Abstract
Essential thrombocythemia (ET) is an acquired myeloproliferative disorder (MPD) characterized by a sustained elevation of platelet number with a tendency for thrombosis and hemorrhage. The prevalence in the general population is approximately 30/100,000. The median age at diagnosis is 65 to 70 years, but the disease may occur at any age. The female to male ratio is about 2:1. The clinical picture is dominated by a predisposition to vascular occlusive events (involving the cerebrovascular, coronary and peripheral circulation) and hemorrhages. Some patients with ET are asymptomatic, others may experience vasomotor (headaches, visual disturbances, lightheadedness, atypical chest pain, distal paresthesias, erythromelalgia), thrombotic, or hemorrhagic disturbances. Arterial and venous thromboses, as well as platelet-mediated transient occlusions of the microcirculation and bleeding, represent the main risks for ET patients. Thromboses of large arteries represent a major cause of mortality associated with ET or can induce severe neurological, cardiac or peripheral artery manifestations. Acute leukemia or myelodysplasia represent only rare and frequently later-onset events. The molecular pathogenesis of ET, which leads to the overproduction of mature blood cells, is similar to that found in other clonal MPDs such as chronic myeloid leukemia, polycythemia vera and myelofibrosis with myeloid metaplasia of the spleen. Polycythemia vera, myelofibrosis with myeloid metaplasia of the spleen and ET are generally associated under the common denomination of Philadelphia (Ph)-negative MPDs. Despite the recent identification of the JAK2 V617F mutation in a subset of patients with Ph-negative MPDs, the detailed pathogenetic mechanism is still a matter of discussion. Therapeutic interventions in ET are limited to decisions concerning the introduction of anti-aggregation therapy and/or starting platelet cytoreduction. The therapeutic value of hydroxycarbamide and aspirin in high risk patients has been supported by controlled studies. Avoiding thromboreduction or opting for anagrelide to postpone the long-term side effects of hydrocarbamide in young or low risk patients represent alternative options. Life expectancy is almost normal and similar to that of a healthy population matched by age and sex.
Disease name and synonyms
Essential thrombocythemia (ET)
Primary thrombocythemia (PT)
Hemorrhagic thrombocythemia
Definition
Essential thrombocythemia (ET) is an acquired Myeloproliferative disorder (MPD) characterized by a sustained elevation of the platelet number with a tendency to thrombosis and hemorrhage. Elevated platelet count is related to an expansion of megakaryocytic lineage and the disorder is usually considered to be a clonal disease arising in a multipotent stem cell.
Etiology
ET shares a molecular pathogenesis leading to overproduction of mature blood cells with other clonal MPDs such as Chronic myeloid leukemia (CML), Polycythemia vera (PV), Myelofibrosis with myeloid metaplasia of the spleen (IMF). CML is now easily recognized by the presence of the Philadelphia (Ph)-positive chromosomal abnormality and/or the evidence of a specific molecular marker, the disrupted protein kinase BCR/ABL. However, despite the recent description of the JAK2 V617F mutation in a subset of patients with PV, ET and IMF, the intimate mechanism underlying molecular pathogenesis of these myeloproliferative disorders is still a matter of discussion. They are therefore generally associated under the common denomination of Ph-negative MPDs. The presence of the mutation confers a proliferative and survival advantage by rendering the cells more sensitive to incoming stimulatory signals, causing clonal expansion of hematopoietic progenitors in myeloproliferative disorders [1].
Epidemiology
The reported annual incidence rates for ET range from 0.59 to 2.53/100,000 (<9/100,000) inhabitants. A population based survey in the city of Göteborg, Sweden, adjusted to standard population reported an incidence rate of 1.55/100,000, just under the incidence rate evaluated in the same conditions for PV (1.97/100,000). The prevalence is around 30/100,000 inhabitants [2]. The diagnosis of ET is more frequently established today than in the past [2,3], the most likely explanation being a wider use of automatic count in routine examination leading to the diagnosis of more non symptomatic ET patients. The median age at diagnosis is 65 to 70 years but the range in age of onset is characteristically wide. ET is often diagnosed during the third or fourth decade of life. Since the diagnosis of ET is frequently recognized early in life and the incidence of the disease is around two times higher in females compared to males, the occurrence of ET associated pregnancies is common [4-6].
Clinical description
The clinical presentation of ET is dominated by a predisposition to vascular occlusive events and hemorrhages.
Vascular occlusive events
Vascular occlusive events include major thrombotic events involving the cerebrovascular, coronary and peripheral arterial circulation. Thromboses of large arteries represent a major cause of mortality associated with the disease or can induce severe neurological, cardiac or peripheral arteries disabilities.
Deep vein thrombosis also represents a potentially serious and eventually life-threatening event due to the risk of pulmonary embolism or related to the region involved as it is the case in hepatic (Budd Chiari syndrome) or portal thrombosis [7].
Vascular occlusive events can also occur in the micro-vessels where they cause a wide range of clinical symptoms, secondary to a transitory suspension of the circulation. They are caused by platelet-mediated transient occlusive thrombosis in the end-arterial circulation [8]. Aspirin-sensitive erythromelalgia, one of the most characteristic microvascular disturbances in ET, is described as burning painful and ulcerative toes. It is often accompanied by a warm, red or violet colored congested limb extremity. The ischemic attacks of digital arteries may subsequently progress towards small zones of limited necrosis or even peripheral gangrene with palpable arterial pulsations.
Headaches are the most common neurological manifestations. Their pathophysiology remains uncertain. Some of them resemble migraines. Sometimes, neurological symptoms in ET show a striking similarity with migraine aura or accompaniments. In contrast, transient typical or atypical ischemic attacks, convulsions and sudden transitory absences seem to result from an ischemic mechanism. Visual dysfunction manifests as attacks of diplopia and sudden reversible attacks of blurred vision. All these symptoms share a specific reversibility or at least sensitivity to anti-aggregating agents and occur, at least in younger ET patients, in the absence of conspicuous atheromatous lesions in the arterial system.
Hemorrhagic manifestations
Bleeding in ET is often limited to recurrent skin manifestations: bruising, subcutaneous hematomas, ecchymoses, and epistaxis or gum bleeding. Petechiae are never seen. A history of gastrointestinal blood loss (melena and/or hematemesis) or biological evidence in favor of chronic occult blood loss may be evidenced at diagnosis. Secondary bleeding, eventually life-threatening can also occur after trauma or surgery. Hemorrhagic complications are rarely observed during the course of the disease when appropriate preventive measures are taken. Bleeding symptoms are primarily observed in patients with the highest platelet counts [9]. The bleeding diathesis is not due to impaired platelet function but rather to an acquired Von Willebrand's disease caused by proteolytic reduction of Von Willebrand Factor (VWF) multimers. There is an inverse relationship between VWF levels and platelet counts. The VWF large multimer deficiency appears at platelet counts of 1000 to 1500 × 109/L and increases thereafter. Aspirin may unmask a latent bleeding diathesis and may result in severe hemorrhagic complications. It is therefore contraindicated in patients with bleeding history and a very high platelet count (in excess of 1500 × 109/L leading to the acquisition of Von Willebrand's deficiency). If indicated, aspirin should be used in the widely accepted low dose regimen (100 mg daily).
Asymptomatic presentation
The frequency of thrombohemorrhagic complications at presentation of ET varies widely in the different retrospective studies. In a group of 809 ET patients diagnosed according to the Polycythemia Vera Study Group (PVSG) criteria from 11 retrospective clinical studies [10], the incidence of thromboembolic events without bleeding was 42%, bleeding symptoms without thrombosis occurred in 1.4%, and both bleeding and thrombosis in 15% of the patients. The arterial thrombotic manifestations were described as microcirculatory disturbances in 41%. However, the most important message of this retrospective compilation was that 36% of ET patients were free of symptoms at diagnosis [11]. It is also important to note that many of them remained free of complications during the evolution of ET.
Maternal and fetal risk in pregnancy with ET
Maternal risk is limited. The increased risk of thrombosis (present in healthy pregnant women as well) may be worsened by ET. Hemorrhagic risk is low, except in patients with acquired Von Willebrand's disease [4]. The fetus seems to be at greater risks. There is an overall increased incidence of first trimester miscarriage in one out of three pregnancies. Late pregnancy loss is far more frequently observed in ET than in normal population (5–9.6% vs. 0.5%). An increased incidence of intrauterine growth retardation (4–5.1%), preterm delivery (5.6–8%) and placental abruption (2.8%) was reported. Placental micro-infarctions due to increased platelet number and to platelet activation seem to be the underlying pathological basis of adverse events for the fetus. Overall live birth rate may be as low as 50 to 57% [5]. A spontaneous decrease in the number of platelets is frequently observed, beginning after the first trimester of pregnancy.
Diagnostic criteria
Due to the lack of a specific molecular marker, the diagnosis of ET can only be settled after a step-by-step elimination of the other clinical situations associated with a protracted elevation of the platelet number. The criteria of this stepwise elimination were initially proposed by the PVSG [12]. More recently, the World Health Organisation (WHO) has proposed an improved new set of criteria where specific abnormalities of megacaryocytopoiesis associated with other bone marrow biopsy findings were proposed as a common positive marker of Ph-negative MPDs [13]. The JAK2 V617F mutation clearly represents a new molecular marker for the Ph-negative patients. Initially described in PV patients, where it has been observed in 65% to 97% of the cases [1,14-17], this mutation has also been detected in subsets of each of the other Ph-negative MPDs: in 23 to 57% of ET and 43 to 67% of IMF patients, as well as in some Ph-negative CML and MDS [18,19]. The presence or absence of the V617F mutation does not strictly correlate with any phenotype of Ph-negative MPD recognized according to either the PVSG criteria or the WHO classification. As the absence of the mutation has been repeatedly confirmed in more than 50% of ET patients, the diagnosis of ET presently remains a mixture of: 1) positive non specific arguments in favor of a Ph-negative MPD, including the JAK2 mutation and the bone marrow (BM) biopsy findings and 2) elimination of PV and IMF according to their currently used and phenotypically based definitions [12,13,20].
Arguments supporting the diagnosis of Ph-negative MPD
• Standardized histological BM features have been introduced in the WHO diagnostic criteria for Ph-negative MPDs. The diagnostic target of histopathology in patients presenting an elevated platelet count according to this classification may be twofold:
1) To confirm the presence of a Ph-negative MPD and exclude long lasting reactive thrombocytoses (Rth). This step can be achieved through a systematic analysis of: a) megacaryocytopoiesis, focusing especially on the proportion of giant vs. small forms, nuclear lobulation, maturation defects, cluster formation by megacaryocytes; b) BM cellularity; c) degree of expansion and of left shifting of granulocyte and erythrocyte lineages; d) densification of the reticulin network and presence of collagen fibrosis in the BM stroma [7,21,22].
2) To offer a specific morphologic description of each of the Ph-negative MPDs. For instance in ET, megakaryocytopoiesis is characterized by the presence of large to giant cells with a predominance of hyperlobulated staghorn nuclei loosely clustered throughout the BM. Cellularity in ET is not significantly increased compared with age-matched normal samples, and neutrophil granulopoiesis and erythropoiesis display no significant change in the distribution and proliferation of both cell lineages. In true ET there is no collagen fibrosis and no increase in reticulin fibers of the myeloid stroma [22].
The relevant parameters in BM introduced by the WHO classification to eliminate not only classical forms of PV or IMF but mainly prefibrotic early stages of IMF or/and early or latent PV will be described conjointly with the differential diagnosis of the other Ph-negative MPDs.
• The unique, acquired, clonal, somatic mutation of JAK2 occurring at the level of hematopoietic stem cell in a large proportion of PV patients and in a lower subset of IMF and ET patients has provided a completely new approach to demonstrate a clonal origin of the cell proliferation, in patients with a protracted platelet number. This new marker unequivocally represents a major improvement upon the previous cytogenetic studies, which were able to demonstrate clonal cytogenetic abnormalities in BM cells in less than 5% of ET patients. Interestingly, results of the direct assessment of a clonal expansion in peripheral blood cell of female ET patients with heterozygoty for X-linked genes do not strictly match the presence of the mutation. The unexpected presence of the mutation in female ET patients with non clonal hematopoiesis, according to X-linked gene studies, may only reflect a lower sensitivity of methods based on quantification of X-linked gene alleles compared to the polymerase chain reaction (PCR) detection of the JAK2 mutation. Absence of the V617F mutation in ET with unquestionable clonal hematopoiesis may however suggest, on the contrary, that other mutation(s) might still explain myeloid expansion in these patients [23-28].
• Prior to the description of the JAK2 V617F mutation, a set of positive markers including 1) "spontaneous" growth of erythrocyte precursors (EEC) in absence of added erythropoietin (EPO) [29]; 2) endogenous megakaryocytic colony formation [30]; 3) decreased expression of c-MPL at platelet or megakaryocyte cell surface; 4) polycythemia rubra vera protein 1 (PRV-1) and 5) nuclear factor erythroid 2 (NF-E2) mRNA over expression in polymorphonuclear cells were initially described in PV patients. However, like the JAK2 mutation, these markers were also observed in subsets of other Ph-negative MPDs, including ET. It was therefore suggested that these biological markers may be used as criteria in favor of a clonal origin for the increased platelet number observed in a given patient [31-35]. There is no longer an indication for these investigations in patients positive for the JAK2 V617F mutation, since it has been demonstrated that this mutation induces activation of the JAK2 kinase directly involved in the intracellular signaling, following the exposure to EPO or thrombopoietin (TPO), in the elevated expression of mRNA for PRV-1 or NF-E2 in granulocytes, or in processes involving MPL maturation and trafficking. However, since the mutation was still not found in some patients who were shown to form EEC [36] and over express PRV-1 [37], testing erythrocyte or megakaryocyte colony formation may represent a non specific alternative to BM biopsy to bring arguments in favor of an MPD in ET and PV patients without JAK2 mutation.
• The presence of decreased circulating EPO levels that is common to PV patients and to a subset of ET patients [38] may also be used as an argument in favor of a MPD-related origin of the elevated platelet number. However, since a strong correlation between low EPO levels and the diagnosis of PV has been demonstrated, there is a controversy over using this criterion either as a biological marker common to several kinds of MPD or as a strong indicator of PV phenotype [39,40].
Diagnostic methods and differential diagnosis
Platelet number
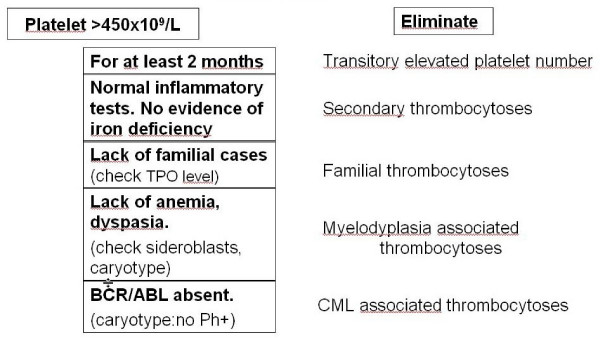
The platelet number required for considering the presence of ET has decreased since the initial definition of the disease, where it was initially fixed to over 1000 × 109 platelets/L (Figure 1). In the last version of the PVSG criteria, it has subsequently been lowered to over 600 × 109 platelets/L observed on two occasions, separated by at least one month (to eliminate a cause of transitory elevation of the platelet number). The availability of positive arguments in favor of a Ph-negative MPD-like bone marrow biopsy findings and, moreover, evidence of the V617F mutation makes it now possible to advocate this diagnosis with a platelet number just over 450 × 109/L, whenever a suggestive clinical context including erythromelalgia or occurrence of arterial or venous thrombosis [7,41] is associated.
Figure 1.
Diagnostic algorithm of thrombocytoses associated with Philadelphia (Ph) negative MPDs (Myeloproliferative disorders).
Elimination of the causes of secondary thrombocytoses
The main causes of secondary thrombocytoses including: iron deficiency, malignancy, chronic inflammatory disease, or histories of splenectomy or of protracted marrow regeneration have to be excluded.
Elimination of iron deficiency includes a normal or increased serum ferritin level in the absence of inflammatory indices (erythrocyte sedimentation rate, fibrinogen, C-reactive protein (CRP)) and normal red cell mean corpuscular volume. This step remains an important one in the diagnosis of ET, both to exclude thrombocytoses related to body iron stock depletion and to recognize PV masked by iron deficiency. The original requirement for stainable marrow iron is for this reason still proposed in one of the most recent set of criteria [36]. If these measurements suggest iron deficiency, a closely monitored trial of iron therapy may be necessary to eliminate progression of hematocrit toward the polycythemic range. Again, the availability of positive arguments in favor of a Ph-negative MPD such as BM biopsy findings and, moreover, evidence of the V617F mutation, represent definitive arguments against secondary thrombocytoses (RhT), although these investigations might be required only during later steps of investigation of patients with protracted thrombocytoses.
Elimination of familial hereditary thrombocytoses
TPO gene mutations, causing elevated TPO levels and therefore leading to familial thrombocytosis, have been described [42]. The context of several cases of thrombocytoses observed in the same offspring or increased TPO levels in sporadic ET may prompt a check for a gain of function mutation of the TPO gene. This eventuality is different from situations where several cases of true MPDs are aggregated in the same family [43] suggesting the presence of a predisposition factor to PV, ET, IMF and CML. In these cases, additional events such as chromosome translocation (9;22) (q34;q11) in CML or several somatic mutations arising in hematopoietic stem cell (including the V617F mutation) may usually be responsible for the disease onset or for the specific characteristics of proliferation leading to the phenotypic classification of the final MPD.
Elimination of primary thrombocytoses associated with chronic myelodysplastic syndromes (MDS)
This step was proposed [12,13] to exclude patients whose hemograms, myelograms or cytogenetic abnormalities were suggestive of myelodysplasia. The presence of the JAK2 mutation in some patients with MDS and sideroblastic anemia [18,19] may justify the individualization in the most recent classification of myeloid neoplasia of a distinct myeloproliferative-myelodysplastic overlap category [44]. However, the distinction of these borderline situations from a "true ET" might still be maintained, at least in studies in which long term evolution or evaluation of treatment are concerned.
Elimination of primary thrombocytoses associated with the full-blown picture or with more subtle presentations of a chronic myeloproliferative syndrome different from ET
1) The elimination of the more or less pure thrombocythemic forms of CML [45] that previously led to the concept of Ph-negative MPD, is now easily achieved and based mainly on the determination of the presence of BCR/ABL transcripts in peripheral blood, rather than on the research of chromosome Philadelphia in BM cells.
Distinction between ET and PV patient
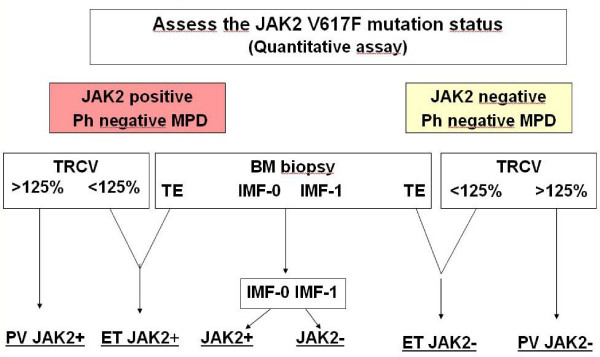
2) The identification of features specific to ET among patients with a primary Ph-negative MPD and an increased platelet number is more controversial. Determination of the total red cell volume (TRCV) in all patients with thrombocytoses and hemoglobin (Hb) or hematocrit (Ht) values equal or superior to normal value using a radioisotopic method [46], after exclusion or treatment of iron deficiency was recommended in the PVSG and WHO criteria for ET. Patients with TRCV>125% of normal value definitively represent PV patients with an increased platelet count (Figure 2). However, there is an unequal availability of the opportunity of TRCV measurement according to the geographical repartition of centers involved in MPD evaluations. The fact that this isotopic measurement is costly, time consuming and non specific (since, alone, it does not differentiate an increased red cell volume in congenital polycythemia (CP), secondary erythrocytosis (SE) or PV) has prompted several alternative strategies for differentiation between PV and ET. None of them can presently be regarded as universally accepted.
Figure 2.
Diagnostic algorithm of Essential thrombocythemia (ET).
• Spontaneous EEC and a low serum EPO level are criteria of high specificity but low exhaustively for the diagnosis of PV. EEC assays are almost always negative in RhT and positive EEC results have been extensively reported in ET patients [40]. The presence of EEC has even been recommended as a minor criterion for the diagnosis of ET. Fifty percent of patients with ET diagnosed according to the PVSG criteria have EPO-independent EEC and a variable proportion of them have low serum EPO levels reflecting a biologically distinct subgroup of ET at risk of progression to PV [35,39].
• Standardized histological BM features (introduced in the WHO diagnostic criteria for Ph-negative MPDs) have been proposed to distinguish initial stages of PV without an obvious increase in Ht level but with an elevated platelet count, mimicking ET on the basis of an increased BM cellularity, an increased and left shifting pattern of both erythropoiesis and granulopoiesis. Megakaryocytes vary in size, generating a pleiomorphic aspect without maturation defects but with a more pronounced tendency toward clustering than in "true" ET.
• Evidence of the JAK2 V617F mutation (found in 90% of PV patients) has been proposed as a prerequisite criterion for the diagnosis of PV [36] but it may be of no help for differentiation of 50% of ET patients positive for JAK2 mutation. However, it is worth noting that differences in the phenotype presentation and clinical events between the JAK2 positive and JAK2 negative ET patients have been demonstrated [47], even if these differences are mainly statistically based and remain small. Furthermore, the presence of EEC and low serum EPO level are still observed in patients that do not carry JAK2 mutation [1,8]. Therefore, disease classification as well as patient management according to the JAK2 status seems to be premature.
Distinction between ET and IMF patients
Patients with a full-blown picture of IMF including large spleen, tear drop shaped red cells, leukoerythroblastic blood picture and collagen fibrosis on BM biopsy are easily eliminated.
• The recognition of patients presenting an increased platelet number that may correspond to a prefibrotic phase of a Ph-negative MPD was one of the first evidences demonstrating the heterogeneity of ET defined according to the PVSG criteria. The identification of these patients is based on BM morphology according to the WHO criteria [20,48] and is associated with a significant loss of life expectancy when their evolution was compared to patients with BM feature specific of ET. Discrimination of prefibrotic or early fibrotic stages of IMF, or IMF-0 and IMF-1 respectively (as it has been proposed to name them) is based on an age-matched increase in marrow cellularity, clustering of megakaryocytes exhibiting dense hyperchromatic cloud-like nuclei and deviation of nuclear cytoplasmic maturation. This stage of prefibrotic Ph-negative MPD is characterized by a pronounced proliferation and left-shifting of the neutrophil granulopoiesis but is devoid of collagen fibrosis and even of an increase in reticulin fibers (at least in IMF-0).
• The JAK2 V617F mutation found in 43 to 67% of classical IMF patients is more frequently homozygous (6 to 18%) compared to ET (3 to 4%). Homozygoty for JAK2 mutation associated with a loss of heterozygoty on chromosome 9p is even more frequent in PV (24 to 27%) and, moreover, almost always present when PV has progressed to myelofibrosis [1,49]. In prefibrotic or early fibrotic patients (IMF-0 or IMF-1), no difference in the frequency of JAK2 mutation has presently been observed [47]. No data on the predictive value of the sequential evaluation of circulating CD34 cells are presently available for detection of this subset of patients whose recognition remains exclusively based on BM biopsy examination.
Management including treatment
Risk categories of patients with ET
Most of the published treatment algorithms [36,50-55] rely on the concept of a risk stratified management. The majority of them distinguished three risk categories based mainly on the estimation of the thrombohemorrhagic risk. This limited ambition is due to the fact that at present there is no drug known to cure the underlying disease or to prevent the risk of clonal evolution. The current rationale for using drugs is therefore either to prevent, or more rarely, to treat a thrombohemorrhagic event.
High risk patients
The criteria recognized as major risk of thrombosis and embolism by almost all investigators [50,51,53,55] are:
• Age: the risk increases significantly in patients older than 60 years.
• Previous thrombotic event.
When the stratification also includes an evaluation of the risk of major hemorrhage [50,51,55], the most frequently considered factors are:
• Platelet count >1500 × 109/L.
• History of major bleeding or, as learned from The European Collaboration on Low-dose aspirin in Polycythemia Vera (ECLAP) experience, a history of minor bleeding with a platelet count >1000 × 109/L, especially in patients with a long disease duration >15 years) [56].
Low risk patients
The most restrictive definition of patients at low risk includes:
• Patients aged less than 40 years.
• Patients presenting with no high risk feature.
• Patient without cardiovascular risk factor and thrombophilia of clinically significant familial expression.
Intermediate risk patient
Intermediate risk patients represent a category without a clear consensus on its definition. The risk of thrombosis is often regarded as intermediate according to:
• Age (patients aged between 40 and 60 years).
• Well established risk factors of cardiovascular disease (hypertension, diabetes, smoking and hypercholesterolemia). In the Medical Research Council (MRC) PT1 study the presence of these factors was even sufficient to include patients in the high risk group.
• An intermediate risk related to a platelet count between 1000 and 1500 × 109/L has also been proposed [50,56]. In this case, the platelet number was regarded as increasing the thrombotic risk when associated with a vascular risk factor or familial thrombophilia [50], and the hemorrhagic risk when associated to either minor bleeding or long term duration of the disease [56].
Therapeutic intervention in ET patients
To reduce the vascular risk in ET patients (except for the management of reversible cardiovascular risk factors) the therapeutic intervention is limited to the decision of decreasing the platelet count or altering the platelet function by aspirin or an equivalent therapy.
Therapeutic options
As a first step in their management, patients should have an assessment of their risk factors for vascular disease and, if necessary, modulation of hypertension, hyperlipemia, smoking habits and overweight.
A. Anti-platelet treatment
Aspirin (and alternative anti-platelet agents)
Aspirin (Asp) is the standard therapy for ischemic manifestations of the microcirculation. Reducing the number of platelets also attenuates the symptoms. Continuous Asp therapy is indicated when the occlusive manifestations of the microcirculation persist despite lowering the number of platelets.
The role of Asp in the prevention of arterial thromboses has been clearly established in the general population. The benefit of a low dose Asp in preventing the risk of thrombotic complications without increasing significantly the hemorrhagic risk in PV patients has recently been demonstrated in the extensive prospective ECLAP study [57]. A preliminary pilot study in polycythemia patients receiving either 40 mg/day of Asp or a placebo showed that this dosage fully inhibited the cyclooxygenase activity and did not cause any major hemorrhage [58]. Anti-platelet therapy has not yet prospectively been shown to reduce the incidence of thrombosis in ET, however, the combination of an anti-aggregating agent with cytoreductive therapy has been found to be safe and to reduce the incidence of thrombosis in ET patients in retrospective studies [59,60]. Low dose Asp is often prescribed as soon as an excess of platelets is discovered by the general practitioners, however, objective data in favor of the preventive role of Asp against the vascular risk in symptom-free patients not taking a platelet-lowering treatment are not presently available and the use of Asp is contraindicated in ET patients with extreme thrombocytosis in whom the presence of Acquired Von Willebrand syndrome might be screened.
ADP receptor antagonists (thienopyridines such as ticlopidine and clopidrogel) have a growing role in patients with ongoing thrombotic events (suggesting resistance to Asp). At present, there are no data to recommend their use in MPD patients [61].
B. Platelet-lowering treatment
Hydroxyurea (HU) has emerged as the reference platelet-lowering agent in high risk ET patients. A valuable platelet lowering as well as anti thrombotic activity has been demonstrated in both of the (only) two published randomized prospective trials concerning the treatment of this category of patients [62,63]. However, the still ongoing controversy (according to whether or not HU is leukemogenic) has prompted the consideration of two main alternative drugs: anagrelide, now authorized for marketing in European countries, and interferon α (IFN-α). Two more medications, pipobroman (available only in certain European countries) and busulfan are also used as platelet-lowering drugs in ET patients, as well as platelet apheresis, which may be the preferred therapeutic option in case of emergency.
Hydroxyurea (Hydroxycarbamide, HU)
Efficacy
The efficiency of HU in controlling the platelet number in high risk ET patients has been further documented by the recently published results of the MRC PT1 trial [63]. A lack of platelet control was observed in less than 4% (15 patients) of the 404 patients treated by HU. At 3 months 90% of the patients had a platelet number <600 × 109/L. The median platelet count at 6 months was <400 × 109/L. Stable reduction of the median platelet number (lower or equal to 400 × 109/L) was obtained with HU for the following 24 months. Importantly, the protection from thrombosis in high risk ET patients already demonstrated by Cortellazzo et al. with HU (plus anti-aggregating agents in almost 70% of the patients) [62] was confirmed by this study with HU associated with Asp in all the patients. After 2 years, the prevalence of thrombotic events was 4%, identical to the former study of Cortelazzo, and significantly lower than the prevalence of 24% found in the control group [62].
Side effects
Clinical and hematological tolerance of HU is usually good even during very long periods of time. Major short term toxic effects are dose limiting hematological impairment and fever. However, oral and leg ulcers and other skin lesions are currently observed but often only after several months or years of treatment, suggesting the role in their occurrence of the cumulative dose received.
Mutagenic potential and long term resistance
HU was initially introduced in the treatment of ET patients because it is supposedly non mutagenic. Its mechanism of action is the inhibition of DNA synthesis by blocking the ribonucleoside reductase activity. As leukemogenic effects of HU have not been definitively eliminated, this molecule should be used cautiously in young individuals. After a continuous use in ET patients, the non selective effect of the drug on platelet reduction may, in the long run decrease, lead to anemia and neutropenia after dosage escalation of HU. This delayed hematological toxicity (leading to a withdrawal of the drug) sometimes seems to be related to a progression of the disease.
A unified definition of the clinical resistance and intolerance to HU in ET is proposed [64] and includes:
1. A platelet count persistently high (>600 × 109/L) after 3 months of treatment by HU at maximum daily possible dosage.
2. Or at any dose of HU: a) A decrease of the white blood cell (WBC) counts under 2.5 × 103/mm3 or of Hb level under 10 g/dl to maintain the platelet number under 400 × 109/L; b) Presence of leg ulcers or other unacceptable muco-cutaneous manifestations; c) Presence of HU related fever. In these situations the risk of leukemic progression in patients requiring treatment by multiple agents should not be underestimated and has implications for the choice of a second agent [51].
The status of ET patients according to the JAK2 V617F mutation might affect the response to HU treatment. A recent study of 640 high risk patients (MRC PT1 trial, JAK2 genotype data available) has demonstrated that HU-treated patients positive for the JAK2 mutation had a persistently lower platelet count than the individuals negative for the JAK2 mutation. Furthermore, ET patients positive for the JAK2 mutation were more sensitive to HU than ET patients without this mutation, as demonstrated by lower doses HU required to control their platelet count and higher decrease in their Hb concentration and WBC counts compared to the pre-treatment levels. These differences in treatment sensitivity according to the mutation status of high risk ET patient was not seen in the group treated with anagrelide (see below) [47].
Anagrelide
Anagrelide (Ana) is an oral imidazoquinazolin compound initially developed for its potent inhibition of platelet aggregation. It causes thrombocytopenia at doses below those exhibiting an anti-aggregating effect.
Efficacy
In the largest study to date, the efficacy of anagrelide in controlling the platelet count was evaluated and demonstrated in 934 ET patients [65]. This was further documented in high risk ET patients by the recently published results of the MRC PT1 trial [63]. In this prospective randomized study, a lack of platelet control was observed in less than 4% (19 patients) of the 405 patients treated by anagrelide. At 3 months the 90th percentile of platelet count was only <800 × 109/L. However, at 6 months the median platelet count was <400 × 109/L and a stable reduction of the median platelet number (lower or equal to 400 × 109/L), similar to what was obtained with HU, was maintained during the following 24 months. A mechanism involving a diminution of the size and ploidy of BM megacaryocytes without modification of their number is supposed at the origin of the platelet number reduction. This may explain why anagrelide does not usually modify the number of white cells or does not cause a significant reduction of the Hb concentration.
The comparison of HU+Asp vs. Ana+Asp in this study restricted to high risk patients [63] has demonstrated that anagrelide and aspirin offer a confirmed protection from thrombosis. The prevalence of thrombotic events for anagrelide-treated patients was 8% (significantly less than 24% in the control study arm, without cytoreduction of the princeps control study of Cortelazzo et al.) [62]. However, in this trial (despite an equivalent control of the platelet number) protection from thrombosis in patients treated by Ana+Asp was less efficient than with HU+Asp (prevalence of thrombotic events 4% at 2 years). Interestingly, protection from arterial thrombosis and especially AIT (transient ischemic attack) was better with HU+Asp; high risk ET patients treated with Ana+Asp had a lower rate of venous thromboembolism. In the same study but in an article published later [47], it was observed that the rates of arterial thrombosis in JAK2 V617F positive patients randomized to anagrelide were higher than in those randomized to HU, whereas equal numbers of arterial thromboses were observed in the two arms of treatment for the patients negative for JAK2 V617F mutation.
Side effects and mutagenic potential
To date, 2251 patients were evaluated for safety of anagrelide [65]. With a maximum follow-up of 7 years, leukemogenic effect has not been demonstrated. Major side effects of anagrelide are consistent with its mechanism of action as a phosphodiesterase inhibitor [51] and include headache (12.6%), tachycardia-type palpitations (15.5%), fluid retention (6%) and gastrointestinal intolerance (14%) leading to premature interruption of the treatment in 22% of the patients [63]. A small number of reversible cardiomyopathies have been described, suggesting that an evaluation of cardiac function should be performed in elderly patients with previous cardiac disorders [53].
The comparative study of HU+Asp vs. Ana+Asp of the MRC PT1 trial has shown that anagrelide was more poorly tolerated than HU (frequency of side effects 21.7% vs. 10.6%). The drop-out rate has been estimated to around 14% [66]. There was more concern in this study from the statement that major hemorrhages occurred more frequently in the Ana+Asp arm than in the HU+Asp arm, suggesting a potentiation of the anti-aggregating effects of aspirin by its association with anagrelide. The question raised by the difference in the rate of progression toward myelofibrosis observed in the anagrelide arm (16 vs. 5 events) needs further evaluation. A previous study comparing BM biopsy findings before and after therapy failed to demonstrate a progression in reticulin or collagen fibrosis during the treatment with anagrelide [67]. Further data may be expected from an on-going study comparing anagrelide to HU in ET patients (newly diagnosed according to the WHO criteria) in whom BM findings will be submitted to a panel revaluation (Study by AOP pharma 007).
Anagrelide has been approved by Food and Drug Administration (FDA) and by European authorities as oral treatment for ET and thrombocytoses associated with PV. Nevertheless, its usage is recommended in case of failure or intolerance to HU.
Interferon-α (IFN-α)
Efficacy
Efficiency of IFN-α administered by subcutaneous injections to reduce the platelet number has been documented in a large number of cohort studies [50]. Only a preliminary report of a multi-center comparative (HU vs. IFN) randomized trial has been published [68]. The overall response rate was 84% and a complete normalization of the platelet count was obtained in 53% of the patients after 3 months. A positive effect on clinical symptoms was associated with normalization of the platelet count; precise information about the reduction of the rate of thrombotic events in high risk patients is not presently available [69].
Side effects and mutagenic potential
No associated leukemogenic effect has been reported with IFN-α. Side effects, mainly flu-like syndrome, are common at the initiation of IFN treatment. They can subside thereafter, mainly when IFN doses are sharply lowered during the subsequent maintenance therapy. However, they lead to discontinuation of the drug in 16.5% of the patients.
Interesting data from phase II studies of pegylated (Peg) IFN-α are now available. The rate of subcutaneous injections, only once per week, improves the compliance to the treatment and increases the number of long lasting hematological responses [70]. A preferential targeting of the JAK2 V617F mutated clone by Peg-IFN-α has been recently suggested by a phase II study in PV [71]. In this prospective study of 27 PV patients positive for the JAK2 mutation, a high rate of complete hematological responses was observed together with a decreased expression of mutated JAK2 in peripheral blood cells in 87% of patients.
C. Alternative cytoreductive therapy
Pipobroman (PI)
Pipobroman (PI) is a piperazine derivative. PI appears to competitively inhibit pyrimidines though it shows a structural resemblance to alkylating agents.
Efficacy
The efficacy of PI in controlling the platelet number in patients with ET is close to that of HU, with an overall response around 95%, documented in several cohort studies [72-74]. In 118 ET patients at high risk for thrombosis treated by PI, protracted hematological remissions and good clinical and hematological tolerances have been reported after a long follow-up [74]. The cumulative risk of thrombosis at year 10 was equal to 14%. Therefore, although not demonstrated by prospective or comparative studies, PI has presumably, like HU, a role in preventing thrombotic events in high risk patients.
Side effects and mutagenic potential
Treatment with either HU or PI was carried out on randomized 292 patients with PV [75]. The risk of leukemia was approximately 10% after13 years of follow-up, with no significant difference between the two arms. Therefore, a long term leukemogenic risk was suspected in PI-treated PV patients, even if this treatment was given alone [75]. In ET, however, the cumulative risk of acute leukemia was 3% at 10 years and 6% at 15 years [73]. A comparison, although retrospective, between PI and HU has been recently assessed. After a median follow-up of 104 months in a cohort study of 155 ET patients, a significantly lower transformation rate was observed in PI-treated patients [76].
Busulfan (BU)
Busulfan (BU) is an alkylating agent formerly used in the treatment of chronic myelocytic leukemia. Despite the reputation of this drug to induce severe and protracted bone marrow aplasias in CML, BU is sometimes proposed to control the platelet number in elderly patients with ET. Rationale for this usage comes from the statement that it is the only treatment able to induce sustained hematological remissions without need of maintenance therapy.
Treatment algorithm
All patients
• Check and treat all the reversible cardiovascular risk factors (hypertension, hypercholesterolemia, diabetes, obesity, smoking).
• Consensus to recommend either a systematic investigation of associated constitutional or acquired factors of thrombophilia in patients without a clinically significant familial history or to screen average ET patients for Acquired Von Willebrand syndrome is lacking.
Hight risk patients
• Cytoreduction is recommended in high risk patients.
In the older patient group (>60) the recommended cytoreductive treatment is HU. In case of resistance or intolerance to HU, consider only non-mutagenic alternative drugs for second line treatment.
There is still reluctance in using HU in young patients. High risk patients under 60 must be informed of the potential side effects occurring after a protracted use of the drug. Non-mutagenic alternative (anagrelide or IFN-α may be considered, although antithrombotic effects of these drugs are regarded presently either as inferior to HU (in term of AIT or arterial thrombosis (for anagrelide) or not yet evaluated (for IFN).
◦ The aims of cytoreduction are to bring the platelet number into the normal range in patients with high risk of thrombosis. In patients whose high risk is mainly hemorrhagic, lowering the absolute platelet number largely under 1000 × 109/L may be the most important goal.
◦ Aspirin therapy is recommended in high risk patients (except when contra-indicated by platelet count >1500 × 109/L, history of major bleeding or presence of three minor risk factors). The benefit of combining of anti-aggregating agent with a cytoreductive therapy (although not demonstrated in randomized studies in ET) is supported by the demonstration of antithrombotic effect of this combination in PV patients and by the presumptive thrombotic role of platelet activation.
Low risk patients
• Abstention of cytoreduction is recommended in low risk patients. The risk of thrombosis in asymptomatic low risk ET patients has been evaluated in several studies [77,78] and may not be high enough to warrant the use of potentially mutagenic or toxic cytoreductive therapy [36,50,53].
• Prescription of low dose aspirin is often proposed [36,53] and may rely again on the presumptive role of platelet activation in vascular risk.
Intermediate risk patients
• Consider cytoreduction in patients in the intermediate age group (40–60), if associated risk factors are present (uncontrolled cardiovascular risk factor, thrombophilia of significant familial expression).
• Prescription of low dose aspirin is supported in this group of patients by uncontrolled observational studies in ET patients.
Management of ET in pregnancy
Information available in the medical literature for the management of ET in pregnancy is still limited, but a large number of pregnancies have been reported in retrospective studies [4-6]. Recommendations for management of ET in pregnancy based on current knowledge have been elaborated [5]. They include an evaluation of the maternal risk of thrombosis and hemorrhages, based on the current characteristics of the disease: previous venous or arterial thrombosis and previous hemorrhages (during previous pregnancies or not), as well as ET induced previous complications: early (first trimester) or late (second or third trimester) pregnancy loss, intrauterine death or still birth, decreased birth weight (of more than 5th centile for gestation), severe pre-eclampsia, ante- or post-partum hemorrhage. Evaluation of the trend in the platelet number in patients without current cytoreduction is mandatory. The threshold for platelet reduction may be over 1500 × 109/L rather than over 1000 × 109/L due to expected natural decline of platelets after the first trimester of pregnancy. A systematic biological screening for associated prothrombotic risk factors is not clearly indicated in patients without personal or familial history of thromboses. Strong coordination between the obstetrician (monitoring the pregnancy) and the hematological department (managing ET) is mandatory.
The therapeutic options include antithrombotic treatment and cytoreductive agents. Low dose aspirin is safe during pregnancy. However, demonstration of a significantly better outcome of pregnancies evolving under continuous aspirin treatment has not been confirmed by a recent report [6]. Some studies have suggested that aspirin from the onset of pregnancy plus heparin from the second trimester might improve pregnancy outcome [79].
Many pregnancies in ET have a successful outcome with minimal therapy. Cytoreductive treatment should preferably be avoided during pregnancy, especially during the first trimester. None of the agents currently used for cytoreduction in ET has a product license for use in pregnancy. As IFN-α does not cross the placental barrier, it is a potential therapeutic solution when platelet reduction is required for high risk ET pregnant patients. A small number of publications is available, showing, up to now, no toxicity for the fetus [5,80]. Breast feeding is however not recommended during cytoreductive treatment including IFN-α. Several retrospective analyses have also led authors to suggest that reducing the platelet number using IFN-α for patients with prior pregnancy events might improve the chance of a successful outcome in subsequent pregnancies.
When ET has been already diagnosed, some important issues concerning the risk of pregnancy should be discussed before conception. For ET female already treated with HU, a wash-out period of 3 months has been recommended. However, for pregnancies where HU had been used during a more or less limited period of time, the toxicity of the product is probably less than it might be anticipated.
Anesthetists prefer aspirin to be stopped two weeks before delivery, to prevent the risk of hematoma after epidural and spinal anesthesia. Low molecular weight heparin (LMWH) is given as an alternative to aspirin. The greatest risk for thromboembolism is post partum and prophylaxis, usually in the form of aspirin and LMWH, should be continued for several weeks.
Prognosis
No substantial differences between life expectancy of 247 patients with ET, according to the PVSG criteria and that of a control population were found by Rozman et al. [76,81]. Nevertheless, the common feeling that ET is a rather benign MPD, affecting more the quality of life than the overall life expectancy, has been challenged by recent publications. An eventual loss of life expectancy may be demonstrated if the group of observed patients is chosen according to selective BM based criteria. Discrimination of prefibrotic (IMF-0) or early fibrotic (IMF-1) stages of IMF from true ET, according to WHO classification, demonstrated a shortening of life expectancy of 32.3% and 21.6% for the first two subgroups compared to only 8.9% for true ET patients [22]. Bazzan et al. compared a cohort of 187 consecutive ET patients to age-matched healthy people living in the same area during a follow-up period of 15 years [82]. This study demonstrated a significantly higher mortality rate and a lower thrombosis-free survival in patients younger than 55 years. On the contrary, in a group of 74 women with ET younger than 50 years at diagnosis, the overall survival was similar to that of an age- and sex-matched control population, with a low incidence of life-threatening thrombohemorrhagic complications or acute leukemia but an increased incidence of first trimester miscarriages [83].
Due to the heterogeneity of the disease, suggested by these prognostic discrepancies, efforts toward understanding the risk of hemostatic complications in ET patients, as well as the risk factors involved in the clonal progression toward PV, IMF, myelodysplasia and acute leukemia have been attempted for a long time.
Risk of thrombosis
The Cortelazzo study [84] in a cohort of 100 historical patients with ET has shown that the overall risk of thrombosis was equal to 6.6 % patients/year compared to 1.2% control individuals/year. Three important risk factors were identified by this study. The risk of thrombosis increases with age (1.7% patients/year before 40 years; 6.3% patients/year between 40 and 60; 15.1% patients/year after 60 years). A previous history of vascular occlusive episode increases the incidence of thrombosis from 3.4 to 31.4% patients/year. Patient's exposure time to elevated platelet number was also, in this study, predictive for the risk of thrombosis, however no document has ever demonstrated proportionality between the thrombotic risk and the degree of elevation of the platelet number. Moreover, 10 to 20% of severe thrombotic complications occur in ET patients with a platelet number under 600 to 700 × 109/L [85].
A second study by Cortelazzo et al. demonstrated that the reduction of the platelet count by HU randomized against abstention of cytoreductive treatment in a group of 114 ET patients with an elevated risk for thrombosis (age over 60 or previous thrombotic event) resulted in a significant prevention of thrombosis or severe ischemia (AIT) even in the background of an anti-aggregation therapy administered in nearly 70% of patients [62].
A number of other features have been claimed to correlate with thrombotic complications in ET:
• The presence of thrombophilia: an increased risk of venous thromboembolism has been associated with a carriership of factor V Leiden [86]; an increased prevalence of antiphospholipid antibodies in ET patients has been also noted [87].
• Cardiovascular risk factors such as hypertension, smoking and hypercholesterolemia have been implicated in the increased risk of thrombosis in ET patients, as shown by retrospective studies [88-90].
• Presence of the JAK2 V617F clonal mutation and platelet and granulocyte activation pattern are still subject to investigations.
Clonal pattern of X chromosome inactivation [25-27], reduced expression of MPL in BM megakaryocytes and over expression of PRV1 mRNA in peripheral blood granulocytes [34,35] (previously proposed as criteria in favor of the diagnosis of clonal MPDs) were all associated with an increased risk of thrombosis. However, the role of the clonal mutation of the JAK2 tyrosine kinase gene in the risk of thrombosis is still controversial. The presence of the mutation may increase the risk of arterial thrombosis [91], may be associated with a global increase of vascular complications [1] or may correlate, but not to a significant level, to the elevated risk of venous thrombosis at diagnosis [47]. In another study, however, the JAK2 mutation status had not been correlated to the occurrence of thrombotic events [92].
The role of platelet hyperactivation [93] and granulocyte activation pattern [94] in the risk of vasculo-occlusive episodes in ET patients have been previously suggested. In more recent studies, a JAK2 gene dosage effect on granulocyte activation pattern was demonstrated in MPDs [49] along with, in ET, the activation of monocytes and platelet being more frequently observed in patients with a history of thrombosis [95]. In this last study, the JAK2 V617F mutation was found associated with the increased platelet activation. Clearly more information is needed regarding gene mutations and peripheral cells activation and their incidence on vasculo-occlusive complications.
Hemorrhagic risk
A risk of major hemorrhage of 0.33% patients/year during follow-up has been deducted from 21 previously published studies [50]. As already mentioned, severe hemorrhages were reported to be more common in patients with platelet count >1500 × 109/L but the risk decreases when platelet number is reduced by treatment, allowing the use of low dose aspirin associated to cytoreduction in these patients. In the ECLAP study of PV patients, a history of previous bleeding problems was also an important predictor of major hemorrhagia. Recently, a tentative categorization of the hemorrhagic risk has been proposed [56]:
A) High: in patients with platelet number >1500 × 109/L or history of major bleeding or presence of all three minor risk factors. Minor risk factors proposed are: 1) Disease duration over 15 years; 2) Platelet count >1000 × 109/L; 3) History of minor bleeding.
B/Intermediate: when patients have two minor risk factors.
C/Low: in all the other cases.
Risk of a clonal progression of ET
• progression or transformation into PV
In ET patients, an increase of the Hb and Ht levels toward values observed in patients with primary erythrocytosis, or even a progression to a full-blown picture of PV (according to the PVSG criteria) has been observed in up to 5–6.5% of large series of patients [96]. A critical revue of the criteria used at diagnosis to differentiate ET and PV may probably explain some of these apparent early progressions. The presence of EEC in BM cells, an increase level of PRV-1 mRNA in granulocytes and low circulating EPO levels in ET patients has already been proposed as an indication of a close relationship between PV and a subset of ET patients [35,38]. More recently, in a large prospective study of 806 ET patients it has been observed that progression to PV was restricted to patients with the JAK2 V617F mutation [47]. In this study, statistically higher Hb and circulating granulocytes levels, lower EPO values and hypercellular BM on biopsy material in JAK2 V617F positive-compared to JAK2 negative ET patients, suggested a continuum in mutated patients between ET and PV phenotypes. A statistically different rate of progression to a PV phenotype in JAK2 mutated ET patients was also observed in a retrospective study with a long term follow-up [92].
• progression into myelofibrosis
The risk of myelofibrotic transformation is shared by all Ph-negative MPDs diagnosed according to the PVSG criteria. Myelofibrosis itself is not a disease but rather a reaction pattern of the BM to cytokines released from the clonal proliferative cells in PV and IMF [97]. In ET (according to the PVSG criteria) myelofibrosis is a delayed event with a cumulative risk of occurrence estimated at 3% after 5 years, 8% after 10 years and 15% after 15 years [98]. No clear predictive factor of the risk in patients diagnosed according to the PVSG criteria has been published. According to the WHO classification based on BM biopsy findings, the risk of fibrotic progression has been estimated to 50% after a median follow-up of 38+/-30 months in IMF-0 or IMF-1 patients. In the description of IMF-0 and IMF-1, the degree of megakaryocyte dysplasia and the level of granulocyte hyperplasia are important features and may confirm their role in the risk of fibrotic progression. Fibrotic progression might rarely and even never be observed in true ET patients [22,48]. In fact, this point needs confirmation coming from long term prospective studies.
The status of the JAK2 mutation, however, was not correlated with the presence of fibrosis in ET patients included in the large prospective MRC PT1 study. In PV patients, although the role of JAK2 V617F mutation in progression toward fibrosis is currently unknown, transition from heterozygoty to homozygoty for JAK2 may represent an important step in the progression toward post-PV myelofibrosis [49].
• Progression to acute leukemia
In the published literature (concerning often small series of ET patients with different lengths of follow-up), the risk of progression to acute leukemia or myelodyplasia during follow-up ranges from 0.6 to 6.1% [99]. Progression to acute leukemia represents the most serious life-threatening complication of ET but is always a late occurring event, with a median delay of occurrence of 6.5 years [99-101]. The predicting factors of leukemic progression risk include cytogenetic abnormalities, previous progression to myelofibrosis or treatment by cytotoxic agent(s) [101].
Untreated patients can transform, but only very rarely, into acute leukemia [99]. A retrospective study of 2316 Italians suggested an incidence of approximately 1% [102]. Patients who received previous therapy with P32 or alkylating agents, or who require more than one cytotoxic agent, have an increased risk of acute myeloid leukemia (AML)/MDS [100-105]. It is not clear whether this reflects the combined effect of the drugs (including HU, which is the most frequently used drug) or the selection (through the necessity of more aggressive treatment modalities) of more evolved and, hence, more progressive forms of the disease.
However, the leukemogenic potential of HU used alone is still a matter of controversy. Prospective randomized studies in ET patients [63,104,105] did not find significant difference in the incidence of acute leukemia between untreated patients, or patients treated by HU or anagrelide alone. However, randomized studies either lack of the long term follow-up (necessary to evaluate the late toxicity of HU) or were conducted according to the PVSG criteria (ignoring the distinction between IMF-0 and IMF-1 and true ET). According to the WHO classification, IMF-0 and IMF-1 are, in fact, early IMF with a higher propensy to leukemic transformation than the true ET. In a large retrospective study of 357 ET patients treated with HU (most of them unbiopsied), progression to AML/MDS was observed in 4.5%, frequently associated with deletion of 17p [101]. In the same study (201 ET patients who had been treated only with HU) the rate of progression to AML/MDS was 3.5%. Nielsen and Hasselbalch [106] reported progression to AML/MDS in about 3.4% in HU-treated ET patients. The most relevant information of leukemogenicity of HU is expected to come from prospective randomized studies of patients classified according to the WHO guidelines.
Information about the risk of progression to leukemia and the JAK2 mutation status of ET patients is still limited. No correlation has been demonstrated. Moreover, the study of blast cells in two JAK2 mutated ET patients with acute leukemia failed to demonstrate the JAK2 mutation in one of them [107].
Unresolved question
Implication of the description of JAK2 V617F mutation on the management of ET
Data on the JAK2 mutation status of ET patients cannot, at present, be used for prognosis assessment. Correlations between the JAK2 status and the vascular risk and eventuality of clonal progression remain to be established. For the same reasons, a therapeutic approach based on the JAK2 positive or negative status of an individual patient has presently no clinical relevance. However, future prospective studies regarding the treatment of ET must include a quantitative approach of JAK2 V617F expression as well as a sophisticated interpretation of the bone marrow findings.
Relevance of therapeutic strategies aiming at reduction or eradication of the clonal disorder
Allogeneic bone marrow transplantation is exclusively discussed for those patients who have transformed into acute leukemia or myelodyplasia or have progressed into high risk myelofibrosis. Even in this context, transplantation should be regarded as experimental therapy [108]. At variance with the model proposed in CML, where a control of the BCR/ABL positive clone is now the goal of treatment, data concerning the incidence of a similar therapeutic approach in ET are lacking. The expected availability of new tyrosine kinase inhibitors may offer opportunities to test this strategy.
Abbreviations
AIT = Transient ischemic attack
Ana = Anagrelide
AML = Acute myeloid leukemia
Asp = Aspirin
BM = Bone marrow
BU = Busulfan
CML = Chronic myeloid leukemia
CP = Congenital polycythemia
CRP = C-Reactive protein
ECLAP = European Collaboration on Low-dose Aspirin in Polycythemia Vera
EEC = Endogenous Erythrocyte Colony formation by erythrocytes progenitors
EPO = Erythropoietin
ET = Essential thrombocythemia
Hb = Hemoglobin
Ht = Hematocrit
HU = Hydroxyurea
INF = Interferon-α
IMF = Myelofibrosis with myeloid metaplasia of the spleen
LMWH = Low molecular weight heparin
MDS = Myelodysplastic syndrome
MPD = Myeloproliferative disorder
MPL = Thrombopoietin receptor
MRC PT1 = Medical Research Council PT1 study
NF-E2 (Nuclear factor erythroid 2)
PCR = Polymerase chain reaction
PI = Pipobroman
PRV-1 = Polycythemia rubra vera protein 1
PT = Primary thrombocythemia
PV = Polycythemia vera
PVSG = Polycythemia Vera Study Group
RhT = Secondary thrombocytoses
Rth = Reactive thrombocytoses
SE = Secondary erythrocytosis
TPO = Thrombopoietin
TRCV = Total red cell volume
VWF = Von Willebrand factor
WBC = White blood bell
WHO = World Health Organization
References
- Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain of function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- Johansson P. Epidemiology of the myeloproliferative disorders polycythemia vera and essential thrombocythemia. Semin Thromb Hemost. 2006;32:171–173. doi: 10.1055/s-2006-939430. [DOI] [PubMed] [Google Scholar]
- Jensen MK, de Nully Brown P, Nielsen OJ, Hasselbalch HC. Incidence, clinical features and outcome of essential thrombocythaemia in a well defined geographical area. Eur J Haematol. 2000;65:132–139. doi: 10.1034/j.1600-0609.2000.90236.x. [DOI] [PubMed] [Google Scholar]
- Griesshammer M, Heimpel H, Pearson T. Essential thrombocythemia and pregnancy. Leuk Lymphoma. 1996;22:57–63. doi: 10.3109/10428199609074361. [DOI] [PubMed] [Google Scholar]
- Harrison C. Pregnancy and its management in the Philadelphia negative myeloproliferative diseases. Br J Haematol. 2005;129:293–306. doi: 10.1111/j.1365-2141.2005.05400.x. [DOI] [PubMed] [Google Scholar]
- Elliott MA, Tefferi A. Thrombocythaemia and pregnancy. Best Pract Res Clin Haematol. 2003;16:227–242. doi: 10.1016/S1521-6926(03)00019-7. [DOI] [PubMed] [Google Scholar]
- Chait Y, Condat B, Cazals-Hatem D, Rufat P, Atmani S, Chaoui D, Guilmin F, Kiladjian JJ, Plessier A, Denninger MH, Casadevall N, Valla D, Brière JB. Relevance of the criteria commonly used to diagnose myeloproliferative disorders in patients with splanchnic thrombosis. Br J Haematol. 2005;129:553–560. doi: 10.1111/j.1365-2141.2005.05490.x. [DOI] [PubMed] [Google Scholar]
- Michiels JJ, Berneman Z, Bockstaele DV, van der Planken M, De Raeve H, Schroyens W. Clinical and laboratory features, pathology of platelet-mediated thrombosis and bleeding complications and the molecular etiology of essential thrombocythemia and polycythemia vera: therapeutic implications. Semin Thromb Hemost. 2006;32:174–207. doi: 10.1055/s-2006-939431. [DOI] [PubMed] [Google Scholar]
- Van Genderen PJJ, Budde U, Michiels JJ, van Strik R, van Vliet HHMD. The reduction of large von Willebrand factor multimères in plama in essential thrombocythemia is related to the platelet count. Br J Haematol. 1996;93:362–965. doi: 10.1046/j.1365-2141.1996.d01-1729.x. [DOI] [PubMed] [Google Scholar]
- Griesshammer M, Bangerter M, van Vliet HH, Michiels JJ. Aspirin in essential thrombocythemia: status quo and quo vadis. Semin Thromb Hemost. 1997;23:371–377. doi: 10.1055/s-2007-996111. [DOI] [PubMed] [Google Scholar]
- Barbui T, Finazzi G, Dupuy E, Kiladjian JJ, Briere J. Treatment strategies in essential thrombocythemia. A critical appraisal of various experiences in different centers. Leuk Lymphoma. 1996;22:149–160. doi: 10.3109/10428199609074373. [DOI] [PubMed] [Google Scholar]
- Murphy S, Peterson P, Illand H, Lazlo J. Experience of the Polycythemia Vera Study Group with essential thrombocythemia: a final report on diagnostic criteria, survival, and leukemic transition by treatment. Semin Hematol. 1997;34:29–39. [PubMed] [Google Scholar]
- Imbert M, Pierre R, Thiele J, Vardiman JW, Brunning RD, Flandrin G. Essential thrombocythaemia. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editor. World Health Organization Classification of Tumours Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon, IARC press; 2001. pp. 39–41. [Google Scholar]
- James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, Garcon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL, Constantinescu SN, Casadevall N, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia Vera. Nature. 2005;434:1444–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR, Cancer Genome Project Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;366:122. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, Adelsperger J, Koo S, Lee JC, Gabriel S, Mercher T, D'Andrea A, Frohling S, Dohner K, Marynen P, Vandenberghe P, Mesa RA, Tefferi A, Griffin JD, Eck MJ, Sellers WR, Meyerson M, Golub TR, Lee SJ, Gilliland DG. Activating mutation in the tyrosine kinase JAK2 in polycythemia, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cel. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Kaushansky K. On the molecular origins of the chronic myeloproliferative disorders: it all makes sense. Blood. 2005;105:4187–4190. doi: 10.1182/blood-2005-03-1287. [DOI] [PubMed] [Google Scholar]
- Jelinek J, Oki Y, Gharibyan V, Bueso-Ramos C, Prchal JT, Verstovsek S, Beran M, Estey E, Kantarjian HM, Issa JP. JAK2 mutation 1849G >T is rare in acute leukemias but can be found in CMML, Philadelphia-chromosome negative CML and megakaryocytic leukemia. Blood. 2005;106:3370–3373. doi: 10.1182/blood-2005-05-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpurka H, Tiu R, Murugesan G, Aboudola S, Hsi ED, Theil KS, Sekeres MA, Maciejewski JP. Refractory anemia with ringed sideroblasts associted with marked thrombocytosis (RAR-T), another myeloproliferative condition characterised by JAK2 V617F mutation. Blood. 2006;108:2173–2181. doi: 10.1182/blood-2006-02-005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele J, Kvasnicka HM. Chronic myeloproliferative disorders with thrombocythemia: a comparative study of two classification systems (PVSG, WHO) on 839 patients. Ann Hematol. 2003;82:148–152. doi: 10.1007/s00277-002-0604-y. [DOI] [PubMed] [Google Scholar]
- Georgii A, Buhr T, Buesche G, Kreft A, Choritz H. Classification and staging of Ph-negative myeloproliferative disorders by histopathology from bone marrow biopsies. Leuk Lymphoma. 1996;22:15–29. doi: 10.3109/10428199609074357. [DOI] [PubMed] [Google Scholar]
- Thiele J, Kvasnika HM. Clinicopathological criteria for differential diagnosis of thrombocythemias in various myeloproliferative disorders. Semin Throm Hemost. 2006;32:219–230. doi: 10.1055/s-2006-939433. [DOI] [PubMed] [Google Scholar]
- Antonioli E, Guglielmelli P, Pancrazzi A, Bogani C, Verrucci M, Ponziani V, Longo G, Bosi A, Vannucchi AM. Clinical implications of the JAK2 V617F mutation in essential thrombocythemia. Leukemia. 2005;19:1847–1849. doi: 10.1038/sj.leu.2403902. [DOI] [PubMed] [Google Scholar]
- El Kassar N, Hetet G, Briere J, Grandchamp B. Clonality analysis of haematopoiesis in essential thrombocythemia: advantages of studying T-lymphocytes and platelets. Blood. 1997;89:128–134. [PubMed] [Google Scholar]
- Harnsson CN, Gale RE, Machin SJ, Linch DC. A large proportion of patients with a diagnosis of essential thrombocythaemia do not have a clonal disorder and may be at lower risk of thrombotic complications. Blood. 1999;93:417–424. [PubMed] [Google Scholar]
- Chiusolo P, La Barbera EO, Laurenti L, Piccirillo N, Sora F, Giordano G, Urbano R, Mazzucconi MG, De Stefano V, Leone G, Sica S. Clonal hematopoiesis and risk of thrombosis in young female patient with essential thrombocythemia. Exp Hematol. 2001;29:670–676. doi: 10.1016/S0301-472X(01)00640-3. [DOI] [PubMed] [Google Scholar]
- Shih LY, Lin TL, Lai CL, Dunn P, Wu JH, Wang PN, Kuo MC, Lee LC. Predictive values of X-chromosome inactivation patterns and clinicohematologic parameters for vascular complications in female patients with essential thrombocythemia. Blood. 2002;100:1596–1601. doi: 10.1182/blood-2002-01-0195. [DOI] [PubMed] [Google Scholar]
- Kladjian JJ, Elkassar N, Cassinat B, Hetet G, Giraudier S, Balitrand N, Conejero C, Briere J, Fenaux P, Chomienne C, Grandchamp B. Essential thrombocythemias without V617F JAK2 mutation are clonal hematopoietic stem cell disorders. Leukemia. 2006;20:1181–1183. doi: 10.1038/sj.leu.2404214. [DOI] [PubMed] [Google Scholar]
- Dobo I, Donnard M, Girodon F, Mossuz P, Boiret N, Boukhari R, Allegraud A, Bascans E, Campos L, Pineau D, Turlure P, Praloran V, Hermouet S. Standardization and comparison of endogenous erythroid colony assays performed with bone marrow or blood progenitors for the diagnosis of polycythemia vera. Hematol J. 2004;5:161–167. doi: 10.1038/sj.thj.6200344. [DOI] [PubMed] [Google Scholar]
- Li Y, El Kassar N, Gardin C, Briere J. Clonality assay and megacaryocyte culture technics in essential thrombocythemia. Leuk Lymphoma. 1996;22:31–40. doi: 10.3109/10428199609074358. [DOI] [PubMed] [Google Scholar]
- Kralovics R, Buser SA, Teo S-S, Coers J, Tichelli A, Skoda R. Comparisonn of molecular markers in a cohort of patients with chronic myeloproliferative disorders. Blood. 2003;102:1869–1871. doi: 10.1182/blood-2003-03-0744. [DOI] [PubMed] [Google Scholar]
- Klippel S, Pahl HL. Molecular markers for the diagnosis of philadelphia chromosome negative myeloproliferative disorders. Pathol Biol (Paris) 2004;52:267–274. doi: 10.1016/j.patbio.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Horikawa Y, Matsumura I, Hashimoto K, Shiraga M, Kosugi S, Tadokoro S, Kato T, Miyazaki H, Tomiyama Y, Kurata Y, Matsuzawa Y, Kanakura Y. Markedly reduced expression of platelet c-mpl receptor in essential thrombocythemia. Blood. 1997;90:4031–4038. [PubMed] [Google Scholar]
- Li J, Xia Y, Kuter DJ. The platelet megakaryocyte receptor number and function are markedly decreased in patients with essential thrombocythemia. Br J Haematol. 2000;111:943–963. doi: 10.1046/j.1365-2141.2000.02430.x. [DOI] [PubMed] [Google Scholar]
- Griesshammer M, Klippel S, Strunck E, Temerinac S, Mohr U, Heimpel H, Pahl HL. PRV-1 mRNA expression discriminates two types of essential thrombocythemia. Ann Hematol. 2004;83:364–370. doi: 10.1007/s00277-004-0864-9. [DOI] [PubMed] [Google Scholar]
- Campbell PJ, Green AR. Management of polycythemia vera and essential thrombocythemia. Hematology Am Soc Hematol Educ Program. 2005:201–208. doi: 10.1182/asheducation-2005.1.201. [DOI] [PubMed] [Google Scholar]
- Goerttler PS, Steimle C, Marz E, Johansson PL, Andreasson B, Griesshammer M, Gisslinger H, Heimpel H, Pahl HL. The Jak2V617F mutation, PRV-1 overexpression and EEC formation define a similar cohort of MPD patients. Blood. 2005;106:2862–2864. doi: 10.1182/blood-2005-04-1515. [DOI] [PubMed] [Google Scholar]
- Messinezy M, Westwood NB, El-Hemaidi I, Marsden JT, Sherwood RS, Pearson TC. Serum erythropoietin values in erythroses and in primary thrombocythaemia. Br J Haematol. 2002;117:47–53. doi: 10.1046/j.1365-2141.2002.03386.x. [DOI] [PubMed] [Google Scholar]
- Messinezy M, Westwood NB, Woodstock SP, Strong RM, Pearson TC. Low serum erythropoietine: a strong diagnostic criterion of primary polycythaemia even at normal haemoglobin levels. Clin Lab Haematol. 1995;17:217–220. [PubMed] [Google Scholar]
- Mossuz P, Groupe d'Etudes Multicentriques des Syndrome MyeloProliferatifs (GEMSMP) Influence of the assays of endogenous colony formation and serum erythropoietin on the diagnosis of polycythemia vera and essential thrombocythemia. Semin Thromb Hemost. 2006;32:246–250. doi: 10.1055/s-2006-939435. [DOI] [PubMed] [Google Scholar]
- Sacchi S, Vinci G, Gugliotta L, Rupoli S, Gargantini L, Martinelli V, Baravelli S, Lazzarino M, Finazzi G. Diagnosis of essential thrombocythemia at platelet counts between 400 and 600 × 10(9)/L. Haematologica. 2000;85:492–495. [PubMed] [Google Scholar]
- Kondo T, Okabe M, Sanada M, Kurosawa M, Suzuki S, Kobayashi M, Hosokawa M, Asaka M. Familial essential thrombocythemia associated with one-base deletion in the 5'-untranslated region of the thrombopoietin gene. Blood. 1998;92:1091–1096. [PubMed] [Google Scholar]
- Bellanne-Chantelot C, Chaumarel I, Labopin M, Bellanger F, Barbu V, De Toma C, Delhommeau F, Casadevall N, Vainchenker W, Thomas G, Najman A. Genetic and clinical implications of the Val617Phe JAK2 mutation in 72 families with myeloproliferative disorders. Blood. 2006;108:346–352. doi: 10.1182/blood-2005-12-4852. [DOI] [PubMed] [Google Scholar]
- Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House. Virginia. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- Michiels JJ, Berneman Z, Schroyens W, Kutti J, Swolin B, Ridell B, Fernando P, Zanetto U. Philadelphia (Ph) chromosome-positive thrombocythemia without features of chronic myeloid leukemia in peripheral blood: natural history and diagnostic differentiation from Ph negative essential thrombocythemia. Ann Hematol. 2004;83:504–512. doi: 10.1007/s00277-004-0877-4. [DOI] [PubMed] [Google Scholar]
- Pearson TC, Guthrie DL, Simpson J, Chinn S, Barosi G, Ferrant A, Lewis SM, Najean Y. Interpretation of measured red cell mass and plasma volume in the adults: expert panel on radionuclides of the International Council for Standardization in Haematology. Br J Haematol. 1995;89:748–756. doi: 10.1111/j.1365-2141.1995.tb08411.x. [DOI] [PubMed] [Google Scholar]
- Campbell PJ, Scott LM, Buck G, Wheatley K, East CL, Marsden JT, Duffy A, Boyd EM, Bench AJ, Scott MA, Vassiliou GS, Milligan DW, Smith SR, Erber WN, Bareford D, Wilkins BS, Reilly JT, Harrison CN, Green AR, United Kingdom Myeloproliferative Disorders Study Group; Medical Research Council Adult Leukaemia Working Party; Australasian Leukaemia and Lymphoma Group Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet. 2005;366:1945–1953. doi: 10.1016/S0140-6736(05)67785-9. [DOI] [PubMed] [Google Scholar]
- Thiele J, Kvasnika HM, Zankhovich R, Diehl V. Relevance of bone marrow features in the differential diagnosis between essential thrombocythemia and early stage idiopathic myelofibrosis. Haematologica. 2000;85:1126–1134. [PubMed] [Google Scholar]
- Passamonti F, Rumi E, Pietra D, Della Porta MG, Boveri E, Pascutto C, Vanelli L, Arcaini L, Burcheri S, Malcovati L, Lazzarino M, Cazzola M. Relation between JAK2 (V617F) mutation status, granulocyte activation, and constitutive mobilization of CD34+ cells into peripheral blood in myeloproliferative disorders. Blood. 2006;107:3676–3682. doi: 10.1182/blood-2005-09-3826. [DOI] [PubMed] [Google Scholar]
- Barbui T, Barosi G, Grossi A, Gugliotta L, Liberato LN, Marchetti M, Mazzucconi MG, Rodeghiero F, Tura S. Practice guidelines for the therapy of essential thrombocythemia. A statement from the Italian Society of Hematology, the Italian Society of Experimental Hematology and the Italian Group for Bone Marrow Transplantation. Haematologica. 2004;89:215–232. [PubMed] [Google Scholar]
- Harrison C. Essential thrombocythaemia: challenges and evidence-based management. Br J Haematol. 2005;130:153–165. doi: 10.1111/j.1365-2141.2005.05543.x. [DOI] [PubMed] [Google Scholar]
- Michiels JJ. Aspirin and platelet-lowering agents for the prevention of vascular complications in essential thrombocythemia. Clin Appl Throm Hemost. 1999;5:247–251. doi: 10.1177/107602969900500408. [DOI] [PubMed] [Google Scholar]
- Elliott MA, Tefferi A. Thrombosis and haemorrhage in polycythemia vera and essential thrombocythaemia. Brh J Haematol. 2005;128:275–290. doi: 10.1111/j.1365-2141.2004.05277.x. [DOI] [PubMed] [Google Scholar]
- Schwarz J, Pytlik R, Doubek M, Brychtova Y, Dulicek P, Campr V, Kren L, Penka M. Analysis of risk factors: the rationale of guidelines of the Czech Hematological Society for diagnosis and treatment of chronic myeloproliferative disorders with thrombocythemia. Sem Throm Hemost. 2006;32:231–245. doi: 10.1055/s-2006-939434. [DOI] [PubMed] [Google Scholar]
- Schafer AI. Molecular basis of the diagnosis and treatment of polycythemia vera and essential thrombocythemia. Blood. 2006;107:4214–4222. doi: 10.1182/blood-2005-08-3526. [DOI] [PubMed] [Google Scholar]
- Landolfi R, Di Gennaro L, Novarese L, Patrono C. Aspirin for the control of platelet activation and prevention of thrombosis in essential thrombocythemia and polycythemia vera: current insights and rationale for futures studies. Semin Thromb Hemost. 2006;32:251–259. doi: 10.1055/s-2006-939436. [DOI] [PubMed] [Google Scholar]
- Landolfi R, Marchioli R, Kutti J, Gisslinger H, Tognoni G, Patrono C, Barbui T, European Collaboration on Low-Dose Aspirin in Polycythemia Vera Investigators Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350:114–124. doi: 10.1056/NEJMoa035572. [DOI] [PubMed] [Google Scholar]
- Low dose aspirin in polycythaemia vera: a pilot study. Gruppo Italiano Studio Policitemia. Br J Haematol. 1997;97:453–456. doi: 10.1046/j.1365-2141.1997.362682.x. [DOI] [PubMed] [Google Scholar]
- Randi ML, Rossi C, Fabris F, Menapace L, Girolami A. Aspirin seems as effective as myelosuppressive agents in the prevention of rethrombosis in essential thrombocythemia. Clin Appl Thromb Hemost. 1999;5:131–135. doi: 10.1177/107602969900500210. [DOI] [PubMed] [Google Scholar]
- Van Genderen PJJ, Mulder PGH, Weleboer M, Van de Moesdijk D, Michiels JJ. Prevention and treatment of thrombotic complications in essential thrombocythaemia: efficacy and safety of aspirin. Br J Haematol. 1997;97:179–84. doi: 10.1046/j.1365-2141.1997.d01-2127.x. [DOI] [PubMed] [Google Scholar]
- Ruggeri M, Castaman G, Rodeghiero F. Is Ticlopidine a safe alternative to management of myeloproliferative disorders. Haematologica. 1993;78:18–21. [PubMed] [Google Scholar]
- Cortelazzo S, Finazzi G, Ruggeri M, Vestri 0, Galli M, Rodeghiero F, Barbui T. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N EngI J Med. 1995;332:1132–1136. doi: 10.1056/NEJM199504273321704. [DOI] [PubMed] [Google Scholar]
- Harrison CN, Campbell PJ, Buck G, Wheatley K, East CL, Bareford D, Wilkins BS, van der Walt JD, Reilly JT, Grigg AP, Revell P, Woodcock BE, Green AR, United Kingdom Medical Research Council Primary Thrombocythemia 1 Study Hydroxyurea compared to Anagrelide in High-risk essential thrombocythemia. N Engl J Med. 2005;353:33–45. doi: 10.1056/NEJMoa043800. [DOI] [PubMed] [Google Scholar]
- Barosi G, Besses C, Birgegard G, et al. A unified definition of clinical resistance/intolerance to Hydroxycarbamide in essential thrombocythemia: Results of a concensus process by an International Working Goup. Leukemia doi: 10.1038/sj.leu.2404473. [DOI] [PubMed] [Google Scholar]
- Fruchtman SM, Petitt RM, Gilbert HS, Fiddler G, Lyne A, Anagrelide Study Group Anagrelide: analysis of long-term efficacy, safety and leukemogenic potential in myeloproliferative disorders. Leuk Res. 2005;29:481–491. doi: 10.1016/j.leukres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Petitt RM, Silverstein MN, Petrone ME. Anagrelide for control of thrombocythemia in polycythemia vera and other myeloproliferative disorders. Semin Hematol. 1997;34:51–54. [PubMed] [Google Scholar]
- Thiele J, Kvasnicka HM, Fuchs N, Brunnbauer K, Volkwein N, Schmitt-Graeff A. Anagrelide-induced bone marrow changes during therapy of chronic myeloproliferative disorders with thrombocytosis. an immunohistochemical and morphometric study of sequential trephine biopsies. Haematologica. 2003;88:1130–1138. [PubMed] [Google Scholar]
- Griesshammer M, Bergmann L, Hafner M, Heimpel H, Seifned E, Bangerter M, Hehlmann R, Lengfelder E. Treatment and clinical course of essential thrombocythaemia (ET): preliminary results of a prospective multicenter trial [abstract] Blood. 1998;92:1750. [Google Scholar]
- Radin AI, Kim HT, Grant BW, Bennett JM, Kirkwood JM, Stewart JA, Hahn RG, Dutcher JP, Wiernik PH, Oken MM, Eastern Cooperative Oncology Group Phase II study of alpha2 interferon in the treatment of the chronic myeloproliferative disorders (E5487): a trial of the Eastern Cooperative Oncology Group. Cancer. 2003;98:100–109. doi: 10.1002/cncr.11486. [DOI] [PubMed] [Google Scholar]
- Samuelsson J, Hasselbalch H, Bruserud O, Temerinac S, Brandberg Y, Merup M, Linder O, Bjorkholm M, Pahl HL, Birgegard G, Nordic Study Group for Myeloproliferative Disorders A phase II trial of pegylated interferon alpha-2b therapy for polycythemia vera and essential thrombocythemia: feasibility, clinical and biologic effects, and impact on quality of life. Cancer. 2006;106:2397–2405. doi: 10.1002/cncr.21900. [DOI] [PubMed] [Google Scholar]
- Kiladjian JJ, Cassinat B, Turlure P, Cambier N, Roussel M, Bellucci S, Menot ML, Massonnet G, Dutel JL, Ghomari K, Rousselot P, Grange MJ, Chait Y, Vainchenker W, Parquet N, Abdelkader-Aljassem L, Bernard JF, Rain JD, Chevret S, Chomienne C, Fenaux P. High molecular response rate of polycythemia vera patients treated with pegylated interferon {alpha}-2a. Blood. 2006;108:2037–2040. doi: 10.1182/blood-2006-03-009860. [DOI] [PubMed] [Google Scholar]
- Bernasconi P, Boni M, Cavigliano PM, Calatroni S, Brusamolino E, Passamonti F, Volpe G, Pistorio A, Giardini I, Rocca B, Caresana M, Lazzarino M, Bernasconi C. Acute myeloid leukemia (AML) having evolved from essential thrombocythemia (ET): distinctive chromosome abnormalities in patients treated with pipobroman or hydroxyurea. Leukemia. 2002;16:2078–2083. doi: 10.1038/sj.leu.2402638. [DOI] [PubMed] [Google Scholar]
- Passamonti F, Malabarba L, Orlandi E, Pascutto C, Brusamolino E, Astori C, Barate C, Canevari A, Corso A, Bernasconi P, Cazzola M, Lazzarino M. Pipobroman is safe and effective treatment for patients with essential thrombocythaemia at high risk of thrombosis. Br J Haematol. 2002;116:855–861. doi: 10.1046/j.0007-1048.2002.03367.x. [DOI] [PubMed] [Google Scholar]
- Passamonti F, Rumi E, Malabarba L, Arcaini L, Orlandi E, Brusamolino E, Pascutto C, Cazzola M, Lazzarino M. Long-term follow-up of young patients with essential thrombocythemia treated with pipobroman. Ann Hematol. 2004;83:495–497. doi: 10.1007/s00277-004-0882-7. [DOI] [PubMed] [Google Scholar]
- Najean Y, Rain JD. Treatment of polycythemia vera: the use of hydroxyurea and pipobroman in 292 patients under the age of 65 years. Blood. 1997;90:3370–3379. [PubMed] [Google Scholar]
- Passamonti F, Rumi E, Pungolino E, Malabarba L, Bertazzoni P, Valentini M, Orlandi E, Arcaini L, Brusamolino E, Pascutto C, Cazzola M, Morra E, Lazzarino M. Life expectancy and prognostic factors for survival in polycythemia vera and essential thrombocythemia. Am J Med. 2004;117:755–761. doi: 10.1016/j.amjmed.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Ruggieri M, Finazzi G, Tosetto A, Riva S, Rodeghiero F, Barbui T. No treatment for low-risk thrombocythaemia: results from a prospective study. Br J Haematol. 1998;103:772–777. doi: 10.1046/j.1365-2141.1998.01021.x. [DOI] [PubMed] [Google Scholar]
- Randi MI, Luzzatto G, Fabris F. Low-risk essential thrombocythemia in patients younger than 40. Br J Haematol. 1999;104:929. doi: 10.1046/j.1365-2141.1999.1331b.x. [DOI] [PubMed] [Google Scholar]
- Pagliaro P, Arrigoni L, Muggiasca ML, Poggio M, Russo U, Rossi E. Primary thrombocythemia and pregnancy: treatment and outcome in fifteen cases. Am J Hematol. 1996;53:6–10. doi: 10.1002/(SICI)1096-8652(199609)53:1<6::AID-AJH2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Martinelli P, Martinelli V, Agangi A, Maruotti GM, Paladini D, Ciancia R, Rotoli B. Interferon alfa treatment for pregnant women affected by essential thrombocythemia: case reports and a review. Am J Obstet Gynecol. 2004;191:2016–2020. doi: 10.1016/j.ajog.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Rozman C, Giralt M, Feliu E. Life expectancy of patients with chronic myeloproliferative disorders. Cancer. 1991;67:2658–2663. doi: 10.1002/1097-0142(19910515)67:10<2658::AID-CNCR2820671042>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Bazzan M, Tamponi G, Schinco P, Vaccarino A, Foli C, Gallone G, Pileri A. Thrombosis-free survival and life expectancy in 187 consecutive patients with essential thrombocythemia. Ann Hematol. 1999;78:539–543. doi: 10.1007/s002770050555. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Fonseca R, Pereira DL, Hoagland HC. A long term retospective study of young women with essential thrombocythemia. Mayo Clin Proc. 2001;76:22–28. doi: 10.4065/76.1.22. [DOI] [PubMed] [Google Scholar]
- Cortelazzo S, Viero P, Finazzi G, D'Emilio A, Rodeghiero F, Barbui T. Incidence and risk factors for thrombotic complications in a historical cohort of 100 patients with essential thrombocythemia. J Clin Oncol. 1990;8:556–562. doi: 10.1200/JCO.1990.8.3.556. [DOI] [PubMed] [Google Scholar]
- Stark P, Regev A, Blickstein D, Lahav M, Shaklai M. Thrombotic complications in essential thrombocythemia with relatively low platelet counts. Am J Hematol. 1997;56:168–172. doi: 10.1002/(SICI)1096-8652(199711)56:3<168::AID-AJH6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Ruggeri M, Gisslinger H, Tosetto A, Rintelen C, Mannhalter C, Pabinger I, Heis N, Castaman G, Missiaglia E, Lechner K, Rodeghiero F. Factor V Leiden mutation carriership and venous thromboembolism in polycythemia vera and essential thrombocythemia. Am J Hematol. 2002;71:1–6. doi: 10.1002/ajh.10153. [DOI] [PubMed] [Google Scholar]
- Harrison CN, Donohoe S, Carr P, Dave M, Mackie I, Machin SJ. Patients with essential thrombocythaemia have an increased prevalence of antiphospholipid antibodies which may be associated with thrombosis. Thromb Haemost. 2002;87:802–807. [PubMed] [Google Scholar]
- Besses C, Cervantes F, Pereira A, Florensa L, Sole F, Hernandez-Boluda JC, Woessner S, Sans-Sabrafen J, Rozman C, Montserrat E. Major vascular complications in essential thrombocythemia: a study of predictive factors in a series of 148 patients. Leukemia. 1993;13:150–154. doi: 10.1038/sj.leu.2401270. [DOI] [PubMed] [Google Scholar]
- Januten R, Juvonen E, Ikkalla E, Oksanen K, Antilla P, Ruutu T. The predictive value of vascular risk factors and gender for the developpement of thrombotic complications in essential thrombocythemia. Ann Hematol. 2001;80:74–78. doi: 10.1007/s002770000244. [DOI] [PubMed] [Google Scholar]
- Watson KV, Key N. Vascular complications of essential thrombocythemia: a link to cardiovascular risk factors. Br J Haematol. 1993;83:198–203. doi: 10.1111/j.1365-2141.1993.tb08272.x. [DOI] [PubMed] [Google Scholar]
- Cheung B, Radia D, Pantelidis P, Yadegarfar G, Harrison C. The presence of the JAK2 V617F mutation is associated with a higher haemoglobin and increased risk of thrombosis in essential thrombocythaemia. Br J Haematol. 2006;132:244–245. doi: 10.1111/j.1365-2141.2005.05858.x. [DOI] [PubMed] [Google Scholar]
- Wolanskyj AP, Lasho TL, Schwager SM, McClure RF, Wadleigh M, Lee SJ, Gilliland DG, Tefferi A. JAK2 mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol. 2005;131:208–213. doi: 10.1111/j.1365-2141.2005.05764.x. [DOI] [PubMed] [Google Scholar]
- Bellucci S, Michiels JJ. The role of JAK2 V617F mutation, spontaneous erythropoiesis and megakaryocytopoiesis, hypersensitive platelets, activated leukocytes, and endothelial cells in the etiology of thrombotic manifestations in polycythemia vera and essential thrombocythemia. Semin Thromb Hemost. 2006;32:381–398. doi: 10.1055/s-2006-942759. [DOI] [PubMed] [Google Scholar]
- Falanga A, Marchetti M, Evangelista V, Vignoli A, Licini M, Balicco M, Manarini S, Finazzi G, Cerletti C, Barbui T. Polymorphonuclear leukocyte activation and hemostasis in patients with essential thrombocythemia and polycythemia vera. Blood. 2000;96:4216–4226. [PubMed] [Google Scholar]
- Arellano-Rodrigo E, Alvarez-Larran A, Reverter JC, Villamor N, Colomer D, Cervantes F. Increased platelet and leukocyte activation as contributing mechanisms for thrombosis in essential thrombocythemia and correlation with the JAK2 mutational status. Haematologica. 2006;91:169–175. [PubMed] [Google Scholar]
- Jantunen R, Juvonen E, Ikkala E, Oksanen K, Antilla P, Ruutu T. Development of erythrocytosis in the course of essential thrombocythemia. Ann Hematol. 1999;78:219–222. doi: 10.1007/s002770050505. [DOI] [PubMed] [Google Scholar]
- Le Bousse-Kerdiles MC, Martyre MC. Dual implication of fibrogenic cytokines in the pathogenesis offibrosis and myeloproliferation in myeloid metaplasia with myelofibrosis. Ann Hematol. 1999;78:362–371. doi: 10.1007/s002770050595. [DOI] [PubMed] [Google Scholar]
- Cervantes F, Alvarez-Larran A, Talarn C, Gomez M, Montserrat E. Myelofibrosis with myeloid metaplasia following essential thrombocythaemia: actuarial probability, presenting characteristics and evolution in a series of 195 patients. Br J Haematol. 2002;118:786–790. doi: 10.1046/j.1365-2141.2002.03688.x. [DOI] [PubMed] [Google Scholar]
- Anderson PO, Ridell B, Wadenvik H, Kutti J. Leukemic transformation of essential thrombocythemia without previous cytoreductive treatment. Ann Hematol. 2000;79:40–42. doi: 10.1007/s002770050007. [DOI] [PubMed] [Google Scholar]
- Shibata K, Shimamoto Y, Suga K, Sano M, Matsuzaki M. Essential thrombocythemia terminating in acute leukemia with minimal myeloid differentiation – a brief review of recent literature. Acta Haematol. 1994;91:84–88. doi: 10.1159/000204260. [DOI] [PubMed] [Google Scholar]
- Sterkers Y, Preudhomme C, Lai JL, Demory JL, Caulier MT, Wattel E, Bordessoule D, Bauters F, Fenaux P. Acute leukemia and myelodysplastic syndromes following essential thrombocythemia treated with hydroxyurea: high proportion of cases with 17p deletion. Blood. 1998;91:616–622. [PubMed] [Google Scholar]
- Spivak JL, Barosi G, Tognoni G, Barbui T, Finazzi G, Marchioli R, Marchetti M. Chronic myeloproliferative disorders. Hematology Am Soc Hematol Educ Program. 2003:200–224. doi: 10.1182/asheducation-2003.1.200. [DOI] [PubMed] [Google Scholar]
- Randi ML, Fabris F, Girolami A. Second malignancies in patients with essential thrombocythemia. Br J Haematol. 2002;116:293–294. doi: 10.1111/j.1365-2141.2002.3346_1.x. [DOI] [PubMed] [Google Scholar]
- Finazzi G, Barbui T. Treatment of essential thrombocythemia with special emphasis on leukemogenic risk. Ann Hematol. 1999;78:389–392. doi: 10.1007/s002770050536. [DOI] [PubMed] [Google Scholar]
- Finazzi G, Ruggieri M, Rodeghiero F, Barbui T. Second malignancies in patients with essential thrombocythemia treated with busulfan and hydroxyurea: long term follow up of a randomised clinical trial. Br J Haematol. 2002;116:924–924. doi: 10.1046/j.1365-2141.2000.02188.x. [DOI] [PubMed] [Google Scholar]
- Nielsen I, Hasselbalch HC. Acute leukemia and myelodysplasia in patients with Philadelphia chromosome negative chronic myeloproliferative disorder treated with hydroxyurea alone or with hydroxyurea after busulphan. Am J Hematol. 2003;74:26–31. doi: 10.1002/ajh.10375. [DOI] [PubMed] [Google Scholar]
- Campbell JP, Baxter EJ, Beer PA, Scott LM, Bench AJ, Huntly BJ, Erber WN, Kusec R, Larsen TS, Giraudier S, Le Bousse-Kerdiles MC, Griesshammer M, Reilly JT, Cheung BY, Harrison CN, Green AR. Mutation of JAK2 in the myeloproliferative disorders: timing, clonality studies, cytogenetic associations and role in leukemic transformation. Blood. [DOI] [PubMed]
- Cervantes F. Modern management of myelofibrosis. Br J Haematol. 2005;128:583–592. doi: 10.1111/j.1365-2141.2004.05301.x. [DOI] [PubMed] [Google Scholar]