Short abstract
The antigenic specificity of an unusual antinuclear antibody pattern in three patient sera was identified after separating HeLa-cell nuclear extracts by two-dimensional (2D) gel electrophoresis and localizing the antigens by immunoblotting with patient serum. Protein spots were excised from the 2D gel and their contents were analyzed by matrix-assisted laser desorption-ionization (MALDI) or nanoelectrospray ionization time-of-flight (TOF) tandem mass spectrometry (MS) after in-gel digestion with trypsin. A database search identified the proteins as the C1 and C2 heterogeneous nuclear ribonucleoproteins. The clinical spectrum of patients with these autoantibodies includes arthritis, psoriasis, myositis, and scleroderma. None of 59 patients with rheumatoid arthritis, 19 with polymyositis, 33 with scleroderma, and 10 with psoriatic arthritis had similar antibodies. High-resolution protein-separation methods and mass-spectrometric peptide mapping in combination with database searches are powerful tools in the identification of novel autoantigen specificities.
Keywords: antinuclear antibodies, autoantibodies, heterogeneous nuclear ribonucleoproteins C1/C2, mass spectrometry
Abstract
Introduction:
The classification of antinuclear antibodies (ANAs) is important for diagnosis and prognosis and for understanding the molecular pathology of autoimmune disease. Many of the proteins that associate with RNA in the ribonucleoprotein (RNP) complexes of the spliceosome have been found to react with some types of ANA [1], including proteins of the heterogeneous nuclear RNP (hnRNP) complex that associate with newly transcribed pre-mRNA. Autoantibodies to the A2, B1, and B2 proteins of hnRNP found in some patients may be markers of several overlap syndromes [2]. However, ANAs with specificity for these proteins as well as for the D protein also appear to occur in many distinct connective-tissue diseases, although epitope specificities may differ [3]. ANAs with specificity for the C component of hnRNP (consisting of the C1 and C2 proteins) have to our knowledge so far been described in only one case [4]. We here describe the approach taken to unambiguously identify the C1/C2 proteins as ANA targets in the sera of some patients.
Aims:
To determine the fine specificity of sera containing an unusual speckled ANA-staining pattern using a combination of 2D gel electrophoresis and MS.
Methods:
Patient sera were screened for ANAs by indirect immunofluorescence microscopy on HEp-2 cells (cultured carcinoma cells). Sera with an unusual, very regular, speckled ANA pattern were tested for reactivity with components of nuclear extracts of HeLa cells that were separated by one-dimensional (1D) or 2D gel electrophoresis or by reversed-phase high-performance liquid chromatography (HPLC). IgG reactivity was assessed by immunoblotting. Reactive protein spots from 2D separations were excised from the gels and subjected to in-gel digestion with trypsin for subsequent peptide mapping, partial peptide sequencing, and protein identification by MS and tandem MS on a hybrid electrospray ionization/quadrupole/time-of-flight (ESI-Q-TOF) mass spectrometer [5,6,7].
Results:
We observed a strong nuclear staining pattern (titer >1280) with the characteristic even-sized coarse speckles and no staining of nucleoli in sera from three patients. On immunoblots of nuclear extracts from HeLa cells, these sera stained two distinct bands, at Mr 42 000 and 41 000. There activity strongly resembled that of the patient originally described by Stanek et al [4]. The antigens were enriched by fractionating the extract using reversed-phase HPLC on a C4 column, and the two reactive spots on 2D separations were excised for identification. The two components appeared to be of approximately the same isoelectric points, although their molecular masses differed by approximately 2000. Peptide-mass mapping was performed by matrix-assisted laser desorption-ionization time-of-flight (MALDI-TOF) MS on the tryptic peptide mixture generated by digestion of the two excised proteins. The database search suggested that the two proteins were C1/C2 hnRNPs (Swissprot accession number P07910). The identity of the proteins was further confirmed by tandem MS using an ESI-Q-TOF instrument. One peptide carrying two positive charges (m/z 580.32 Da), corresponding to a peptide mass of 1158.7 Da, was selected as a precursor ion and partially sequenced by collisional fragmentation. The fragmented peptide was found to represent the tryptic fragment VDSLLENLEK, ie amino acids 207-216 (C2 protein numbering). Four other peptides were partially sequenced and all of them matched the human C1/C2 hnRNP sequence. The theoretical masses of C1 and C2 are 32.0 and 33.3 kDa, respectively. The difference between the two sequences is a 13-amino-acid insert in C2 between positions 107 and 108 of C1. The presence of a specific tryptic fragment in the MALDI-TOF peptide-mass map from the higher-molecular-mass spot containing a 13-amino-acid insert that was not present in the lower-molecular-mass spot, further demonstrated that the two components represented the two isoforms of the C class of hnRNPs.
The patient whose case prompted us to investigate the specificities of these antibodies was a 72-year-old man who had arthralgias and oligoarthritis but did not fulfill the criteria for rheumatoid arthritis and did not have dermatological complaints. The reactivity of various patient groups to the C1/C2 hnRNP autoantigens was subsequently tested by immunoblotting of HeLa-cell nuclear extracts. Of 59 patients with rheumatoid arthritis, 19 with polymyositis, 33 with scleroderma, and 10 with psoriatic arthritis, none had IgG antibodies reacting with the two bands. Of sera from 139 consecutive patients who had moderately to strongly positive speckled ANA patterns shown by indirect immunofluorescence on HEp-2 cells, only two reacted with the C1/C2 hnRNP bands in immunoblotting. One of these was from a young woman (22 years old) whose complaints of muscle tenderness were not explained by objective findings or abnormal laboratory test results. The third patient that we identified through ANA screening followed by immunoblotting was a 54-year-old male who was being treated with methotrexate for long-standing polymyositis in addition to psoriasis and possible osteoporosis.
Discussion:
The results confirm the existence of anti-C1/C2 antibodies in some patients with speckled ANAs. The antigens were identified through the use of biochemical methods using high-resolution separation techniques combined with mass-spectrometry peptide mapping and database searches. As a general approach, this is a powerful way to identify new antigens using small amounts of material without the need for conventional protein sequencing. The approach does require, however, that the proteins can be found in databases, that they are not extensively post-translationally modified, that they can be digested enzymatically, and that they can be isolated in appropriately pure form by the separation technique used.
It is not known at present if the C1/C2 antibodies may have pathogenic relevance and/or relate to specific diagnoses or subsets within the group of connective-tissue diseases. It does appear that the reactivity is quite rare among ANA-positive patients, and therefore many patients will have to be examined to determine these issues. The fact that the antibodies to the C1/C2 hnRNPs are revealed by indirect immunofluorescence would indicate that the epitopes are accessible in intact, fixed HEp-2 cells and thus probably reside outside the nucleic-acid-binding domains that would be expected to be covered by RNA.
Introduction
The determination of the molecular and submolecular specificity of ANAs is important for diagnosis and prognosis in clinical medicine and for understanding the molecular pathology of autoimmune disease. Different antigenic specificities of ANA are associated with different diseases and different patterns of disease manifestations. The various protein antigens associated with ANAs characteristically represent molecules that bind nucleic acids. Thus, many of the proteins that associate with RNA in the RNP complexes of the spliceosome have been found to react with some types of ANA [1]. Also, the hnRNP complex that associates with newly transcribed pre-mRNA contains proteins that have been shown to be targets of ANAs in some rheumatic diseases. The protein components of hnRNP are the most abundant nonhistone proteins of the cell nucleus and are classified roughly on the basis of their molecular masses into at least 21 protein groups (A through U). Autoantibodies to the A2, B1, and B2 proteins of hnRNP found in some patients may be markers of several overlap syndromes [2]. However, ANAs with specificity for these proteins as well as for the D protein and the I protein also appear to occur in many distinct connective-tissue diseases, although epitope specificities may differ [3,8]. Despite the discovery of the A, B, and R hnRNP ANA specificities in patients [2,9,10], antibodies against the C component of hnRNP (consisting of the C1 and C2 proteins) have to our knowledge so far been described in only one case [4]. The identification in this study was based on the colocalization of patient immunoreactivity on immunoblots of nuclear extracts with the band developed with a monoclonal anti-C1/C2 antibody. In the present study, we identify the C1/C2 specificity of circulating ANAs in three patients by high-resolution separation techniques (2D gel electrophoresis) combined with the assignment of protein identities by mass-spectrometric (MS and tandem MS) peptide mapping. This protein was also found to be the target of the ANA found in the patient described earlier [4].
Materials and methods
Reagents
Trifluoroacetic acid was from Fluka (Buchs, Switzerland), HPLC-grade water and acetonitrile from Merck (Darmstadt, Germany), sequencing-grade trypsin from Promega (Madison, WI, USA). The bicinchonic acid assay for protein was from Pierce (Rockford, IL, USA). All other chemicals were of analytical grade from Sigma Chemical Co (St Louis, MO, USA).
Cells and antibodies
HeLa-cell nuclei were obtained from 4C (Mons, Belgium). Cultured carcinoma (HEp-2)-cell slides were from Immunoconcepts, Inc (Sacramento, CA, USA). Fluorescein- and alkaline-phosphatase-labeled rabbit anti-human IgG antibodies (F0202 and D0336, respectively) were from Dako (Glostrup, Denmark). A patient serum sample previously described as containing antibodies reacting with C1/C2 hnRNP [4] was generously provided by Dr J Vencovský, Prague.
Indirect immunofluorescence
Patient sera were screened for ANAs by indirect immunofluorescence microscopy on slides of acetone-fixed HEp-2 cells using a cutoff dilution of 1:160 for the patient sera.
Preparation of HeLa-cell nuclear extracts
To establish the best conditions for extracting the antigens, the nuclear material was subjected to five different procedures: high-salt extraction using either 0.5 M NaCl or 6 M guanidinium hydrochloride in 125 mM Tris/HCl, pH 6.8 (sonicated for 3×5 s at 20 000 Hz on ice); detergent extraction in 125 mM Tris/HCl, pH 6.8, with 5% β-octyl glucoside or with 2.5% Triton X-100, using sonication as above; or extraction with 2.5% SDS for 10 min on ice after mixing. All samples were subsequently centrifuged at 16 000× g for 5 min and the supernatants were then analyzed by gel electrophoresis and immunoblotting (high-salt samples after being diluted 10 times in water). Extracts were either stored at -80°C or processed immediately for 1D or 2D gel electrophoresis or for reversed-phase HPLC.
Reversed-phase HPLC
HeLa-cell nuclear extracts were separated by reversed-phase HPLC on an analytical C4 column (4.6×250 mm, 300 Å, 5 μm particle size) from Vydac (Hesperia, CA, USA) using an Äkta chromatography system (Pharmacia, Lund, Sweden). The column was eluted with a 50-min gradient of 0-70% acetonitrile in 0.1% aqueous trifluoroacetic acid at 1 ml/min with monitoring at 210 and 280 nm. Fractions of 1 ml were collected. Prior to testing for reactivity with patient sera, the collected fractions were dried down in a Savant SpeedVac (Farmingdale, NY, USA) and resolubilized in 100 μl water. Dots of 2 μl of these fractions were subsequently placed on nitrocellulose, dried, and developed with patient sera as detailed below.
One- and 2D gel electrophoresis and immunoblotting
SDS-PAGE was performed in 10% precast gels from Novex (San Diego, CA, USA). Samples were solubilized at a protein concentration of 1-2 mg/ml by boiling 20-μl aliquots for 3 min in the presence of 2.5% (w/v) SDS and 25 mM dithiothreitol in 10 mM Tris/HCl, pH8, 10% (v/v) glycerol. The sample buffer also contained 50 μg/ml Pyronin G as a marker. The electrophoresis running buffer was 25 mM Tris, 192 mM glycine, 0.1% SDS, pH8.3 (Novex), and separation took place at 120 V for 1-2 h until the marker reached 1 cm from the bottom edge of the gel.
Two-dimensional electrophoresis was used to analyze crude HeLa-cell nuclear extracts and HPLC-purified sub-fractions of the extracts. Prior to isoelectric focusing, samples were solubilized at a protein concentration of approximately 1 mg/ml in 1.5% (v/v) Ampholine 3.5-9.5 (Pharmacia), 0.75% (w/v) SDS, 12 mM dithiothreitol, 2 M urea, 1% CHAPS (3- [{3-cholamidopropyl}-dimethylammonio]-1-propane sulfonate), and an aliquot of bromphenol blue. Isoelectric focusing of 17-ml samples took place in 1-mm×130-mm tube gels (4% acrylamide [C=2.7%] containing 9 M urea, 1% [w/v] CHAPS, and 0.5% [v/v] Nonidet P40). The first-dimension isoelectric focusing was performed at 400 V for 17-18 h and then at 800 V for 1 h. After the isoelectric focusing, the tube gels were extruded and placed on top of a large (13×13 cm) 11% polyacrylamide gel (C=2.7%) prepared in a Protean Cell (BioRad, Hercules, CA, USA). After equilibration for about 1 min in the presence of 500 μl transfer buffer (6.25 mM Tris/HCl, pH6.8, 10% [v/v] glycerol, 2% [w/v] SDS, 200 mM dithiothreitol, and an aliquot of bromophenol blue), the second-dimension SDS-PAGE took place at a constant 15 W/gel for 4-5 h. One- and 2D gels were either silver-stained using a modified protocol of Heukeshoven and Dernick [11], in which glutaraldehyde had been left out, or they were electroblotted for 1 h at 1 mA/cm2 onto nitrocellulose of pore size 0.2 μm in a semidry electroblotting cell, according to standard procedures. Blots were blocked with non-ionic detergent (2% Tween 20) for 5 min and developed with patient sera (diluted 1:100 unless otherwise noted) and alkaline-phosphatase-labeled anti-human IgG antibodies at 1:2000 using Nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate for the color reaction as described elsewhere [12].
In-gel digestion of proteins separated by gel electrophoresis
In-gel digestion was performed as described previously [6]. The excised gel plugs were washed in 50 mM NH4HCO3/acetonitrile (60/40) and dried by vacuum centrifugation. The trypsin (sequencing grade), dissolved in 50 mM NH4HCO3 at 12 ng/μl, was added to the dry gel pieces and incubated on ice for 1 h for reswelling. The supernatant was removed, 60 μl digestion buffer was added, and the digestion was continued at 37°C for 4-18 h. The peptide mixture was analyzed directly by MALDI-MS without extraction by depositing a 0.8-μl 1:1 mixture of analyte solution in 2% trifluoroacetic acid and matrix solution (15-20 g/l α-cyano-4-hydroxycinnamic acid in 70% acetonitrile) directly on the MALDI target.
In other cases, the samples were desalted and concentrated as described elsewhere [13], and for analysis by MALDI-MS, the peptides were eluted with 0.5 μl matrix solution (15-20 g/l of α-cyano-4-hydroxycinnamic acid in 70% acetonitrile) and deposited directly on the MALDI target. For tandem MS, the peptides on the column were eluted with 1.5 μl 50% methanol/49% H2O/1% formic acid directly into the nanoelectrospray needle.
Peptide-mass mapping by MALDI-MS
A Bruker REFLEX model MALDI-TOF mass spectrometer (Bruker-Franzen Analytik GmbH, Bremen, Germany) equipped with the Scout source and variable detector bias gating was used in positive-ion reflector mode for mass analysis of peptide mixtures (peptide-mass mapping). The ion acceleration voltage was 20 kV.
ESI-Q-TOF MS
Tandem MS of peptides generated by in-gel digestion was performed on a nano-ESI-Q-TOF mass spectrometer (Micromass, Manchester, UK). Ions were produced in an atmospheric pressure ionization (API)/ESI ion source at 40°C. The flow rate of the drying gas was 50 l/h. A potential of 1 kV was applied to the precoated borosilicate nanoelectrospray needles (Protana A/S, Odense, Denmark), and a stable flow rate (10-30 nl/min) was produced with a nitrogen back-pressure of 0-5 psi (0-34.5 kPa). The cone voltage was 50 V. A quadrupole analyzer was used to select the precursor ion for fragmentation in the hexapole collision cell. The collision gas was argon at a pressure of 6×10-5 mbar (6×10-3 Pa) and the collision voltage was 20-25 V. Product ions were analyzed using an orthogonal TOF analyzer. Data were processed with a Mass Lynx Windows NT-based program.
Protein identification
Protein identification by peptide-mass fingerprinting was performed by searching the peptide masses in a comprehensive non-redundant protein sequence database (NRDB; European Bioinformatics Institute, Hinxton, UK) containing approximately 420 000 protein sequences, using the database search program PepSea (Protana, Odense, Denmark). The peptide-mass maps and the protein identifications were evaluated as described in [6]. The partial amino acid sequences of the peptides generated by tandem MS together with the associated masses were used to construct the peptide sequence tags [7] that were used to identify the proteins in the NRDB.
Results
Indirect immunofluorescence on HEp-2 cells
The starting point for the investigation into the specificity of the antibodies in the present study was the observation of an unusual staining pattern in a patient serum being tested for IgG reactivity on HEp-2 cells. A strong nuclear staining pattern (titer >1280) with very even-sized, coarse speckles and no staining of nucleoli was observed (Fig. 1). This staining pattern did not resemble the well-known variably sized spliceosomal speckled pattern seen with antibodiesto Sm/U1RNP or the fine speckled pattern characteristic of SSA/SSB-antibody-positive sera. In accordance with this, the patient had none of these antibody specificities when tested by specific assays (data not shown).
Figure 1.
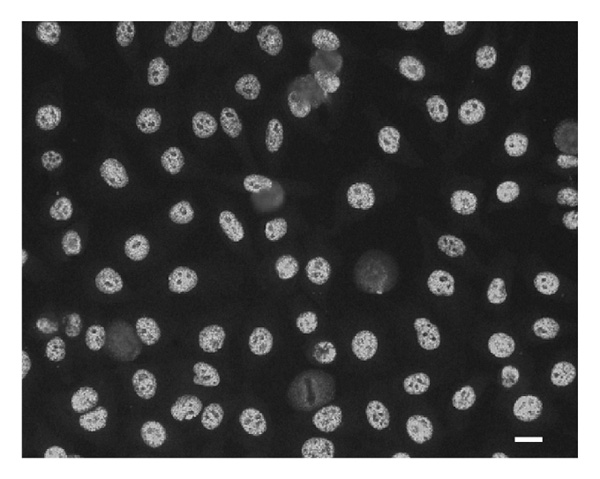
Indirect immunofluorescence pattern of anti-C1/C2-positive patient serum on HEp-2 cells. Cells were incubated with diluted patient serum (1:160), and bound IgG was detected by fluorescence microscopy after incubation with fluoresceinylated rabbit anti-human IgG. Strongly positive, very regular, coarse nuclear staining that spares nucleoli is observed. Original magnification is 200×. Scale bar is 10 μm.
Determination of antigen molecular mass in HeLa-cell nuclear extracts
The IgG reactivity of the serum shown in Fig. 1 was tested on an SDS-PAGE-separated nuclear extract from HeLa cells as shown in Fig. 2. A double band in the Mr 40 000-42 000 range and a high-molecular-mass band (around 200 000) were observed as the sole reactivities of this particular patient (Fig. 2, lane 2). Sera from two other patients, with a similar atypical ANA-staining pattern, also stained components of this doublet in immunoblotting (Fig. 2, lanes 3 and 4). The reactivity strongly resembled that of the patient originally described by Stanek et al [4] (Fig. 2, lane 5). The two different, more weakly reacting, sera appear to react preferentially with either the lower (lane 3) or the higher (lane 4) band, respectively. There was no reactivity with a normal serum (lane 1, Fig. 2). The antigens were present both in high-salt, SDS, and non-ionic detergent extracts of nuclei but appeared to be extracted to the highest degree in the β-octyl glucoside extracts (data not shown).
Figure 2.
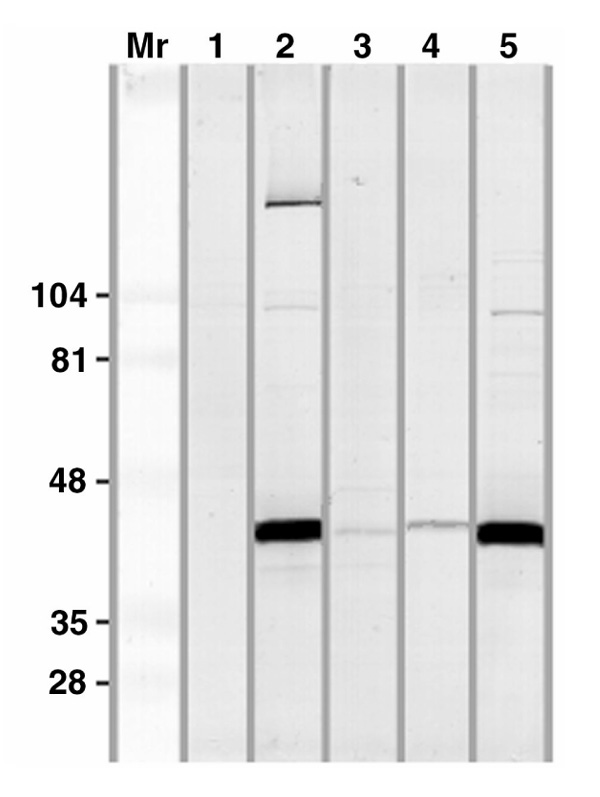
Immunoblotting using different patient sera (2-5) and a normal control serum (1) diluted 1:100 on blots of HeLa-cell nuclear extracts separated on SDS-PAGE. The serum examined in lane 2 is identical to the one shown in Fig. 1, and the serum in lane 5 is a sample from the original patient described by Stanek et al [4]. Developed with anti-IgG antibodies. Molecular masses (×10-3) of standard proteins are indicated on the left.
Enrichment of antigen preparation
After verification by immunoblotting that antibody reactivity was present in crude HeLa-cell nuclear extracts obtained by treatment with β-octyl glucoside (Fig. 2), the antigen was enriched by fractionating the extract by reversed-phase HPLC on a C4 column. Two of the collected fractions contained material with a molecular mass around 40 000 that was reactive with the patient antibody as judged by SDS-PAGE immunoblotting (data not shown).
Micropreparative separation of antigens
The contents of the crude nuclear extract obtained after β-octyl glucoside treatment were separated by 2D gel electrophoresis into several hundred components (Fig. 3a). However, the patient antibodies only reacted with two components in the mixture on immunoblots of the 2D separation (Fig. 3b). The two components appeared to be of approximately the same isoelectric points, but they differed in molecular mass by approximately 2000. The purified fractions from the C4 reversed-phase HPLC separation gave a less complicated 2D separation pattern (Fig. 3c), in which the two reactive spots (encircled) could be clearly identified and were positive on immunoblotting with patient serum (Fig. 3d). These two spots were excised for individual characterization.
Figure 3.
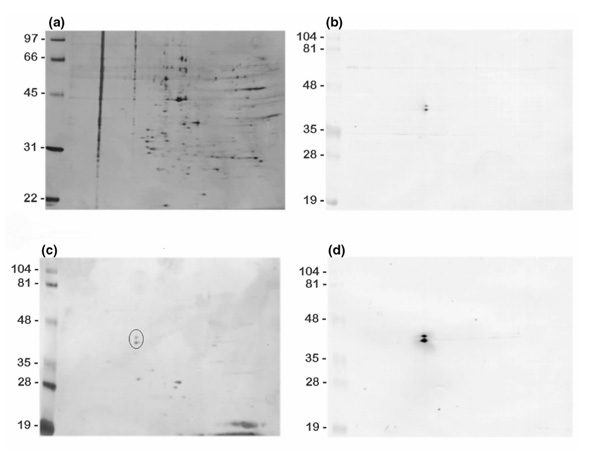
Isolation of antibody-reactive proteins by 2D gel electrophoresis and immunoblotting. (a) Two-dimensional gel electrophoresis of HeLa-cell nuclear extract. Molecular-weight marker positions are indicated on the left. Isoelectric focusing was in the horizontal dimension with the anode on the left. The gel is silver-stained. (b) Immunoblot from a separation similar to the one shown in (a), developed with the patient serum diluted 1:200. (c) Two-dimensional electrophoresis of a fraction of the nuclear extract purified by reversed-phase HPLC. (d) The immunoblot of the separation in (c), developed with a 1:200 dilution of patient serum. The two prominent spots marked with a circle in (c) were cut out separately and analyzed by MS after in-gel trypsin digestion.
Protein identification
MALDI-TOF peptide-mass mapping was performed on the tryptic peptide mixture generated by in-gel digestion of the two components after excision from 2D gels in two independent experiments. The database search compared the detected peptide masses with the theoretical tryptic peptide masses of the protein sequences present in the database. This approach in both sets of experiments suggested that the two proteins were hnRNPs C1/C2 (Swissprot accession number P07910), a conclusion suggested by the matching of 8 of 27 peptide masses entered (data not shown). The low number of matched peptides relative to the number of peptides included in the search, together with the small difference in the number of matching peptides to the next hit in the database (three peptides), make this identification only tentative. Therefore, the identity of the proteins was further confirmed by tandem MS using an ESI-Q-TOF instrument. One peptide carrying two positive charges (m/z 580.32 Da) corresponding to a peptide mass of 1158.7 Da was selected as a precursor ion and fragmented in the collision cell. The fragment ion spectrum is illustrated in Fig. 4a. A peptide sequence tag [7] consisting of a partial sequence and its associated masses was constructed -(632.36)LLSD(1060.70) - and was used to search the NRDB. The database search subsequently unambiguously identified the protein as human C1/C2 hnRNP (data not shown). The fragmented peptide was found to represent the tryptic fragment VDSLLENLEK, ie amino acids 207-216 (C2 protein numbering). Four other peptides were partially sequenced, and all were found to match the human C1/C2 hnRNP sequence (data not shown). Several of the peptide masses that were not matched to the sequence could be matched to peptides derived from keratin and from autoproteolysis of trypsin (data not shown).
Figure 4.
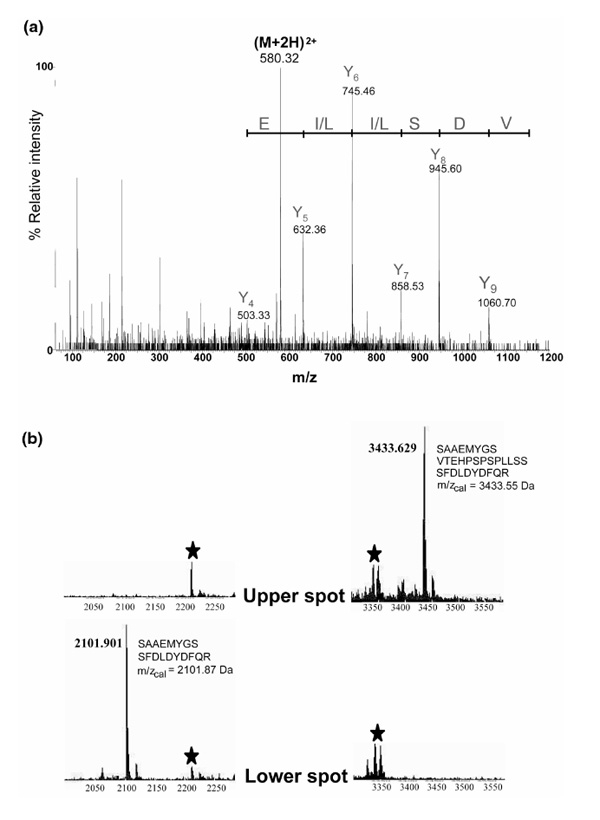
Identification of C1/C2 hnRNPs by MS and by tandem MS sequencing. (a) Collision-induced dissociation spectrum of a doubly charged ion at m/z 580.32 Da corresponding to the sequence 207-216 (VDSLLENLEK) of the C2 hnRNP. The Y ion signal series is shown together with the deduced sequence giving the peptide sequence tag (632.36)LLSD(1060.70). (b) Two sections of the MALDI-MS peptide-mass maps showing the difference between the upper (C2) hnRNP and the lower (C1) hnRNP spots excised from 2D gels (cf Fig. 3c). The signal at m/z 3433.6 Da detected in the trypsin-digested material from the upper spot could be identified as the tryptic peptide that includes the 13-amino-acid insert in C2 hnRNP (illustrated in the figure). Only the corresponding peptide without the insert could be detected (at m/z 2101.9 Da) in the peptide-mass map from the lower spot. Signals derived by autoproteolysis of trypsin are marked with stars.
The theoretical masses of C1 and C2 are 32.0 and 33.3 kDa, respectively. The difference between the two sequences is a 13-amino-acid insert in C2 between positions 107 and 108 of C1. The presence of a specific tryptic fragment in the MALDI-TOF peptide-mass map from the upper spot but not from the lower spot containing a 13-amino-acid insert in the protein sequence (SAAEMYGSVT-EHPSPSPLLSSSFDLDYDFQR) clearly demonstrates that the two components in the gel represent the two isoforms of the C-class of hnRNPS (Fig. 4b). The peptide-mass map of C1 contains a peptide signal at m/z 2101.9 Da, corresponding to the tryptic peptide without the 13-amino-acid insert - SAAEMYGSSFDLDYDFQR.
Patients and clinical data
The patient whose case prompted us to investigate the specificities of these antibodies was a 72-year-old man with a speckled ANA titer of >1280, who had arthralgias and oligoarthritis but did not fulfill the criteria for rheumatoid arthritis and did not have dermatological complaints. The reactivity of sera from various patient groups towards the C1/C2 hnRNP autoantigens was subsequently tested by immunoblotting of HeLa-cell nuclear extracts. None of 59 patients with rheumatoid arthritis, 19 with polymyositis, 33 with scleroderma, and 10 with psoriatic arthritis had IgG antibodies reacting with the two bands. Among 139 consecutive patients who had moderately to strongly positive speckled ANA patterns by indirect immunofluorescence on HEp-2 cells, only two additional patients had sera that reacted with the C1/C2 hnRNP bands in immunoblotting (Fig. 2, lanes 3 and 4). The serum of one of these patients reacted preferentially with the C1 hnRNP, and that of the other reacted with the C2 hnRNP and thus may be specific for the C2 insert. One patient was a young woman (22 years old) with complaints of muscle tenderness but without objective findings or other abnormal laboratory test findings, whose sera showed speckled ANAs, with a titer of >1280. A third patient whose case we identified through ANA screening followed by immunoblotting was a 54-year-old man, whose sera also showed speckled ANAs, with a titer of >1280. This man was receiving methotrexate and had long-standing polymyositis in addition to psoriasis and possible osteoporosis.
Discussion
Although the prototype serum found in our study strongly matched the reactivity of the serum from the report by Stanek et al [4] with respect to the singular staining pattern shown in Fig. 1, it probably would not be recognized as such in routine diagnostic laboratory tests.
The present study confirms the existence of anti-C1/C2 antibodies in some patients with speckled ANAs. The antigens were identified through the use of biochemical methods using high-resolution separation techniques combined with mass-spectrometry peptide mapping and database searches. As a general approach, this is a powerful way to identify new antigens using small amounts of material without the need for conventional protein sequencing and consequently with no problems related to blocked N-termini. The approach does require, however, that the proteins can be found in databases, that they are not extensively post-translationally modified, that they can be digested enzymatically, and that they can be isolated in appropriately pure form by the separation technique used.
The C1 and C2 proteins are identical except for an insert of 13 amino acids after serine 107 in the 303-amino-acid-containing C2 protein [14,15]. The presence of this insert accounts for the separation of the C1 and C2 proteins by SDS-PAGE. Like other hnRNPs, they contain consensus sequence RNA-binding domains and nuclear localization signals [15]. The C1/C2 proteins form anisotropic([C1]3 [C2]1) tetramers that bind strongly but apparentlynonspecifically to RNA and probably form a basic structural unit in pre-mRNA-processing and RNA-packaging complexes [14,16,17,18]. Together with the A and B groups of hnRNPs, they constitute the core proteins of the 40S hnRNP particles [19]. C1 and C2 are phosphorylated, contain unusually acidic C-terminal domains, and, accordingly, are the most acidic of the hnRNPs.
It is not known at present if the C1/C2 antibodies are pathogenically relevant and/or relate to specific diagnoses or subsets within the group of connective-tissue diseases. Although ELISA with native antigen may increase the number of positive findings, reactivity does seem to be quite rare among ANA-positive patients. Therefore, clarification of these issues would require the study of many patients.
Autoantibodies to many of the core proteins of the hnRNP complex have now been described, including the A1, A2/RA33, A3, B1, and B2 proteins as well as D/AUF-1 and, recently, also the hnRNP R protein [2,3,9,10]. These proteins are primarily involved in packaging and processing of pre-mRNA and in the regulation of alternative splicing. They may also be involved in RNA transport between the nucleus and the cytoplasm. ANAs directed against these proteins do not give clear-cut ANA reactivity on commonly used ANA substrates such as HEp-2 cells [20,21]. This is probably because the antibodies specifically target RNA-binding domains that are shielded by bound RNA in intact cells. The fact that these antibodies often appear together with other ANAs in systemic lupus erythematosus, in mixed connective-tissue diseases, and in adult and juvenile rheumatoid arthritis has made it difficult to study their presence except by immunoblotting of extracts treated with DNase and RNase to remove nucleic acids. The fact that antibodies to the C1/C2 hnRNPs are revealed by indirect immunofluorescence indicates that the epitopes are accessible in intact, fixed HEp-2 cells and thus are probably outside nucleic-acid-binding domains that would be expected to be covered by RNA.
Acknowledgments
Acknowledgements
Supported in part by The Danish Biotechnology Program (MRL). The present study is part of the activities of the Center for Experimental Bioinformatics, sponsored by the Danish National Research Foundation. We thank J Vencovský for providing a serum sample from his C1/C2 antibody-positive patient, EM Jensen and J Petersen for providing clinical information on patients, and B Jensen and C Ingwersen for expert technical assistance.
References
- Hassfeld W, Steiner G, Studnicka-Benke A, Skriner K, Graninger W, Fischer I, Smolen JS. Autoimmune response to the spliceosome: an immunologic link between rheumatoid arthritis, mixed connective tissue disease, and systemic lupus erythematosus. Arthritis Rheum. 1995;6:777–785. doi: 10.1002/art.1780380610. [DOI] [PubMed] [Google Scholar]
- Zimmermann C, Steiner G, Skriner K, Hassfeld W, Petera P, Smolen JS. The concurrence of rheumatoid arthritis and limited systemic sclerosis: clinical and serologic characteristics of an overlap syndrome. . Arthritis Rheum. 1998;41:1938–1945. doi: 10.1002/1529-0131(199811)41:11<1938::AID-ART7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Steiner G, Skriner K, Smolen JS. Autoantibodies to the A/B proteins of the heterogeneous nuclear ribonucleoprotein complex: novel tools for the diagnosis of rheumatic diseases. Int Arch Allergy Appl Immunol. 1996;111:314–319. doi: 10.1159/000237386. [DOI] [PubMed] [Google Scholar]
- Stanek D, Vencovský J, Kafková J, Raska I. Heterogenous nuclear RNP C1 and C2 core proteins are targets for an autoantibody found in the serum of a patient with systemic sclerosis and psoriatic arthritis. . Arthritis Rheum. 1997;40:2172–2177. doi: 10.1002/art.1780401211. [DOI] [PubMed] [Google Scholar]
- Nawrocki A, Larsen MR, Podtelejnikov AV, Jensen ON, Mann M, Roepstorff P, Görg A, Fey SJ, Mose-Larsen P. Correlation of acidic and basic carrier ampholyte and immobilized pH gradient two-dimensional gel electrophoresis patterns based on mass spectrometric protein identification. . Electrophoresis. 1998;19:1024–1035. doi: 10.1002/elps.1150190618. [DOI] [PubMed] [Google Scholar]
- Jensen ON, Larsen MR, Roepstorff P. Mass spectrometric identification and microcharacterization of proteins from electrophoretic gels: strategies and applications. Proteins. 1998;Suppl 2:74–89. doi: 10.1002/(sici)1097-0134(1998)33:2+<74::aid-prot9>3.3.co;2-2. [DOI] [PubMed] [Google Scholar]
- Mann M, Wilm M. Error-tolerant identification of peptides in sequence databases by peptide sequence tags. Anal Chem. 1994;66:4390–4399. doi: 10.1021/ac00096a002. [DOI] [PubMed] [Google Scholar]
- Montecucco C, Caporali R, Cobianchi F, Biamonti G. Identification of autoantibodies to the I protein of the heterogeneous nuclear ribonucleoprotein complex in patients with systemic sclerosis. Arthritis Rheum. 1996;39:1669–1676. doi: 10.1002/art.1780391009. [DOI] [PubMed] [Google Scholar]
- Hassfeld W, Chan EKL, Mathison DA, Portman D, Dreyfuss G, Tan EM. Molecular definition of heterogeneous nuclear ribonucleoprotein R (hnRNP R) using autoimmune antibody: immunological relationship with hnRNP P. Nucleic Acids Res. 1998;26:439–445. doi: 10.1093/nar/26.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriner K, Sommergruber WH, Tremmel V, Fischer I, Barta A, Smolen JS, Steiner G. Anti-A2/RA33 autoantibodies are directed to the RNA binding region of the A2 protein of the heterogeneous nuclear ribobucleoprotein complex: differential epitope recognition in rheumatoid arthritis, systemic lupus erythematosus, and mixed connective tissue disease. J Clin Invest. 1997;100:127–135. doi: 10.1172/JCI119504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heukeshoven J, Dernick R. Neue ergebnisse zum mechanismus der silberfärbung. In Elektrophorese Forum `86 Edited by Radola BJMunich: Technische Universität München, 1986:22–27. [Google Scholar]
- Heegaard NHH, Bjerrum OJ. Immunoblotting - general principles and procedures. In Handbook of Immunoblotting of Proteins Edited by Bjerrum OJ, Heegaard NHH Boca Raton, FL: CRC Press, Inc, 1988:1–25. [Google Scholar]
- Gobom J, Nordhoff E, Mirgorodskaya E, Ekman R, Roepstorff P. A sample purification and preparation technique based on nano-scale RP-columns for the sensitive analysis of complex peptide mixtures by MALDI-MS. . J Mass Spectrom. 1998;34:105–116. doi: 10.1002/(SICI)1096-9888(199902)34:2<105::AID-JMS768>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Merrill BM, Barnett SF, LeStourgeon WM, Williams KR. Primary structure differences between proteins C1 and C2 of HeLa 40S nuclear ribonucleoprotein particles. Nucleic Acids Res. 1989;17:8441–8449. doi: 10.1093/nar/17.21.8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd CG, Swanson MS, Görlach M, Dreyfuss G. Primary structures of the heterogeneous nuclear ribonucleoprotein A2, B1, and C2 proteins: a diversity of RNA binding protein is generated by small peptide inserts. . Proc Natl Acad Sci USA. 1989;86:9788–9792. doi: 10.1073/pnas.86.24.9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlach M, Wittekind M, Beckman RA, Mueller L, Dreyfuss G. Interaction of the RNA-binding domain of the hnRNP C proteins with RNA. EMBO J. 1992;11:3289–3295. doi: 10.1002/j.1460-2075.1992.tb05407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahied-Milam L, Soltaninassab SR, Iyer GV, LeStourgeon WM. The heterogeneous nuclear ribonucleoprotein C protein tetramer binds U1, U2, and U6 snRNAs through its high affinity RNA binding domain (the bZIP-like motif). J Biol Chem. 1998;273:21359–21367. doi: 10.1074/jbc.273.33.21359. [DOI] [PubMed] [Google Scholar]
- Soltaninassab SR, McAfee JG, Shahied-Milam L, LeStourgeon WM. Oligonuceotide binding specificities of the hnRNP C protein tetramer. Nucleic Acids Res. 1998;26:3410–3417. doi: 10.1093/nar/26.14.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer AL, Christensen ME, Walker BW, LeSturgeon WM. Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell. 1977;11:127–138. doi: 10.1016/0092-8674(77)90323-3. [DOI] [PubMed] [Google Scholar]
- Hassfeld W, Steiner G, Harmuth K, Kolarz G, Scherak O, Graninger W, Thumb N, Smolen JS. Demonstration of a new antinuclear antibody (anti-RA33) that is highly specific for rheumatoid arthritis. Arthritis Rheum. 1989;32:1515–1520. doi: 10.1002/anr.1780321204. [DOI] [PubMed] [Google Scholar]
- Steiner G. The A2 protein of the heterogeneous nuclear ribonucle-oprotein. In Handbook of Biological Markers of Disease Edited by Maini RN, van Venrooij W Amsterdam: Kluwer Academic Publishers, 1994:1–9. [Google Scholar]


