Abstract
Challenges associated with the efficient and effective preparation of micro- and nano-scale (micro- and nano-gram) clinical specimens for proteomic applications include the unmitigated sample losses that occur during the processing steps. Herein we describe a simple “single-tube” preparation protocol appropriate for small proteomic samples using the organic co-solvent, trifluoroethanol (TFE) that circumvents the loss of sample by facilitating both protein extraction and protein denaturation without requiring a separate cleanup step. The performance of the TFE-based method was initially evaluated by comparisons to traditional detergent-based methods on relatively large scale sample processing using human breast cancer cells and mouse brain tissue. The results demonstrated that the TFE-based protocol provided comparable results to the traditional detergent-based protocols for larger, conventionally-sized proteomic samples (>100 μg protein content), based on both sample recovery and peptide/protein identifications. The effectiveness of this protocol for micro- and nano-scale sample processing was then evaluated for the extraction of proteins/peptides and shown effective for small mouse brain tissue samples (∼30 μg total protein content) and also for samples of ∼5 000 MCF-7 human breast cancer cells (∼500 ng total protein content), where the detergent-based methods were ineffective due to losses during cleanup and transfer steps.
Keywords: micro- and nano-scale protein extraction, sample preparation, organic co-solvent, LC-MS/MS, proteomics
Introduction
Recent advances in high resolution liquid chromatography (LC) and mass spectrometry (MS) have enabled the analysis of complex peptide mixtures,1, 2 and the use of sophisticated bioinformatics tools3-6 has empowered broad and confident protein identifications. Efficient and effective sample preparation methods are essential to achieving good protein/peptide recovery and to producing high quality mass spectra; however, current sample preparation methodologies are often accompanied by significant sample losses and are problematic for processing micro- to nano-scale complex samples. Micro-biopsies,7-9 laser capture micro-dissection (LCM),10-12 and stem cell therapy13, 14 typically provide only small, non-conventional sample sizes, and thus require improved sample processing to minimize sample losses in order to enable quantitative proteomic measurements.
A number of protocols have been commonly used for proteomic sample processing from cells, tissues, or biological fluids.15, 16 One traditional cell lysis method employs a strong denaturant (urea or guanidine hydrochloride) with different types of detergents, either ionic detergents such as sodium dodecylsulfate (SDS), or zwitterionic detergents such as 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), or non-ionic detergents such as octyl-β-glucopyranoside (OG) 17, 18, or the proprietary product RapiGest (Waters, Cambridge, MA). The detergent-based methods typically provide rapid cell lysis and protein solubilization; however, it is necessary to deplete or entirely remove the detergents or salts from the sample prior to the LC-MS analysis in order to achieve satisfying detection sensitivity for peptides. Although various peptide sample purification procedures, for example, solid phase extraction (SPE),19, 20 dialysis21, 22 and chromatography techniques,23 have been applied to purify samples prior to LC-MS analysis, a fraction of the sample is inevitably lost during the clean-up process despite careful handling. The detergent-based methods are particularly ineffective when handling sub-microgram or smaller amounts of complex proteomic samples due to the issue of sample loss during clean-up and sample transferring steps.
In order to overcome the limitations associated with detergent-based preparations for micro- and nano-scale samples, here we report a “single-tube” sample preparation protocol that uses an organic co-solvent, trifluoroethanol (TFE), with hypotonic aqueous buffer for cell lysis and protein denaturation. The use of organic solvents as alternatives to detergents in proteomic sample processing has been previously reported.24, 25 Organic solvents improve protein solubility and assist protein denaturation, and the application of this method has the distinct advantage over detergent-based protocols because organic solvents readily evaporate during the lyophilization process; thus no cleanup or tube transferring step is required prior to LC-MS analysis, minimizing sample loss. This TFE-based protocol was initially evaluated by comparing to the traditional detergent-based protocols for protein extraction from relatively large scale samples of human breast cancer MCF-7 cells and mouse brain tissues. It was demonstrated that this detergent-free, single-tube protocol provided results comparable to or better than the traditional detergent-based methods when handling relatively large, conventional (> 100 μg) quantities of protein samples based on peptide sample recovery and protein identification results from LCMS/MS analyses. The effectiveness of this TFE-based protocol for micro- and nano-scale sample processing was then demonstrated in processing mouse brain single voxel samples (a concept originated from biomedical imaging, referring to a spatially registered 3-D cube with (0.75 mm)3 of volume26, 27) containing ∼ 30 μg of proteins and also ∼ 5 000 human MCF-7 cells containing ∼ 500 ng of proteins. While traditional detergent-based protocols were proved ineffective in handling sub-microgram amounts of sample due to sample losses during the cleanup and transfer steps prior to the analysis, the preparation of 5 000 cell MCF-7 samples using the TFE-based protocol was effective, resulting in an average of 246 different peptides and 104 proteins identified from LC-MS/MS analysis, and > 5000 features (i.e., a peak with unique mass and elution time) detected from a single LC-FTICR analysis. The overall increased sensitivity in sample processing using this new protocol may have broad applications for micro-to nano-scale proteomic samples.
Experimental
Preparation of MCF-7 cell samples
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless stated otherwise. The use of the MCF-7 human breast cancer cells was reviewed by the Pacific Northwest National Laboratory IRB for human subjects research in accordance with federal regulations. MCF-7 breast cancer cells were cultured in DMEM containing 5% fetal bovine serum and 10 μg/mL bovine insulin (Gibco, Gaithersburg, MD) until achieving 90% confluence. One million MCF-7 cells were used to evaluate two different lysis conditions: traditional SDS-based protocol and the new TFE-based protocol. For the SDS-based protocol, 1 million MCF-7 cells were resuspended in 60 μL of 10 mM PBS (pH 7.0) with 0.5% SDS (Research Organics Inc., Cleveland, OH) and sonicated in an ice-water bath for 5 min. The lysates were subsequently denatured in 8 M urea and reduced by 5 mM dithiothreitol (DTT) at 37 °C for 1 h. The reduced samples were diluted 8-fold using 50 mM NH4HCO3 (pH 7.8) and digested using sequencing grade trypsin (Promega, Madison, WI) at a 1:50 enzyme to protein ratio overnight at 37 °C. Both digests were purified using a SPE SCX column (Supelco, Bellefonte, PA). Peptides were eluted with 1 mL of 500 mM ammonium formate in 25% acetonitrile, pH 6.8. Following lyophilization, peptides were resuspended in 100 μL 50 mM NH4HCO3 for LC-MS/MS analysis using an LCQ Deca XP ion trap mass spectrometer (ThermoFinnigan, San Jose, CA).
For the TFE-based protocol, trifluoroethanol (TFE) was introduced in place of SDS during cell lysis. One million MCF-7 cells were incubated with 100 μL of 5 mM PBS at room temperature for 2 h with gentle shaking, followed by a 5 min sonication in an ice-water bath. 100 μL of TFE was then added to the sample. The sample was incubated at 60 °C for 1 h, and then sonicated in an ice-water bath for 2 min. 5 mM tributyl phosphine (TBP) was used to reduce proteins and the sample was diluted 5-fold using 50 mM NH4HCO3 to reduce the final TFE concentration to 10% (v/v). The sample was digested by trypsin at an enzyme:protein ratio of 1:50 at 37 °C overnight. Digests were lyophilized and redissolved in 100 μL 50 mM NH4HCO3 for LC-MS/MS analysis using an ion trap (LCQ) mass spectrometer. In both methods, peptide concentrations were determined using the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL). The TFE-based protocol was also used for the lysis and digestion of four MCF-7 samples that each contained approximately 5 000 cells. Peptides were analyzed by both LC-MS/MS using a linear ion trap mass spectrometer LTQ (ThermoElectron Corp) and LC-MS using an 11 tesla FTICR instrument.
Preparation of mouse brain tissue and voxel samples
Brain tissue samples from C57BL6/J male mice were prepared as previously described.26 To evaluate the performance of the TFE protocol for processing tissue samples, mouse brain tissues were lysed either in CHAPS- containing buffer or in hypotonic buffer with TFE. Six small pieces of brain (4.5-5.0 mg per piece) were sliced from a whole mouse brain using a spatula. Three pieces of brain tissues were lysed using a buffer containing 7 M urea, 2 M thiourea, 40 mM Tris base and 4% CHAPS in 50 mM NH4HCO3 (pH 8.0). Following sonication in an ice-water bath, samples were incubated at 37 °C with shaking for 2 h followed by another sonication for 5 min in an ice-water bath. The tissue lysates were then reduced by incubating in 5 mM TBP at 37 °C for 1 h. Each sample was diluted 8-fold using 50 mM NH4HCO3 and digested by trypsin overnight. Each digest was cleaned using an SPE SCX column and then lyophilized. The other three pieces of brain tissue were lysed in 80 μL of 5 mM PBS with 80 μL TFE with intermittent sonication in an ice-water bath. The lysates were reduced by 5 mM TBP and digested by trypsin overnight. The digests were lyophilized immediately after digestion without further cleanup steps. Peptide samples prepared by both protocols were re-dissolved in 100 μL 50 mM NH4HCO3 and the peptide concentrations were measured using the BCA protein assay. The peptides were then analyzed by LC-MS/MS. In addition, the TFE protocol was also applied in the sample preparation of single voxel samples. After digestion, peptides were analyzed by both LCMS/MS and the 11 tesla LC-FTICR.
Capillary LC-MS/MS and LC-FTICR analysis
Approximately 10 μg of peptides were injected onto a fully automated in-house built capillary HPLC system, which was coupled online with either an LCQ or an LTQ ion trap mass spectrometer, or an 11 Tesla FTICR mass spectrometer using an in-house manufactured ESI interface. The technical details and separation performance of this LC platform have been described previously.28 In brief, the capillary column was made by slurry packing 3-μm Jupiter C18 particles (Phenomenex, Torrence, CA) into a 65-cm length of 150 μm i.d. fused silica capillary (Polymicro Technologies Inc., Phoenix, AZ). The separation was performed using an exponential gradient, which started with 100% mobile phase A (0.2% acetic acid and 0.05% TFA in water) and increased the mobile-phase composition to ∼60% B (0.1% TFA in 90% acetonitrile) in the mixing chamber over the duration of 100 min. The LCQ and LTQ ion trap instruments were operated using similar settings. Both instruments were operated in data dependent MS/MS with m/z ranging from 400 to 2000. Dynamic exclusion was used to discriminate against previously analyzed ions. Nano-gram scale peptide samples were also analyzed by LC-MS on the 11 tesla LC-FTICR mass spectrometer.
Data analysis
For MS/MS data, peptides were identified by searching against either human or mouse International Protein Index (IPI) databases (available online at http://www.ebi.ac.uk/IPI), using the SEQUEST algorithm (ThermoElectron, San Jose, CA). The raw SEQUEST results were filtered with the following criteria to obtain highly confident peptide/protein identifications: correlation score (Xcorr) ≥ 1.5 for charge state +1 full tryptic peptides, Xcorr ≥ 3.1 for partial tryptic peptides; Xcorr ≥ 1.9 for charge state +2 full tryptic peptides, Xcorr ≥ 3.8 for partial tryptic peptides; Xcorr ≥ 2.9 for charge state +3 full tryptic peptides, and Xcorr ≥ 4.5 for partial tryptic peptides. All with DelCn ≥ 0.1. The stringent filtering criteria guidelines applied in this study were developed to achieve highly confident peptide identifications with < 5% false positive rate based upon sequence-reversed human protein database searching.29
For LC-FTICR data, the data analysis was performed using in-house software tools to generate a list of unique features (a feature is equivalent to a peptide that is waiting to be assigned a specific identification from the AMT tag database) with the accurate mass measurement and LC-normalized elution time (NET) for each observed feature. Peptide elution times were normalized into a 0 to 1 scale for more accurate comparison among LC-MS data. The accurately measured masses and NET values for each feature were then matched to the corresponding accurate mass and time (AMT) tag in the AMT tag database to identify peptide sequences. The peptide AMT tag database was initially generated using the highly confident, filtered peptide identifications from LC-MS/MS analyses. The peptide sequence for a given LC-FTICR feature was assigned when the measured mass and NET for each given feature matched the calculated theoretical mass and NET of a peptide in the AMT tag database within a 5 ppm mass error and a 5% NET error. Details about the AMT tag strategy for identifying LC-FTICR features have been described previously30-32.
Results and Discussion
In the studies of protein or peptide structure using spectroscopic techniques (e.g. NMR) TFE has often been used to stabilize or disrupt three-dimensional protein structures based on its concentration;33 however, its application for proteomics has only recently been recognized. Deshusses and co-workers34 investigated a phase partition system using 2:1 TFE/chloroform to extract proteins from E. coli membranes. A large number of membrane proteins from the TFE-abundant aqueous phase were detected in one dimensional gel electrophoresis and identified by MALDI-TOF MS. While the principle of the co-solvent effect is still not clear, it was speculated that a high concentration of TFE (40-50% v/v) can reduce the dielectric constant of the solubilization medium and improve the protein extraction efficiency. The preliminary success of employing TFE protein extraction in a proteomics application led us to explore the use of this organic solvent for micro- and nano-scale proteomic sample preparation for LC-MS analysis.
In this study a TFE-based protocol was initially evaluated by comparing to traditional detergent-based protocols for the preparation of relatively large scale (100 μg-400 μg) samples from tissues and cultured cells. The performance of this new protocol was then demonstrated for processing of small mouse brain samples and 5 000 human MCF-7 cell samples.
Comparison of the TFE-based protocol with traditional detergent-based protocols
The TFE-based protocol was first compared to the traditional CHAPS-based protocol for processing ∼5 mg of mouse brain tissue samples, containing ∼350 μg proteins (∼7% w/w). Mouse brain tissues were disrupted and lysed in either a buffer containing a high concentration of denaturants and 4% CHAPS or in an aqueous hypotonic buffer with 50% (v/v) TFE. Figure 1 shows the base peak chromatograms of LC-MS/MS analyses of two brain tissue samples prepared using either the CHAPS protocol (A) or the TFE protocol (B). Even after SCX SPE cleanup, a large detergent peak (elution time: ∼ 50 min) was still observed as shown in the chromatogram (Fig. 1A). The presence of detergent significantly suppressed signals for peptides that co-eluted with the detergent. In contrast, the chromatogram of the sample prepared by the TFE protocol shows a large number of peptide peaks demonstrating the high resolution of the LC separation. The peptide recoveries and the peptide/protein identifications resulting from both methods are given in Table 1. The average quantities of recovered peptides from the CHAPS-based and TFE-based protocols were 244 μg and 231 μg, respectively, suggesting comparable sample recovery for these two protocols. In terms of peptide/protein identification results from LC-MS/MS analyses, a total of 491 proteins were identified from 1286 different peptides from the samples prepared using the TFE protocol and 399 proteins were identified based on 1009 different peptides from samples prepared using the CHAPS protocol, showing a modest increase in the number of identifications using the TFE-based protocol. Similar comparisons were performed when brain tissues were prepared by the TFE-based method versus other methods using different detergents, such as RapiGest and OG (data not shown). The results consistently showed that the TFE protocol provided comparable or slightly better results compared to traditional detergent-based protocols for brain tissue sample processing.
Figure 1.
Base peak chromatograms of LCQ LC-MS/MS results of mouse brain tissues prepared using, (A) 7 M urea, 2 M thiourea, 40 mM Tris, 4% CHAPS in 50 mM NH4HCO3 buffer, and (B) 5 mM PBS with 50% (v/v) TFE buffer.
Table 1.
The quantities of recovered peptidesa and the numbers of different peptides and proteins identifiedb by LC-MS/MS from 4.5-5.0 mg of mouse brain tissue prepared by two different lysis protocols.
| Tissue amount (mg) | Recovered peptide (μg) | Different peptides Identified | Proteins Identified | ||
|---|---|---|---|---|---|
| 1 | 4.6 | 261 | 644 | 303 | |
| CHAPS method | 2 | 4.7 | 257 | 579 | 241 |
| 3 | 4.7 | 215 | 495 | 226 | |
| Combinedc | 1009 | 399 | |||
| | |||||
| 1 | 4.7 | 181 | 724 | 319 | |
| TFE method | 2 | 4.8 | 315 | 787 | 322 |
| 3 | 4.6 | 196 | 768 | 329 | |
| | |||||
| Combined | 1286 | 491 | |||
| Overlapd | 604 | 297 | |||
Peptides were resuspended in 100 μL of 50 mM NH4HCO3 and their concentrations were measured using BCA protein assay.
All peptides identified passed the filtering criteria described in the experimental section.
Total number of different peptides and proteins combined from three datasets obtained from the same protocol.
The number of peptides and proteins observed in samples prepared using both protocols.
Next, we compared the performances of the TFE-based protocol and traditional detergent-based protocol for processing one million cultured MCF-7 cells, with a protein content of ∼150 μg. Three replicate LC-MS/MS analyses were performed for each sample. Comparable results from these two protocols were again observed in terms of both the amounts of peptides recovered and the number of peptides/proteins identified using LC-MS/MS, as shown in Table 2. When peptide/protein identifications from the triplicate experiments for each protocol were combined, the protocol using SDS resulted in 332 proteins identified based upon 694 different peptides, while the new TFE-based method resulted in 378 proteins identified based on 755 different peptides. A total of 228 proteins (∼65% overlap) were in common to both sets of samples, an observation that likely reflects both somewhat different selectivity and the “under-sampling” inherent in this LC-MS/MS approach. In summary, the comparison results demonstrated that the new TFE-based protocol provided comparable or slightly better efficiency than the traditional detergent-based protocols for the processing of relatively large sample sizes of brain tissues and cultured cells.
Table 2.
The quantities of recovered peptidesa and the numbers of different peptides and proteins identifiedb by LC-MS/MS from ∼1 million MCF-7 cells prepared by two different lysis protocols.
| Recovered peptide (μg) | Different peptides identified | Proteins identified | ||
|---|---|---|---|---|
| 1 | 439 | 242 | ||
| SDS method | 2 | 122 | 282 | 159 |
| 3 | 467 | 242 | ||
| Combined | 694 | 332 | ||
| | ||||
| 1 | 443 | 247 | ||
| TFE method | 2 | 125 | 491 | 268 |
| 3 | 436 | 237 | ||
| | ||||
| Combined | 755 | 378 | ||
| Overlap | 369 | 228 | ||
Peptides were resuspended in 100 μL of 50 mM NH4HCO3 and their concentrations were measured using BCA protein assay.
All peptides identified passed the filtering criteria discussed in detail in the experimental section.
Micro- and nano-scale sample processing using the TFE-based protocol
Having demonstrated that the TFE protocol performed as effectively as the traditional detergent-based protocols for processing relatively large amount of cells or tissues, we further explored its application in preparing micro-scale mouse brain voxel samples and nano-scale cultured cell samples (aliquots of 5 000 MCF-7 cells). When handling smaller samples, the TFE-based method becomes advantageous since the sample remains in the same tube during the entire process and is ready for LC-MS analyses without the necessity of any transfer or cleanup steps, and thereby minimizing sample losses.
Mouse brain voxelation has enabled the 3-D mapping of gene expression35, and we are presently exploring similar 3-D profiling of protein abundances based upon the high throughput quantitative proteomic analyses of large sets of the small tissue “voxels”. Each “voxel” is a spatially registered volume element, in the present case having a volume of ∼ (0.75 mm)3 per mouse brain voxel. Approximately 600 voxels can be generated from a whole adult mouse brain using high resolution voxelation.35 The sample preparation for such a large number of micro-scale samples presents a two-fold challenge: (1) repetitive manual manipulation of such samples will likely introduce significant variation in sample quality; (2) highly sensitive quantitative proteomic measurements require the minimization of sample loss. We plan to address these challenges using an automated process for sample preparation using the TFE-based lysis/extraction method. Table 3 shows the peptide recovery and LC-MS/MS analysis results for four single voxel samples prepared by the TFE-based protocol. As shown, ∼ 2000 different peptides and 600-800 proteins were identified from the small voxel samples, providing good proteome coverage. The average amount of peptide recovered from single voxels after digestion was ∼20 μg, although deviations were observed from the amounts recovered from different voxel samples due to the inhomogeneous dimension of individual voxels. These results demonstrate the effectiveness of the TFE-based protocol for handling very small proteome samples. Compared to the detergent-based methods, the TFE-based protocol provides not only better sample recovery, but also better reproducibility, because the extra clean-up steps in detergent-based methods introduce additional variabilities during sample processing and added technical difficulties for automation. Initial success for automated sample processing with multiple single voxel samples applying the TFE-based protocol in a 96-well platform has been achieved (data not shown).
Table 3.
The quantities of recovered peptidesa and peptide/protein identificationsb from four single mouse brain voxels prepared by the TFE-based protocol and analyzed using LC-MS/MS with a linear ion trap mass spectrometer (LTQ).
| Recovered Peptide (μg) | Different peptides | Proteins | |
|---|---|---|---|
| Voxel-3F5 | 23.0 | 2595 | 855 |
| Voxel-3F7 | 15.5 | 1762 | 587 |
| Voxel-3F8 | 15.5 | 2446 | 757 |
| Voxel-3F9 | 25.0 | 1982 | 664 |
Peptides were resuspended in 50 μL of 50 mM NH4HCO3 and their concentrations were measured using BCA protein assay.
All peptides identified passed the filtering criteria discussed in detail in the experimental section.
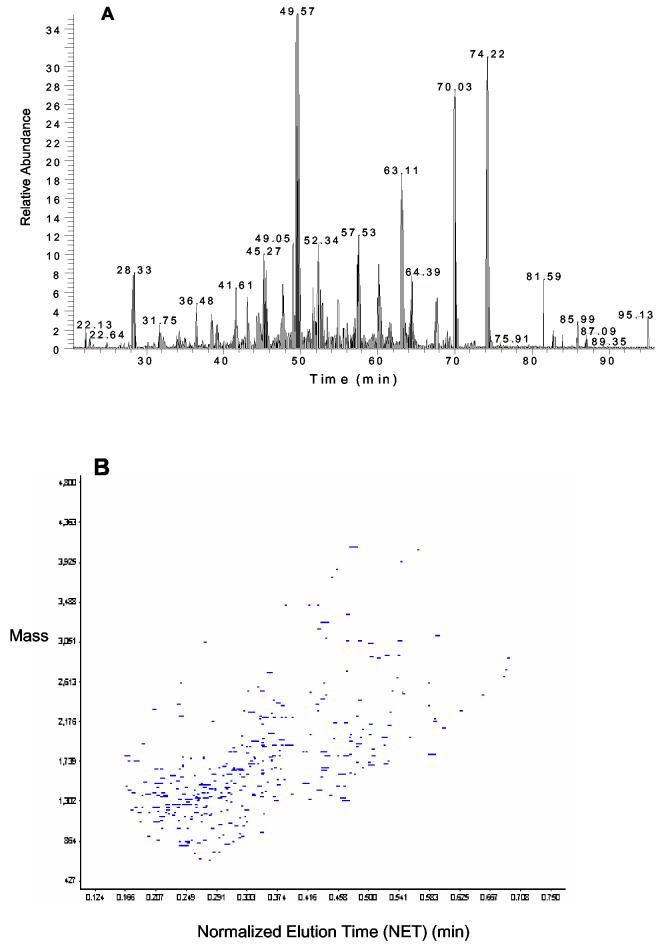
Additionally, the utility of the TFE-based protocol was demonstrated for processing submicrogram size of complex proteome samples. Five samples of 5 000 MCF-7 cells, each containing ∼500 ng of total protein were prepared using this protocol, a case where traditional detergent-based methods consistently failed to allow effective subsequent analysis. Following the TFE preparation steps, four samples were analyzed by LC-MS/MS analysis on the LTQ ion trap MS while one was analyzed by LC-FTICR, which was expected to offer higher sensitivity. Figure 2A shows the base peak chromatogram obtained from one LC-MS/MS analysis. Although the overall peak intensities were relatively low due to the low peptide concentration, a number of peptide peaks were detected. Table 4 gives the number of peptide/protein identification results obtained from the four LC-MS/MS runs. Due to the limitations of both sample amount and instrument sensitivity, only 83-133 proteins were identified from different samples with 40 proteins in common; however, these results clearly demonstrate that the TFE-based protocol is efficient for processing sub-microgram scale samples. Figure 2B shows the 2-D display of LC-FTICR identified features from the 5 000 cell sample. Peptides were displayed based on their monoisotopic masses and LC-normalized elution times (NET). From the LCFTICR analysis, >5000 unique features (putative peptides) were detected. By matching against a limited-sized AMT tag database for MCF-7 cells, which contains ∼ 3000 peptide identifications generated from LC-MS/MS analysis of non-fractionated MCF-7 whole cell lysates, 525 different peptides corresponding to 224 proteins were identified using the AMT tag approach.30, 31 These results further demonstrated the capability of this TFE-based protocol for micro- and nano-scale proteomic sample preparation and the overall sensitivity in sample preparation, where conventional detergent-based methods have significant limitations.
Figure 2.
(A) Base peak chromatogram of LTQ LC-MS/MS analysis of 5000 MCF-7 human breast cancer cells prepared using the TFE protocol. (B) 2-D plot of identified peptides from 5000 MCF-7 cancer cells using the TFE protocol. Peptides observed in the LC-FTICR analysis were displayed based on their monoisotopic masses and normalized elution times (NET).
Table 4.
Number of peptide and protein identificationsa from four MCF-7 samples, each containing ∼ 5 000 cells, prepared by the TFE-based protocol and analyzed using LC-MS/MS with a linear ion trap mass spectrometer (LTQ).
| Different peptides | Proteins | |
|---|---|---|
| #1 | 305 | 113 |
| #2 | 211 | 85 |
| #3 | 290 | 133 |
| #4 | 179 | 83 |
| | ||
| Overlap | 82 | 40 |
All peptides identified passed the filtering criteria discussed in detail in the experimental section.
Conclusion
The growing interest in the proteomic profiling of biological samples with small quantities of proteins requires the development of more effective sample preparation methods capable of minimizing sample loss and amenable for automation. Traditional sample preparation methods typically employ various detergents with a high concentration of denaturing solution to disrupt cells and denature proteins. The presence of detergent and salt creates significant interference with subsequent LC-MS analysis and extra cleanup steps have to be taken prior to LC-MS analysis, often leading to significant sample losses. In this study, we developed and evaluated an alternative sample preparation method using TFE that improves protein solubilization, facilitates protein denaturation, and can be readily removed following tryptic digestion without an extra clean-up step. The utility of this protocol in micro- and nano-scale sample processing was demonstrated in the effective preparation of micro-gram scale single mouse brain voxel samples and sub-microgram size 5 000 MCF-7 human breast cancer cell samples, where traditional detergent-based methods generally failed due to significant sample losses. The protocol can also be easily coupled to stable isotope labeling approach such as 16O/18O peptide labeling for quantitative studies of small biological samples.36 The simple TFE-based protocol is expected to have broad applications for the processing of a diversity of micro-and nano-scale biological samples.
Acknowledgements
The authors thank the NIH National Center for Research Resources (RR018522) for support of this research and the Environmental Molecular Sciences Laboratory (EMSL) at Pacific Northwest National Laboratory (PNNL) for use of the instrumentation applied in this research. EMSL is a national scientific user facility sponsored by the U.S. Department of Energy's (DOE) Office of Biological and Environmental Research. PNNL is a multi-program national laboratory operated by Battelle Memorial Institute for the DOE under Contract DEAC05-76RL01830.
Abbreviations
- AMT tag
accurate mass and time tag
- FTICR
Fourier transform-ion cyclotron resonance mass spectrometry
- NET
normalized elution time
- SCX
strong cation exchange
- SPE
solid phase extraction
- TFE
trifluoroethanol
References
- 1.Shen Y, Tolic N, Masselon C, Pasa-Tolic L, Camp DG, Hixson KK, Zhao R, Anderson GA, Smith RD. Anal. Chem. 2004;76:144–154. doi: 10.1021/ac030096q. [DOI] [PubMed] [Google Scholar]
- 2.Aebersold R, Mann M. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 3.Eng JK, McCormack AL, Yates JR. J. Am. Soc. Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 4.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Field H, Fenyo D, Beavis R. Proteomics. 2002;2:36–47. [PubMed] [Google Scholar]
- 6.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. Anal. Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 7.Wotherspoon AC, Norton AJ, Lees WR, Shaw P, Isaacson PG. J. Pathol. 1989;158:115–121. doi: 10.1002/path.1711580206. [DOI] [PubMed] [Google Scholar]
- 8.Mahlstedt K, Ussmuller J, Donath K. J. Otolaryngol. 2002;31:299–303. doi: 10.2310/7070.2002.34324. [DOI] [PubMed] [Google Scholar]
- 9.Moyer CD, Gilchrist IC. Catheter. Cardiovasc. Interv. 2005;64:134–137. doi: 10.1002/ccd.20270. [DOI] [PubMed] [Google Scholar]
- 10.Liang G, Zhang XD, Wang LJ, Sha YS, Zhang JC, Miao SY, Zong SD, Wang LF, Koide SS. Cell Res. 2004;14:507–512. doi: 10.1038/sj.cr.7290254. [DOI] [PubMed] [Google Scholar]
- 11.Marko-Varga GA, Fehniger TE. J. Chromatogr. A. 2004;1053:279–290. doi: 10.1016/j.chroma.2004.08.115. [DOI] [PubMed] [Google Scholar]
- 12.Malhotra PS, Malekfzali A, Bonner RF, Juhn S, Van Waes C, Chen Z. Laryngoscope. 2004;114:2123–2128. doi: 10.1097/01.mlg.0000149446.14770.52. [DOI] [PubMed] [Google Scholar]
- 13.Jensen M. Biol. Blood Marrow Transplant. 2005;11:34–39. doi: 10.1016/j.bbmt.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Singh SK, Clarke ID, Hide T, Dirks PB. Oncogene. 2004;23:7267–7273. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- 15.Celis JE. Cell Biology: A Laboratory Handbook. 2nd ed. Vol. 1. Academic Press; San Diego, California: 1998. [Google Scholar]
- 16.Spector DL, Goldman RD, Leinwand LA. Cells: A laboratory Manual. 1-3. Cold Spring Harbor Press; Cold Spring Harbor, New York: 1998. [Google Scholar]
- 17.Stubbs GW, Litman BJ. Biochemistry. 1978;17:215–219. doi: 10.1021/bi00595a003. [DOI] [PubMed] [Google Scholar]
- 18.Gould RJ, Ginsberg BH, Spector AA. Biochemistry. 1981;20:6776–6781. doi: 10.1021/bi00527a006. [DOI] [PubMed] [Google Scholar]
- 19.Janini GM, Conrads TP, Veenstra TD, Issaq HJ. J. Chromatogr. B. 2003;787:43–51. doi: 10.1016/s1570-0232(02)00616-5. [DOI] [PubMed] [Google Scholar]
- 20.Gilar M, Belenky A, Wang BH. J. Chromatogr. A. 2001;921:3–13. doi: 10.1016/s0021-9673(01)00833-0. [DOI] [PubMed] [Google Scholar]
- 21.Brooks DA, Bradford TM, Hopwood JJ. J. Immunol. Methods. 1992;155:129–132. doi: 10.1016/0022-1759(92)90279-3. [DOI] [PubMed] [Google Scholar]
- 22.Kachlany SC, Fine DH, Figurski DH. Protein Expr. Purif. 2002;25:465–471. doi: 10.1016/s1046-5928(02)00037-2. [DOI] [PubMed] [Google Scholar]
- 23.Asenjo JA, Andrews BA. J. Mol. Recognit. 2004;17:236–247. doi: 10.1002/jmr.676. [DOI] [PubMed] [Google Scholar]
- 24.Chertov O, Biragyn A, Kwak LW, Simpson JT, Boronina T, Hoang VM, Prieto DA, Conrads TP, Veenstra TD, Fisher RJ. Proteomics. 2004;4:1195–1203. doi: 10.1002/pmic.200300677. [DOI] [PubMed] [Google Scholar]
- 25.Ferro M, Seigneurin-Berny D, Rolland N, Chapel A, Salvi D, Garin J, Joyard J. Electrophoresis. 2000;21:3517–3526. doi: 10.1002/1522-2683(20001001)21:16<3517::AID-ELPS3517>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 26.Brown VM, Ossadtchi A, Khan AH, Yee S, Lacan G, Melega WP, Cherry SR, Leahy RM, Smith DJ. Genome Res. 2002;12:868–884. doi: 10.1101/gr.229002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson AS. Genome Res. 2002;12:217–218. doi: 10.1101/gr.227802. [DOI] [PubMed] [Google Scholar]
- 28.Shen Y, Zhao R, Belov ME, Conrads TP, Anderson GA, Tang K, Pasa-Tolic L, Veenstra TD, Lipton MS, Smith RD. Anal. Chem. 2001;73:1766–1775. doi: 10.1021/ac0011336. [DOI] [PubMed] [Google Scholar]
- 29.Qian W-J, Liu T, Monroe ME, Strittmatter EF, Jacobs JM, Kangas LJ, Petritis K, II DGC, Smith RD. J. Proteome Res. 2005;4:53–62. doi: 10.1021/pr0498638. [DOI] [PubMed] [Google Scholar]
- 30.Qian WJ, Camp DG, Smith RD. Expert Rev. Proteomics. 2004;1:89–97. doi: 10.1586/14789450.1.1.87. [DOI] [PubMed] [Google Scholar]
- 31.Smith RD, Anderson GA, Lipton MS, Pasa-Tolic L, Shen Y, Conrads TP, Veenstra TD, Udseth HR. Proteomics. 2002;2:513–523. doi: 10.1002/1615-9861(200205)2:5<513::AID-PROT513>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 32.Liu T, Qian WJ, Strittmatter EF, Camp DG, Anderson GA, Thrall BD, Smith RD. Anal. Chem. 2004;76:5345–5353. doi: 10.1021/ac049485q. [DOI] [PubMed] [Google Scholar]
- 33.Buck M. Q. Rev. Biophys. 1998;31:297–355. doi: 10.1017/s003358359800345x. [DOI] [PubMed] [Google Scholar]
- 34.Deshusses JM, Burgess JA, Scherl A, Wenger Y, Walter N, Converset V, Paesano S, Corthals GL, Hochstrasser DF, Sanchez JC. Proteomics. 2003;3:1418–1424. doi: 10.1002/pmic.200300492. [DOI] [PubMed] [Google Scholar]
- 35.Singh RP, Brown VM, Chaudhari A, Khan AH, Ossadtchi A, Sforza DM, Meadors AK, Cherry SR, Leahy RM, Smith DJ. J. Neurosci. Methods. 2003;125:93–101. doi: 10.1016/s0165-0270(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 36.Qian WJ, Monroe ME, Liu T, Jacobs JM, Anderson GA, Shen Y, Moore RJ, Anderson DJ, Zhang R, Calvano SE, Lowry SF, Xiao W, Moldawer LL, Davis RW, Tompkins RG, Camp DG, Smith RD. Mol. Cell. Proteomics. 2005;4:700–709. doi: 10.1074/mcp.M500045-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]