Abstract
Thy-1 is a 25–37 kDa glycosylphosphatidylinositol (GPI)-anchored protein involved in T cell activation, neurite outgrowth, apoptosis, tumor suppression, wound healing, and fibrosis. To mediate these diverse effects, Thy-1 participates in multiple signaling cascades. In this review, we discuss Thy-1 signaling primarily in non-immunologic cell types, including neurons, mesangial cells, ovarian cancer cells, nasopharyngeal carcinoma cells, endothelial cells, and fibroblasts. We review the current literature regarding Thy-1 signaling via integrins, protein tyrosine kinases, and cytokines and growth factors; and the roles of these signaling pathways in cellular adhesion, apoptosis, cell proliferation, and cell adhesion and migration. We also discuss the role of Thy-1 localization to lipid rafts, and of the GPI anchor in Thy-1 signaling. Ongoing research on the mechanisms of Thy-1 signaling will add to our understanding of the diverse physiologic and pathologic processes in which Thy-1 plays a role.
Keywords: Thy-1, GPI anchor, cell signaling, integrins, tyrosine kinases, lipid rafts
Abbreviations: COX, cyclooxygenase; FAK, focal adhesion kinase; Gadd 45 γ, DNA-damage-inducible protein 45 gamma; GAP, GTPase activating protein; GPI, glycosylphosphatidylinositol; LAT, linker for activation of T cells; MAPK, mitogen- activated protein kinase; NCAM, neural cell adhesion molecule; NGFI-B, nerve growth factor induced protein I-B; NPC, nasopharyngeal carcinoma; PDGF, platelet-derived growth factor; PG, prostaglandin; PI 3-kinase, phosphatidylinositol 3-kinase; PKC, protein kinase C; PTK, protein tyrosine kinase; SFK, Src family kinase; SMA, smooth muscle actin; STAT3, signal transducer and activator of transcription 3; TGF, transforming growth factor; TNF, tumor necrosis factor; TUNEL, TdT-mediated dUTP nick-end labeling
1. Introduction
Thy-1 is a 25–37 kDa GPI-anchored cell surface protein expressed in various cell types, including fibroblasts, ovarian cancer cells, endothelial cells, neurons, and hematopoietic cells [1, 2]. Thy-1 expression is developmentally regulated in neurons and ovarian follicular cells [3–6]. For example, it is expressed on mature neurons, but not on growing neurons [7]. The expression of Thy-1 can be regulated transcriptionally in nasopharyngeal carcinoma and T cell lymphoma, potentially by promoter hypermethylation [8, 9], or post-transcriptionally in neurons due to an unidentified transacting suppressor, or by shedding [10–14].
Based on studies using Thy-1 knockout mice, Thy-1 appears to be important for certain neurologic and immunologic functions, and for tissue remodeling in respone to injury. Thy-1 knockout mice are viable, with no apparent major abnormalities. However, they display inhibition of hippocampal long term potentiation in the dentate gyrus but not in the CA1 region [15]. Although these mice have no impairments in spatial learning assessed by a water maze, they fail to base their food choices on socially-transmitted cues, indicating that specific regions of the hippocampus are involved in different types of learning and memory [15, 16]. Interestingly, anti-Thy-1 antibody administration abolishes both immediate and long-term memory in 2-day-old chicks [17]. Thy-1 knockout mice also have impaired cutaneous immune responses and abnormal retinal development [18, 19]. Our laboratory has studied the susceptibility of Thy-1 knockout mice to pulmonary fibrosis. Following intratracheal bleomycin (see section 5, below),Thy-1 knockout mice develop more severe fibrosis, as evidenced by histopathologic scoring, increased collagen deposition, and increased activation of latent transforming growth factor (TGF)-β, without significant differences in the early inflammatory response [14]. Ongoing phenotypic characterization of the Thy-1 null mouse using this and other disease models will likely elucidate additional roles for Thy-1 in vivo.
Thy-1 has been reported to function in T cell activation, neurite outgrowth, apoptosis, tumor suppression, and wound healing and fibrosis [20]. To mediate these diverse effects, Thy-1 signals through multiple pathways. Thy-1 is involved in T cell activation, and the role for Thy-1 in T cells is extensively reviewed elsewhere [21–23]. Anti-Thy-1 antibody induces T cell proliferation and IL-2 synthesis when co-stimulated by dendritic cells [24]. To mediate T cell proliferation, Thy-1 signals via tyrosine kinases and MAPK [21, 25]. Thy-1 can signal via integrins, focal adhesion kinase (FAK), and Rho to mediate cell adhesion [26, 27]. Thy-1 can also activate cell death and inhibit tumorigenic growth of cancer cells [28–35]. Expression of Thy-1 modulates the proliferative responses of fibroblasts to cytokines and growth factors [36–40]. Lastly, like other GPI-anchored molecules, Thy-1 localizes to lipid rafts, and this localization appears critical for Thy-1 signaling.
2. Thy-1 and cellular adhesion signaling
The formation and disassembly of focal adhesion structures, as well as other cell-cell and cell-matrix interactions, primarily involve integrin-related signaling [41, 42]. Thy-1-integrin interactions are primarily involved in heterotypic interactions between cells. Thy-1 expressed on neurons and endothelial cells interacts with β2 and β3 integrins on astrocytes, leukocytes, and melanoma cells [43–48] (Table 1). The interaction of neuronal Thy-1 with integrin β3 on astrocytes induces recruitment of FAK, paxillin, and vinculin to focal adhesions (Table 2). FAK and p130Cas activation are increased, stimulating focal adhesion formation and cell adhesion [27] (Fig. 1A). The Thy-1-induced focal adhesion formation is dependent on β3 clustering and RhoA activation [26]. Because Thy-1 expression on neurons inhibits neurite outgrowth [49], it has been suggested that the interaction between Thy-1 and β3 may activate bidirectional signaling inducing structural changes in β3-expressing astrocytes and potentially modulating neurite outgrowth of Thy-1-expressing neurons [45]. The mechanism and effects of bidirectional signaling between Thy-1 and β3 are still not fully characterized but warrant further investigation, as downregulation of Thy-1 expression or inhibition of Thy-1 signaling on mature neurons may facilitate nerve regeneration.
Table 1.
Summary of Thy-1/Integrin Signaling
| Cell type expressing Thy-1 | Integrin Receptor | Signaling Pathway | Effect |
|---|---|---|---|
| Endothelial cell | αXβ2, αMβ2 on leukocytes | unknown | Transendothelial cell migration, potential role in leukocyte extravasation |
| Endothelial cell | αvβ3 on melanoma cells | unknown | Transendothelial cell migration, potential role in melanoma metastasis |
| Neuron | β3 on astrocytes | FAK, p130Cas, RhoA, vinculin and paxillin recruitment | Focal adhesion and stress fiber formation in astrocytes |
Table 2.
Summary of Thy-1 signaling via tyrosine kinases
| Cell type | Signaling pathway | Effect |
|---|---|---|
| Mesangial cells | Fyn, lyn | Apoptosis induction |
| Mesangial cells | Smad1, STAT3 | Cell proliferation |
| Pulmonary fibroblasts | ↓SFK, ↓p190RhoGAP, RhoA activation | Focal adhesion and stress fiber formation, Decreased migration |
| Neurons | Fyn, lyn, Gαi, α- & β-tubulin, reggie-1 &-2 | Unknown |
| T cells | Calcineurin, PTKs, PI 3- kinase, PKC, and MAPK | Cell proliferation |
| Astrocytes | FAK, p130Cas, RhoA | Focal adhesion and stress fiber formation |
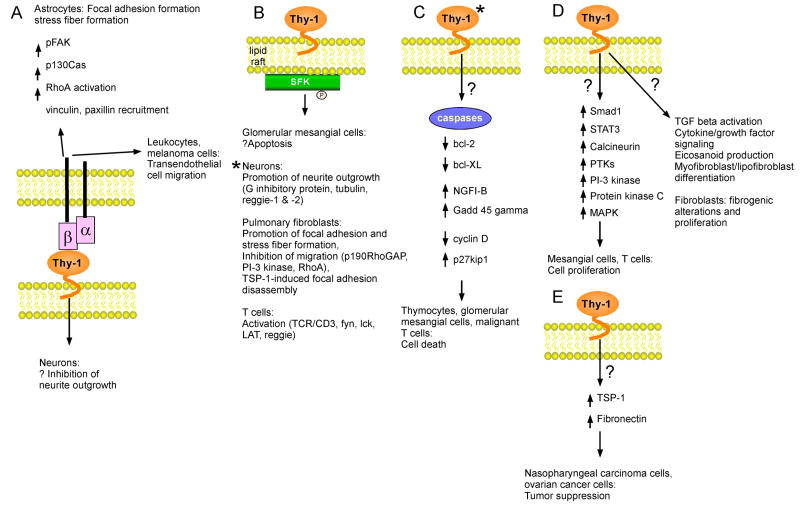
Fig. 1.
Schematic of Thy-1-associated signaling pathways. (A) Thy-1 interacts with both β2 and β3 integrins. The association of neuronal Thy-1 with β3 on astrocytes induces activation of FAK, vinculin, paxillin, p130Cas, and RhoA, promoting focal adhesion and stress fiber formation. This interaction may induce bidirectional signaling by concurrently signaling an inhibition of neurite outgrowth in mature neurons. Endothelial cell Thy-1 also interacts with β2 on leukocytes and β3 on melanoma cells to promote transendothelial cell migration in vitro. (B) Thy-1 associates with protein tyrosine kinases such as SFK within lipid rafts. This interaction may induce apoptosis of glomerular mesangial cells, promote neurite outgrowth in neurons, inhibit migration of pulmonary fibroblasts by altering the cytoskeleton, and promote T cell activation. (C) Thy-1 activation induces apoptosis in thymocytes, glomerular mesangial cells, and mouse malignant T cells. In malignant T cells, Thy-1 activation activates caspases and downregulates anti-apoptotic bcl-2 and bcl-XL. In an anti-Thy-1-induced model of glomerulonephritis, the apoptosis-related genes NGFI-B and Gadd 45 γ are upregulated. In this same model, the expression of cell cycle regulatory proteins is also modulated (see text for details). (D) Thy-1 promotes proliferation of mesangial cells via activation of Smad1 and STAT3, and proliferation of T cells through a pathway involving calcineurin, protein tyrosine kinases, PI-3 kinase, protein kinase C, and MAPK. Thy-1 also promotes TGF-β activation, cytokine/growth factor signaling, eicosanoid production, and myofibroblasts/lipofibroblast differentiation. (E) Thy-1 functions as tumor suppressor in nasopharyngeal carcinoma and ovarian cancer cells, potentially due to its upregulation of thrombospondin-1 and fibronectin. * indicates that Thy-1 cross-linking is necessary for effect.
Endothelial cell Thy-1 interacts with αvβ3 on melanoma cells and αXβ2 and αMβ2 on leukocytes [43, 44, 46–48] (Table 1). These interactions promote melanoma cell and leukocyte migration through an endothelial cell monolayer, suggesting that Thy-1 signaling may be important for melanoma metastasis and leukocyte recruitment and extravasation [44, 46]. Whether Thy-1 interacts with integrins within the same cell is unknown. It will be interesting to examine whether promotion of transendothelial cell migration by Thy-1 observed in vitro also occurs in vivo and to determine whether Thy-1 knockout mice have abnormalities of leukocyte recruitment or are less susceptible to melanoma metastases.
Adhesive signaling affects cell migration [50]. In the rat brain, Thy-1 is expressed at highest concentrations in the striatum and hippocampus [51]. Thy-1 is reported to function in the brain by either inhibiting or promoting neurite outgrowth [49, 52]. Thy-1 expression on a neural cell line inhibits neurite outgrowth over an astrocyte substrate, but not over a substrate composed of Schwann cells or embryonic glial cells [49]. As Thy-1 expression is upregulated on mature neurons, and neurite outgrowth is necessary for neuronal growth and synapse formation [3, 7, 53], Thy-1 may stabilize neuronal synapses and inhibit neuronal regeneration of mature neurons.
Thy-1-induced neurite outgrowth requires calcium influx, activation of L- and N-type calcium channels, and G-protein signaling [52]. In avian neurons, Thy-1 interacts with fyn, Gαi family members, and α- and β-tubulin within lipid rafts (Table 2; Fig. 1B). Addition of an anti-Thy-1 antibody decreased the overall kinase activity within the isolated lipid rafts [54], and these changes in signaling may contribute to the effects of Thy-1 on neurite outgrowth. Additionally, using primary rat cerebellar neurons, Thy-1 was isolated from lipid rafts enriched with prion protein, lyn, and fyn [55]. In fish retinal ganglion cell axons and fish and rat growth cones, Thy-1 co-immunoprecipitates with reggie-1 and reggie-2 in noncaveolar lipid rafts, suggesting that Thy-1 may modulate axon regeneration [56, 57]. Interestingly, Thy-1 knockout mice develop grossly normal brains and spinal cords, and axon regeneration following spinal cord injury was not detected [58], perhaps indicating species-specific functions of Thy-1 in neural tissue. Further investigation will be necessary to elucidate the exact role for Thy-1 in neuronal network formation.
The effects of Thy-1 expression on tyrosine kinase activity relevant to cell morphology and cell migration have also been investigated in fibroblasts. SFK activation differs in Thy-1 (+) and (−) pulmonary fibroblasts. At baseline, SFK and p190 RhoGAP activities are increased in Thy-1 (−) fibroblasts, resulting in inactive Rho. Thy-1 (+) pulmonary fibroblasts, however, have decreased SFK and p190 RhoGAP activity and active Rho [59] (Table 2; Fig. 1B). SFK signaling modulates focal adhesion turnover, and Rho activation can promote focal adhesion formation [60, 61]. Consistent with the different baseline levels of these kinases, Thy-1 (+) and (−) fibroblasts differ in cytoskeletal morphology and migration. Thy-1 (−) pulmonary fibroblasts are polygonal, and contain small peripheral focal adhesions and fewer actin stress fibers [59, 62]. Thy-1 (−) pulmonary fibroblasts are also more migratory at baseline in a wound healing assay [59]. Thy-1 (+) pulmonary fibroblasts are spindle-shaped, have long focal adhesions and well-organized stress fibers, and are less migratory at baseline [59]. Following treatment with the matricellular protein thrombospondin-1, only Thy-1 (+) pulmonary fibroblasts undergo focal adhesion disassembly. This differential response to Thy-1 is due to selective activation of phosphatidylinositol 3-kinase (PI 3-kinase) and SFK in only Thy-1 (+) cells [63] (Fig. 1B). Perhaps Thy-1 is important in regulating cell migration signaling pathways activated in response to matricellular proteins such as thrombospondin-1, which are present during early wound healing following injury. Loss or absence of Thy-1 expression, such as in a fibrotic condition, leads to dysregulated cell migration.
Because the GPI anchor of Thy-1 does not traverse the cell membrane, it is unclear how Thy-1 activates cytoplasmic tyrosine kinases. The GPI anchor of Thy-1 may directly interact with palmitoylated and myristylated cysteines on these kinases. Other GPI-anchored proteins, such as CD55 and CD59, also interact with cytoplasmic tyrosine kinases, and site-directed mutagenesis of palmitoylation sites on the SFK members lck and fyn inhibits their interaction with these GPI-anchored proteins [64]. Alternatively, Thy-1 may interact with cytoplasmic tyrosine kinases through an adapter protein. Co-activation of Thy-1 and CD3 activates the lipid raft transmembrane adapter protein linker for activation of T cells (LAT) [65] (Fig. 1B). Thy-1 also interacts with an 85–90 kDa transmembrane phosphorylated protein that contains binding sites for SH2 domain-containing proteins, including fyn, csk, PI 3-kinase, ras GTPase activating protein (GAP), vav, and lck [66]. Investigation of the mechanism by which Thy-1 activates these intracellular signaling molecules will further our understanding of the functions of Thy-1, as well as those of other GPI-anchored proteins.
3. Thy-1 and apoptosis
Thy-1 signals cell death in malignant mouse T cells, thymocytes and glomerular mesangial cells [31–34]. In vitro, the addition of cross-linking anti-Thy-1 antibodies to rat glomerular mesangial cells induces apoptosis, characterized by TdT-mediated dUTP nick-end labeling (TUNEL) and annexin V assays [32]. In vivo, injection of an anti-Thy-1 antibody into rats induces kidney mesangial cell death and glomerulonephritis, suggesting Thy-1 may play a role in glomerular fucntion [34, 67]. Aged Thy-1 deficient mice are more susceptible to T cell lymphomas, suggesting that Thy-1 may function in negative selection possibly by regulating apoptosis [68].
Cell death induced by the anti-Thy-1 antibody involves both apoptotic proteins and cell cycle regulators. Following antibody-induced Thy-1 aggregation, caspases are activated and the anti-apoptotic bcl-2 family members bcl-2 and bcl-XL are downregulated in malignant mouse T cell lymphoma cells [33] (Fig. 1C). The apoptosis-related genes nerve growth factor induced protein I-B (NGFI-B) and DNA-damage-inducible protein 45 gamma (Gadd 45 γ) are also upregulated in the anti-Thy-1-induced glomerulonephritis model [69].
Thy-1 associates with Src family kinase (SFK) members fyn and lyn in rat mesangial cells and fyn in T cells [70, 71]. This signaling may be necessary for Thy-1-induced apoptosis (Fig. 1B). Thy-1 induction of apoptosis in glomerular mesangial cells requires inositol triphosphate production and protein tyrosine kinase signaling, as well as an increase in intracellular calcium [72].
Electron microscopy studies suggested that the mesangial cell death induced by anti-Thy-1 antibodies may be necrotic rather than apoptotic, as no chromatin condensation, nuclear membrane disruption, cell swelling, or organelle degradation were detected [73]. However, more recent studies have determined that anti-Thy-1-induced nephritis is a combination of complement-mediated necrosis and apoptosis [35]. As Thy-1 is also expressed on human mesangial cells [74], understanding the role of Thy-1 in inducing mesangial cell death in mice may have implications in the understanding of the pathogenesis and treatment of renal diseases in humans. To date, changes in expression of Thy-1 in human renal disease have not been reported. Because loss of Thy-1 expression has been associated with cancer and fibrosis, it will be interesting to determine whether its effects on cellular apoptosis and survival are important in these conditions.
4. Thy-1 and cell proliferation
Hematopoietic stem cells sorted based on Thy-1 expression differ in cell cycle distribution. Following cytokine stimulation for 6 days, Thy-1 (+) stem cells entered the cell cycle, whereas Thy-1 (−) cells remained quiescent. Because proliferation of gene-expressing cells is necessary for a therapeutic effect in gene therapy, these differences may be useful in targeting stem cells that are better suited for gene therapy [75]. Anti-Thy-1-induced T cell proliferation requires signaling via calcinuerin, protein tyrosine kinases, PI-3 kinase, protein kinase C, and MAPK [25] (Table 2). Thy-1 (−) fibroblasts are more proliferative in response to fibrogenic cytokines and growth factors, as discussed below.
In the anti-Thy-1-induced nephritis model, as stated above, mesangial cells undergo cell death by a mechanism combining apoptosis and necrosis during the early phase [35], but then secondarily proliferate via activation of Smad1 and STAT3 during the late phase [76] (Table 2; Fig. 1D). Treatment with mycophenolate mofetil, an inhibitor of de novo guanosine nucleotide synthesis, or roscovitine, a cyclin-dependent kinase inhibitor, reduced mesangial cell proliferation by downregulating cyclin D expression and upregulating the cyclin inhibitor p27kip1, suggesting these drugs may be useful in preventing mesangial proliferative glomerulonephritis [77]. Antibody to Thy-1 induces mesangial cell apoptosis or proliferation in a time-dependent manner that appears to be due to activation of different signaling pathways.
On the other hand, Thy-1 may inhibit proliferation and thus function as a tumor suppressor in ovarian cancer and nasopharyngeal carcinoma. Thy-1 is expressed on non-tumorigenic human ovarian cancer cell lines [30]. Addition of Thy-1 antisense into a non-tumorigenic clone restored tumorigenicity. Furthermore, SCID mice injected subcutaneously with ovarian cancer cells expressing Thy-1 formed tumors that were smaller and propagated more slowly than ovarian cancer cells not expressing Thy-1 [28]. Additionally, Thy-1 may function as a tumor suppressor by up-regulating fibronectin and the anti-angiogenic molecule thrombospondin-1 [29] (Fig. 1E).
Epigenetic suppression of Thy-1 expression due to promoter hypermethylation has been detected in many nasopharyngeal cell carcinoma (NPC) cell lines, as well as in NPC tumor samples. Colony formation of NPC HONE1 cells is decreased following re-expression of Thy-1 [8]. Oncogenic transformation of NIH 3T3 cells by ras oncoproteins, resulting in anchorage-independent growth and soft agar colony formation, is associated with loss of Thy-1 surface expression [78]. As with proliferation, the role of Thy-1 in tumorigenesis is unclear. Thy-1 facilitates melanoma cell migration through a transendothelial cell monolayer [47], yet functions as a tumor suppressor in ovarian cancer and NPC [8, 28–30]. Differences in the role of Thy-1 in cell proliferation may be cell type-specific, and the effects of Thy-1 on tumorigenicity may be mediated through non-proliferative mechanisms. It will be interesting to examine whether Thy-1 knockout mice are more susceptible to tumor invasion and metastasis.
5. Thy-1 and cytokine/growth factor signaling
Normal lung fibroblasts are heterogeneous, and the most extensively characterized in vitro model of fibroblast heterogeneity is based on the cell surface expression of Thy-1 [37, 62]. Fibroblasts sorted based on Thy-1 expression differ in their response to and/or production of many cytokines and growth factors (Table 3; Fig. 1D). Thy-1 (+) splenic fibroblasts secrete greater levels of interleukin (IL)-6 at baseline, but only Thy-1 (−) pulmonary fibroblasts secrete IL-1 following tumor necrosis factor (TNF)-α stimulation [36, 79]. Following IL-1β stimulation, Thy-1 (−) pulmonary fibroblasts have increased proliferation and IL-6 expression as compared to Thy-1 (+) fibroblasts [38]. Interestingly, both subsets express IL-1 receptor components and activate NFκB-1 in response to IL-1β, suggesting that Thy-1 may affect non-canonical IL-1 signaling pathways. Thy-1 (−) pulmonary fibroblasts express higher levels of platelet-derived growth factor (PDGF)-α and are selectively responsive to PDGF-AA-induced proliferation [39]. Furthermore, PDGF stimulation of human smooth muscle cells increases the levels of Thy-1 localized to lipid rafts [80].
Table 3.
Summary of Thy-1 and cytokine/growth factor signaling in fibroblasts
| Cytokine/Growth Factor stimulation | Differential fibroblast response |
|---|---|
| At baseline | Thy-1 (+) splenic fibroblasts secrete higher levels of IL-6 |
| At baseline | Thy-1 (+) myometrial fibroblasts express COX-1 and secrete high levels of PGE2, and Thy-1 (−) fibroblasts express COX-2 |
| TNFα | Only Thy-1 (−) pulmonary fibroblasts secrete IL-1 and activate latent TGF-β |
| IL-1β | Only Thy-1 (−) pulmonary fibroblasts have increased IL-6 secretion, increased proliferation, and activation of latent TGF-β |
| PDGF | Only Thy-1 (−) pulmonary fibroblasts express PDGF-α, undergo PDGF-AA-induced proliferation, and activate latent TGF-β |
| Fibrogenic stimuli | Thy-1 (−) pulmonary fibroblasts produce more TGF-β, and only Thy- 1 (−) pulmonary fibroblasts are able to activate latent TGF-β |
Non-lung fibroblasts can also be divided into heterogeneous populations based on the expression of Thy-1. Fibroblasts isolated from the human female reproductive tract differ in cyclooxygenase (COX) expression and prostaglandin (PG) release. Thy-1 (+) myometrial fibroblasts express high levels of COX-1 and produce high levels of PGE2, whereas Thy-1 (−) fibroblasts constitutively express COX-2 and produce low levels of PGE2 [81] (Fig. 1D).
The differing responses of Thy-1 (+) vs. (−) fibroblast subpopulations to cytokines and growth factors suggest that Thy-1 may affect fibroblast function during wound healing and fibrosis. In response to fibrogenic stimuli, Thy-1 (−) pulmonary fibroblasts produce more latent TGF-β than Thy-1 (+) fibroblasts and are selectively able to activate latent TGF-β, suggesting Thy-1 expression may provide protection from a fibrogenic response [82, 83] (Fig. 1D). The role for Thy-1 in fibrosis has been confirmed in vivo. Intratracheal bleomycin administration induces pulmonary fibrosis in animal models characterized by pulmonary parenchymal inflammation, epithelial cell injury, interstitial and intra-alveolar fibrosis, and formation of fibroblastic foci [84, 85]. Following intratracheal bleomycin, Thy-1 knockout mice develop more severe pulmonary fibrosis than wild type mice. Fibrotic lesions from bleomycin-exposed wild-type mice contain mostly Thy-1 (−) myofibroblasts with active TGF-β. Furthermore, tissue sections from patients diagnosed with idiopathic pulmonary fibrosis contain fibroblastic foci composed of Thy-1 (−) myofibroblasts, whereas most normal human lung fibroblasts are Thy-1 (+) [14].
Myofibroblasts are contractile fibroblasts expressing α-smooth muscle actin (SMA), and these cells often persist in fibrotic lesions [86, 87]. It remains unclear how Thy-1 expression affects the ability of fibroblasts to differentiate into myofibroblasts (Fig. 1D). In pulmonary fibrosis, lack of Thy-1 expression seems to correlate with myofibroblast differentiation in the mouse bleomycin model and in human idiopathic pulmonary fibrosis [14]. Furthermore, Thy-1 (−) pulmonary fibroblasts have higher α-SMA protein expression at baseline and in response to TGF-β [83]. IL-1 and TNFα treatment of pulmonary fibroblasts promotes a loss of Thy-1 expression and differentiation to a myofibroblast phenotype [14]. However, the relationship of Thy-1 to the myofibroblasts phenotype appears to be tissue-dependent. Following treatment with TGF-β or supernatant from platelets, only Thy-1 (+) human myometrial and orbital fibroblasts differentiated into myofibroblasts expressing α-SMA. Thy-1 (−) cells instead differentiated into lipofibroblasts [88]. The observed differences may be a result of different roles for Thy-1 in different tissues.
6. Thy-1 and lipid raft signaling
The GPI anchor of Thy-1 localizes the protein to specific lipid raft microdomains. When the GPI anchor of Thy-1 was replaced with sequences from the transmembrane domains of neural cell adhesion molecule (NCAM) or of CD8, Thy-1-mediated inhibition of neurite outgrowth was blocked. In cells expressing a transmembrane domain in place of the GPI anchor, Thy-1 was localized to different lipid raft microdomains than was wild type Thy-1 [89]. Both wild type/GPI-anchored Thy-1 and transmembrane Thy-1 localized predominantly to filopodia, but GPI-Thy-1 was excluded from coated pits [89]. Moreover, Thy-1 localizes to a lipid raft microdomain distinct from that of the GPI-anchored prion protein [90, 91]. Lipid rafts containing prion protein contained significantly higher levels of unsaturated, longer chain lipids, whereas lipid rafts containing Thy-1 had 5-fold higher levels of hexosylceramide. The authors suggest that the differences in localization of these two GPI-anchored proteins reflect protein-specific trafficking and solubility [90]. Furthermore, localization to specific domains within lipid rafts may place Thy-1 in proximity to other signaling molecules such as cytoplasmic tyrosine kinases. For example, Thy-1 associates with reggie-1 and -2, prion protein, and fyn and lyn within lipid rafts [55–57]. Crosslinking of prion protein in Jurkat and human peripheral blood T cells induces recruitment of Thy-1, TCR/CD3, fyn, lck, and LAT to reggie-containing lipid rafts [92]. There is also evidence that Thy-1 signaling requires lipid raft integrity. Disruption of lipid rafts blocks thrombospondin-1-induced focal adhesion disassembly in Thy-1 (+) pulmonary fibroblasts [63]. Much work still needs to be done in determining the role of Thy-1 in lipid raft signaling. Thy-1 may signal and recruits other signaling molecules via interaction with a transmembrane adapter protein or via lipid interactions with palmitoylated and myristylated cytoplasmic tyrosine kinases. Alterantively, Thy-1 may not signal directly, but may function as a scaffolding or partitioning protein within lipid rafts.
7. Concluding Remarks
Thy-1 has diverse cellular functions and activates multiple signaling pathways, affecting cell interactions with the extracellular environment or with other cells, and influencing cell proliferation, differentiation, and survival. Further exploration of the role of Thy-1 signaling in these cell processes in vitro and in animal models may advance our understanding of nerve regeneration, glomerulonephritis, tumorigenesis, wound healing and fibrosis in humans. Thy-1 interacts with both integrins and cytoplasmic tyrosine kinases to promote cell adhesion. Thy-1 appears to inhibit cellular migration at baseline, but it may facilitate regulated migration in response to injury, via both tyrosine kinase- and lipid raft-dependent mechanisms. The interaction with cytoplasmic tyrosine kinases may also be required for Thy-1-induced apoptosis, and potentially for the development of glomerulonephritis. Thy-1 also affects proliferation of tumor cells and fibroblasts, suggesting a role for Thy-1 in tumorigenesis and fibrogenesis. It is important to reiterate that the effects of Thy-1 are tissue- and cell type-specific.
Although Thy-1 signaling has been presented in this review as activating multiple signaling pathways, it is possible that Thy-1 signals through few pathways or a single pathway, and that the other signaling cascades discussed in this article are connected via cross-talk. For example, there is evidence that Thy-1 signals via the MAPK pathway. Anti-Thy-1 induced T cell proliferation requires MEK1 signaling, and MAPK signaling was required for IL-1β-induced COX-2 expression in Thy-1 (+) myometrial fibroblasts [25, 81]. MAPK signaling is involved in many of the processes that Thy-1 modulates, including apoptosis, cell proliferation, focal adhesion disassembly [93]. It will be necessary to further dissect signaling molecules activated downstream of Thy-1 to determine if Thy-1 is indeed part of a single signaling pathway or multiple pathways.
Although much work has been done in elucidating the role of Thy-1 within signaling pathways, the exact mechanism(s) by which Thy-1 signals remains unclear. Potentially, Thy-1 may directly interact with signaling molecules such as SFK and integrins. Additionally, Thy-1 may be part of a larger signaling complex including transmembrane adapter proteins and cytoplasmic signaling molecules. Regardless of the mechanism(s), there is an established role for Thy-1 in multiple cellular processes with broad clinical significance.
Acknowledgments
Supported in part by National Institutes of Health (NIH) grant HL065348 to J.S.H. and fellowship NS49674 to T.A.R., and Research Facilities Improvement Program Grant No. C06 RR 15490 from the National Center for Research Resources.
References
- 1.Craig W, Kay R, Cutler RL, Lansdorp PM. Expression of Thy-1 on human hematopoietic progenitor cells. J Exp Med. 1993;177:1331–42. doi: 10.1084/jem.177.5.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saalbach A, Wetzig T, Haustein UF, Anderegg U. Detection of human soluble Thy-1 in serum by ELISA. Fibroblasts and activated endothelial cells are a possible source of soluble Thy-1 in serum. Cell Tissue Res. 1999;298:307–15. doi: 10.1007/s004419900079. [DOI] [PubMed] [Google Scholar]
- 3.Xue GP, Calvert RA, Morris RJ. Expression of the neuronal surface glycoprotein Thy-1 is under post-transcriptional control, and is spatially regulated, in the developing olfactory system. Development. 1990;109:851–64. doi: 10.1242/dev.109.4.851. [DOI] [PubMed] [Google Scholar]
- 4.Barlow JZ, Huntley GW. Developmentally regulated expression of Thy-1 in structures of the mouse sensory-motor system. J Comp Neurol. 2000;421:215–33. doi: 10.1002/(sici)1096-9861(20000529)421:2<215::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.Bukovsky A, Presl J, Zidovsky J, Mancal P. The localization of Thy-1.1, MRC OX 2 and Ia antigens in the rat ovary and fallopian tube. Immunology. 1983;48:587–96. [PMC free article] [PubMed] [Google Scholar]
- 6.Presl J, Bukovsky A. Role of Thy-1+ and Ia+ cells in ovarian function. Biol Reprod. 1986;34:159–69. doi: 10.1095/biolreprod34.1.159. [DOI] [PubMed] [Google Scholar]
- 7.Xue GP, Rivero BP, Morris RJ. The surface glycoprotein Thy-1 is excluded from growing axons during development: a study of the expression of Thy-1 during axogenesis in hippocampus and hindbrain. Development. 1991;112:161–76. doi: 10.1242/dev.112.1.161. [DOI] [PubMed] [Google Scholar]
- 8.Lung HL, Bangarusamy DK, Xie D, Cheung AK, Cheng Y, Kumaran MK, Miller L, Liu ET, Guan XY, Sham JS, Fang Y, Li L, Wang N, Protopopov AI, Zabarovsky ER, Tsao SW, Stanbridge EJ, Lung ML. THY1 is a candidate tumour suppressor gene with decreased expression in metastatic nasopharyngeal carcinoma. Oncogene. 2005;24:6525–32. doi: 10.1038/sj.onc.1208812. [DOI] [PubMed] [Google Scholar]
- 9.Sneller MC, Gunter KC. DNA methylation alters chromatin structure and regulates Thy-1 expression in EL-4 T cells. J Immunol. 1987;138:3505–12. [PubMed] [Google Scholar]
- 10.Almqvist P, Carlsson SR. Characterization of a hydrophilic form of Thy-1 purified from human cerebrospinal fluid. J Biol Chem. 1988;263:12709–15. [PubMed] [Google Scholar]
- 11.Xue GP, Morris R. Expression of the neuronal surface glycoprotein Thy-1 does not follow appearance of its mRNA in developing mouse Purkinje cells. J Neurochem. 1992;58:430–40. doi: 10.1111/j.1471-4159.1992.tb09740.x. [DOI] [PubMed] [Google Scholar]
- 12.Saleh M, Barlett PF. Evidence from neuron/lymphoma heterokaryons for a common trans-acting factor suppressing Thy-1 expression. J Neuroimmunol. 1989;23:203–14. doi: 10.1016/0165-5728(89)90052-0. [DOI] [PubMed] [Google Scholar]
- 13.Freimuth WW, Esselman WJ, Miller HC. Release of thy-1.2 and thy-1.1 from lymphoblastoid cells: partial characterization and antigenicity of shed material. J Immunol. 1978;120:1651–8. [PubMed] [Google Scholar]
- 14.Hagood JS, Prabhakaran P, Kumbla P, Salazar L, Macewen MW, Barker TH, Ortiz LA, Schoeb T, Siegal GP, Alexander CB, Pardo A, Selman M. Loss of fibroblast thy-1 expression correlates with lung fibrogenesis. Am J Pathol. 2005;167:365–79. doi: 10.1016/S0002-9440(10)62982-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nosten-Bertrand M, Errington ML, Murphy KP, Tokugawa Y, Barboni E, Kozlova E, Michalovich D, Morris RG, Silver J, Stewart CL, Bliss TV, Morris RJ. Normal spatial learning despite regional inhibition of LTP in mice lacking Thy-1. Nature. 1996;379:826–9. doi: 10.1038/379826a0. [DOI] [PubMed] [Google Scholar]
- 16.Mayeux-Portas V, File SE, Stewart CL, Morris RJ. Mice lacking the cell adhesion molecule Thy-1 fail to use socially transmitted cues to direct their choice of food. Curr Biol. 2000;10:68–75. doi: 10.1016/s0960-9822(99)00278-x. [DOI] [PubMed] [Google Scholar]
- 17.Feng H, Zhao WQ, Bernard CC, Ng KT, Sedman G. Purified antichick Thy-1 IgG abolishes intermediate and long-term memory. Physiol Behav. 1993;53:215–9. doi: 10.1016/0031-9384(93)90196-m. [DOI] [PubMed] [Google Scholar]
- 18.Beissert S, He HT, Hueber AO, Lellouch AC, Metze D, Mehling A, Luger TA, Schwarz T, Grabbe S. Impaired cutaneous immune responses in Thy-1-deficient mice. J Immunol. 1998;161:5296–302. [PubMed] [Google Scholar]
- 19.Simon PD, McConnell J, Zurakowski D, Vorwerk CK, Naskar R, Grosskreutz CL, Dreyer EB. Thy-1 is critical for normal retinal development. Brain Res Dev Brain Res. 1999;117:219–23. doi: 10.1016/s0165-3806(99)00123-6. [DOI] [PubMed] [Google Scholar]
- 20.Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. Faseb J. 2006;20:1045–54. doi: 10.1096/fj.05-5460rev. [DOI] [PubMed] [Google Scholar]
- 21.Marmor MD, Julius M. The function of GPI-anchored proteins in T cell development, activation and regulation of homeostasis. J Biol Regul Homeost Agents. 2000;14:99–115. [PubMed] [Google Scholar]
- 22.Marmor MD, Bachmann MF, Ohashi PS, Malek TR, Julius M. Immobilization of glycosylphosphatidylinositol-anchored proteins inhibits T cell growth but not function. Int Immunol. 1999;11:1381–93. doi: 10.1093/intimm/11.9.1381. [DOI] [PubMed] [Google Scholar]
- 23.Haeryfar SM, Hoskin DW. Thy-1: more than a mouse pan-T cell marker. J Immunol. 2004;173:3581–8. doi: 10.4049/jimmunol.173.6.3581. [DOI] [PubMed] [Google Scholar]
- 24.Haeryfar SM, Al-Alwan MM, Mader JS, Rowden G, West KA, Hoskin DW. Thy-1 signaling in the context of costimulation provided by dendritic cells provides signal 1 for T cell proliferation and cytotoxic effector molecule expression, but fails to trigger delivery of the lethal hit. J Immunol. 2003;171:69–77. doi: 10.4049/jimmunol.171.1.69. [DOI] [PubMed] [Google Scholar]
- 25.Haeryfar SM, Hoskin DW. Selective pharmacological inhibitors reveal differences between Thy-1- and T cell receptor-mediated signal transduction in mouse T lymphocytes. Int Immunopharmacol. 2001;1:689–98. doi: 10.1016/s1567-5769(01)00002-9. [DOI] [PubMed] [Google Scholar]
- 26.Avalos AM, Arthur WT, Schneider P, Quest AF, Burridge K, Leyton L. Aggregation of integrins and RhoA activation are required for Thy-1-induced morphological changes in astrocytes. J Biol Chem. 2004;279:39139–45. doi: 10.1074/jbc.M403439200. [DOI] [PubMed] [Google Scholar]
- 27.Leyton L, Schneider P, Labra CV, Ruegg C, Hetz CA, Quest AF, Bron C. Thy-1 binds to integrin beta(3) on astrocytes and triggers formation of focal contact sites. Curr Biol. 2001;11:1028–38. doi: 10.1016/s0960-9822(01)00262-7. [DOI] [PubMed] [Google Scholar]
- 28.Abeysinghe HR, Pollock SJ, Guckert NL, Veyberman Y, Keng P, Halterman M, Federoff HJ, Rosenblatt JP, Wang N. The role of the THY1 gene in human ovarian cancer suppression based on transfection studies. Cancer Genet Cytogenet. 2004;149:1–10. doi: 10.1016/S0165-4608(03)00234-6. [DOI] [PubMed] [Google Scholar]
- 29.Abeysinghe HR, Li LQ, Guckert NL, Reeder J, Wang N. THY-1 induction is associated with up-regulation of fibronectin and thrombospondin-1 in human ovarian cancer. Cancer Genet Cytogenet. 2005;161:151–8. doi: 10.1016/j.cancergencyto.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Abeysinghe HR, Cao Q, Xu J, Pollock S, Veyberman Y, Guckert NL, Keng P, Wang N. THY1 expression is associated with tumor suppression of human ovarian cancer. Cancer Genet Cytogenet. 2003;143:125–32. doi: 10.1016/s0165-4608(02)00855-5. [DOI] [PubMed] [Google Scholar]
- 31.Fujita N, Kodama N, Kato Y, Lee SH, Tsuruo T. Aggregation of Thy-1 glycoprotein induces thymocyte apoptosis through activation of CPP32-like proteases. Exp Cell Res. 1997;232:400–6. doi: 10.1006/excr.1997.3505. [DOI] [PubMed] [Google Scholar]
- 32.Sato T, van Dixhoorn MG, Schroeijers WE, Huizinga TW, Reutelingsperger CP, van Es LA, Daha MR. Apoptosis of cultured rat glomerular mesangial cells induced by IgG2a monoclonal anti-Thy-1 antibodies. Kidney Int. 1996;49:403–12. doi: 10.1038/ki.1996.59. [DOI] [PubMed] [Google Scholar]
- 33.Fujita N, Kato Y, Naito M, Tsuruo T. A novel anti-Thy-1 (CD90) monoclonal antibody induces apoptosis in mouse malignant T-lymphoma cells in spite of inducing bcl-2 expression. Int J Cancer. 1996;66:544–50. doi: 10.1002/(SICI)1097-0215(19960516)66:4<544::AID-IJC20>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 34.Morita H, Isobe K, Cai Z, Miyazaki T, Matsumoto Y, Shinzato T, Yoshikai Y, Kimata K, Maeda K. Thy-1 antigen mediates apoptosis of rat glomerular cells in vitro and in vivo. Nephron. 1996;73:293–8. doi: 10.1159/000189054. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu A, Masuda Y, Kitamura H, Ishizaki M, Ohashi R, Sugisaki Y, Yamanaka N. Complement-mediated killing of mesangial cells in experimental glomerulonephritis: cell death by a combination of apoptosis and necrosis. Nephron. 2000;86:152–60. doi: 10.1159/000045734. [DOI] [PubMed] [Google Scholar]
- 36.Borrello MA, Phipps RP. Differential Thy-1 expression by splenic fibroblasts defines functionally distinct subsets. Cell Immunol. 1996;173:198–206. doi: 10.1006/cimm.1996.0268. [DOI] [PubMed] [Google Scholar]
- 37.Fries KM, Blieden T, Looney RJ, Sempowski GD, Silvera MR, Willis RA, Phipps RP. Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin Immunol Immunopathol. 1994;72:283–92. doi: 10.1006/clin.1994.1144. [DOI] [PubMed] [Google Scholar]
- 38.Hagood JS, Mangalwadi A, Guo B, MacEwen MW, Salazar L, Fuller GM. Concordant and discordant interleukin-1-mediated signaling in lung fibroblast thy-1 subpopulations. Am J Respir Cell Mol Biol. 2002;26:702–8. doi: 10.1165/ajrcmb.26.6.4547. [DOI] [PubMed] [Google Scholar]
- 39.Hagood JS, Miller PJ, Lasky JA, Tousson A, Guo B, Fuller GM, McIntosh JC. Differential expression of platelet-derived growth factor-alpha receptor by Thy-1(−) and Thy-1(+) lung fibroblasts. Am J Physiol. 1999;277:L218–24. doi: 10.1152/ajplung.1999.277.1.L218. [DOI] [PubMed] [Google Scholar]
- 40.Hagood JS, Lasky JA, Nesbitt JE, Segarini P. Differential expression, surface binding, and response to connective tissue growth factor in lung fibroblast subpopulations. Chest. 2001;120:64S–66S. doi: 10.1378/chest.120.1_suppl.s64. [DOI] [PubMed] [Google Scholar]
- 41.ffrench-Constant C, Colognato H. Integrins: versatile integrators of extracellular signals. Trends Cell Biol. 2004;14:678–86. doi: 10.1016/j.tcb.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 43.Choi J, Leyton L, Nham SU. Characterization of alphaX I-domain binding to Thy-1. Biochem Biophys Res Commun. 2005;331:557–61. doi: 10.1016/j.bbrc.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Wetzel A, Chavakis T, Preissner KT, Sticherling M, Haustein UF, Anderegg U, Saalbach A. Human Thy-1 (CD90) on activated endothelial cells is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) J Immunol. 2004;172:3850–9. doi: 10.4049/jimmunol.172.6.3850. [DOI] [PubMed] [Google Scholar]
- 45.Avalos AM, Labra CV, Quest AF, Leyton L. Signaling triggered by Thy-1 interaction with beta 3 integrin on astrocytes is an essential step towards unraveling neuronal Thy-1 function. Biol Res. 2002;35:231–8. doi: 10.4067/s0716-97602002000200015. [DOI] [PubMed] [Google Scholar]
- 46.Saalbach A, Wetzel A, Haustein UF, Sticherling M, Simon JC, Anderegg U. Interaction of human Thy-1 (CD 90) with the integrin alphavbeta3 (CD51/CD61): an important mechanism mediating melanoma cell adhesion to activated endothelium. Oncogene. 2005;24:4710–20. doi: 10.1038/sj.onc.1208559. [DOI] [PubMed] [Google Scholar]
- 47.Saalbach A, Hildebrandt G, Haustein UF, Anderegg U. The Thy-1/Thy-1 ligand interaction is involved in binding of melanoma cells to activated Thy-1- positive microvascular endothelial cells. Microvasc Res. 2002;64:86–93. doi: 10.1006/mvre.2002.2401. [DOI] [PubMed] [Google Scholar]
- 48.Saalbach A, Haustein UF, Anderegg U. A ligand of human thy-1 is localized on polymorphonuclear leukocytes and monocytes and mediates the binding to activated thy-1-positive microvascular endothelial cells and fibroblasts. J Invest Dermatol. 2000;115:882–8. doi: 10.1046/j.1523-1747.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- 49.Tiveron MC, Barboni E, Pliego Rivero FB, Gormley AM, Seeley PJ, Grosveld F, Morris R. Selective inhibition of neurite outgrowth on mature astrocytes by Thy-1 glycoprotein. Nature. 1992;355:745–8. doi: 10.1038/355745a0. [DOI] [PubMed] [Google Scholar]
- 50.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–69. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 51.Seki M, Nawa H, Morioka T, Fukuchi T, Oite T, Abe H, Takei N. Establishment of a novel enzyme-linked immunosorbent assay for Thy-1; quantitative assessment of neuronal degeneration. Neurosci Lett. 2002;329:185–8. doi: 10.1016/s0304-3940(02)00654-7. [DOI] [PubMed] [Google Scholar]
- 52.Doherty P, Singh A, Rimon G, Bolsover SR, Walsh FS. Thy-1 antibody-triggered neurite outgrowth requires an influx of calcium into neurons via N- and L-type calcium channels. J Cell Biol. 1993;122:181–9. doi: 10.1083/jcb.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo L, O'Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–56. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- 54.Henke RC, Seeto GS, Jeffrey PL. Thy-1 and AvGp50 signal transduction complex in the avian nervous system: c-Fyn and G alpha i protein association and activation of signalling pathways. J Neurosci Res. 1997;49:655–70. doi: 10.1002/(SICI)1097-4547(19970915)49:6<655::AID-JNR1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 55.Loberto N, Prioni S, Bettiga A, Chigorno V, Prinetti A, Sonnino S. The membrane environment of endogenous cellular prion protein in primary rat cerebellar neurons. J Neurochem. 2005;95:771–83. doi: 10.1111/j.1471-4159.2005.03397.x. [DOI] [PubMed] [Google Scholar]
- 56.Deininger SO, Rajendran L, Lottspeich F, Przybylski M, Illges H, Stuermer CA, Reuter A. Identification of teleost Thy-1 and association with the microdomain/lipid raft reggie proteins in regenerating CNS axons. Mol Cell Neurosci. 2003;22:544–54. doi: 10.1016/s1044-7431(03)00028-9. [DOI] [PubMed] [Google Scholar]
- 57.Lang DM, Lommel S, Jung M, Ankerhold R, Petrausch B, Laessing U, Wiechers MF, Plattner H, Stuermer CA. Identification of reggie-1 and reggie-2 as plasmamembrane-associated proteins which cocluster with activated GPI-anchored cell adhesion molecules in non-caveolar micropatches in neurons. J Neurobiol. 1998;37:502–23. doi: 10.1002/(sici)1097-4695(199812)37:4<502::aid-neu2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 58.Barlow JZ, Kelley KA, Bozdagi O, Huntley GW. Testing the role of the cell-surface molecule Thy-1 in regeneration and plasticity of connectivity in the CNS. Neuroscience. 2002;111:837–52. doi: 10.1016/s0306-4522(02)00023-4. [DOI] [PubMed] [Google Scholar]
- 59.Barker TH, Grenett HE, MacEwen MW, Tilden SG, Fuller GM, Settleman J, Woods A, Murphy-Ullrich J, Hagood JS. Thy-1 regulates fibroblast focal adhesions, cytoskeletal organization and migration through modulation of p190 RhoGAP and Rho GTPase activity. Exp Cell Res. 2004;295:488–96. doi: 10.1016/j.yexcr.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 60.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 61.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–79. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 62.Phipps RP, Penney DP, Keng P, Quill H, Paxhia A, Derdak S, Felch ME. Characterization of two major populations of lung fibroblasts: distinguishing morphology and discordant display of Thy 1 and class II MHC. Am J Respir Cell Mol Biol. 1989;1:65–74. doi: 10.1165/ajrcmb/1.1.65. [DOI] [PubMed] [Google Scholar]
- 63.Barker TH, Pallero MA, MacEwen MW, Tilden SG, Woods A, Murphy-Ullrich JE, Hagood JS. Thrombospondin-1-induced focal adhesion disassembly in fibroblasts requires Thy-1 surface expression, lipid raft integrity, and Src activation. J Biol Chem. 2004;279:23510–6. doi: 10.1074/jbc.M402169200. [DOI] [PubMed] [Google Scholar]
- 64.Shenoy-Scaria AM, Gauen LK, Kwong J, Shaw AS, Lublin DM. Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Mol Cell Biol. 1993;13:6385–92. doi: 10.1128/mcb.13.10.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leyton L, Quest AF, Bron C. Thy-1/CD3 coengagement promotes TCR signaling and enhances particularly tyrosine phosphorylation of the raft molecule LAT. Mol Immunol. 1999;36:755–68. doi: 10.1016/s0161-5890(99)00086-3. [DOI] [PubMed] [Google Scholar]
- 66.Durrheim GA, Garnett D, Dennehy KM, Beyers AD. Thy-1 associated pp85--90 is a potential docking site for SH2 domain-containing signal transduction molecules. Cell Biol Int. 2001;25:33–42. doi: 10.1006/cbir.2000.0675. [DOI] [PubMed] [Google Scholar]
- 67.Jefferson JA, Johnson RJ. Experimental mesangial proliferative glomerulonephritis (the anti-Thy-1.1 model) J Nephrol. 1999;12:297–307. [PubMed] [Google Scholar]
- 68.Hueber AO, Bernard AM, Battari CL, Marguet D, Massol P, Foa C, Brun N, Garcia S, Stewart C, Pierres M, He HT. Thymocytes in Thy-1−/− mice show augmented TCR signaling and impaired differentiation. Curr Biol. 1997;7:705–8. doi: 10.1016/s0960-9822(06)00300-9. [DOI] [PubMed] [Google Scholar]
- 69.Xu JH, Qiu W, Wang YW, Xu J, Tong JX, Gao LJ, Xu WH, Wu YQ. Gene expression profile and overexpression of apoptosis-related genes (NGFI-B and Gadd 45 gamma) in early phase of Thy-1 nephritis model. Cell Tissue Res. 2006 doi: 10.1007/s00441-006-0214-4. [DOI] [PubMed] [Google Scholar]
- 70.Narisawa-Saito M, Yamanashi Y, Morioka T, Oite T, Shimizu F. Thy-1 molecule associates with protein tyrosine kinase(s) in rat mesangial cells. Clin Exp Immunol. 1996;106:86–90. doi: 10.1046/j.1365-2249.1996.d01-800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomas PM, Samelson LE. The glycophosphatidylinositol-anchored Thy-1 molecule interacts with the p60fyn protein tyrosine kinase in T cells. J Biol Chem. 1992;267:12317–22. [PubMed] [Google Scholar]
- 72.Narisawa-Saito M, Kimura S, Fujiwara N, Oite T, Shimoji K, Shimizu F. Thy-1-mediated phosphatidylinositol turnover in cultured rat glomerular mesangial cell. J Cell Physiol. 1996;168:705–10. doi: 10.1002/(SICI)1097-4652(199609)168:3<705::AID-JCP23>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 73.Mosley K, Collar J, Cattell V. Mesangial cell necrosis in Thy 1 glomerulonephritis--an ultrastructural study. Virchows Arch. 2000;436:567–73. doi: 10.1007/s004289900167. [DOI] [PubMed] [Google Scholar]
- 74.Sengoelge G, Perschl A, Ferrara I, Horl WH, Sunder-Plassmann G. Surface antigens of human mesangial cells: impact of growth surface or IL-1alpha. Tissue Antigens. 2002;60:383–95. doi: 10.1034/j.1399-0039.2002.600505.x. [DOI] [PubMed] [Google Scholar]
- 75.Takeda H, Yamamoto M, Morita N, Tanizawa T. Relationship between Thy-1 expression and cell-cycle distribution in human bone marrow hematopoietic progenitors. Am J Hematol. 2005;79:187–93. doi: 10.1002/ajh.20362. [DOI] [PubMed] [Google Scholar]
- 76.Takahashi T, Abe H, Arai H, Matsubara T, Nagai K, Matsuura M, Iehara N, Yokode M, Nishikawa S, Kita T, Doi T. Activation of STAT3/Smad1 is a key signaling pathway for progression to glomerulosclerosis in experimental glomerulonephritis. J Biol Chem. 2005;280:7100–6. doi: 10.1074/jbc.M411064200. [DOI] [PubMed] [Google Scholar]
- 77.Chiara M, Menegatti E, Di Simone D, Davit A, Bellis D, Sferch D, De Rosa G, Giachino O, Sena LM, Roccatello D. Mycophenolate mofetil and roscovitine decrease cyclin expression and increase p27(kip1) expression in anti Thy1 mesangial proliferative nephritis. Clin Exp Immunol. 2005;139:225–35. doi: 10.1111/j.1365-2249.2004.02684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sugimoto Y, Ikawa Y, Nakauchi H. Thy-1 as a negative growth regulator in ras-transformed mouse fibroblasts. Cancer Res. 1991;51:99–104. [PubMed] [Google Scholar]
- 79.Phipps RP, Baecher C, Frelinger JG, Penney DP, Keng P, Brown D. Differential expression of interleukin 1 alpha by Thy-1+ and Thy-1- lung fibroblast subpopulations: enhancement of interleukin 1 alpha production by tumor necrosis factor-alpha. Eur J Immunol. 1990;20:1723–7. doi: 10.1002/eji.1830200815. [DOI] [PubMed] [Google Scholar]
- 80.Maclellan DL, Steen H, Adam RM, Garlick M, Zurakowski D, Gygi SP, Freeman MR, Solomon KR. A quantitative proteomic analysis of growth factor-induced compositional changes in lipid rafts of human smooth muscle cells. Proteomics. 2005 doi: 10.1002/pmic.200500044. [DOI] [PubMed] [Google Scholar]
- 81.Koumas L, Phipps RP. Differential COX localization and PG release in Thy-1(+) and Thy-1(−) human female reproductive tract fibroblasts. Am J Physiol Cell Physiol. 2002;283:C599–608. doi: 10.1152/ajpcell.00065.2002. [DOI] [PubMed] [Google Scholar]
- 82.Silvera MR, Sempowski GD, Phipps RP. Expression of TGF-beta isoforms by Thy-1+ and Thy-1− pulmonary fibroblast subsets: evidence for TGF-beta as a regulator of IL-1-dependent stimulation of IL-6. Lymphokine Cytokine Res. 1994;13:277–85. [PubMed] [Google Scholar]
- 83.Zhou Y, Hagood JS, Murphy-Ullrich JE. Thy-1 expression regulates the ability of rat lung fibroblasts to activate transforming growth factor-beta in response to fibrogenic stimuli. Am J Pathol. 2004;165:659–69. doi: 10.1016/s0002-9440(10)63330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chua F, Gauldie J, Laurent GJ. Pulmonary fibrosis: searching for model answers. Am J Respir Cell Mol Biol. 2005;33:9–13. doi: 10.1165/rcmb.2005-0062TR. [DOI] [PubMed] [Google Scholar]
- 85.Usuki J, Fukuda Y. Evolution of three patterns of intra-alveolar fibrosis produced by bleomycin in rats. Pathol Int. 1995;45:552–64. doi: 10.1111/j.1440-1827.1995.tb03503.x. [DOI] [PubMed] [Google Scholar]
- 86.Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 87.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–3. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 88.Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003;163:1291–300. doi: 10.1016/S0002-9440(10)63488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tiveron MC, Nosten-Bertrand M, Jani H, Garnett D, Hirst EM, Grosveld F, Morris RJ. The mode of anchorage to the cell surface determines both the function and the membrane location of Thy-1 glycoprotein. J Cell Sci. 1994;107(Pt 7):1783–96. doi: 10.1242/jcs.107.7.1783. [DOI] [PubMed] [Google Scholar]
- 90.Brugger B, Graham C, Leibrecht I, Mombelli E, Jen A, Wieland F, Morris R. The membrane domains occupied by glycosylphosphatidylinositol-anchored prion protein and Thy-1 differ in lipid composition. J Biol Chem. 2004;279:7530–6. doi: 10.1074/jbc.M310207200. [DOI] [PubMed] [Google Scholar]
- 91.Madore N, Smith KL, Graham CH, Jen A, Brady K, Hall S, Morris R. Functionally different GPI proteins are organized in different domains on the neuronal surface. Embo J. 1999;18:6917–26. doi: 10.1093/emboj/18.24.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stuermer CA, Langhorst MF, Wiechers MF, Legler DF, Von Hanwehr SH, Guse AH, Plattner H. PrPc capping in T cells promotes its association with the lipid raft proteins reggie-1 and reggie-2 and leads to signal transduction. Faseb J. 2004;18:1731–3. doi: 10.1096/fj.04-2150fje. [DOI] [PubMed] [Google Scholar]
- 93.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]