Abstract
We investigated the protein-binding sites in a DNAse I hypersensitive site associated with bcl-2 gene expression in human B cells. We mapped this hypersensitive site to the coding sequence of exon 2 of the bcl-2 gene in the bcl-2-expressing REH B-cell line. Electrophoretic mobility shift assays (EMSAs) with extracts from REH cells revealed three previously unrecognized B-Myb-binding sites in this sequence. The protein was identified as B-Myb by using a specific antibody and EMSAs. Accordingly, the levels of B-Myb and bcl-2 proteins, and of Myb EMSA activity, were correlated over a wide range of cell lines, representing different stages of B-cell development. Transfection of REH cells with antisense B-myb down-regulated EMSA activity and the level of bcl-2, and led to the apoptosis of REH cells. Transfection of the bcl-2-non-expressing RPMI 8226 cell line with a B-Myb expression vector induced B-Myb EMSA activity and the expression of bcl-2. Reporter assays indicated that the HSS8 sequence containing the three B-Myb sites may act as an enhancer when it is linked to the bcl-2 gene promoter. Interaction of B-Myb with HSS8 may enhance bcl-2 gene expression by co-operating with positive regulatory elements (e.g. previously identified B-Myb response elements) or silencing negative response elements in the bcl-2 gene promoter.
Keywords: apoptosis, B lymphocytes, Bcl-2 gene, B-Myb, enhancer
Introduction
bcl-2, the archetypal member of a family of genes that control programmed cell death, plays a vital role in determining the lifespan of lymphocytes.1 Bcl-2 is expressed in stem cells and in memory B and T cells, and is overexpressed in lymphocytic tumours.2 Real-time quantitative polymerase chain reaction (PCR) reveals a 20-fold higher level of expression of bcl-2 in follicular lymphoma than in normal germinal centre B cells.2 The elevated level of bcl-2 after translocations that appose bcl-2 to the immunoglobulin genes t(14;18) increases the lifespan of these cells and thus promotes the development of follicular lymphoma.3 Transcriptional activation occurs upon translocation at the bcl-2 locus within either the major break point (mbr)4 or the 5′ region.5 The B cells in transgenic mice that constitutively overexpress bcl-2 undergo polyclonal expansion and increased survival, but no increase in proliferation. Progression to high-grade lymphoma also occurs in these mice.6 Elevated bcl-2 expression probably explains the emergence of resistance to cytotoxic anticancer drugs and radiation.7,8 Down-regulation of bcl-2 gene expression with antisense phosphorothioate oligonucleotides is effective in the treatment of patients with non-Hodgkin's lymphoma.9,10 It is not surprising therefore that the majority of successful strategies for tumour reduction are accompanied by the down-regulation of bcl-2 and apoptosis of the tumour cells. A deeper exploration of therapeutic possibilities along these lines will demand a fuller understanding of bcl-2 gene regulation.11
The human bcl-2 gene is unusually large, comprising three exons, extending over ≈ 200 kb (Fig. 1a). Two promoters (P1 and P2) mediate transcriptional control of the bcl-2 gene. P1, the promoter found further 5′, is located 1386–1423 bp upstream of the translational start site.12 This is a GC-rich, TATA-devoid promoter that displays numerous start sites. The P2 start sites are located 1·3-kb downstream of the P1 promoter. Between P1 and P2 there are multiple non-overlapping negative regulatory elements, which co-localize with regions of increased DNAse I sensitivity.13 An Rb protein-responsive element,14 as well as a Cdx protein-binding site,15 have been identified within this region; their contribution to regulated transcription is unclear, as neither factor binds directly to DNA, but they are recruited into complexes with other factors that bind to the bcl-2 gene. The 3′-mbr of the bcl-2 gene locus also contains a matrix attachment site (MAR), which upon t(14;18) translocation exhibits altered cell cycle expression of the bcl-2 gene.4Bcl-2 gene amplification is also implicated in the aetiology of B-cell lymphoma.16
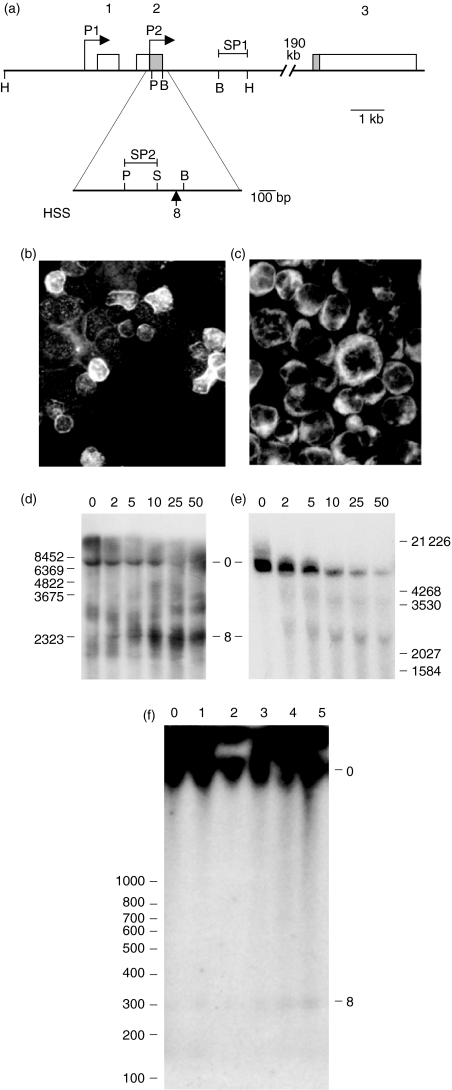
Figure 1.
Structure, expression and DNAse I hypersensitive site mapping of the bcl-2 gene in human B cells. (a) Map of the bcl-2 gene. Transcription start sites (P1) and (P2), exons 1–3, and the position of the DNAse hypersensitive site 8 are shown. The non-coding regions of exons 1–3 are shown in white, and the coding regions within exons 2 and 3 are shown in grey. Restriction sites (B = BamHI; H = HindIII; P = PstI; and S = SacI) and the position of the fragments used as probes in Southern blots (SP1 and SP2) are also indicated. (b) and (c) Bcl-2 protein expression (white) in germinal centre tonsil B cells and in the REH cell line, respectively, was detected by using a fluorescent antibody in cytospins. (d) and (e) DNAse I hypersensitive site mapping of the bcl-2 gene in tonsil B cells and in the REH cell line, respectively. DNAse I concentrations (units) are as shown above each lane. The parental 7·8-kb HindIII fragment (0) and HSS8 fragment (8) are indicated. The probe used was SP1. (f) High-resolution DNAse I hypersensitive site mapping of the bcl-2 gene in the REH B-cell line. DNAse I concentrations (units) are as shown above each lane. The undigested 2·9-kb PstI/HindIII fragment (0) and HSS8 fragment (8) are indicated. The probe used was SP2.
A plethora of trans-acting factors have been associated with bcl-2 transcriptional regulation at both P1 and P2 in many different cells types. Early results suggested that P1 was the dominant promoter in B cells, because it gives rise to the overwhelming majority of transcripts.12,13 A positive regulator of P1 activity is a cAMP response element (CRE) in B cells.17 Ji et al.18 examined the promoter activities of the normal and translocated bcl-2 alleles in the DHL-4 cell line with the t(14;18) translocation, and found that the cAMP response-binding protein binds to a CRE in the bcl-2 5′-flanking region of the translocated allele. The obstruction of this CRE site in the normal bcl-2 allele led them to infer that the CRE site could act only on the translocated bcl-2 allele, functioning as a positive regulatory site in t(14;18) lymphomas. Later studies, however, revealed that the CRE site may be important for bcl-2 expression in neuronal cells19 and for oestrogen receptor activation of bcl-2 in mammary cells.20 The P1 promoter also contains WT1-binding sites, which act as negative regulators of bcl-2 expression in t(14;18) and HeLa cells21,22 and as positive regulators in sporadic Wilm's tumours.23 The activation of bcl-2 by nuclear factor-κB (NF-κB) in (t14;18) lymphoma cells has been shown to be mediated through the CRE and Sp1-binding sites at the P1 promoter.24
The Myb family of transcription factors have been shown to be positive regulators of the bcl-2 P2 promoter in chicken and murine T cells.25,26 Furthermore, A-Myb was able to activate P2 activity in t(14;18) cells, acting through a binding site for the homeodomain protein, Cdx.15 The contribution of enhanced P2 activity in normal B cells, in which most transcripts originate at P1, is unclear, nor is it known whether such activity reflects a shift from P1 to P2 at the translocated allele. Another positive regulator of the bcl-2 gene is the Ikaros family member, Alios. This transcription factor becomes phosphorylated by Ras after interleukin-2 (IL-2) stimulation of T cells and localizes to the nucleus, where it binds to the P2 promoter.27 Negative regulators of P2 activity include p53, which acts through the TATA element,28 and the factors that interact with π binding sites, which are essential for bcl-2 down-regulation in immature B cells.29
Another unusual feature of the bcl-2 gene is the presence of an unusually large second intron, of ≈ 190 kb, separating exon 2 and exon 3. Sequences in exon 3 are essential for the anti-apoptotic activity of bcl-2 protein. Although low levels of a truncated bcl-2 mRNA lacking exon 3 were observed in one study,30 the corresponding protein seems not to have been detected.
Studies on the regulation of the bcl-2 gene have been confined mainly to the promoter region. Young & Korsmeyer identified a series of eight DNAse I hypersensitive sites (HSS1–8) at the 5′ end of the human bcl-2 gene.13 HSS1, 2 and 3 were located in the 5′ flanking sequence of P1, HSS5–7 were found between P1 and P2, and HSS8 was mapped approximately in the region of the exon 2 and intron 2 junction. HSS1–7 were found in both the bcl-2-expressing REH and in a bcl-2-non-expressing DHL9 B-cell line. HSS8 occurred only in the REH cell line, suggesting that this region of the DNA may be important in bcl-2 gene activation, but so far no such activity has been demonstrated.13
As chromatin remodelling plays a central role in gene regulation, and DNAse I sites reveal interruptions in the nucleosomal structure of chromatin caused by the binding of regulatory proteins,31 we decided to investigate this region further. We have mapped HSS8 in the bcl-2 gene to higher resolution and examined the functions of the DNA sequence in protein binding and transcription. We show here that HSS8 marks a bcl-2 gene regulatory region containing three Myb-binding sites, activated by B-Myb.
Materials and methods
Cell culture
Cell lines REH, BL-2, Ramos, RPMI 8226, and Namalwa, Raji and RPMI 8866, were obtained from Prof. M. Greaves (Institute of Cancer Research, London, UK), Dr D. Vercelli (Arizona Respiratory Center, University of Arizona, Tucson, AZ), Dr J. Murphy (Kings College London, London, UK), the ECACC (Health Protection Agency, Porton Down, Wiltshire, UK) or the ATCC (Queens Road, Teddington, Middlesex, UK), respectively. Cells were grown in RPMI-1640 supplemented with 10% (v/v) fetal calf serum (Invitrogen Ltd, Paisley, UK), 2 mm l-glutamine and 100 U of penicillin/streptomycin (Invitrogen) at a density of 0·5–1·0 × 106 ml−1. Tonsil cells were teased from palatine tonsils within 3 hr of surgery, and T cells were removed by rosetting with sheep red blood cells. B cells were collected from the interface of Ficoll–paque (Pfizer Ltd, Tadworth, Surrey, UK) and washed twice in phosphate-buffered saline (PBS) to yield a population of ≥ 70% B cells.
Cell staining for Bcl-2 protein
Cytospin preparations were fixed in acetone for 10 min at room temperature, washed in PBS, incubated for 45 min at room temperature with 10 µl of anti-bcl-2 antibody (DAKO, Glostrup, Denmark), washed with PBS and then incubated with fluorescein isothiocyanate (FITC) or tetramethylrhodamine isothiocyanate (TRITC) goat anti-mouse immunoglobulin G1 (IgG1) for 45 min at room temperature. Immunofluorescence was observed by using a confocal microscope (Leica Camera Ltd, Milton Keynes, UK).
DNAse I hypersensitivity site mapping
Low-resolution DNAse I hypersensitivity mapping in REH and primary tonsil B cells was carried out using a 0·7% agarose gel essentially as described by Young & Korsmeyer.13 High-resolution mapping was performed by using the PstI/SacI (SP2) probe rather than by using the BamHI/HindII (SP1) probe (Fig. 1a) using a 1·6% agarose gel.
Isolation of nuclear or total cell proteins
To isolate nuclei, pelleted cells were washed with PBS, resuspended in 10 mm HEPES, pH 8, containing 1·5 mm MgCl2, 10 mm KCl and 0·5 mm dithiothreitol, incubated on ice for 10 min, pelleted and then resuspended in the same buffer which also included 0·23 m sucrose. Cells were disrupted using a glass homogenizer and the nuclei were pelleted by centrifugation. Nuclei were resuspended in three pellet volumes of 10 mm HEPES, pH 8, containing 1·5 mm MgCl2, 10 mm KCl, 0·5 mm dithiothreitol, 25% (v/v) glycerol and 320 mm KCl, incubated on ice for 30 min and the nuclear extract harvested by centrifugation. Total proteins (for Western blotting experiments) were isolated by resuspending cells in 10 mm HEPES, pH 8, containing 1·5 mm MgCl2, 10 mm KCl, 0·5 mm dithiothereitol, 25% glycerol and 320 mm KCl, incubated on ice for 30 min, and harvested by centrifugation.
Electrophoretic mobility shift assays (EMSAs)
Sequences of the oligonucleotides, in addition to those shown in Fig. 2(a), are as follows:
Mim: CTAGGACATTATAACGGTTTTTTAGTCTAG.
m-mim: CTAGGACATTATCACGGTTTTTTAGTCTAG.
NF-κB: GGCGCCAGATTTGTATGGAGGGGGAGTGTCCAAACCTCTT.32
Figure 2.
Electrophoretic mobility shift assays (EMSA). (a) Sequences α, β and γ within the DNAse hypersensitive site 8 (HSS8) of the bcl-2 gene, used in EMSAs, are indicated. Consensus transcription binding sites are boxed and labelled in bold type; the myb consensus is based on Weston.35 Additional myb-binding sites (see the Discussion) are presented in grey boxes. (b) EMSA patterns with nuclear extracts from REH and RPMI 8226 cells. The probes and competitors (comp) are indicated. (c) EMSA patterns with nuclear extracts from REH cells, with probes and competitors indicated. The specific complex is indicated with an arrow and the free probe with an arrowhead.
Ten femtomol (fmol) of 32P-labelled double-stranded oligonucleotide was incubated with 1 µg of nuclear extract on ice for 20 min in 2 mm Tris–HCl, pH 7·4, containing 1 mm MgCl2, 100 mm NaCl, 4% (w/v) Ficoll and 5 µg of poly(dI.dC) plus competitor, as indicated, and then separated on a 4% (w/v) polyacrylamide gel in 1 × TBE (89·18 mm Tris Base, 88·95 mm boric acid, 2·5 mm EDTA). To examine the effect of anti-B-Myb, a 1-µg aliquot of nuclear extract was preincubated on ice for 20 min with non-immune rabbit serum or with rabbit anti-human-B-Myb (provided by Dr R. Lewis),33 as indicated in the above buffer in the absence of DNA. Five micrograms of poly (dI.dC), 10 fmol of reverse transcription-labelled oligonucleotide and any unlabelled competitor DNA was added. The mixture was incubated on ice for 20 min and separated on a 4% (w/v) polycrylamide gel in 0·25× TBE. Quantification of DNA–protein complexes was carried out on files produced by using the Vuego Scan (Brisa, Domingos de Rana, Portugal) scanner and analysed using the Phoretix 1D Quantifier (Phoretix International, Newcastle upon Tyne, UK).
UV crosslinking and Western blotting
Bromodeoxyuridine (BrdU)-substituted oligonucleotides were provided by the DNA:RNA Synthesis Laboratory (Beckman Research Institute of the City of Hope, Duarte, CA). Gel shift assays were performed by using 32P-labelled BrdU-substituted α (Fig. 2a) and nuclear extracts from REH cells. The labelled complex was visualized by autoradiography, and the DNA–protein complex formed was excised, UV crosslinked at 300 nm by using a spectorlinker XL1000 [AMS Biotech (UK) Ltd, Abingdon, Oxon, UK], analysed by electrophoresis on a 10% (w/v) sodium dodecyl sulphate (SDS)-polyacrylamide gel and visualized by autoradiography.
Western blotting of B-Myb and Bcl-2
A total of 10 µg of each extract was analysed by electrophoresis in a 10% (w/v) SDS-polyacrylamide gel. Protein was transferred to 0·2-mm nitrocellulose paper (Schleicher and Schuell, London, UK). A rabbit anti-human B-Myb monoclonal antibody (sc-724; Santa Cruz Biotechnology, Inc., Heidelberg, Germany) or a rabbit anti-human B-Myb polyclonal antibody (a gift of Dr R. Lewis33) and conjugated anti-rabbit horseradish peroxidase (DAKO) were used, together with the enhanced chemiluminescence (ECL) system (Amersham, Little Chalfont, Buckinghamshire, UK), to visualize B-Myb protein. The Santa Cruz and Lewis antibodies were raised against overlapping peptides (586–700 and 553–611, respectively), unique to B-Myb, at least not present in A-Myb or c-myb. A mouse anti-human bcl-2 (DAKO) and conjugated anti-mouse horseradish peroxidase (DAKO), together with the Amersham ECL system, were used to visualize bcl-2 protein. Quantification was carried out on files produced with the Vuegu Scan (Brisa) scanner and analysed by using the Phoretix 1D Quantifier (Phoretix International).
Antisense experiments
A total of 5 µg of each antisense oligonucleotide sequence was transfected into 2 × 106 cells using lipofectamine 2000 (Invitrogen) following the manufacturer's instructions. The phosphorothioate oligonucleotide sequences used were:
Antisense B-myb: GCAGCGCGTCCGCCGAGA.
Sense B-myb: TCTCGGCGGACGCGCTGC.
Mismatched B-myb: GCACCGCCTCGGCGGAGA.
Antisense c-myb: GTGCCGGGGTCTTCGGGC.
Antisense A-myb: ACTGCGCGACCTCTTCGC.
The number of viable cells was determined every 48 hr, and apoptosis was assayed on day 4. The number of apoptotic cells in the cultures was assayed by using an in situ TUNEL Detection Kit (Boehringer Mannheim, Lewes, East Sussex, UK), following the manufacturer's instructions.
Reporter constructs
The α, β, γ sequences (Fig. 2a) and mut-γ (GGATCCAGGATCTGGGAGGCTGGGTAGGTGCACGGATCC) double-stranded oligonucleotide sequences were cloned 3′ of the chloramphenicol acetyltransferase (CAT) reporter gene in the pCAT-promoter vector by using BamHI/XbaI, XbaI/SalI, SalI/PstI and BamHI/BamHI, respectively. Other constructs were created as described below:
Intermediate Construct A. Bcl-2 cDNA34 (accession number M14745) was subcloned into Bluescript vector pBSKSII using PvuII and ClaI restriction enzyme digestion.
Construct 1. Bcl-2 cDNA, which included the transcription termination signal, was deleted from Construct A by using SphI located 618 bp 3′ of the start of transcription (accession number M14745) and SalI in Bluescript. The resultant cDNA was subcloned into pGFP-N1 using the flanking KpnI restriction enzyme sites. The orientation of the inserted cDNA was determined by using restriction enzyme digestion.
Construct 2. A 132-bp fragment, bases 486–618 3′ of the start of transcription (accession number M14745), of the bcl-2 cDNA, which included αβγ, was deleted from Construct 1 using the HincII restriction enzyme sites at 486 (accession number M14745) and within the Bluescript polylinker.
Intermediate Construct B. 4·8 kb of the 5′ region of a genomic clone30 containing the P1 promoter, the P2 promoter/exon 1, intron 1 and part of exon 2 was subcloned into Bluescript vector pBSKSII using HindIII/PstI restriction enzyme digestion.
Construct 3. The 5′ portion of the bcl-2 cDNA in Construct 1 was replaced with the 4·8-kb HindIII/PstI genomic fragment containing the bcl-2 promoter region, exon 1, intron 1 and part of exon 2 from Construct B.
Construct 4. The 5′ portion of the bcl-2 cDNA in Construct 2 was replaced with a HindIII–PstI fragment containing the bcl-2 promoter region, exon 1, intron 1 and part of exon 2 from Construct B.
Transfection studies with CAT reporter genes
A total of 106 cells were transfected with a total of 1·5 µg of DNA (0·5 µg per construct) using Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions. Cells were harvested at 48 hr and lysed in 150 µl of 1 × reporter lysis buffer (Promega, Madison WI). Fifty microlitres of the lysate was used for the β-galactosidase assay and 100 µl of lysate was used for the CAT assay (E1000; Promega) following the manufacturer's instructions. β-galactosidase was measured at 405 nm by using a Wallac 1420 multilable HTS counter (Perkin Elmer, Wellesley, MA). CAT activity was measured by using a liquid scintillation counter. CAT activity measurements were normalized for transfection efficiency using the level of β-galactosidase activity from the co-transfected IE–β-gal reporter construct.
Transfection studies with the green fluorescent protein (GFP) reporters
Transient transfections were carried out using DOTAP reagent (Boehringer Mannheim) following the manufacturer's instructions. A total of 2·5 µg of each reporter construct was transfected into 5 × 106 cells, which were harvested at 48 hr. Green fluorescent protein was detected by using a UVStat ultrablock fluorimeter at an excitation wavelength of 395 nm and an emission wavelength of 509 nm. Measurements were normalized for transfection efficiency by using the level of β-galactosidase activity from the co-transfected pCMV/β-Gal (Tropix Applied Biosystems, Warrington, UK) reporter construct determined using Galacto-Light Plus (Tropix) following the manufacturer's instructions.
Stable transfections
Stable transfections were carried out using DOTAP reagent (Boehringer Mannheim) following the manufacturer's instructions. A total of 5 µg of pCMV-B-MYB (a gift of Dr R. Watson) was transfected into 5 × 106 RPMI 8226 cells. Selection (1 mg/ml geneticin) was introduced at 48 hr, and individual clones were analysed for B-Myb expression at 2 months.
Results
HSS8 in the REH B-cell line and in tonsil B cells
We first confirmed the expression of bcl-2 protein and the presence of DNAse I HSS8 in the REH B-cell line, and investigated whether both also occur in primary tonsil B cells. Both tonsil B cells (Fig. 1b) and the REH B-cell line (Fig. 1c) express bcl-2 protein, as indicated by immunohistochemical staining with a specific antibody. HSS8 is detected in both cell populations by the appearance of a 2·5-kb fragment in DNAse I hypersensitive site assays, using the SP1 probe to detect cleavage in the PstI–HindIII fragment (Fig. 1d, 1e). This locates HSS8 to the exon 2/intron 2 border of the bcl-2 gene, in agreement with previous results.13 To locate the position of HSS8 more accurately, we repeated the assay using the SP2 probe (Fig. 1a). Release of a 310-bp fragment places the centre of HSS8 at ≈ 40 bp upstream from the BamHI restriction site and 62 nucleotides upstream of the exon 2/intron 2 border (Fig. 1a, 1f). Thus, all or a major part of HSS8 is contained within exon 2 of the bcl-2 gene.
EMSAs
We analysed protein binding in the region corresponding to HSS8 using the electrophoretic mobility shift assay (EMSA). The region of DNAse I hypersensitivity contains putative binding sites for a number of transcription factors, including myb (YAACNG), Sp1 (TGCAC) and GATA (GATA/C), on the antisense strand of the DNA (Fig. 2a) and for ets (GGAA/T) on the sense strand. However, we found evidence of only three strong binding sites when EMSA was performed with REH nuclear extracts (results not shown). REH extracts formed one major complex of the same mobility with α, β and γ oligonucleotides of similar length (Fig. 2b). Only traces of this complex could be seen in EMSA with RPMI 8226 cell extracts (Fig. 2b). The formation of complexes with the REH protein was competed by a 100-fold excess of the same unlabelled sequence (Fig. 2b), demonstrating the specificity of protein binding.
Experiments with chicken c-Myb led to the designation of PyAAC(G/T)G as the consensus binding site,35 but later experiments indicated that the sequences recognized by members of the Myb family of transcription factors is even more degenerate (see the Discussion). The essential AAC core of the myb consensus sequence is present in α, β and γ, and a protein of similar size binds to these oligonucleotides. To test whether the REH protein binding to α, β and γ oligonucleotides is a member of the Myb family, we used the oligonucleotide mim from the mim-1 promoter and also m-mim, containing a mutation that no longer binds myb protein,36 as competitors in EMSA. As shown for the α oligonucleotide in Fig. 2(c), these experiments revealed that the same (α, β or γ) oligonucleotides and mim, but not m-mim, are effective competitors. When the mim oligonucleotide itself is labelled and mixed with REH extracts, it forms a specific complex of the same mobility as that observed with the α, β or γ oligonucleotides (Fig. 3b). These results suggest that the protein binding to the α, β and γ oligonucleotides is a member of the Myb family of transcription factors.
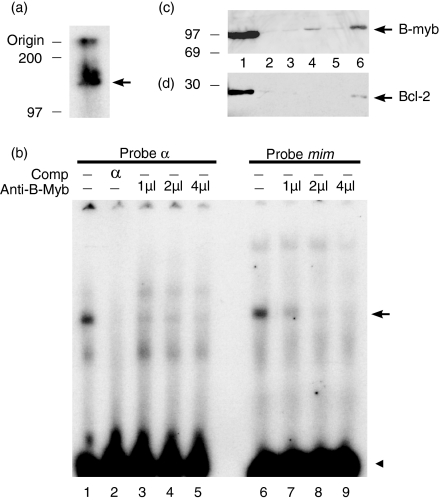
Figure 3.
Identification of the protein in the REH protein complex with α. (a) Apparent molecular weight determination. REH nuclear proteins UV crosslinked to bromodeoxyuridine (BrdU)-substituted 32P-labelled α, UV crosslinked and visualized by autoradiography. The UV-crosslinked product is marked with an arrow. Molecular weight markers are shown on the left. (b) EMSA with the labelled α and mim probes, competitors and increasing amounts of a specific anti-B-myb antibody. Non-specific antiserum (4 µl) was included in lane 1. The specific complex is indicated with an arrow and the free probe indicated with an arrowhead. (c) and (d) Western blotting analysis of total proteins. Ten micrograms of total cellular extract from REH (lane 1), RPMI 8226 (lane 2), RPMI 8866 (lane 3), Raji (lane 4), Namalwa (lane 5) and Ramos (lane 6) B-cell lines were probed with the anti-human-B-myb immunoglobulin (c) or with an anti-bcl-2 immunoglobulin (DAKO) (d). Protein size markers are on the left.
In an attempt to demonstrate protein binding to α, β and γ within our cell lines, we performed in vivo footprinting and chromatin immunopreciptation (ChIP) assays. Three in vivo footprints across α, β and γ were observed in the bcl-2-expressing REH cell line; these footprints were absent in non-expressing RPMI 8226.37 In order to identify the protein bound in vivo to α, β and γ, ChIP was performed on the bcl-2-expressing REH, non-expressing RPMI 8226 and RPMI 8226 stably transfected with a B-Myb expression vector, CMV-B-Myb. Protein was fixed to chromatin in vivo by using formaldehyde, chromatin was sonicated to an average size of 300 bp and immunoprecipitated by using anti-A-Myb, anti-B-Myb, anti-c-Myb or a control antibody. No chromatin spanning HSS8 was immunoprecipitated with antibody. However, the region of chromatin containing α, β and γ was not found in chromatin prior to immunoprecipitation, indicating that this site had been preferentially destroyed by sonication. In order to control for quality and quantity of the ChIP output, we compared input and output of the ChIP assay on a known target gene by PCR, using anti-B-Myb and primers spanning the relevant myb-binding site. Both the input and the output exhibited the appropriate PCR product (results not shown). Thus we could not use ChIP to analyse proteins bound to the α, β and γ sequences.
Which member of the Myb family?
To characterize the protein further, its apparent molecular weight was measured by UV crosslinking of the REH protein bound to BrdU-substituted and radioactively labelled α oligonucleotide; the specific complex was isolated from an agarose gel and electrophoresed in an SDS-polyacrylamide gel alongside protein molecular-weight (MW) markers (Fig. 3a). Allowing for the size of the DNA (30 000 MW), the molecular weight of the protein was found to be ≈ 100 000 MW. This size would be consistent with the protein being A-Myb or B-Myb, which are 90 000–110 000 MW.38 As DNA does not run like protein in SDS–polyacrylamide gel electrophoresis (PAGE), estimates of MW are inaccurate, but the results appear to exclude c-Myb, which has a MW of 75 000.
To distinguish between A-myb and B-myb, we used a B-myb-specific antiserum.33 Titration with this antiserum, but not with a non-specific antiserum, inhibited the formation of REH protein complexes with labelled α and mim oligonucleotides in a concentration-dependent manner (Fig. 3b). Specificity of protein binding to the α oligonucleotide was demonstrated by self-competition with the α oligonucleotide. B-Myb antibody did not interfere with NF-κB binding to a labelled oligonucleotide containing an NF-κB binding site (results not shown). These results indicate that the REH protein which binds to the α and mim oligonucleotides is B-Myb.
Correlation between the levels of B-Myb and bcl-2 in a wide range of B-cell lines
An anti-B-Myb antibody was used to measure the relative levels of B-Myb (Fig. 3c) and of bcl-2 (Fig. 3d) in a wide range of B-cell lines: the REH pro-B (lane 1), RPMI 8226 plasma (lane 2), RPMI 8866 lymphoblastoid (lane 3) and Raji, Namalwa and Ramos Burkitt's lymphoma (lanes 4–6) cell lines. It can be seen that there is a general correspondence between the levels of B-Myb and bcl-2 in these cell lines. The inability to detect B-Myb in RPMI 8226, compared to REH cells, is consistent with the α, β and γ DNA-binding activities of the two cell extracts in the EMSAs (Fig. 2b).
Transfection of cells with antisense oligonucleotides
To investigate the role of Myb family members in bcl-2 gene expression in REH cells, we employed antisense phosphorothioate oligonucleotides, corresponding to A-myb, B-myb and c-myb, transfected into REH cells. The transfected cells were examined for bcl-2 protein levels, apoptosis and the ability of proteins in REH extracts to bind to the γ oligonucleotide.
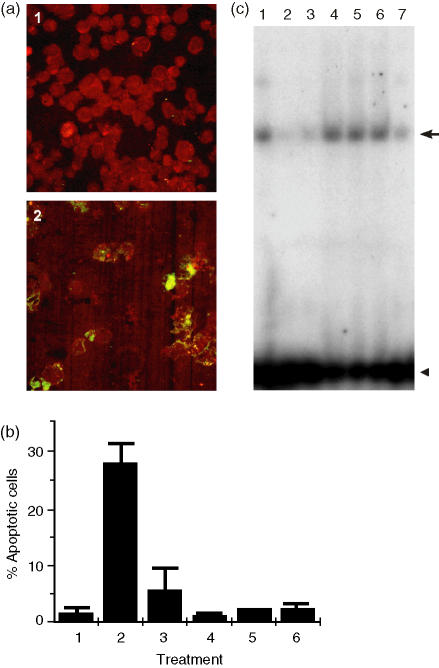
Bcl-2 protein was visualized by staining cells with an antibody against bcl-2 and apoptosis by the TUNEL assay (red and green, respectively, in Fig. 4a). The disappearance of bcl-2 protein and accumulation of DNA fragments in the cytoplasm of cells are the expected results of inhibiting bcl-2 gene expression. Bcl-2 protein levels were decreased by antisense B-myb (Fig. 4a, cf. panels 1 and 2), but not to a significant extent by sense B-myb, mismatched B-myb, antisense A-myb or antisense c-myb (results not shown). The level of apoptosis observed with antisense B-myb was ≈ 30%, compared to < 5% for all the other oligonucleodies (Fig. 4b). These results implicate B-Myb alone in determining REH cell viability.
Figure 4.
Antisense treatment of REH cells. (a) Confocal analysis of (1) untreated REH cells and (2) REH cells treated with antisense B-myb stained for apoptosis (green) and bcl-2 protein (red). (b) The percentage of apoptotic cells in (1) untreated REH cells and in REH cells treated with (2) antisense B-myb, (3) sense B-myb, (4) mismatched B-myb, (5) antisense c-myb and (6) antisense A-myb. (c) Electrophoretic mobility shift assays (EMSAs) with labelled γ and extracts from (1) untreated REH cells and from REH cells treated with (2) antisense B-myb, (3) sense B-myb, (4) mismatched B-myb, (5) antisense c-myb, (6) antisense A-myb and (7) a 10-fold excess of unlabelled γ.
Next, we carried out EMSA with the labelled γ oligonucleotide and extracts of the antisense-treated REH cells. Treatment with antisense B-myb and, to a lesser extent, sense B-myb, but not mismatched B-myb, antisense c-myb or antisense A-myb, resulted in the loss of γ oligonucleotide-binding activity (Fig. 4c). In agreement with the above results (Fig. 3b), this data identifies B-Myb, rather than A-Myb or c-Myb, in γ oligonucleotide-binding activity. Taken together, the results in Fig. 4 reveal a strong correlation between the B-Myb binding to specific sites in exon-2 of the bcl-2 gene in vitro, the level of bcl-2 protein and cell survival.
Up-regulation of the endogenous bcl-2 gene by expression of B-Myb in RPMI 8226 cells
To determine whether expression of the endogenous bcl-2 gene in RPMI 8226 cells can be up-regulated by Myb, we examined the effects of stable transfection of these cells with an expression vector for B-Myb (pCMV-B-MYB). Using extracts from the transfected cells, we observed that complex formation with α in the gel-shift assay was raised to the level observed with the REH cell extract, consistent with B-Myb synthesis and activation in the transfected cells (Fig. 5a). The results of Western blotting with the bcl-2 antibody confirm the low level of expression in untransfected RPMI 8226 cells, relative to REH cells (Fig. 5b). Following transfection with the B-Myb expression vector, however, we observe bcl-2 protein at the same level as that in untransfected REH cells (Fig. 5c). These experiments suggest that B-Myb is sufficient to induce endogenous bcl-2 gene expression in RPMI 8226 cells.
Figure 5.
B-myb induction of bcl-2 protein in RPMI 8226 cells. (a) Electrophoretic mobility shift assays (EMSAs) with labelled α oligonucleotide and various cell extracts: (1) REH cells, (2) RPMI 8226 cells and (3) RPMI 8226 cells transfected with pCMV-B-MYB. (b) Relative amounts of bcl-2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein detected with anti-bcl-2 and anti-GAPDH immunoglobulin, respectively, in Western blots of the total proteins described in (a). (c) Relative amounts of bcl-2 protein, described in (a), as measured by densitometry.
Enhancer activity of α, β and γ sequences
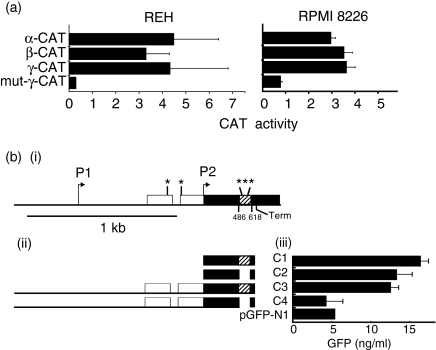
To test the enhancer activity of the individual α, β and γ sequences, and to analyse their dependence upon the Myb protein, we linked these sequences to the 3′ end of the CAT gene in the p-CAT promoter vector. The IE-β-Gal vector was co-transfected with the p-CAT-promoter vectors for normalization of the results to transfection efficiencies. When REH cells were transfected with each of the constructs, we observed a 3·3- to 4·5-fold stimulation of CAT activity (Fig. 6a). No increase in synthesis of CAT was seen on transfection of RPMI 8226 cells with any of the constructs (results not shown). When RPMI 8226 cells were co-transfected with each of these constructs, together with an expression vector for B-Myb (pCMV-B-myb), however, we observed a 3·0- to 3·6-fold stimulation of CAT activity (Fig. 6a). The mutant γ sequence was inactive in response to B-Myb. These results indicate that α, β and γ can enhance gene expression in combination with B-Myb.
Figure 6.
Enhancer activities of α, β and γ sequences. (a) Chloramphenicol acetyltransferase (CAT) assays. Left: activity of α, β and γ enhancer constructs in REH cells relative to that of the original pCAT-promoter vector. Right: activity of each construct co-transfected with pCMV-B-Myb into RPMI 8226 cells relative to the original pCAT-promoter vector co-transfected with pCMV-B-myb. (bi) The bcl-2 5′ noncoding sequences (white) and coding sequences containing the 132-bp sequence encompassing α, β and γ (black and cross-hatched respectively), cloned between the HCMV promoter and the green fluorescent protein (GFP) gene. Also indicated: Myb-binding sites (*), transcriptional start sites (arrows) and point of RNA chain termination (Term). (bii) Regions of the bcl-2 gene cloned into pGFP-N1 to produce constructs C1-4. (biii) Levels of GFP observed in REH cells transfected with constructs C1-4 or with the empty pGFP-N1 vector (pGFP-N1).
To test whether the region encompassing all three myb-binding sites in the coding sequence of the bcl-2 gene itself stimulates gene expression, we created four constructs (see the Materials and methods). Construct 1 (C1 in Fig. 6b) contained the entire coding sequence (cDNA) of the bcl-2 gene with a deletion of 3′ untranslated sequence up to and including the transcription termination site, linked in-frame to the GFP gene in the vector pGFP-N1. In construct 2 (C2 in Fig. 6b) 132 bp of the bcl-2 cDNA containing the αβγ sequence in C1 was deleted, leaving GFP in-frame. In construct 3 (C3 in Fig. 6b), the 5′ end of the bcl-2 cDNA in C1 was replaced with the corresponding genomic fragment of the bcl-2 gene containing the P1 and P2 promoters, exon 1, intron 1 and exon 2. The same substitution in C2 was made in C3 to produce C4 (Fig. 6b).
These constructs, or pGFPN-1, were co-transfected into REH cells along with pCMV/β-Gal vector (to normalizse transfection efficiencies) and the levels of GFP in cell extracts were measured after 48 hr (Fig. 6b). Both C1 and C2 were transcribed at a level three times higher than the empty vector (pGFP-N1), showing that the presence of the αβγ sequence had no effect upon expression in the absence of bcl-2 5′ sequences. C3, which contains the 5′ untranslated promoter sequences plus exon 2 with the αβγ sequence, was equally active. Deletion of the αβγ sequence in C4, however, reduced the activity to the level of the vector alone. These results reveal that the αβγ sequence can act as an enhancer in the context of the 5′ sequences in the bcl-2 gene in REH cells.
Discussion
We have investigated the protein-binding sites in a DNAse I hypersensitive site that is associated with bcl-2 gene activation in human B cells.13 The presence of the hypersensitive site in bcl-2-expressing tonsil B indicates that it probably has a function in normal B cells. Mapping of the hypersensitive site to the 3′ end of exon 2 facilitated identification of the DNA-binding proteins and their activities.
We observed that a protein of similar size binds to three separate regions (α, β and γ) at the 3′ end of exon 2. The fact that these sequences each contained the AAC myb core consensus sequence suggested that the protein might be a member of the Myb family of transcription factors. Early work led to the designation of PyAAC(G/T)G as the myb consensus sequence.35 Nuclear magnetic resonance structures demonstrate that the core AAC and a G residue in one of the two 3′ positions are contact residues in complexes with Myb DNA-binding domains.39,40 Binding and transactivation studies have demonstrated that myb-binding sites may contain AT or AC in the last two positions, indicating even greater degeneracy in the consensus sequence.41 The putative myb-binding site in the γ oligonucleotide (TAACGG) corresponds to the original consensus sequence,35 but those in the α and β oligonucleotides would be admissible, according to more recent studies.
There are three members of the Myb family of transcription factors (A-Myb, B-Myb and c-Myb), all of which are expressed at some stage of B-cell development. B-Myb is ubiquitous in cells and is expressed in a cell cycle-dependent manner in late G1 and S phases of the cell cycle (reviewed in refs 42 and 43).
The size of the protein binding to the α oligonucleotide is consistent with its identification as either A-Myb or B-Myb. Formation of the α-REH DNA–protein complex formation is inhibited by a specific antiserum against B-Myb. The antibody recognizes a C-terminal sequence that is unique to this member of the family of Myb transcription factors.33 Although the DNA-binding domain of Myb proteins is at the opposite end of the protein, the C-terminal sequence of B-Myb is well known to modulate its activity (reviewed in refs 42 and 43).
Experiments with antisense RNA were performed to test the function of various members of the Myb family in the expression of the bcl-2 gene. Antisense B-myb down-regulated the expression of the bcl-2 protein and led to the apoptosis of REH cells. Antisense A-myb and c-myb had no effect on bcl-2 protein levels or on REH cell viability. The effects of these sequences on bcl-2 protein levels and apoptosis were reflected in the γ oligonucleotide-binding activity of cell extracts in vitro and indicated that antisense B-myb depleted the cells of all detectable Myb-binding activity. Previous authors have reported that antisense B-myb induced apoptosis in cell lines representing neurobastoma and several haematopoietic lineages, including a B-cell line.44,45 Our results, unlike earlier results on the survival of T cells26 and myeloid cells,25 revealed that antisense c-myb had little effect on the survival of REH cells. This suggests that B-Myb may play a more prominent role in B-cell survival.
Expression of B-Myb in the bcl-2 non-expressing RPMI 8226 cell line stimulated both the synthesis (or activity) of a protein that was active in binding to the α oligonucleotide in vitro, probably B-Myb itself, and bcl-2 expression. The level of bcl-2 protein in the transfected cells was comparable to that seen in untransfected REH cells. These results provide further confirmation that B-Myb is involved in bcl-2 gene activation.
Reporter assays with α, β or γ oligonucleotides linked 3′ to the CAT gene revealed that each of the sites can act as an enhancer in REH cells. These sequences are inactive in RPMI 8226 cells, but can be activated by co-expression with B-Myb. These results demonstrate that each individual site is a transcriptional enhancer in both REH and RPMI 8226 cells, and that B-Myb stimulates their activity in the bcl-2 non-expressing RPMI 8226 cell line.
Experiments using the native bcl-2 gene constructs were carried out to test the effect of the αβγ sequences in this context. Interestingly, our results revealed that the sequence worked as an enhancer only in the context of the 5′ flanking sequence of the bcl-2 gene and suggests that the myb-binding sites in αβγ may either co-operate with positive regulatory elements (e.g. other myb-binding sites)46 or counteract negative regulatory elements13,29 in the promoter. The 30-bp spacing between each of the three myb-binding sites, α, β and γ, aligns these sequences along one face of the DNA, allowing for the potential co-operative binding of B-Myb to this bcl-2 enhancer.
Other workers have shown that bcl-2 gene activation requires c-Myb in murine T cells26 and v-Myb in chicken myeloid cells.25 However immunodepletion experiments have shown that B-Myb, rather than c-Myb binding, is required for bcl-2 gene activation in murine CTLL-2 T cells.47 At least one other gene, the anti-apoptotic apolipoprotein J (Clusterin) gene, is a target gene for B-Myb in neuroblastoma cells.48 Different members of the Myb family of transcription factors are known to have overlapping sequence specificity and operate with different accessory protein factors, underpinning distinctive cellular functions (reviewed in refs 42 and 43).
All three former studies of essential myb-binding sites in the murine26 and chicken25,47 bcl-2 genes focused on myb-binding sites in the sequence homologous to the region between P1 and the first codon in the human bcl-2 gene. Thus, the enhancer sequences described in the present study contain novel myb-binding sites that have not been examined previously. Single myb-binding sites have been shown to act as enhancers in other genes, e.g. the SV-40 gene49 and the T-cell-receptor δ gene.50 The SV-40 gene contains several myb-binding sites that are preferentially bound by either c-Myb or B-Myb, while the myb-binding site in the δ-gene requires c-Myb in T cells. Sequence specificity, post-translational modifications (phosphorylation) and association with cell-specific accessory proteins, modulate the activity of Myb proteins. B-Myb is actively repressed prior to G1, phosphorylated by cyclin A or E/cdc2 in late G1/S and degraded during the M phase of the cell cycle.42,43
Our work suggests that B-Myb is essential for B-cell survival, as indeed it appears to function in the germinal centres of lymphoid tissue during the clonal selection of B cells by antigen.51 B-Myb may activate the bcl-2 gene directly through its binding sites in the exon-2 enhancer sequence, perhaps in conjunction with other sites between P1 and P2. Further experiments are necessary to test whether the enhancer is active in other cell types and whether the myb-binding sites in exon 2 are necessary for activation of the endogenous gene in vivo. Their presence in an accessible region of chromatin structure, revealed by the hypersensitivity of a region encompassing the αβγ sequence to DNAse I digestion, is consistent with this function in vivo.
Acknowledgments
We are grateful to Prof. Y. Tsujimoto for the 5′Bcl-2 genomic clone λ1821, Dr K. Weston for vector pCMCEF-c-myb, Dr R. Watson for vector pCMV-B-MYB and Dr R. Lewis for the anti-B-myb antiserum. The hospitality and assistance to W.M.G. by Dr A. R. Riggs in pilot studies on the Bcl-2 gene, and the gift of a BrdU-substituted oligonucleotide, is gratefully acknowledged. This work was supported by a Wellcome Trust Programme Grant (H.J.G. and B. J. Sutton), a Wellcome Trust PhD Studentship (W.M.G.), and Cancer Research UK (G.L.).
Abbreviations
- BrdU
bromodeoxyuridine
- ChIP
chromatin immune precipitation
- CRE
cAMP response element
- ECL
enhanced chemiluminescence
- EMSA
electrophoretic mobility shift assay
- HSS
hypersensitive site
- mbr
major break point
- NF-κB
nuclear factor-κB
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- SDS
sodium dodecyl sulphates
References
- 1.Ranger AM, Malynn BA, Korsmeyer SJ. Mouse models of cell death. Nat Genet. 2001;28:113–8. doi: 10.1038/88815. [DOI] [PubMed] [Google Scholar]
- 2.Husson H, Carideo EG, Neuberg D, Schultze J, Munoz O, Marks PW, Donovan JW, Chillemi AC, O'Connell P, Freedman AS. Gene expression profiling of follicular lymphoma and normal germinal center B cells using cDNA arrays. Blood. 2002;99:282–9. doi: 10.1182/blood.v99.1.282. [DOI] [PubMed] [Google Scholar]
- 3.Graninger WB, Seto M, Boutain B, Goldman P, Korsmeyer SJ. Expression of Bcl-2 and Bcl-2-Ig fusion transcripts in normal and neoplastic cells. J Clin Invest. 1987;80:1512–5. doi: 10.1172/JCI113235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Y, Wyatt RT, Bigley A, Krontiris TG. Expression and repliciation timing patterns of wildtype and translocated BCL2 genes. Genomics. 2001;73:161–70. doi: 10.1006/geno.2000.6479. [DOI] [PubMed] [Google Scholar]
- 5.Yonetani N, Ueda C, Akasaka T, Nishikori M, Uchiyama T, Ohno H. Heterogeneous breakpoints on the immunoglobulin genes are involved in fusion with the 5′ region of BCL2 in B-cell tumors. Jpn J Cancer Res. 2001;92:933–40. doi: 10.1111/j.1349-7006.2001.tb01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonnell TJ, Korsmeyer SJ. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14;18) Nature. 1991;349:254–6. doi: 10.1038/349254a0. [DOI] [PubMed] [Google Scholar]
- 7.Reed JC. Balancing cell life and death: bax, apoptosis, and breast cancer. J Clin Invest. 1996;97:2403–4. doi: 10.1172/JCI118684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolowiec D, Mekki Y, Ffrench P, Manel A-M, Bertrand Y, Rimokh R, Philippe N, Bryon P-A, Ffrench M. Differential expression of cell proliferation regulatory proteins in B- and T-lineage acute lymphoblastic leukaemias. Br J Haematol. 1996;95:518–23. doi: 10.1046/j.1365-2141.1996.d01-1930.x. [DOI] [PubMed] [Google Scholar]
- 9.Webb A, Cunningham D, Cotter F, Clarke PA, di Stefano F, Ross P, Corbo M, Dziewanowska Z. BCL-2 antisense therapy in patients with non-Hodgkin lymphoma. Lancet. 1997;349:1137–41. doi: 10.1016/s0140-6736(96)11103-x. [DOI] [PubMed] [Google Scholar]
- 10.Manion MK, Hockenbery DM. Targeting BCL-2-related proteins in cancer therapy. Cancer Biol Ther. 2003;2:S105–14. [PubMed] [Google Scholar]
- 11.Reed JC. Dysregulation of apoptosis in cancer. J Clin Oncol. 1999;17:2941–53. doi: 10.1200/JCO.1999.17.9.2941. [DOI] [PubMed] [Google Scholar]
- 12.Seto M, Jaeger U, Hockett RD, Graninger W, Bennett S, Goldman P, Korsmeyer SJ. Alternative promoters and exons, somatic mutation and deregulation of the Bcl-2-Ig fusion gene in lymphoma. EMBO J. 1988;7:123–31. doi: 10.1002/j.1460-2075.1988.tb02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young RL, Korsmeyer SJ. A negative regulatory element in the bcl-2 5′-untranslated region inhibits expression from an upstream promoter. Mol Cell Biol. 1993;13:3686–97. doi: 10.1128/mcb.13.6.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Decary S, Decesse JT, Ogryzko V, Reed JC, Naguibneva I, Harel-Bellan A, Cremisi CE. The retinoblastoma protein binds the promoter of the survival gene bcl-2 and regulates its transcription in epithelial cells through transcription factor AP-2. Mol Cell Biol. 2002;22:7877–88. doi: 10.1128/MCB.22.22.7877-7888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heckman CA, Mehwe JW, Ying GG, Introna M, Golay J, Boxer LM. A-Myb up-regulates Bcl-2 through a Cdx binding site in t(14;18) lymphoma cells. J Biol Chem. 2000;275:6499–508. doi: 10.1074/jbc.275.9.6499. [DOI] [PubMed] [Google Scholar]
- 16.Wessendorf S, Schwaenen C, Kohlhammer H, Kienle D, Wrobel G, Barth TF, Nessling M, Moller P, Dohner H, Lichter P, Bentz M. Hidden gene amplifications in aggressive B-cell non-Hodgkin lymphomas detected by microarray-based comparative genomic hybridization. Oncogene. 2003;22:1425–9. doi: 10.1038/sj.onc.1206297. [DOI] [PubMed] [Google Scholar]
- 17.Wilson BE, Mochon E, Boxer LM. Induction of bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Mol Cell Biol. 1996;16:5546–56. doi: 10.1128/mcb.16.10.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji L, Mochon E, Arcinas M, Boxer LM. CREB proteins function as positive regulators of the translocated bcl-2 allele in t(14;18) lymphomas. J Biol Chem. 1996;271:22687–91. doi: 10.1074/jbc.271.37.22687. [DOI] [PubMed] [Google Scholar]
- 19.Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 1999;286:2358–61. doi: 10.1126/science.286.5448.2358. [DOI] [PubMed] [Google Scholar]
- 20.Dong L, Wang W, Wang F, Stoner M, Reed JC, Harigai M, Samudio I, Kladde MP, Vyhlidal C, Safe S. Mechanisms of transcriptional activation of bcl-2 gene expression by 17beta-estradiol in breast cancer cells. J Biol Chem. 1999;274:32099–107. doi: 10.1074/jbc.274.45.32099. [DOI] [PubMed] [Google Scholar]
- 21.Heckman C, Mochon E, Arcinas M, Boxer LM. The WT1 protein is a negative regulator of the normal bcl-2 allele in t(14;18) lymphomas. J Biol Chem. 1997;272:19609–14. doi: 10.1074/jbc.272.31.19609. [DOI] [PubMed] [Google Scholar]
- 22.Hewitt SM, Hamada S, McDonnell TJ, Rauscher FJ, III, Saunders GF. Regulation of the proto-oncogenes bcl-2 and c-myc by the Wilms' tumor suppressor gene WT1. Cancer Res. 1995;55:5386–9. [PubMed] [Google Scholar]
- 23.Mayo MW, Wang CY, Drouin SS, Madrid LV, Marshall AF, Reed JC, Weissman BE, Baldwin AS. WT1 modulates apoptosis by transcriptionally upregulating the bcl-2 proto-oncogene. EMBO J. 1999;18:3990–4003. doi: 10.1093/emboj/18.14.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heckman CA, Mehew JW, Boxer LM. NF-kappaB activates Bcl-2 expression in t(14;18) lymphoma cells. Oncogene. 2002;21:3898–908. doi: 10.1038/sj.onc.1205483. [DOI] [PubMed] [Google Scholar]
- 25.Frampton J, Ramqvist T, Graf T. v-Myb of E26 leukemia virus up-regulates bcl-2 and suppresses apoptosis in myeloid cells. Genes Dev. 1996;10:2720–31. doi: 10.1101/gad.10.21.2720. [DOI] [PubMed] [Google Scholar]
- 26.Taylor D, Badiani P, Weston K. A dominant interfering Myb mutant causes apoptosis in T cells. Genes Dev. 1996;10:2732–44. doi: 10.1101/gad.10.21.2732. [DOI] [PubMed] [Google Scholar]
- 27.Romero F, Martinez AC, Camonis J, Rebollo A. Aiolos transcription factor controls cell death in T cells by regulating Bcl-2 expression and its cellular localization. EMBO J. 1999;18:3419–30. doi: 10.1093/emboj/18.12.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Y, Mehew JW, Heckman CA, Arcinas M, Boxer LM. Negative regulation of bcl-2 expression by p53 in hematopoietic cells. Oncogene. 2001;20:240–51. doi: 10.1038/sj.onc.1204067. [DOI] [PubMed] [Google Scholar]
- 29.Chen H-M, Boxer LM. Pi 1 binding sites are negative regulators of bcl-2 expression in pre-B cells. Mol Cell Biol. 1995;15:3840–7. doi: 10.1128/mcb.15.7.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsujimoto Y, Croce CM. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci USA. 1986;83:5214–8. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonifer C. Long-distance chromatin mechanisms controlling tissue-specific gene locus activation. Gene. 1999;238:277–89. doi: 10.1016/s0378-1119(99)00340-6. [DOI] [PubMed] [Google Scholar]
- 32.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–3. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 33.Johnson TK, Schweppe RE, Septer J, Lewis RE. Phosphorylation of B-Myb regulates its transactivation potential and DNA binding. J Biol Chem. 1999;274:36741–9. doi: 10.1074/jbc.274.51.36741. [DOI] [PubMed] [Google Scholar]
- 34.Cleary ML, Smith SD, Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986;47:19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- 35.Weston K. Extension of the DNA binding consensus of the chicken c-Myb and v-Myb proteins. Nucleic Acids Res. 1992;20:3043–9. doi: 10.1093/nar/20.12.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ness SA, Marknell A, Graf T. The v-myb oncogene product binds to and activates the promyelocyte-specific mim-1 gene. Cell. 1989;59:1115–25. doi: 10.1016/0092-8674(89)90767-8. [DOI] [PubMed] [Google Scholar]
- 37.Gombert WM. PhD Thesis. London: London University; 1998. Regulation of the Bcl-2 gene in B cells. [Google Scholar]
- 38.Nomura N, Takahashi M, Matsui M, Ishii S, Date T, Sasamoyo S, Ishizati R. Isolation of human cDNA clones of myb-related genes, A-Myb and B-myb. Nucleic Acids Res. 1988;16:11075–89. doi: 10.1093/nar/16.23.11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogata K, Morikawa S, Nkamura H, Sekikawa A, Inoue T, Kanai H, Sarai A, Ishii S, Nishimura Y. Solution structure of a specific DNA complex of the Myb DNA-binding domain with cooperative recognition helices. Cell. 1994;79:639–48. doi: 10.1016/0092-8674(94)90549-5. [DOI] [PubMed] [Google Scholar]
- 40.McIntosh PB, Frenkiel TA, Wollborn U, McCormick JE, Klempnauer K-H, Feeney J, Carr MD. Solution structure of the B-Myb DNA-binding domain: a possible role for protein in DNA binding and control of gene expression. Biochemistry. 1998;37:9619–29. doi: 10.1021/bi972861z. [DOI] [PubMed] [Google Scholar]
- 41.Cogswell JP, Cogswell PC, Kuehl WM, Cuddihy AM, Bender TM, Engelke U, Marcu KB, Ting JP-Y. Mechanism of c-myc regulation by c-Myb in different cell lineages. Mol Cell Biol. 1993;13:2858–69. doi: 10.1128/mcb.13.5.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weston KM. Myb proteins in life, death and differentiation. Curr Opin Gen Dev. 1998;8:76–81. doi: 10.1016/s0959-437x(98)80065-8. [DOI] [PubMed] [Google Scholar]
- 43.Oh I-H, Reddy EP. The myb gene family in cell growth, differentiation and apoptosis. Oncogene. 1999;18:3017–33. doi: 10.1038/sj.onc.1202839. [DOI] [PubMed] [Google Scholar]
- 44.Arsura M, Introna M, Passerini F, Mantovani A, Golay J. B-myb antisense oligonucleotides inhibit proliferation of human hematopoietic cell lines. Blood. 1992;79:2708–16. [PubMed] [Google Scholar]
- 45.Raschella G, Negroni A, Sala A, Pucci S, Romeo A, Calabretta B. Requirement of B-myb function for survival and differentiative potential of human neuroblastoma cells. J Biol Chem. 1995;270:8540–5. doi: 10.1074/jbc.270.15.8540. [DOI] [PubMed] [Google Scholar]
- 46.Kim T, Jung H, Min S, Kim K-T, Ha H. B-myb proto-oncogene products interact in vivo with each other via the carboxy-terminal conserved region. FEBS Lett. 1999;460:363–8. doi: 10.1016/s0014-5793(99)01375-7. [DOI] [PubMed] [Google Scholar]
- 47.Grassilli E, Salomoni P, Perrotti D, Franceschi C, Calabretta B. Resistance to apoptosis in CTLL-2 cells overexpressing B-myb is associated with B-Myb-dependent bcl-2 induction. Cancer Res. 1999;59:2451–6. [PubMed] [Google Scholar]
- 48.Cervellera M, Raschella G, Santilli G, Tanno B, Venura A, Mancini C, Sevignani C, Calabretta B, Sala A. Direct transactivation of the anti-apoptotic gene apolipoprotein J (clusterin) by B-MYB. J Biol Chem. 2000;275:21055–60. doi: 10.1074/jbc.M002055200. [DOI] [PubMed] [Google Scholar]
- 49.Mizuguchi G, Nakagoshi H, Nagase T, Nomura N, Date T, Ueno Y, Ishii S. DNA binding activity and transcriptional activator function of the human B-Myb protein compared with c-myb. J Biol Chem. 1990;265:9280–4. [PubMed] [Google Scholar]
- 50.Hernandez-Munain C, Krangel MS. c-Myb and core-binding factor/PEBP2 display functional synergy but bind independently to adjacent sites in the T-cell receptor delta enhancer. Mol Cell Biol. 1995;15:3090–9. doi: 10.1128/mcb.15.6.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yokoyama T, Tanahashi M, Kobayashi Y, Yamakawa Y, Maeda M, Inaba T, Kiriyama M, Fukai I, Fujii Y. The expression of Bcl-2 family proteins (Bcl-2, Bcl-x, Bax, Bak and Bim) in human lymphocytes. Immunol Lett. 2002;81:107–13. doi: 10.1016/s0165-2478(02)00003-2. [DOI] [PubMed] [Google Scholar]