep, which is exaggerated by obesity, could cause upper airway occlusion (49).
In conclusion, the results of the present study suggest that PVN stimulation affects central regulatory mechanisms controlling both respiratory and cardiovascular functions, resulting in parallel changes mediated mainly by an OT-OT receptor signaling pathway. Hence, drugs that potentiate central OT dependent mechanisms, via the activation of OT receptors expressed by these neurons, may represent targets for the development of new treatments for respiratory disorders associated with obesity and respiratory depression. For example, subtype-selective agonists directed toward OT receptors may represent a successful therapeutic intervention for some forms of obesity associated with obstructive seep apneas and CO2 retention.
Table 3.
Effect of PVN stimulation on cardiorespiratory variables after microinjection of GABAA R agonist into preBötC.
| Drug | DEMG (Au) | Min DEMG | Ti (s) | Te (s) | f (breaths/min) | GGEMG (Au) | Min GGEMG | BP (mmHg) | HR (bpm) |
|---|---|---|---|---|---|---|---|---|---|
| ControlBaseline | 0.40 ± 0.09 | 18.0 ± 5.8 | 0.33 ± 0.03 | 1.19 ± 0.11 | 41 ± 4 | 0.54 ± 0.09 | 21.2 ± 3.5 | 117 ± 6 | 408 ± 11 |
| Muscimol | 0.35 ± 0.15 | 17.3 ± 8.8 | 0.27 ± 0.05 | 1.19 ± 0.16 | 45 ± 6 | 0.31 ± 0.09 | 13.1 ± 4.7 | 102 ± 7 | 398 ± 8 |
| Bicuculline | 0.42 ± 0.17 | 17.5 ± 7.2 | 0.40 ± 0.16 | 1.14 ± 0.16 | 43 ± 6 | 0.41 ± 0.14 | 14.9 ± 3.7 | 107 ± 12 | 401 ± 9 |
Values are means ± SE; n=6. Administration of muscimol at the adjusted the adjusted ventilator rate were slightly lower than baseline values, however differences were not significant (P >0.05). Differences between values post muscimol and bicciucllinebicuculline were not Significant (P >0.05) for all analyzed variables.There were no significant differences (P > 0.05) between control and treatment values for all variables.
Acknowledgments
We thank Makeda Williams and Kerry Cadogan for their technical support. Supported by NIH NS45859, NINDS/NCRR U54 NS39407, HL50527
References
- 1.Akaneya Y, Jiang B, Tsumoto T. RNAi-induced gene silencing by local electroporation in targeting brain region. J Neurophysiol. 2005;93:594–602. doi: 10.1152/jn.00161.2004. [DOI] [PubMed] [Google Scholar]
- 2.Arletti R, Benelli A, Bertolini A. Oxytocin inhibits food and fluid intake in rats. Physiol Behav. 1990;48:825–830. doi: 10.1016/0031-9384(90)90234-u. [DOI] [PubMed] [Google Scholar]
- 3.Bailer UF, Kaye WH. A review of neuropeptide and neuroendocrine dysregulation in anorexia and bulimia nervosa. Curr Drug Targets CNS Neurol Disord. 2003;2:53–59. doi: 10.2174/1568007033338689. [DOI] [PubMed] [Google Scholar]
- 4.Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- 5.Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Crit Rev Neurobiol. 1996;10:119–154. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- 6.Benarroch EE. Paraventricular nucleus, stress response, and cardiovascular disease. Clin Auton Res. 2005;15:254–263. doi: 10.1007/s10286-005-0290-7. [DOI] [PubMed] [Google Scholar]
- 7.Benelli A, Bertolini A, Arletti R. Oxytocin-induced inhibition of feeding and drinking: no sexual dimorphism in rats. Neuropeptides. 1991;20:57–62. doi: 10.1016/0143-4179(91)90040-p. [DOI] [PubMed] [Google Scholar]
- 8.Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol. 2004;287:R87–96. doi: 10.1152/ajpregu.00604.2003. [DOI] [PubMed] [Google Scholar]
- 9.Blevins JE, Schwartz MW, Baskin DG. Society for Neuroscience. Orlando, FL: 2002. Fourth ventricular administration of an oxytocin receptor antagonist stimulates food intake in rats. [Google Scholar]
- 10.Bruce EN, Cherniack NS. Central chemoreceptors. J Appl Physiol. 1987;62:389–402. doi: 10.1152/jappl.1987.62.2.389. [DOI] [PubMed] [Google Scholar]
- 11.Buijs RM. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res. 1978;192:423–435. doi: 10.1007/BF00212323. [DOI] [PubMed] [Google Scholar]
- 12.Caba M, Rovirosa MJ, Silver R. Suckling and genital stroking induces Fos expression in hypothalamic oxytocinergic neurons of rabbit pups. Brain Res Dev Brain Res. 2003;143:119–128. doi: 10.1016/s0165-3806(03)00064-6. [DOI] [PubMed] [Google Scholar]
- 13.Charpak S, Armstrong WE, Muhlethaler M, Dreifuss JJ. Stimulatory action of oxytocin on neurones of the dorsal motor nucleus of the vagus nerve. Brain Res. 1984;300:83–89. doi: 10.1016/0006-8993(84)91342-8. [DOI] [PubMed] [Google Scholar]
- 14.Coote JH. A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp Physiol. 2005;90:169–173. doi: 10.1113/expphysiol.2004.029041. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham ET, Jr., Sawchenko PE. Dorsal medullary pathways subserving oromotor reflexes in the rat: implications for the central neural control of swallowing. J Comp Neurol. 2000;417:448–466. [PubMed] [Google Scholar]
- 16.Dreifuss JJ, Raggenbass M. Oxytocin-responsive cells in the mammalian nervous system. Regul Pept. 1993;45:109–114. doi: 10.1016/0167-0115(93)90191-a. [DOI] [PubMed] [Google Scholar]
- 17.Enquist LW. Exploiting circuit-specific spread of pseudorabies virus in the central nervous system: insights to pathogenesis and circuit tracers. J Infect Dis. 2002;186(Suppl 2):S209–214. doi: 10.1086/344278. [DOI] [PubMed] [Google Scholar]
- 18.Grill WM, Erokwu BO, Hadziefendic S, Haxhiu MA. Extended survival time following pseudorabies virus injection labels the suprapontine neural network controlling the bladder and urethra in the rat. Neurosci Lett. 1999;270:63–66. doi: 10.1016/s0304-3940(99)00462-0. [DOI] [PubMed] [Google Scholar]
- 19.Hadziefendic S, Haxhiu MA. CNS innervation of vagal preganglionic neurons controlling peripheral airways: a transneuronal labeling study using pseudorabies virus. J Auton Nerv Syst. 1999;76:135–145. doi: 10.1016/s0165-1838(99)00020-x. [DOI] [PubMed] [Google Scholar]
- 20.Haxhiu MA, Jansen AS, Cherniack NS, Loewy AD. CNS innervation of airway-related parasympathetic preganglionic neurons: a transneuronal labeling study using pseudorabies virus. Brain Res. 1993;618:115–134. doi: 10.1016/0006-8993(93)90435-p. [DOI] [PubMed] [Google Scholar]
- 21.Haxhiu MA, Mitra J, van Lunteren E, Bruce EN, Cherniack NS. Hypoglossal and phrenic responses to cholinergic agents applied to ventral medullary surface. Am J Physiol. 1984;247:R939–944. doi: 10.1152/ajpregu.1984.247.6.R939. [DOI] [PubMed] [Google Scholar]
- 22.Haxhiu MA, Mitra J, van Lunteren E, Prabhakar N, Bruce EN, Cherniack NS. Responses of hypoglossal and phrenic nerves to decreased respiratory drive in cats. Respiration. 1986;50:130–138. doi: 10.1159/000194919. [DOI] [PubMed] [Google Scholar]
- 23.Haxhiu MA, Van Lunteren E, Van de Graaff WB, Strohl KP, Bruce EN, Mitra J, Cherniack NS. Action of nicotine on the respiratory activity of the diaphragm and genioglossus muscles and the nerves that innervate them. Respir Physiol. 1984;57:153–169. doi: 10.1016/0034-5687(84)90090-2. [DOI] [PubMed] [Google Scholar]
- 24.Higa KT, Mori E, Viana FF, Morris M, Michelini LC. Baroreflex control of heart rate by oxytocin in the solitary-vagal complex. Am J Physiol Regul Integr Comp Physiol. doi: 10.1152/ajpregu.00806.2000. [DOI] [PubMed] [Google Scholar]
- 25.Hiiemae KM, Palmer JB. Tongue movements in feeding and speech. Crit Rev Oral Biol Med. 2003;14:413–429. doi: 10.1177/154411130301400604. [DOI] [PubMed] [Google Scholar]
- 26.Hosoya Y, Matsukawa, Okado N, Sugiura Y, Kohno K. Oxytocinergic innervation to the upper thoracic sympathetic preganglionic neurons in the rat. A light and electron microscopical study using a combined retrograde transport and immunocytochemical technique. Exp Brain Res. 1995;107:9–16. doi: 10.1007/BF00228011. [DOI] [PubMed] [Google Scholar]
- 27.Jansen AS, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science. 1995;270:644–646. doi: 10.1126/science.270.5236.644. [DOI] [PubMed] [Google Scholar]
- 28.Kc P, Haxhiu MA, Tolentino-Silva FP, Wu M, Trouth CO, Mack SO. Paraventricular vasopressin-containing neurons project to brain stem and spinal cord respiratory-related sites. Respir Physiol Neurobiol. 2002;133:75–88. doi: 10.1016/s1569-9048(02)00131-3. [DOI] [PubMed] [Google Scholar]
- 29.Kerman IA, Enquist LW, Watson SJ, Yates BJ. Brainstem substrates of sympatho-motor circuitry identified using trans-synaptic tracing with pseudorabies virus recombinants. J Neurosci. 2003;23:4657–4666. doi: 10.1523/JNEUROSCI.23-11-04657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiss A, Mikkelsen JD. Oxytocin--anatomy and functional assignments: a minireview. Endocr Regul. 2005;39:97–105. [PubMed] [Google Scholar]
- 31.Kita I, Yoshida Y, Nishino S. An activation of parvocellular oxytocinergic neurons in the paraventricular nucleus in oxytocin-induced yawning and penile erection. Neurosci Res. 2006;54:269–275. doi: 10.1016/j.neures.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Leibowitz SF, Hammer NJ, Chang K. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol Behav. 1981;27:1031–1040. doi: 10.1016/0031-9384(81)90366-8. [DOI] [PubMed] [Google Scholar]
- 33.Lipski J, Bellingham MC, West MJ, Pilowsky P. Limitations of the technique of pressure microinjection of excitatory amino acids for evoking responses from localized regions of the CNS. J Neurosci Methods. 1988;26:169–179. doi: 10.1016/0165-0270(88)90166-5. [DOI] [PubMed] [Google Scholar]
- 34.Loewy AD. Viruses as transneuronal tracers for defining neural circuits. Neurosci Biobehav Rev. 1998;22:679–684. doi: 10.1016/s0149-7634(98)00006-2. [DOI] [PubMed] [Google Scholar]
- 35.Loewy AD, Burton H. Nuclei of the solitary tract: efferent projections to the lower brain stem and spinal cord of the cat. J Comp Neurol. 1978;181:421–449. doi: 10.1002/cne.901810211. [DOI] [PubMed] [Google Scholar]
- 36.Loewy AD, Haxhiu MA. CNS cell groups projecting to pancreatic parasympathetic preganglionic neurons. Brain Res. 1993;620:323–330. doi: 10.1016/0006-8993(93)90174-l. [DOI] [PubMed] [Google Scholar]
- 37.Lokrantz CM, Uvnas-Moberg K, Kaplan JM. Effects of central oxytocin administration on intraoral intake of glucose in deprived and nondeprived rats. Physiol Behav. 1997;62:347–352. doi: 10.1016/s0031-9384(97)00021-8. [DOI] [PubMed] [Google Scholar]
- 38.Loup F, Tribollet E, Dubois-Dauphin M, Pizzolato G, Dreifuss JJ. Localization of oxytocin binding sites in the human brainstem and upper spinal cord: an autoradiographic study. Brain Res. 1989;500:223–230. doi: 10.1016/0006-8993(89)90317-x. [DOI] [PubMed] [Google Scholar]
- 39.Mack SO, Kc P, Wu M, Coleman BR, Tolentino-Silva FP, Haxhiu MA. Paraventricular oxytocin neurons are involved in neural modulation of breathing. J Appl Physiol. 2002;92:826–834. doi: 10.1152/japplphysiol.00839.2001. [DOI] [PubMed] [Google Scholar]
- 40.McCann MJ, Rogers RC. Oxytocin excites gastric-related neurones in rat dorsal vagal complex. J Physiol. 1990;428:95–108. doi: 10.1113/jphysiol.1990.sp018202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olivan MV, Bonagamba LG, Machado BH. Involvement of the paraventricular nucleus of the hypothalamus in the pressor response to chemoreflex activation in awake rats. Brain Res. 2001;895:167–172. doi: 10.1016/s0006-8993(01)02067-4. [DOI] [PubMed] [Google Scholar]
- 42.Olson AL, Zwillich C. The obesity hypoventilation syndrome. Am J Med. 2005;118:948–956. doi: 10.1016/j.amjmed.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 43.Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides. 1991;12:113–118. doi: 10.1016/0196-9781(91)90176-p. [DOI] [PubMed] [Google Scholar]
- 44.Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology. 1991;129:785–791. doi: 10.1210/endo-129-2-785. [DOI] [PubMed] [Google Scholar]
- 45.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Orlando, FL: 1998. [Google Scholar]
- 46.Pettibone DJ, Clineschmidt BV, Kishel MT, Lis EV, Reiss DR, Woyden CJ, Evans BE, Freidinger RM, Veber DF, Cook MJ, et al. Identification of an orally active, nonpeptidyl oxytocin antagonist. J Pharmacol Exp Ther. 1993;264:308–314. [PubMed] [Google Scholar]
- 47.Raggenbass M, Charpak S, Dubois-Dauphin M, Dreifuss JJ. Electrophysiological evidence for oxytocin receptors on neurones located in the dorsal motor nucleus of the vagus nerve in the rat brainstem. J Recept Res. 1988;8:273–282. doi: 10.3109/10799898809048992. [DOI] [PubMed] [Google Scholar]
- 48.Reddy MK, Patel KP, Schultz HD. Differential role of the paraventricular nucleus of the hypothalamus in modulating the sympathoexcitatory component of peripheral and central chemoreflexes. Am J Physiol Regul Integr Comp Physiol. 2005;289:R789–797. doi: 10.1152/ajpregu.00222.2005. [DOI] [PubMed] [Google Scholar]
- 49.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 50.Rinaman L. Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. J Comp Neurol. 1998;399:101–109. doi: 10.1002/(sici)1096-9861(19980914)399:1<101::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 51.Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- 52.Schlenker E, Barnes L, Hansen S, Martin D. Cardiorespiratory and metabolic responses to injection of bicuculline into the hypothalamic paraventricular nucleus (PVN) of conscious rats. Brain Res. 2001;895:33–40. doi: 10.1016/s0006-8993(01)02011-x. [DOI] [PubMed] [Google Scholar]
- 53.Schlenker EH. Integration in the PVN: another piece of the puzzle. Am J Physiol Regul Integr Comp Physiol. 2005;289:R653–655. doi: 10.1152/ajpregu.00386.2005. [DOI] [PubMed] [Google Scholar]
- 54.Shintani T, Anker AR, Billig I, Card JP, Yates BJ. Transneuronal tracing of neural pathways influencing both diaphragm and genioglossal muscle activity in the ferret. J Appl Physiol. 2003;95:1453–1459. doi: 10.1152/japplphysiol.00558.2003. [DOI] [PubMed] [Google Scholar]
- 55.Smith BN, Banfield BW, Smeraski CA, Wilcox CL, Dudek FE, Enquist LW, Pickard GE. Pseudorabies virus expressing enhanced green fluorescent protein: A tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc Natl Acad Sci U S A. 2000;97:9264–9269. doi: 10.1073/pnas.97.16.9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stone M, Epstein MA, Iskarous K. Functional segments in tongue movement. Clin Linguist Phon. 2004;18:507–521. doi: 10.1080/02699200410003583. [DOI] [PubMed] [Google Scholar]
- 58.Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- 59.Xia H, Mao Q, Paulson HL, Davidson BL. siRNA-mediated gene silencing in vitro and in vivo. Nat Biotechnol. 2002;20:1006–1010. doi: 10.1038/nbt739. [DOI] [PubMed] [Google Scholar]
- 60.Yeh ER, Erokwu B, LaManna JC, Haxhiu MA. The paraventricular nucleus of the hypothalamus influences respiratory timing and activity in the rat. Neurosci Lett. 1997;232:63–66. doi: 10.1016/s0304-3940(97)00579-x. [DOI] [PubMed] [Google Scholar]