SYNOPSIS
Objective
American Indians and Alaska Natives (AI/AN) adults ≥65 years of age (older adults) have the second highest age group-specific infectious disease (ID) hospitalization rate. To assess morbidity and disparities of IDs for older AI/AN adults, this study examined the epidemiology of overall and specific infectious disease hospitalizations among older AI/AN adults.
Methods
ID hospitalization data for older AI/AN adults were analyzed by using Indian Health Service hospital discharge data for 1990 through 2002 and comparing it with published findings for the general U.S. population of older adults.
Results
ID hospitalizations accounted for 23% of all hospitalizations among older AI/AN adults. The average annual ID hospitalization rate increased 5% for 1990–1992 to 2000–2002; however, the rate increased more than 20% in the Alaska and the Southwest regions. The rate for older AI/AN adults living in the Southwest region was greater than that for the older U.S. adult population. For 2000–2002, lower respiratory tract infections accounted for almost half of all ID hospitalizations followed by kidney, urinary tract, and bladder infections, and cellulitis.
Conclusions
The ID hospitalization rate increased among older AI/AN adults living in the Southwest and Alaska regions, and the rate for the older AI/AN adults living in the Southwest region was higher than that for the U.S. general population. Prevention measures should focus on ways to reduce ID hospitalizations among older AI/AN adults, particularly those living in the Southwest and Alaska regions.
Historically, American Indians and Alaska Natives (AI/ANs) have experienced a disproportionate share of infectious disease (ID) morbidity and mortality in the United States1–3 and reducing this disparity remains an important public health challenge. During the early 1990s, the rate of ID hospitalizations among AI/ANs was higher than that for the general U.S. population.2,4 Furthermore, mortality rates for some IDs among AI/ANs were higher than those for the general U.S. population.5–7
Infectious disease morbidity and mortality has disproportionately affected older adults (those ≥65 years of age) in the U.S.4,8–10 The ID hospitalization rate increased significantly among older adults during the 1980s and early 1990s, while the rate for individuals younger than 25 years of age decreased significantly.4 Moreover, ID hospitalization rates among older U.S. adults increased by 13% for 1990 through 2002.8 ID hospitalization rates for older AI/AN adults for 1980 through 1994 were second only to those for children <1 year of age.2,8 In addition, the ID hospitalization rate for older AI/ANs remained stable for 1980 through 1994, while the ID hospitalization rates for AI/ANs <65 years of age declined.
To further assess and describe the epidemiology of infectious diseases among older AI/ANs, we examined ID hospitalizations among older AI/ANs who received medical care through the Indian Health Service (IHS) and tribally administered health facilities for 1990 through 2002. We also compared the ID hospitalization trends and rates for older AI/ANs to those of the general older U.S. adult population.
METHODS
For this study, we analyzed hospital discharge data for AI/AN older adults from the IHS Direct and Contract Health Service Inpatient Dataset for calendar years 1990 through 2002.11 This dataset is maintained by the IHS and consists of all patient discharge records from IHS and tribally operated hospitals and from hospitals that have contracted with the IHS or tribes to provide health care services to federally recognized AI/ANs within the U.S.12 Approximately 1.6 million AI/ANs are eligible for IHS-funded medical care.12 We excluded the IHS California and Portland administrative areas from the analysis because neither had any IHS- or tribally operated hospitals.13 In addition, California did not report contract health services inpatient data by diagnosis, and Portland had limited contract health service funds for inpatient care.14 Thus, this study represents AI/ANs who received direct or contract health care through IHS- or tribally operated inpatient or ambulatory care facilities across the continental U.S. and Alaska, excluding those in the California and Portland administrative areas.12,15
IHS hospital discharge records for older AI/ANs with an ID listed as a discharge diagnosis were selected by using a comprehensive list of IDs and conditions defined by using the International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) codes.2,8,16 A hospitalization with an ID listed as the first diagnosis was considered an ID hospitalization for our analyses.
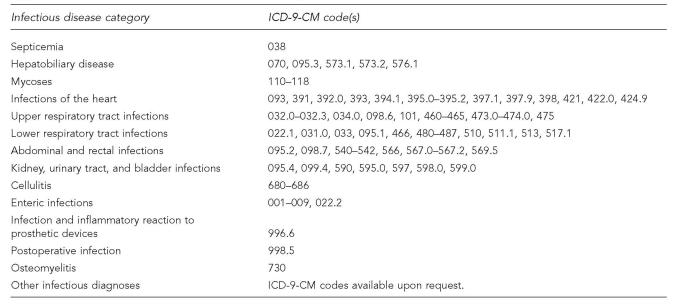
We examined hospitalizations by gender, geographic region, and specific ID diagnoses. We used the IHS Administrative Areas to define IHS regions as follows: East (Nashville), Northern Plains (Aberdeen, Bemidji, and Billings), Alaska, Oklahoma, and Southwest (Albuquerque, Navajo, Phoenix, and Tucson).13 Specific ID diagnoses included septicemia, hepatobiliary disease, mycoses, infections of the heart, upper respiratory tract infections, lower respiratory tract infections (LRTIs), abdominal and rectal infections, kidney, urinary tract, and bladder infections (KUB), cellulitis, enteric infections, infection and inflammatory reactions to prosthetic devices, postoperative infections, osteomyelitis, and other infectious diagnoses (Figure 1).2,4,8 Month of discharge, patient status at discharge, and length of stay were also examined. Hospitalizations could not be identified by patient. The unit of analysis in this study was a hospitalization.
Figure 1.
Classifications of infectious disease categories
Annual hospitalization rates with 95% confidence intervals (CIs) for older AI/ANs were calculated for ID hospitalizations, specific ID diagnoses hospitalizations, and all-cause hospitalizations and expressed as the number of hospitalizations per 100,000 AI/AN older adults. We also calculated annual hospital fatality rates, expressed as the number of hospital deaths per 100 hospitalizations. The population denominator for IHS hospitalization rates was determined for each year of the study by multiplying the IHS fiscal year 2003 user population estimates by the proportion of the estimated IHS service population for previous years, based on the 2003 service population, excluding the California and Portland Areas.12,15 The user population consisted of all registered AI/AN patients who received IHS-funded health care service at least once in the prior three years.12 The service population is an annual estimate of AI/ANs eligible for IHS-funded health care; the population denominator is considered an estimate since it is not an actual census count of AI/ANs eligible for IHS-funded health care.
Comparisons of hospitalization rates by time period (1990–1992 vs. 2000–2002) and characteristics for the most recent period were made by chi-square tests. Due to the year-to-year variability in hospitalization rates, the two three-year time periods were used for rate comparisons. Risk ratios (RRs) and 95% CIs for the comparisons of characteristics during the most recent period were also calculated.17 Comparisons of hospital length of stay by different characteristics were made by using the Wilcoxon rank-sum test.18 Infectious disease and specific ID diagnoses hospitalization trends and rates for older AI/ANs were compared with those previously published for the general U.S. older adult population.8
RESULTS
Characteristics and trends
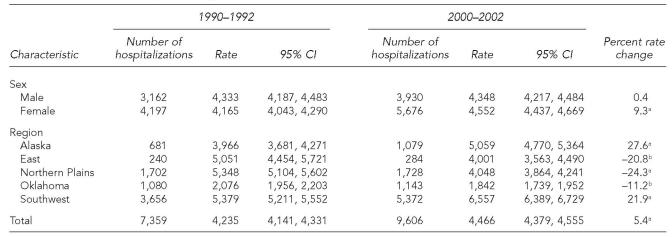
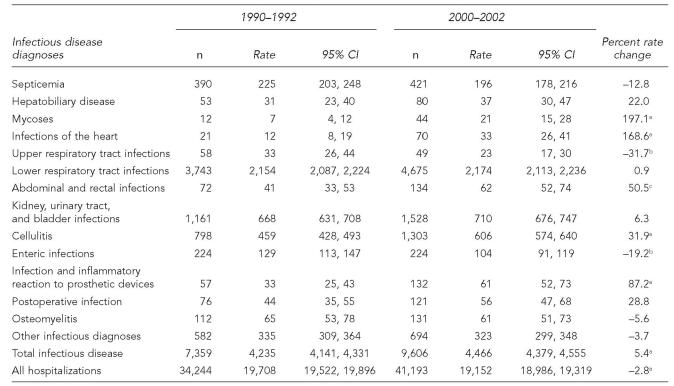
There were approximately 38,000 ID hospitalizations among older AI/ANs for 1990 through 2002, and hospitalizations with an ID as the primary diagnosis accounted for 59% of all hospitalizations with an ID diagnosis listed on the discharge record. The average annual hospitalization rate for IDs increased 5.4%, from 4,235 during 1990–1992 to 4,466 during 2000–2002 per 100,000 older AI/ANs (p<0.001, Table 1, Figure 2). Older AI/ANs accounted for 19% of ID hospitalizations among AI/ANs of all ages for 2000–2002, while representing about 6% of the AI/AN population.12 The ID hospitalization rate for older females increased by 9% during the study period (p<0.001), while the rate did not change for older males. As a result of the ID hospitalization rate increase among older females, their rate was slightly higher for 2000 to 2002 than that for older AI/AN males (RR=1.05; 95% CI 1.00, 1.09; Table 3).
Table 1.
Infectious disease hospitalizations and hospitalization rates per 100,000 among American Indians and Alaska Natives ≥65 years of age, Indian Health Service, 1990–1992 and 2000–2002
p<0.001
0.001≤p<0.01
CI = confidence interval
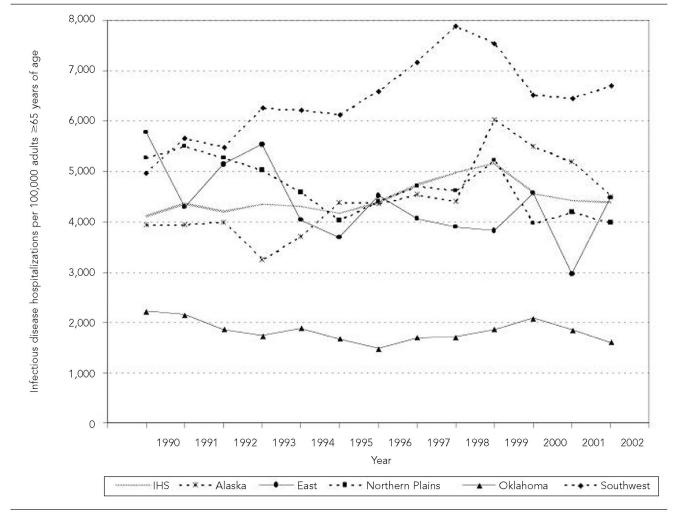
Figure 2.
Annual infectious disease hospitalization rates by region among American Indians and Alaska Natives ≥65 years of age, Indian Health Service, 1990–2002
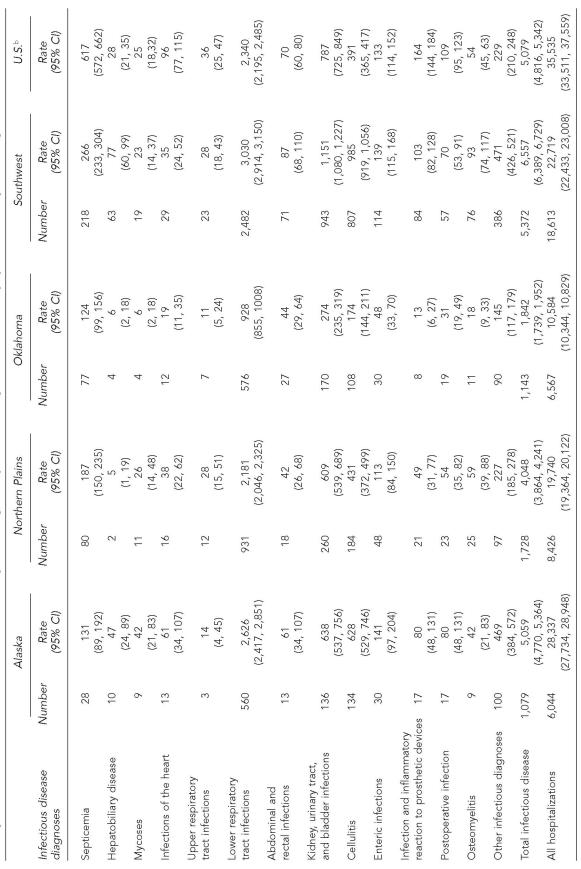
Table 3.
Number of hospitalizations and hospitalization rates per 100,000 by infectious disease diagnoses and Indian Health Service regiona among American Indians and Alaska Natives ≥65 years of age compared to the general U.S. adult population ≥65 years of age, 2000–2002
The Indian Health Service East region was not included due to the region's small number of ID hospitalizations.
Curns AT, Holman RC, Sejvar JJ, Owings MF, Schonberger LB. Infectious disease hospitalizations among older adults in the United States from 1990 through 2002. Arch Intern Med 2005;165:2514-20.
CI = confidence interval
The overall ID hospitalization rate increase was primarily attributed to the Alaska region, where the ID hospitalization rate increased significantly during the study period by 27.6%, and the Southwest region, where it increased by 21.9% (p<0.001, both regions; Table 1, Figure 2). The rate decreased by more than 20% in the East (p=0.006) and Northern Plains regions (p<0.001), and by more than 10% in the Oklahoma region (p=0.004). Older AI/ANs in the Southwest region had the highest ID hospitalization rate among the IHS regions throughout the study period; the 2000–2002 rate was 1.30 times (95% CI 1.21, 1.38) the rate for older AI/ANs living in Alaska, the region with the second highest rate. ID hospitalization rates were significantly lower for each of the remaining IHS regions when compared to the Alaska region. The Oklahoma region had the lowest ID hospitalization rate for both time periods, which was less than half of the ID hospitalization rate for the East and Northern Plains for 2000 through 2002. The ID hospitalization rates for 2000–2002 for older AI/ANs in all IHS regions except for those living in the Southwest and Alaska regions (Table 1) were lower than the rate for the general U.S. older adult population (5,079/100,000; 95% CI 4,816, 5,342).8 Compared to the ID hospitalization rate for the general U.S. older adult population, the rate for older AI/ANs living in the Alaska region was similar, while the rate for older AI/ANs living in the Southwest region was higher.
Proportion of hospitalizations with infectious disease
The all-cause hospitalization rate for older AI/ANs declined by approximately 3% during the study period—from 19,708 to 19,152 hospitalizations per 100,000 older AI/ANs (p<0.001; Table 2). ID hospitalizations among older AI/ANs accounted for 21.5% of all hospitalizations in this age group for 1990–1992 and 23.3% for 2000–2002. This increase was due primarily to the increasing proportion of hospitalizations associated with ID in the Southwest (26.2% to 28.9%; p<0.001). The proportion also increased for the East region (15.6% to 18.6%; p=0.02), but did not significantly increase during the study period in the Alaska, Northern Plains, and Oklahoma regions, where the proportions were 17.9%, 20.5% and 17.4%, respectively, for 2000–2002. Older AI/ANs accounted for 14.4% of ID hospitalizations among AI/ANs of all ages for 1990–1992 and 18.9% for 2000–2002, an increase of more than 30% (p<0.001). The proportion of ID hospitalizations out of all hospitalizations among older U.S. adults for both time periods was approximately 14%.8
Table 2.
Hospitalization rates per 100,000 by infectious disease diagnoses among American Indians and Alaska Natives ≥65 years of age, Indian Health Service, 1990–1992 and 2000–2002
p<0.001
0.01≤p<0.05
0.001≤p<0.01
CI = confidence interval
Hospital fatality rate and hospital stay
The ID hospital fatality rate for older AI/ANs did not change significantly during the study period (four per 100 hospitalizations from 2000 through 2002). The fatality rate for 2000–2002 was similar by region. Older AI/AN males had a slightly higher ID hospital fatality rate (4.5 per 100 hospitalizations) than that for older AI/AN females (3.6 per 100 hospitalizations; RR=1.24; 95% CI 1.02, 1.51). The ID hospital fatality rate for older AI/ANs was approximately half that of the 2000–2002 estimate for the older general U.S. population (seven per 100 hospitalizations; standard error [SE]=0.2).8
The median length of stay for ID hospitalizations among older AI/ANs decreased from five days (quartiles three and eight days) for 1990–1992 to four days (quartiles three and seven days) for 2000–2002 (p<0.001). The 2000–2002 median hospital stay did not differ by gender. For 2000–2002, the median length of stay for the Southwest and Northern Plains regions was four days; it was five days for the Alaska, Oklahoma, and East regions. For 2000–2002, 57,907 days of ID hospitalization occurred among older AI/ANs, accounting for 22.8% of all hospitalization days for older AI/ANs.
Infectious disease diagnoses
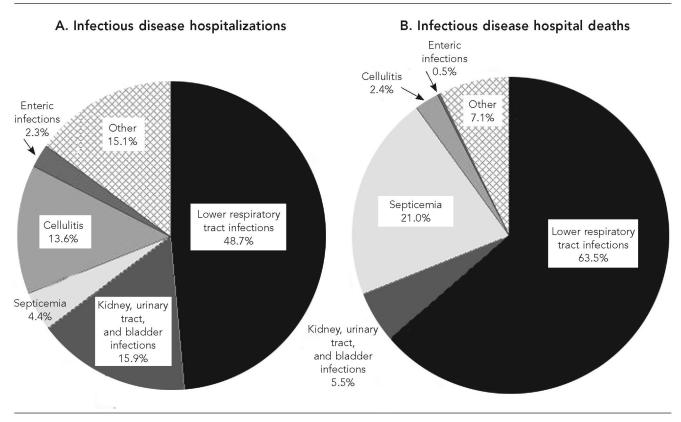
Among the ID diagnoses for older AI/ANs, lower respiratory tract infections (LRTIs) were associated with the highest ID hospitalization rates during the study period (Table 2). LRTIs were associated with nearly half (48.7%) of ID hospitalizations for 2000–2002 (Figure 3A), and nearly two-thirds (63.5%) of ID hospital deaths (Figure 3B). The rate for LRTI hospitalizations did not differ for males and females (RR=0.96; 95% CI 0.91, 1.02) for 2000–2002. As observed for the general U.S. older adult population,8 pneumonia (ICD-9-CM codes 480–486)16 accounted for nearly all of the LRTI hospitalizations (95.4%) and LRTI hospital deaths (97.9%) among older AI/ANs.
Figure 3.
Proportion of infectious disease (ID) hospitalizations (A) and deaths (B) by ID diagnoses among American Indians and Alaska Natives ≥65 years of age, Indian Health Service, 2000–2002
Kidney, urinary tract, and bladder infections (KUB), and cellulitis each accounted for approximately 15% of ID hospitalizations among older AI/ANs (Figure 3A). The hospitalization rate for cellulitis increased by more than 30% during the study period (p<0.001), while the KUB hospitalization rate did not change significantly (Table 2). For 2000–2002, the KUB hospitalization rate for females was almost double that for males (880 and 477 per 100,000, respectively; RR=1.84; 95% CI 1.65, 2.06).
Among other ID diagnoses, the hospitalization rates increased for infections and inflammatory reactions to prosthetic devices (p<0.001), abdominal and rectal infections (p=0.005), mycoses (p<0.001), and infections of the heart (p<0.001) during the study period (Table 2). The hospitalization rates for upper respiratory tract infections (p=0.047) and enteric infections (p=0.02) decreased and the rates for septicemia, hepatobiliary disease, postoperative infection, osteomyelitis, and other infectious diagnoses did not significantly change.
The significant increase of the overall ID hospitalization rate among older AI/AN females could not be attributed to any one ID group. Significant increases were found for hepatobiliary disease, mycoses, infections of the heart, abdominal and rectal infections, cellulitis, and infections and inflammatory reactions to prosthetic devices. Among older AI/AN males, the rates for infections of the heart, cellulitis, and infections and inflammatory reactions to prosthetic devices significantly increased; however, these increases did not result in an increase in the overall ID hospitalization rate for older males.
For 2000–2002, the proportion of ID hospitalizations associated with LRTIs (49%) among older AI/ANs was slightly higher than the estimate for the U.S. older adults (46% [SE=0.7%]).8 By region, the proportion of ID hospitalizations associated with LRTI ranged from 52% in the Alaska region to 46% in the Southwest region. Only in the Southwest region was the LRTI hospitalization rate significantly higher than that for the general U.S. population of older adults (Table 3).8 In addition, the Southwest region's hospitalization rates for KUB, osteomyelitis, and hepatobiliary disease diagnoses were higher than those for U.S. older adults. The rates for cellulitis and the other infectious diagnoses for both the Southwest and Alaska regions were higher than those for U.S. older adults.
DISCUSSION
The present study found that the ID hospitalization rate for older AI/ANs increased from 1990–1992 to 2000–2002. This rate increase occurred only among older AI/ANs living in the Alaska and Southwest regions. Elsewhere, the rates decreased among older AI/ANs (IHS East, Northern Plains, and Oklahoma regions). For 2000–2002, older AI/ANs living in the Southwest region had the highest ID hospitalization rate, followed by those living in the Alaska region. Not only was the ID hospitalization rate higher in the Southwest region than in the other IHS regions, but the rate was also higher than that for the general U.S. older adult population in 2000–2002.8 High rates of overall and specific IDs among AI/ANs of all ages and AI/AN children living in the Southwest and Alaska regions were previously described.2,3,19,20 The increased ID hospitalization rates among AI/ANs living in these regions may be associated with household crowding and a lack of sanitation facilities.21–23 In addition, it may be difficult for AI/ANs living in rural areas to seek preventive medical care or treatment of illnesses before requiring inpatient care. Improvement of these conditions should assist in reducing ID morbidity and mortality among older AI/ANs.
Older AI/ANs in the Oklahoma region had a lower ID hospitalization rate than those living in the other IHS regions and also compared with the general U.S. older adult population. Lower ID and respiratory hospitalization rates among AI/ANs in Oklahoma have been reported previously.2,20,24 There are several factors that may contribute to the low ID hospitalization rate. A large proportion of the AI/AN population in Oklahoma lives primarily in non-reservation settings that could predispose AI/ANs to seek care at non-IHS or tribally administered facilities, resulting in the low ID hospitalization rate. In addition, there are favorable socioeconomic and health indicators, such as a higher percentage of college graduates, lower birth rates, and lower infant mortality rates, compared with populations in other IHS regions.13
LRTIs accounted for almost half of ID hospitalizations and more than 60% of ID hospitalization deaths among older AI/ANs for 2000–2002. The LRTI hospitalization rate for older AI/ANs did not change during the study period and most LRTI hospitalizations were for pneumonia. The trends in LRTI hospitalization rates and the relative contribution of LRTIs to all ID hospitalizations were similar to those observed among the general U.S. older adult population.8 However, the proportion of ID deaths associated with LRTIs was over 60% among older AI/ANs compared with slightly less than 50% for the general U.S. older adult population. The prevalence of ID hospitalizations and death from LRTIs among older AI/ANs reinforces the importance of immunization against vaccine-preventable causes of respiratory infection among this population in reducing overall ID morbidity.25–28
The hospitalization rate for cellulitis increased during the study period and was significantly greater among older AI/ANs living in the Southwest and Alaska regions than the rate for U.S. older adults.8 The increased hospitalization rates for cellulitis in these populations may be attributable to the concurrent increases in rates of chronic medical conditions predisposing to cellulitis, namely obesity and diabetes.29–31 AI/ANs were among the first populations reported to have community-onset methicillin-resistant Staphylococcus aureus (MRSA) infections.32,33 In one Alaska Native community, the predominant MRSA strain carried a virulence determinant associated with higher risk of serious skin and soft tissue infections.34 The influence of MRSA infection on the elevated cellulitis hospitalization rate for older AI/ANs living in the Southwest and the Alaska regions is not known. Managing risk factors for chronic conditions among AI/ANs is an important public health objective, and may have the additional benefit of reducing ID hospitalization rates.
As observed for U.S. older adults in general,8 older AI/AN females had a higher rate for KUB hospitalizations than that for older AI/AN males. In the Southwest region, the rate of KUB hospitalizations was almost 1.5 times that in the general U.S. population and also higher than those in the other IHS regions. Being female, being elderly, and having diabetes are known risk factors for KUB infection.35 However, the proportion of older AI/AN females in the Southwest region was similar to those of other IHS regions during the study period and the Southwest region was reported to have a similar or lower diabetes prevalence compared to the other IHS regions.12,15,36 Further study is required to determine the factors contributing to this observed disparity in KUB hospitalization rates among older AI/ANs.
The hospitalization rates for infections of the heart and infection and inflammatory reaction to prosthetic devices diagnoses increased among older AI/ANs during the study period. While the hospitalization rates for these ID diagnoses were similar to or lower than those for older U.S. adults for 2000–2002,8 the increasing rates may be attributed to more invasive cardiac procedures and knee and hip replacement surgeries. The more frequent use of prosthetic devices, chronic iatrogenic immunosuppression, and indwelling medical devices in older U.S. adults in general would be expected to occur in parallel in the care of older AI/ANs, and to carry with them the associated risks for the development of infection.37–40 In addition, the increasing prevalence of antimicrobial-resistant organisms associated with these types of infections may contribute to increased morbidity in these ID diagnoses.41
There are potential limitations with our study. Due to data reporting limitations, hospitalizations for AI/AN older adults living in the IHS California or Portland areas were not included in our study; these AI/ANs account for approximately 10% of the older adult AI/AN population eligible for IHS care. There are also inherent limitations with the use of hospital discharge data. Although AI/ANs are likely to seek IHS/tribal medical care because prepaid IHS-funded care health care is provided to eligible AI/ANs,15,42 it is possible that older AI/ANs eligible for IHS/tribal services could receive inpatient care outside of this system, resulting in underestimates of hospitalization rates and hospital fatality rates. In addition, hospital admission criteria, patterns for seeking health care, and diagnostic criteria may vary within the IHS/tribal system as well as between the IHS/tribal and general U.S. healthcare systems. Furthermore, our comparisons of older AI/ANs and the older adult U.S. general population might be affected by the lower life expectancy among AI/ANs.43 Finally, due to data-use restrictions, we could not identify readmissions for IDs among individuals. However, the U.S. hospitalization rates that were used for comparison also used the hospitalization as the unit of analysis.8
In general, we found that the ID hospitalization rate among older AI/ANs was similar to the general U.S. older adult population.8 The notable exception was older AI/ANs living in the Southwest, who had a higher ID hospitalization rate than both older AI/ANs in other IHS regions and the general older adult U.S. population. We also found that the ID hospitalization rates for the Southwest and Alaska regions increased substantially during the study period. Future studies should attempt to identify the reasons for these increases. Due to the significant increase in the ID hospitalization rate among older AI/ANs in the Southwest during the study period, the disparity between the ID rates for older AI/ANs living in the Southwest and the general U.S. older adult population increased. Studies and prevention measures should focus on ways to reduce ID morbidity and mortality among older AI/ANs, particularly those living in the Southwest and Alaska regions.
Acknowledgments
The authors thank Claudia Chesley, Krista Yorita, and Kathleen Murray for manuscript review.
REFERENCES
- 1.Young TK. New York: Oxford University Press; 1994. The health of Native Americans. [Google Scholar]
- 2.Holman RC, Curns AT, Kaufman SF, Cheek JE, Pinner RW, Schonberger LB. Trends in infectious disease hospitalizations among American Indians and Alaska Natives. Am J Public Health. 2001;91:425–31. doi: 10.2105/ajph.91.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holman RC, Curns AT, Cheek JE, Singleton RJ, Anderson LJ, Pinner RW. Infectious disease hospitalizations among American Indian and Alaska Native infants. [cited 2006 Jun 30];Pediatrics. 2003 111:E176–82. doi: 10.1542/peds.111.2.e176. Available from: URL: http://www.pediatrics.org/cgi/content/fall/111/2/e176. [DOI] [PubMed] [Google Scholar]
- 4.Simonsen L, Conn LA, Pinner RW, Teutsch S. Trends in infectious disease hospitalizations in the United States, 1980–1994. Arch Intern Med. 1998;158:1923–8. doi: 10.1001/archinte.158.17.1923. [DOI] [PubMed] [Google Scholar]
- 5.Mahoney MC, Michalek AM. Health status of American Indians/Alaska Natives: general patterns of mortality. Fam Med. 1998;30:190–5. [PubMed] [Google Scholar]
- 6.Becker TM, Wiggins C, Peek C, Key CR, Samet JM. Mortality from infectious diseases among New Mexico's American Indians, Hispanic whites, and other whites, 1958–87. Am J Public Health. 1990;80:320–3. doi: 10.2105/ajph.80.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young TK. Recent health trends in the Native American population. Popul Res Policy Rev. 1997;16:147–67. [Google Scholar]
- 8.Curns AT, Holman RC, Sejvar JJ, Owings MF, Schonberger LB. Infectious disease hospitalizations among older adults in the United States from 1990 through 2002. Arch Intern Med. 2005;165:2514–20. doi: 10.1001/archinte.165.21.2514. [DOI] [PubMed] [Google Scholar]
- 9.DeFrances CJ, Hall MJ. 2002 National Hospital Discharge Survey. Adv Data. 2004;342:1–29. [PubMed] [Google Scholar]
- 10.Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. JAMA. 1999;281:61–6. doi: 10.1001/jama.281.1.61. [DOI] [PubMed] [Google Scholar]
- 11.Indian Health Service. National Patient Information Reporting System. Albuquerque: Indian Health Service; 2004. Inpatient/CHS inpatient data fiscal years 1990–2003. [Google Scholar]
- 12.Indian Health Service. Rockville (MD): Indian Health Service; 2004. Trends in Indian health—2000–2001. [Google Scholar]
- 13.Indian Health Service. Rockville (MD): Indian Health Service; 2003. Regional differences in Indian health 2000–2001. [Google Scholar]
- 14.Kaufman SF. Rockville (MD): Indian Health Service; 1998. Utilization of IHS and tribal direct and contract general hospitals, FY 1996, and U.S. non-federal short-stay hospitals, 1996. [Google Scholar]
- 15.Rhoades DA, D'Angelo AJ, Rhoades ER. Data sources and subsets of the Indian population. In: Rhoades ER, editor. American Indian health: innovations in health care, promotion, and policy. Baltimore: Johns Hopkins University Press; 2000. pp. 93–102. [Google Scholar]
- 16.Public Health Service and Health Care Financing Administration (US) Washington: U.S. Department of Health and Human Services; 1997. International classification of diseases, 9th revision, clinical modification, 6th ed. (CD-ROM) [Google Scholar]
- 17.Kleinbaum DG, Kupper LL, Morgenstern H. Belmont (CA): Lifetime Learning Pub; 1982. Epidemiologic research: principles and quantitative methods. [Google Scholar]
- 18.Lehmann EL. San Francisco: Holden-Day, Inc; 1975. Nonparametrics: statistical methods based on ranks. [Google Scholar]
- 19.Wainwright RB. The US Arctic Investigations Program: infectious disease prevention and control research in Alaska. Lancet. 1996;347:517–20. doi: 10.1016/s0140-6736(96)91143-5. [DOI] [PubMed] [Google Scholar]
- 20.Peck AJ, Holman RC, Curns AT, Lingappa JR, Cheek JE, Singleton RJ, et al. Lower respiratory tract infections among American Indian and Alaska Native children and the general population of U.S. children. Pediatr Iinfect Dis J. 2005:342–51. doi: 10.1097/01.inf.0000157250.95880.91. [DOI] [PubMed] [Google Scholar]
- 21.Bulkow LR, Singleton RJ, Karron RA, Harrison LH. Alaska RSV Study Group. Risk factors for severe respiratory syncytial virus infection among Alaska Native children. Pediatrics. 2002;109:210–6. doi: 10.1542/peds.109.2.210. [DOI] [PubMed] [Google Scholar]
- 22.Indian Health Service. Annual report for 2002. Washington: US Public Health Service; 2003. The sanitation facilities construction program of the Indian Health Service, Public Law 86-121. [Google Scholar]
- 23.Census Bureau (US) Washington: US Census Bureau; 1995. US Census statistical briefs: housing of American Indians on reservations plumbing equipment and fuels. [Google Scholar]
- 24.Holman RC, Curns AT, Cheek JE, Bresee JS, Singleton RJ, Carver K, Anderson LJ. Respiratory syncytial virus hospitalizations among American Indian and Alaska Native infants and the general United States infant population. Pediatrics. 2004;114:e437–44. doi: 10.1542/peds.2004-0049. [DOI] [PubMed] [Google Scholar]
- 25.Davidson M, Parkinson AJ, Bulkow LR, Fitzgerald MA, Peters HV, Parks DJ. The epidemiology of invasive pneumococcal disease in Alaska, 1986-1990–ethnic differences and opportunities for prevention. J Infect Dis. 1994;170:368–76. doi: 10.1093/infdis/170.2.368. [DOI] [PubMed] [Google Scholar]
- 26.Buchwald D, Sheffield J, Furman R, Hartman S, Dudden M, Manson S. Influenza and pneumococcal vaccination among Native American elders in a primary care practice. Arch Intern Med. 2000;160:1443–8. doi: 10.1001/archinte.160.10.1443. [DOI] [PubMed] [Google Scholar]
- 27.Watt JP, O'Brien KL, Benin AL, Whitney CG, Robinson K, Parkinson AJ, et al. Invasive pneumococcal disease among Navajo adults, 1989–1998. Clin Infect Dis. 2004;38:496–501. doi: 10.1086/381198. [DOI] [PubMed] [Google Scholar]
- 28.Whitney CG, Harper SA. Lower respiratory tract infections: prevention using vaccines. Infect Dis Clin North Am. 2004;18:899–917. doi: 10.1016/j.idc.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Gohdes D, Oser CS, Harwell TS, Moore KR, McDowell JM, Helgerson SD. Diabetes in Montana's Indians: the epidemiology of diabetes in the Indians of the Northern Plains and Canada. Curr Diab Rep. 2004;4:224–9. doi: 10.1007/s11892-004-0028-2. [DOI] [PubMed] [Google Scholar]
- 30.Rith-Najarian SJ, Gohdes DM, Shields R, Skipper B, Moore KR, Tolbert B, et al. Regional variation in cardiovascular disease risk factors among American Indians and Alaska Natives with diabetes. Diabetes Care. 2002;25:279–83. doi: 10.2337/diacare.25.2.279. [DOI] [PubMed] [Google Scholar]
- 31.Scheinfeld NS. Obesity and dermatology. Clin Dermatol. 2004;22:303–9. doi: 10.1016/j.clindermatol.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Groom AV, Wolsey DH, Naimi TS, Smith K, Johnson S, Boxrud D, et al. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA. 2001;286:1201–5. doi: 10.1001/jama.286.10.1201. [DOI] [PubMed] [Google Scholar]
- 33.Baggett HC, Hennessy TW, Leman R, Hamlin C, Bruden D, Reasonover A, et al. An outbreak of community-onset methicillin-resistant Staphylococcus aureus skin infections in southwestern Alaska. Infect Control Hosp Epidemiol. 2003;24:397–402. doi: 10.1086/502221. [DOI] [PubMed] [Google Scholar]
- 34.Baggett HC, Hennessy TW, Rudolph K, Bruden D, Reasonover A, Parkinson A, et al. Community-onset methicillin-resistant Staphylococcus aureus associated with antibiotic use and the cytotoxin Panton-Valentine leukocidin during a furunculosis outbreak in rural Alaska. J Infect Dis. 2004;189:1565–73. doi: 10.1086/383247. [DOI] [PubMed] [Google Scholar]
- 35.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113(Suppl 1A):5S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 36.Burrows NR, Geiss LS, Engelgau MM, Acton KJ. Prevalence of diabetes among Native Americans and Alaska Natives, 1990–1997: an increasing burden. Diabetes Care. 2000;23:1786–90. doi: 10.2337/diacare.23.12.1786. [DOI] [PubMed] [Google Scholar]
- 37.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–54. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 38.Califf RM, Fowler V, Jr, Cabell CH, Corey GR. Novel approaches to clinical trials: device-related infections. Am Heart J. 2004;147:599–604. doi: 10.1016/j.ahj.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 39.Bernstein AG, Hing E, Moss AJ, Allen KF, Siller AB, Tiggle RB. Hyattsville (MD): National Center for Health Statistics; 2003. Healthcare in America: trends in utilization. [Google Scholar]
- 40.Cabell CH, Heidenreich PA, Chu VH, Moore CM, Stryjewski ME, Corey GR, Fowler VG. Increasing rates of cardiac device infections among Medicare beneficiaries: 1990–1999. Am Heart J. 2004;147:582–6. doi: 10.1016/j.ahj.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Fowler VG, Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubenstein E, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293:3012–21. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 42.Cunningham PJ. Access to care in the Indian Health Service. Health Aff (Millwood) 1993;12:224–33. doi: 10.1377/hlthaff.12.3.224. [DOI] [PubMed] [Google Scholar]
- 43.Indian Health Service. Facts on Indian health disparities. [cited 2005 May 20]; Available from: URL: http://info.ihs.gov/Health/11_DisparitiesFacts-Jan2005.doc. [Google Scholar]