Abstract
Adenovirus (Ad)-based cancer gene therapy is a promising, novel approach for treating cancer resistant to established treatment modalities. Unfortunately, the efficacy of nonreplicative first generation Ads was low and data from clinical trials were disappointing. To address this problem, conditionally replicating Ads have been constructed. Infection of tumor cells with conditionally replicating Ads results in tumor-specific replication, subsequent oncolysis and release of the virus progeny. Recently, it has been suggested that the low expression of the coxsackie–Ad receptor is the rate-limiting factor for infectivity with serotype 5 (Ad5). Unfortunately, coxsackie–Ad receptor expression is highly variable and often low on many tumor types. Consequently, molecular strategies have been applied for the development of coxsackie–Ad receptor-independent oncolytic Ads. This review describes recent developments of Ad-based cancer gene therapy, including novel engineering techniques of the Ad capsid for efficient tumor targeting, as well as targeting techniques, to restrict transgene expression to cancer cells.
Keywords: adenovirus, cancer, oncolytic virus, review, vector targeting, virotherapy
Gene therapy for cancer represents a novel treatment approach for patients resistant to traditional therapies. The concept of gene therapy is gene delivery as a therapeutic molecular intervention to selectively correct or eradicate defective tissues. Adenoviruses (Ads), in particular are a useful approach for effective gene delivery and expression since they demonstrate several advantages over other vectors, such as the ability to transduce dividing and nondividing cells, the ease of manipulation and the capability to produce high titers of current good manufacturing practice quality. Infection of Ads is mediated by binding of the fiber knob region to the receptor of the target cell, which is the coxsackie–Ad receptor (CAR) for most serotypes, including the most commonly used, serotype 5 (Ad5) (Figure 1). Internalization of the virus is mediated by the interaction of a penton-base Arg–Gly–Asp (RGD) motif and cellular αvβ integrins [1], which leads to endocytosis of the virion via clathrin-coated pits. After dissembling of the virus in the endosome, viral DNA is transported to the nucleus and viral genes or transgenes are expressed. Ad DNA is not integrated in the host genome and therefore the risk of mutagenesis is low. Recent reports have found evidence regarding secondary receptor interactions, such as heparan sulfate proteoglycans (HSPGs) [2,3]. Recently, it has been suggested that expression of CAR is a rate-limiting factor for infectivity with Ad5. A growing number of studies have demonstrated that expression of CAR on primary human tumor cells is highly variable. This has been reported for ovarian, cervical, prostate, head and neck, and bladder cancer, melanoma, glioma and others [4-14]. Furthermore, loss of CAR expression may be associated with malignant progression and increased tumor aggressiveness [15]. The function of CAR is not fully understood, but it is localized in the tight junctions, which suggests a role in cell adhesions [16]. Despite exciting preclinical data, anticancer molecular therapies have been proposed as a treatment alternative for advanced cancers resistant to conventional treatment modalities. To this end, Ad vectors have been used for a wide variety of gene therapy applications [17]. Unfortunately, the efficacy of gene transfer to solid tumors has been limited and thus there is little evidence supporting significant clinical benefit; only Phase I and II trials have been reported to date [10,18]. The low efficacy of Ad-based gene therapy trials has been attributed to insufficient transduction of tumor cells. Consequently, various approaches have been evaluated to modify or circumvent CAR deficiency, including genetic capsid modifications or retargeting complexes.
Figure 1.
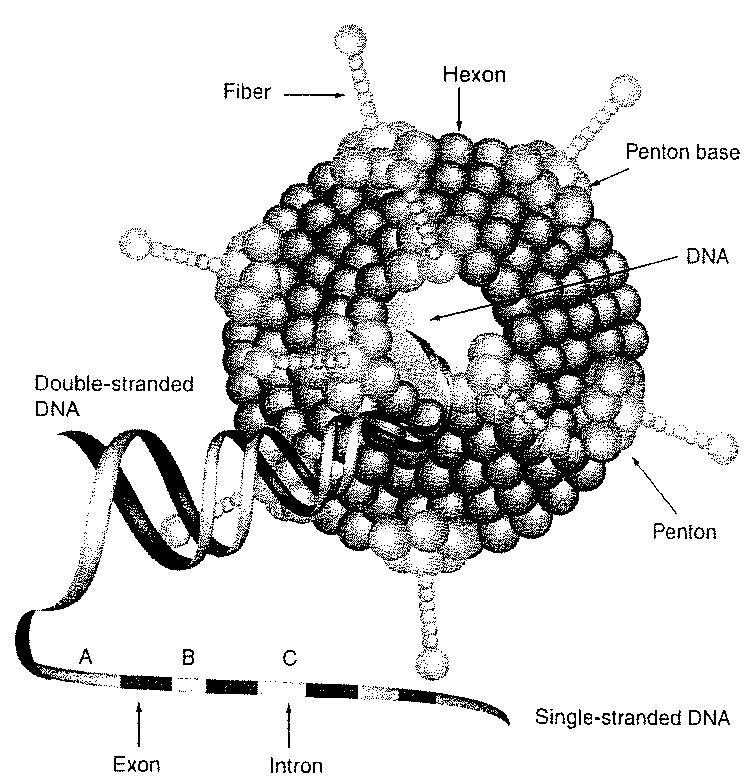
Structure of adenovirus depicting major structural components of a wild-type adenovirus capsid.
Adenovirus capsids contain an up to 36-kilobase double-stranded DNA genome. Selected tumor-specific promoters for gynecological cancer in serotype 5 (Ad5).
Infection with Ads results in a humoral and cellular immune response towards the vector and infected cells. This effect might be potentially beneficial, as it might activate the immune system for recognition of tumor antigens. However, administration of large doses of a viral vector might result in acute toxicity. In general, side effects of Ad gene therapy are rare and mild. However, effective targeting strategies are necessary to further increase target transduction and reduce the risk of unspecific side effects to nontarget tissues.
Genetic targeting approaches
To improve the utility of Ad-based gene transfer vectors in the context of clinically applicable cancer gene therapy, it is essential to increase targeting to tumor cells and/or decrease targeting to the liver. A number of strategies have been developed to alter the tropism of Ad to improve its utility as a gene transfer vector. Increased transduction of low CAR tumor cells can be achieved via genetic retargeting approaches. Ad fiber pseudotyping means alteration of the virus tropism by substituting the receptor-binding proteins (knob/fiber) with those from other serotypes. One such approach is the substitution of Ad5 with a knob from Ad serotype 3 (Ad3). Ad5/3 chimeras have displayed enhanced infectivity in ovarian cancer cells without increasing gene delivery to murine livers [14,19]. Furthermore, Ad5/3 biodistribution, liver toxicity and rate of blood clearance appear to be no different from Ad5 when administered intravenously or intraperitoneally, suggesting an excellent safety profile for Ad5/3 in preclinical tests.
Rigorous structural analysis of the fiber knob domain have led to alternative strategies based on the incorporation of targeting ligands into the Ad knob domain without ablating native CAR-binding. One option is the genetic incorporation of an RGD-4C peptide into the HI loop or the C-terminal end of the Ad5 fiber knob [11,20,21]. This allows Ad-mediated gene transduction through the binding of the RGD peptide to integrins on target cells. A different approach is tumor cell targeting to HSPGs via positively charged polylysine (pK7) motifs [22]. HSPGs are common constituents of cell surfaces and the extracellular matrix, and they are overexpressed in several different cancer types including cervical cancer [23-26]. Double modified vectors containing both RGD and pK7 motifs increase infectivity in different cell types [27,28].
However, systemic administration of tropism-modified adenovirus vectors results mostly in hepatic gene delivery [29,30]. Recent studies have shown that the Ad5 fiber shaft is responsible for liver gene delivery [3,31]. In this regard, strategies to genetically replace the Ad5 fiber shaft can reduce hepatic vector delivery in vivo. The length of the fiber plays an important role in viral infectivity and tropism [32-34]. It is well known that Ad5 internalization requires an additional interaction of RGD motifs on the penton base with αv integrins on the cell surface [35]. Ad5 vectors with short-shafted fibers do not bind efficiently to αv integrins. However, in vivo vector delivery to the liver seems to occur independently of CAR and αv integrins, whereas binding to heparan sulfate glycosaminoglycans (HSGs) seems to be the major determinant for hepatic gene transfer [2].
Conditionally replicative adenoviruses
The first generation of Ad vectors were able to infect cancer cells but were unable to replicate within the cell. Unfortunately, most cancer trials with replication defective Ads demonstrated that transduction rates of tumor cells were too low for significant therapeutic efficacy [36-38]. It is likely that strategies to enhance infectivity of nonreplicating vectors could improve results dramatically [5,39,40]. However, for advanced disease with increased tumor masses, tumor penetration is the key to efficacy. A new and powerful approach for increasing transduction of tumor cells is the use of replication-competent oncolytic agents, such as conditionally replicative Ads (CRAds).
Most approaches for the development of CRAds have focused on the genetic engineering of early 1 (E1) genes. The major tasks of E1 proteins are to induce expression of further viral genes, and to orchestrate modifications of cellular gene expression and protein activity that favor viral replication. Two strategies have been used to restrict virus replication to target cells and to spare normal tissue. Genetic complementation-type (type 1) CRAds [41,42], such as Ad524, have a mutation in the immediately early (E1A) or early (E1B) adenoviral region, which is complemented in tumor cells but not in normal cells. In transcomplementation-type (type 2) CRAds, virus replication is controlled via a tumor/tissue-specific promoter [43]. However, in both instances, ectopic liver transduction is the major predicate of Ad vector-induced toxicity.
The prototype of Type 1 CRAds is dl1520 or ONYX-015 [41]. ONYX-015 has two inactivating mutations in the gene that codes for the E1B55k protein. One function of E1B55k is to bind and inactivate the tumor-suppressor protein p53. Thus, the rationale for this strategy is that the virus can only replicate in cells in which p53 or p14ARF is mutated. However, it is apparent that this CRAd does not replicate in tumor cells to a larger degree than in normal cells. This is probably due to of the lack of E1B55k functions different from p53 activation. Another type-1 CRAd was derived by mutation of E1A. E1A mutant CRAds, such as Ad5Δ24 or CB016, contain specific mutations in individual domains of the protein [42,44,45]. This strategy aims to ablate S phase induction by the CRADs, which is not required in proliferating tumor cells or in dysplastic cervical cells transformed by human papillomavirus (HPV) viruses.
Type 2 CRAds are designed to contain a tissue-specific promoter (TSP), upstream of the E1A gene for specific expression in target cells. Critical for the development of Type 2 CRAds is the availability of promoter fragments with specific activity. To date, the structure–function relationship of promoter regions is not well understood and construction of TSPs is relatively empirical. However, data from preclinical studies are promising.
Tissue specific promoters
For cancer gene therapy, it is rational to choose a TSP that is expressed highly in the tumor but has potentially low activity in the liver. One of the most promising promoters for ovarian cancer is the promoter for secretory leukoprotease inhibitor (SLPI). SLPI is a 11.7-kDa serine protease inhibitor, which is highly expressed in different human carcinomas, including breast, lung, endometrium and ovarian cancer. Gene expression analysis has been used to demonstrate that SLPI is 60-fold upregulated in ovarian carcinomas compared with normal ovarian epithelium [46,47]. In addition, SLPI is expressed minimally in the normal liver [48].
Recently, a chimeric 5/3-fiber-modified SLPI CRAd was shown to have a strong antitumor effect compared with wild-type Ad. Furthermore, the chimeric SLPI CRAd demonstrated very high efficacy in an orthotopic murine model of ovarian cancer.
Another attractive candidate for targeted therapy of ovarian cancer is mesothelin (MSLN), a glycosylphosphatidylinositol-linked cell surface glycoprotein that is overexpressed in different tumor types, such as ovarian cancer, mesotheliomas and several different squamous cell cancers [49,50]. MSLN is not present on normal tissues, except mesothelial cells and is also not shed into the bloodstream in significant amounts. The high tumor specificity of a MSLN-targeted Ad has been demonstrated recently in an ovarian cancer mouse model [51]. Another promising promoter in the context of ovarian cancer gene therapy is cyclooxygenase (COX)-2. Recently, an infectivity-enhanced COX-2 CRAd, which has an RGD-4C motif in the Ad fiber knob region, was shown to have a strong antitumor effect compared with wild-type Ad5.
The promoter of vascular endothelial growth factor (VEGF) is an effective target molecule for the treatment of non-small cell lung cancer (NSCLC). VEGF is one of the most important inducers of angiogenesis and is upregulated in various cancer types, including carcinoma of the cervix uteri [52-54]. Midkine is a heparin-binding growth factor identified as a retinoic acid-responsive gene. Midkine has been reported to be over-expressed in a wide number of malignant tumors. The Midkine promoter has been used successfully for vector gene delivery [55,56] and CRAD targeting approaches [57]. The promoters of the CXC chemokine CXCR4, as well as Survivin, exhibit an optimal profile of activity and specificity in different tumor types, such as breast cancer. It was demonstrated recently that CXCR4 expression is upregulated in breast cancer cells but is not detectable in normal mammary epithelial cells [58]. Survivin is a member of the inhibitor of apoptosis (IAP) protein family and the expression levels of the Survivin gene have been shown to be of clinical value in the detection of human gliomas [59]. Both genes expressing CXCR4 and Survivin play a major role in progression and metastasis of various tumor types [60,61] and are therefore rational targets for cancer therapies. Survivin has been shown to be overexpressed in cervical intraepithelial neoplasias (CINs) and squamous cell carcinomas (SCC) of the uterine cervix [62]. Selected tumor-specific promoters are summarized in Table 1.
Table 1.
Selected tumor-specific promoters for gynecological cancer in Ad5-based gene therapy vectors.
| Author | Tumor-specific promoter | Disease application | Transgene |
|---|---|---|---|
| Kanerva et al. (2004) | COX-2 | Ovarian cancer | CRAD |
| Casado et al. (2001) | HSVtk | ||
| Breidenbach et al. (2004) | Mesothelin | Ovarian cancer | luciferase |
| Rein et al. (2005) | SLPI | Ovarian cancer | CRAD |
| Barker et al. (2003) | HSVtk | ||
| Luciferase | |||
| Zhu et al. (2004) | CXCR4 | Breast cancer | Luciferase |
| Zhu et al. (2004) | Survivin | Cervical cancer | Luciferase |
Ad: Adenovirus; Ad5: Serotype 5; COX: Cyclooxygenase; CRAd: Conditionally replicative Ads; CXCR: Chemokine (CXC) motif receptor; HSVtk: Herpes simplex virus thymidine kinase; SLPI: Secretory leukoprotease inhibitor.
Conclusion
Ad-based gene therapy vectors are the most widely used platform for gene delivery. Their potential utility for cancer treatment is high, although results of clinical trials with nonreplicative first generation Ads were disappointing. Replicating Ad vectors offer high promise as agents for cancer treatment and have been used safely in human clinical trials (Table 2) [63,64]. In addition, molecular modifications, such as genetic targeting approaches or chimeric-fibers, have substantially improved the efficiency of these second-generation virotherapeutics in preclinical trials. However, the outcome of clinical trials using tropism-modified CRAds will be of special interest. Until today, preclinical efficacy and safety data are preliminary owing to the limitations of currently available animal models. To address these shortcomings, we have developed a novel in vitro toxicity assay based on precision cut human liver slices. Preliminary data demonstrate the effect of transcriptional targeting to restrict virus replication to target cells.
Table 2.
Outcome of clinical Phase 1/2 trials with replication-competent Ads.
| Replication-competent Ads | Disease | Outcome | Refs |
|---|---|---|---|
| ONYX-015 | Head and neck cancer (n = 24) | Rapid tumor progression in most patients | [63] |
| ONYX-015 | Head and neck cancer (n = 37) | Combination of gene therapy and chemotherapy with cisplatin and 5-FU, reasonable response rates | [64] |
| ONYX-015 | Pancreatic cancer (n = 23) | No responses after intratumoral virus application | [65] |
| ONYX-015 | Metastatic liver cancer (n = 27) | Moderate responses with a combination of gene therapy and chemotherapy with 5-FU and leucovorin | [66] |
| ONYX-015 | Metastatic colon cancer (n = 18) | Stable disease in 7 patients, moderate side effects | [67] |
| ONYX-015 | Pancreatic cancer (n = 21) | Stable disease in 6 patients, combination of gene therapy and gemcitabine | [68] |
| CG7060 | Prostate cancer (n = 20) | Intratumoral treatment, decrease of PSA levels | [69] |
| Ad5-CD/TKrep | Prostate cancer (n = 15) | Combination of radiotherapy and double-suicide gene therapy | [70] |
Ad: Adenovirus; Ad5: Serotype 5; CD: Cytosine deaminase; CG7060: A PSA-specific, replication-competent adenovirus; FU: Fluorouracil; ONYX-015: An oncolytic adenovirus; PSA: Prostate-specific antigen; Ad5-CD/TKrep: Replication competent Ad.
Executive summary.
Adenovirus-based gene therapy is a promising approach for cancer therapy
Viral gene delivery has been optimized by evolution.
Advantages of adenovirus (Ad) vectors include the ability to transduce dividing and nondividing cells, the ease of manipulation and the capability of producing high titers.
A disadvantage of Ad vectors is natural liver tropism, which can lead to severe side effects after systemic vector delivery.
The low expression of the primary Ad receptor coxsackie-Ad receptor on tumor cells is the rate limiting factor for cancer genetherapy
The coxsackie–Ad receptor (CAR) is the primary receptor of most Ad serotypes, including the most commonly used serotype 5 receptor.
Unfortunately, CAR expression is highly variable and often low on the surface of most tumor cells.
Effective tumor targeting can be achieved via genetic retargeting strategies
Various strategies have been evaluated to modify Ad tropism in order to circumvent CAR deficiency.
Ad fiber pseudotyping is an approach to substitute receptor-binding proteins to alter the virus tropism.
Incorporation of targeting ligands into the Ad knob domain can increase tumor transduction without ablating native CAR-binding.
Specific tumor targeting can be achieved via tumor-specific targeting strategies
Targeting of viral vectors to cellular target structures that are highly and exclusively expressed on tumor cells.
Tissue-specific promoters (TSPs) upstream of the viral E1A gene allow specific transduction of target cells.
It is rational to choose a TSP that is highly expressed in the tumor but has low activity in healthy organs, such as the liver.
Virus replication is needed to face advanced solid tumor masses
Conditionally replicating Ads (CRAds) take advantage of tumor-specific features that allow preferential replication in tumor cells.
Virus replication causes oncolysis of the cell, resulting in the release of the virus progeny and subsequent infection of neighboring cells.
Replication-competent viruses can be armed with therapeutic transgenes, such as suicide genes or antiangiogenic moieties.
Radio–chemo–gene therapy
The lack of overlapping toxicities allows the development of multimodality treatment approaches, which offer great promise in the near future.
Future perspective
Gene therapy strategies have the potential to increase the survival of cancer patients if gene therapy is included in a multimodality treatment approach. The major challenge is the development of effective gene therapy vectors since the vectors available currently mostly result in insufficient gene transfer. Targeting of viral vectors to cellular target structures that are highly and exclusively expressed on tumor cells is a promising approach for the next 5–10 years. Such modified vectors would be able to effectively penetrate solid tumor masses. The current generation of replication-competent Ads demonstrates superior tumor penetration and oncolysis when compared with first generation viruses. In the near future, replication competent viruses will be armed with therapeutic transgenes, such as suicide genes, or cytokines. Since solid tumors can only be destroyed step by step, successful gene therapy approaches will require several rounds of virus administration, whose efficacy may be inhibited by neutralizing antibodies. Strategies to reduce the effect of neutralizing antibodies, which might limit the efficacy of a virotherapy, include alternating related viruses with different capsids or cotreatment with immunosuppressive drugs for temporary abrogation of neutralizing antibodies.
Recent studies have demonstrated the synergistic effect of combination therapies consisting of radio- and/or chemo-therapy and virotherapy. The lack of overlapping toxicities allows the development of multimodality treatment approaches, which offer great promise in the near future. The rapidly growing number of molecular targets will allow us to design individualized Ad vectors according to individual tumor characteristics.
Acknowledgements
The authors wish to acknowledge the following grants that supported this work: NIH 5RO1CA083821-06, NIH 1RO1CA111569-01A1 and DOD W81XWH-05-1-0035.
Bibliography
- 1.Wickham TJ. Targeting adenovirus. Gene Ther. 2000;7:110–114. doi: 10.1038/sj.gt.3301115. [DOI] [PubMed] [Google Scholar]
- 2.Smith TA, Idamakanti N, Marshall-Neff J, et al. Receptor interactions involved in adenoviral-mediated gene delivery after systemic administration in non-human primates. Hum. Gene Ther. 2003;14:1595–1604. doi: 10.1089/104303403322542248. [DOI] [PubMed] [Google Scholar]
- 3.Smith TA, Idamakanti N, Rollence ML, et al. Adenovirus serotype 5 fiber shaft influences in vivo gene transfer in mice. Hum. Gene Ther. 2003;14:777–787. doi: 10.1089/104303403765255165. [DOI] [PubMed] [Google Scholar]
- 4.Breidenbach M, Rein DT, Wang M, et al. Genetic replacement of the adenovirus shaft fiber reduces liver tropism in ovarian cancer gene therapy. Hum. Gene Ther. 2004;15:509–518. doi: 10.1089/10430340460745829. [DOI] [PubMed] [Google Scholar]
- 5.Rein DT, Breidenbach M, Wu H, et al. Gene transfer to cervical cancer with fiber-modified adenoviruses. Int. J. Cancer. 2004;111:698–704. doi: 10.1002/ijc.20295. [DOI] [PubMed] [Google Scholar]
- 6.Cripe TP, Dunphy EJ, Holub AD, et al. Fiber knob modifications overcome low, heterogeneous expression of the coxsackievirus–adenovirus receptor that limits adenovirus gene transfer and oncolysis for human rhabdomyosarcoma cells. Cancer Res. 2001;61:2953–2960. [PubMed] [Google Scholar]
- 7.Fechner H, Wang X, Wang H, et al. Transcomplementation of vector replication versus coxsackie–adenovirus-receptor overexpression to improve transgene expression in poorly permissive cancer cells. Gene Ther. 2000;7:1954–1968. doi: 10.1038/sj.gt.3301321. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Pong RC, Bergelson JM, et al. Loss of adenoviral receptor expression in human bladder cancer cells: a potential impact on the efficacy of gene therapy. Cancer Res. 1999;59:325–330. [PubMed] [Google Scholar]
- 9.Miller CR, Buchsbaum DJ, Reynolds PN, et al. Differential susceptibility of primary and established human glioma cells to adenovirus infection: targeting via the epidermal growth factor receptor achieves fiber receptor-independent gene transfer. Cancer Res. 1998;58:5738–5748. [PubMed] [Google Scholar]
- 10.Bauerschmitz GJ, Barker SD, Hemminki A. Adenoviral gene therapy for cancer: from vectors to targeted and replication competent agents (review) Int. J Oncol. 2002;21:1161–1174. [PubMed] [Google Scholar]
- 11.Dmitriev I, Krasnykh V, Miller CR, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J. Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemmi S, Geertsen R, Mezzacasa A, Peter I, Dummer R. The presence of human coxsackievirus and adenovirus receptor is associated with efficient adenovirus-mediated transgene expression in human melanoma cell cultures. Hum. Gene Ther. 1998;9:2363–2373. doi: 10.1089/hum.1998.9.16-2363. [DOI] [PubMed] [Google Scholar]
- 13.Hemminki A, Alvarez RD. Adenoviruses in oncology: a viable option? BioDrugs. 2002;16:77–87. doi: 10.2165/00063030-200216020-00001. [DOI] [PubMed] [Google Scholar]
- 14.Kanerva A, Mikheeva GV, Krasnykh V, et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin. Cancer Res. 2002;8:275–280. [PubMed] [Google Scholar]
- 15.Okegawa T, Pong RC, Li Y, Bergelson JM, Sagalowsky AI, Hsieh JT. The mechanism of the growth-inhibitory effect of coxsackie and adenovirus receptor (CAR) on human bladder cancer: a functional analysis of CAR protein structure. Cancer Res. 2001;61:6592–6600. [PubMed] [Google Scholar]
- 16.Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl Acad. Sci. USA. 2001;98:15191–15196. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemminki A. From molecular changes to customised therapy. Eur. J. Cancer. 2002;38:333–338. doi: 10.1016/s0959-8049(01)00368-9. [DOI] [PubMed] [Google Scholar]
- 18.Kanerva A, Hemminki A. Modified adenoviruses for cancer gene therapy. Int. J. Cancer. 2004;110:475–480. doi: 10.1002/ijc.20129. [DOI] [PubMed] [Google Scholar]
- 19.Kanerva A, Wang M, Bauerschmitz GJ, et al. Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol. Ther. 2002;5:695–704. doi: 10.1006/mthe.2002.0599. [DOI] [PubMed] [Google Scholar]
- 20.Krasnykh V, Dmitriev I, Mikheeva G, Miller CR, Belousova N, Curiel DT. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J. Virol. 1998;72:1844–1852. doi: 10.1128/jvi.72.3.1844-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michael SI, Hong JS, Curiel DT, Engler JA. Addition of a short peptide ligand to the adenovirus fiber protein. Gene Ther. 1995;2:660–668. [PubMed] [Google Scholar]
- 22.Wickham TJ, Roelvink PW, Brough DE, Kovesdi I. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nature Biotechnol. 1996;14:1570–1573. doi: 10.1038/nbt1196-1570. [DOI] [PubMed] [Google Scholar]
- 23.Blackhall FH, Merry CL, Davies EJ, Jayson GC. Heparan sulfate proteoglycans and cancer. Br. J. Cancer. 2001;85:1094–1098. doi: 10.1054/bjoc.2001.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shriver Z, Liu D, Sasisekharan R. Emerging views of heparan sulfate glycosaminoglycan structure/activity relationships modulating dynamic biological functions. Trends Cardiovasc. Med. 2002;12:71–77. doi: 10.1016/s1050-1738(01)00150-5. [DOI] [PubMed] [Google Scholar]
- 25.Sanderson RD. Heparan sulfate proteoglycans in invasion and metastasis. Semin. Cell Dev. Biol. 2001;12:89–98. doi: 10.1006/scdb.2000.0241. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi Y. Heparan sulfate proteoglycans in the nervous system: their diverse roles in neurogenesis, axon guidance, and synaptogenesis. Semin. Cell Dev. Biol. 2001;12:99–106. doi: 10.1006/scdb.2000.0238. [DOI] [PubMed] [Google Scholar]
- 27.Wu H, Seki T, Dmitriev I, et al. Double modification of adenovirus fiber with RGD and polylysine motifs improves coxsackievirus–adenovirus receptor-independent gene transfer efficiency. Hum. Gene Ther. 2002;13:1647–1653. doi: 10.1089/10430340260201734. [DOI] [PubMed] [Google Scholar]
- 28.Contreras JL, Wu H, Smyth CA, et al. Double genetic modification of adenovirus fiber with RGD polylysine motifs significantly enhances gene transfer to isolated human pancreatic islets. Transplantation. 2003;76:252–261. doi: 10.1097/01.TP.0000066361.02042.CA. [DOI] [PubMed] [Google Scholar]
- 29.Alemany R, Curiel DT. CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Ther. 2001;8:1347–1353. doi: 10.1038/sj.gt.3301515. [DOI] [PubMed] [Google Scholar]
- 30.Einfeld DA, Schroeder R, Roelvink PW, et al. Reducing the native tropism of adenovirus vectors requires removal of both CAR and integrin interactions. J. Virol. 2001;75:11284–11291. doi: 10.1128/JVI.75.23.11284-11291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koizumi N, Mizuguchi H, Sakurai F, Yamaguchi T, Watanabe Y, Hayakawa T. Reduction of natural adenovirus tropism to mouse liver by fiber-shaft exchange in combination with both CAR- and αv integrin-binding ablation. J. Virol. 2003;77:13062–13072. doi: 10.1128/JVI.77.24.13062-13072.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shayakhmetov DM, Lieber A. Dependence of adenovirus infectivity on length of the fiber shaft domain. J. Virol. 2000;74:10274–10286. doi: 10.1128/jvi.74.22.10274-10286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura T, Sato K, Hamada H. Reduction of natural adenovirus tropism to the liver by both ablation of fiber-coxsackievirus and adenovirus receptor interaction and use of replaceable short fiber. J. Virol. 2003;77:2512–2521. doi: 10.1128/JVI.77.4.2512-2521.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vigne E, Dedieu JF, Brie A, et al. Genetic manipulations of adenovirus Type 5 fiber resulting in liver tropism attenuation. Gene Ther. 2003;10:153–162. doi: 10.1038/sj.gt.3301845. [DOI] [PubMed] [Google Scholar]
- 35.Nemerow GR, Stewart PL. Role of α(v) integrins in adenovirus cell entry and gene delivery. Microbiol. Mol. Biol. Rev. 1999;63:725–734. doi: 10.1128/mmbr.63.3.725-734.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swisher SG, Roth JA, Nemunaitis J, et al. Adenovirus-mediated p53 gene transfer in advanced non-small-cell lung cancer. J. Natl Cancer Inst. 1999;91:763–771. doi: 10.1093/jnci/91.9.763. [DOI] [PubMed] [Google Scholar]
- 37.Koeneman KS, Kao C, Ko SC, et al. Osteocalcin-directed gene therapy for prostate-cancer bone metastasis. World J. Urol. 2000;18:102–110. doi: 10.1007/s003450050181. [DOI] [PubMed] [Google Scholar]
- 38.Schuler M, Rochlitz C, Horowitz JA, et al. A Phase I study of adenovirus-mediated wild type p53 gene transfer in patients with advanced non-small cell lung cancer. Hum. Gene Ther. 1998;9:2075–2082. doi: 10.1089/hum.1998.9.14-2075. [DOI] [PubMed] [Google Scholar]
- 39.Hemminki A, Zinn KR, Liu B, et al. In vivo molecular chemotherapy and noninvasive imaging with an infectivity-enhanced adenovirus. J. Natl Cancer Inst. 2002;94:741–749. doi: 10.1093/jnci/94.10.741. [DOI] [PubMed] [Google Scholar]
- 40.Hemminki A, Belousova N, Zinn KR, et al. An adenovirus with enhanced infectivity mediates molecular chemotherapy of ovarian cancer cells and allows imaging of gene expression. Mol. Ther. 2001;4:223–231. doi: 10.1006/mthe.2001.0446. [DOI] [PubMed] [Google Scholar]
- 41.Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 42.Fueyo J, Gomez-Manzano C, Alemany R, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces antiglioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto M, Davydova J, Wang M, et al. Infectivity enhanced, cyclooxygenase-2 promoter-based conditionally replicative adenovirus for pancreatic cancer. Gastroenterology. 2003;125:1203–1218. doi: 10.1016/s0016-5085(03)01196-x. [DOI] [PubMed] [Google Scholar]
- 44.Balague C, Noya F, Alemany R, Chow LT, Curiel DT. Human papillomavirus E6E7-mediated adenovirus cell killing: selectivity of mutant adenovirus replication in organotypic cultures of human keratinocytes. J. Virol. 2001;75:7602–7611. doi: 10.1128/JVI.75.16.7602-7611.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heise C, Hermiston T, Johnson L, et al. An adenovirus E1A mutant that demonstrates potent and selective systemic antitumoral efficacy. Nature Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 46.Hough CD, Sherman-Baust CA, Pizer ES, et al. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 2000;60:6281–6287. [PubMed] [Google Scholar]
- 47.Hough CD, Cho KR, Zonderman AB, Schwartz DR, Morin PJ. Co-ordinately upregulated genes in ovarian cancer. Cancer Res. 2001;61:3869–3876. [PubMed] [Google Scholar]
- 48.Abe T, Tominaga Y, Kikuchi T, et al. Bacterial pneumonia causes augmented expression of the secretory leukoprotease inhibitor gene in the murine lung. Am. J. Respir. Crit. Care Med. 1997;156:1235–1240. doi: 10.1164/ajrccm.156.4.9701075. [DOI] [PubMed] [Google Scholar]
- 49.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc. Natl Acad. Sci. USA. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang K, Pastan I. Molecular cloning and expression of a cDNA encoding a protein detected by the K1 antibody from an ovarian carcinoma (OVCAR-3) cell line. Int. J. Cancer. 1994;57:90–97. doi: 10.1002/ijc.2910570117. [DOI] [PubMed] [Google Scholar]
- 51.Breidenbach M, Rein DT, Everts M, et al. Mesothelin-mediated targeting of adenoviral vectors for ovarian cancer gene therapy. Gene Ther. 2005;12:187–193. doi: 10.1038/sj.gt.3302404. [DOI] [PubMed] [Google Scholar]
- 52.Takayama K, Reynolds PN, Adachi Y, et al. VEGF promoter-based conditionally replicative adenovirus are useful for the treatment of lung cancer. Cancer Res. 2003 In press. [PubMed] [Google Scholar]
- 53.Lopez-Ocejo O, Viloria-Petit A, Bequet-Romero M, Mukhopadhyay D, Rak J, Kerbel RS. Oncogenes and tumor angiogenesis: the HPV-16 E6 oncoprotein activates the vascular endothelial growth factor (VEGF) gene promoter in a p53 independent manner. Oncogene. 2000;19:4611–4620. doi: 10.1038/sj.onc.1203817. [DOI] [PubMed] [Google Scholar]
- 54.Smith-McCune K, Zhu YH, Hanahan D, Arbeit J. Cross-species comparison of angiogenesis during the premalignant stages of squamous carcinogenesis in the human cervix and K14-HPV16 transgenic mice. Cancer Res. 1997;57:1294–1300. [PubMed] [Google Scholar]
- 55.Adachi Y, Reynolds PN, Yamamoto M, et al. A midkine promoter-based conditionally replicative adenovirus for treatment of pediatric solid tumors and bone marrow tumor purging. Cancer Res. 2001;61:7882–7888. [PubMed] [Google Scholar]
- 56.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 57.Chakravarti A, Noll E, Black PM, et al. Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J. Clin. Oncol. 2002;20:1063–1068. doi: 10.1200/JCO.2002.20.4.1063. [DOI] [PubMed] [Google Scholar]
- 58.Moore MA. The role of chemoattraction in cancer metastases. Bioessays. 2001;23:674–676. doi: 10.1002/bies.1095. [DOI] [PubMed] [Google Scholar]
- 59.Murakami T, Maki W, Cardones AR, et al. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 2002;62:7328–7334. [PubMed] [Google Scholar]
- 60.Kim HS, Shiraki K, Park SH. Expression of survivin in CIN and invasive squamous cell carcinoma of uterine cervix. AntiCancer Res. 2002;22:805–808. [PubMed] [Google Scholar]
- 61.Yoshida H, Sumi T, Hyun Y, et al. Expression of survivin and matrix metalloproteinases in adenocarcinoma and squamous cell carcinoma of the uterine cervix. Oncol. Rep. 2003;10:45–49. [PubMed] [Google Scholar]
- 62.Frost M, Jarboe EA, Orlicky D, et al. Immunohistochemical localization of survivin in benign cervical mucosa, cervical dysplasia, and invasive squamous cell carcinoma. Am. J. Clin. Pathol. 2002;117:738–744. doi: 10.1309/6V09-38K3-JQ40-UR50. [DOI] [PubMed] [Google Scholar]
- 63.Nemunaitis J, Ganly I, Khuri F, et al. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a Phase II trial. Cancer Res. 2000;60:6359–6366. [PubMed] [Google Scholar]
- 64.Khuri FR, Nemunaitis J, Ganly I, et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nature Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 65.Mulvihill S, Warren R, Venhook A, et al. Safety and feasibility of injection with and E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: a Phase I trial. Gene Ther. 2001;8(4):308–315. doi: 10.1038/sj.gt.3301398. [DOI] [PubMed] [Google Scholar]
- 66.Reid T, Galanis E, Abbruzzese J, et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): Phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002;62(21):6070–6079. [PubMed] [Google Scholar]
- 67.Hamid O, Varterasian ML, Wadler S, et al. Phase II trial of intravenous C1-1-42 in patietns with metastatic colorectal cancer. J. Clin. Oncol. 2003;21(8):1498–1504. doi: 10.1200/JCO.2003.09.114. [DOI] [PubMed] [Google Scholar]
- 68.Hecht JR, Bedford R, Abbruzzese JL, et al. DA Pahse I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin. Cancer Res. 2003;9(2):555–561. [PubMed] [Google Scholar]
- 69.Li S, Simons J, Detorie N, O'Rourke B, Hamper U, DeWeese TL. Dosimetric and technical considerations for interstitial adenoviral gene therapy as applied to prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 55(1):204–214. doi: 10.1016/s0360-3016(02)03862-2. Comment in 3–4; Int. J. Radiat. Oncol. Biol. Phys. 57(2), 596–597, author reply 599–600 (2003) [DOI] [PubMed] [Google Scholar]
- 70.Freytag SO, Stricker H, Pegg J, et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiaion therapy for the treatment of newly diagnosed, intermediate-to-high-risk prostate cancer. Cancer Res. 2003;63(21):7497–7506. [PubMed] [Google Scholar]


