Abstract
Diverse immunotherapy approaches have achieved success in controlling individual aspects of immune responses in animal models. Transfer of such immunotherapies to clinical trials has obtained some success in patients, with clinical responses observed or effective antigen specific immune responses achieved, but has had limited impact on patient survival. Key elements required to generate de novo cell-mediated antitumour immune responses in vivo include recruitment of antigen-presenting cells to the tumour site, loading these cells with antigen, and their migration and maturation to full antigen-presenting function. In addition, it is essential for antigen-specific T cells to locate the tumour to mediate cytotoxicity, emphasizing the need for local inflammation to target effector cell recruitment. We review those therapies that involve the tumour site as a target and source of antigen for the initiation of immune responses, and discuss strategies to generate and co-ordinate an optimal cell-mediated immune response to control tumours locally.
Keywords: chemokine, dendritic cell, immunotherapy, T cell, tumour
When would intratumoral immunotherapy be clinically applicable?
The majority of human tumours grow in an immunocompetent host and elicit minimal, if any, clinically relevant antitumour immune responses at the point at which they present. However, various tumour-associated antigens that are recognized by specific immune cells are identifiable in patients with cancer, indicating that the immune system is capable of recognizing tumours.1–6 Evidence in animal models suggests that tumour formation occurs at a higher frequency in immunodeficient mice.7 Animals that have a deficiency in T cells or in the interferon (IFN) pathway have a higher incidence of tumour formation when compared to controls.8 Furthermore, those tumours that develop in immunodeficient animals are highly immunogenic when transplanted into immunocompetent hosts, suggesting that immune shaping of the tumour phenotype has not occurred in these tumours.7,8 Circumstantial evidence that similar immune selection occurs in spontaneous human tumours, comes from studies demonstrating reduced major histocompatibility complex (MHC) class I expression,9 deficiencies in antigen processing and presentation machinery10 and, importantly, selection against mutations that occur in MHC-binding epitopes.11
Any intratumoral therapy requires access to the tumour site. Initial studies will be further limited to accessible sites in appropriate clinical scenarios. The accessibility of primary melanomas, and the standard use of surgical excision, provide interesting examples. New chemotherapy drug combinations and radiation therapy doses are beginning to show some promise.12–18 Nonetheless, the low efficacy of traditional treatment modalities has resulted in many alternative immunotherapeutic treatment modalities being tested.19 The most promising immunotherapeutic approach, high-dose IFN-α, is now approved by the Food and Drug Administration (FDA) for high-risk melanoma, including both thick (> 4 mm) node-negative patients and node-positive patients (AJCC Stage II and Stage III, respectively). However, high dose IFN-α is not without its risks: in one study it was associated with grade III toxicity in 67% of patients; 9% of patients experienced life-threatening toxicity and two patients died from hepatotoxicity.20 While a disease-free survival benefit was shown in studies of high-dose IFN, the initial overall survival benefit reported in the ECOG 1684 trial appears to be lost with longer follow-up. Given the conflicting data, as well as the high toxicity, there is controversy among investigators as to whether high-dose IFN should be the standard of care. In Europe, high-dose IFN is not the accepted adjuvant treatment for patients with Stage II and Stage III disease, and among investigators, both in Europe and elsewhere, other adjuvant approaches, such as vaccines and non-toxic long-term IFN schedules, are being explored. In addition to such non-specific approaches, a number of immunodominant and commonly shared melanoma tumour antigens have been identified. Vaccine approaches using either autologous tumours or allogeneic tumour cells expressing shared antigens, or alternatively using purified defined tumour antigens or epitopes, has shown some promise in small trials.21–24 The use of ex vivo cultured autologous tumour cells, which should include multiple target antigens, is highly labour-intensive and such cells are not always successfully grown from the primary tumour material. Use of defined tumour-associated antigen has been associated with a loss of specific antigen expression in tumours following repeated treatment.25 However, for the purpose of this review, one relevant criticism of the majority of vaccine-based approaches is that they are performed at sites distant from the tumour. Antigen-directed vaccination therapies generally result in an accumulation of specific cells at the vaccination site.26–28 Thus, intratumoral approaches are appealing because they may direct antigen-specific responses back to the tumour site, and also exploit the presence of multiple undefined tumour antigens present in the endogenous tumour. Furthermore, the local aspect of the immune reaction within the tumour would be expected to reduce systemic toxicity, analogous to the use of isolated limb perfusion compared to systemic drug delivery.29
As the standard of care for grading and staging in melanoma requires surgical removal of the primary lesion, typically with tumour-free margins, intratumoral therapy is currently only practical in patients with local recurrence or surgically accessible metastases. Eventually, for those therapies that look promising, it may be possible to use a neoadjuvant approach and treat the primary lesion prior to surgical excision. As systemic gene therapy improves, it may also be possible reach metastases or primary tumours that are not accessible for direct injection. We will argue that such neoadjuvant therapies would provide broadly tumour reactive, immune-mediated control of emerging tumours in patients.
Life cycle of the optimal cell-mediated immune response
From the wealth of published literature on those immune responses that are most capable of controlling tumour growth in vivo, we hypothesize that there are certain features that must be incorporated to initiate effective cell-mediated antitumour immunity. We also believe that there must be local provision of antigen, in a form highly accessible to immature dendritic cells.30 This antigen must be provided in conjunction with molecules capable of activating these dendritic cells plus resident immune-modulating cells, such as macrophages.31–37 These criteria are hardly new, and are shown in Fig. 1. Almost any effective vaccination strategy of the previous 200 years incorporates antigen in some form of vehicle and accompanied by classical adjuvants, whether non-specific bacterial DNA and cell-wall products or the inherent properties of an attenuated viral vector.38 However, one critical difference between the goals of infectious disease vaccination and tumour immunotherapy is that the former is intended to protect systemically against a subsequent potentially pathogenic challenge, whereas the latter aims to redirect immune responses to an existing tumour in a specific location, or locations, within the host. Pathogenic challenge with an infectious agent requires local immune activation, even in vaccinated hosts, to identify the infection site.39,40 The location specificity of vaccinations is clear, particularly in classical models such as the delayed-type hypersensitivity response, where potent responses occur solely at the antigen site.26 For these reasons we will discuss immunotherapeutic approaches that act in concert with current understanding of effective cellular immune responses, and consider the spatial regulation required of therapies to both initiate and maintain immune activity against tumours.
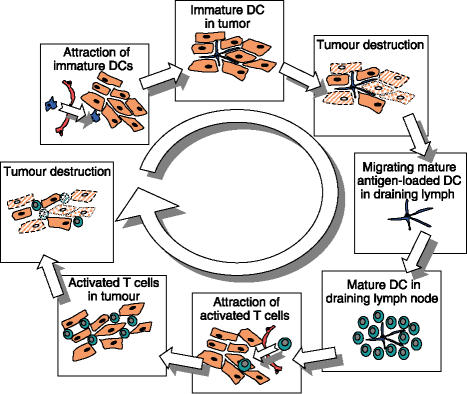
Figure 1.
Life cycle of the optimal cell-mediated antitumour immune response. Local attraction of immature dendritic cells (DCs), which can subsequently be loaded with tumour antigens via cytotoxic therapy and activated to emigrate to regional lymph nodes, effectively prime antitumour effector cells and return to the tumour site for further tumour destruction.
Lessons from tactics applied in existing tumour immunotherapies
Attracting dendritic cells to tumours
Dendritic cells have critical importance in priming effective, antigen-specific T-cell activation within the secondary lymphoid organs.30 Therefore, the first step towards an optimal initiation of antigen-specific antitumour responses involves the attraction of dendritic cells to the tumour. Several studies have documented the presence and prognostic significance of dendritic cells within various human tumours.41–43 These observations support the notion that increasing the number of dendritic cells at a tumour site could have desirable antitumour effects for a host.
Distinct from the systemic and distant vaccination applications of ex vivo-derived dendritic cells to initiate antigen-specific immune responses, various studies have applied such dendritic cells directly to the tumour site (Fig. 2). Table 1 lists the advantages and disadvantages of such an approach. Direct intratumoral application is particularly relevant in view of evidence demonstrating that few dendritic cells administered at vaccination sites reach the draining lymph node,44,45 and the dendritic cells accumulate antigen-specific T cells at the site of vaccination at the expense of peripheral circulating T-cell numbers.28 Intratumoral injection of syngeneic dendritic cells into malignant gliomas in rats led to infiltration of the tumour by CD4 and CD8 cells, and ultimately a prolongation in survival and immunity to subsequent tumour rechallenge.46 In a murine colorectal tumour model, mice receiving primary tumour challenges followed by excision and rechallenge were better protected against rechallenge when the primary tumour was co-administered with syngeneic immature dendritic cells.47 Significant protection against rechallenge was also observed in this model where the dendritic cells were injected into established tumours.47 In a preliminary clinical study, a small cohort of patients with metastatic dermal or subcutaneous breast and melanoma tumours received autologous dendritic cells intratumorally. Regression of injected tumours was observed in six out of 10 patients, and biopsies of the regressing tumours showed the presence of dendritic cells.48 To enhance the efficacy of intratumoral dendritic cells, these dendritic cells have also been engineered to express proinflammatory cytokines.49–51 The data relating to dendritic cell transfer requires careful interpretation because it is unclear in such models whether the transferred dendritic cells are behaving exactly as anticipated. It has been shown that ex vivo-derived dendritic cells have poor viability in vivo, and that host dendritic cells are required for the therapeutic effect.52 The mechanism of antigen transfer to host dendritic cells is at present unclear, and it is not understood whether the death of trafficking dendritic cells is a normal process in the draining lymph node.
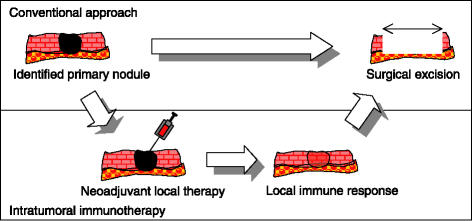
Figure 2.
Example of intratumoral immunotherapy in a neoadjuvant setting.
Table 1.
Advantages and disadvantages of intratumoral immunotherapy
| Using the tumour as a site of vaccination | |
|---|---|
| Advantages | Disadvantages |
| No need to identify antigens or MHC | Limited accessibility of tumours |
| Ability to manipulate environment | Tolerogenic tumour environment |
| Identification of tumour site for effector cells | Acceptability of delay before tumour excision |
MHC, major histocompatibility complex.
An alternative approach to the ex vivo generation of dendritic cells is to exploit the endogenous precursor population, via the chemokine-orchestrated migratory and homing properties of dendritic cells, to increase the number of endogenous dendritic cells at a tumour site.53,54 Several options have been tested to attract endogenous dendritic cells to tumours, including the genetic modification of tumour cells and direct intratumoral injection of recombinant proteins.55–59 Dendritic cell attraction has been proposed as a critical component of the widely studied expression of granulocyte–macrophage colony-stimulating factor (GM-CSF) in tumours.55,56,60–65 However, it is important to note that GM-CSF is not a chemoattractive cytokine, but does induce secretion of chemokines from resident macrophages that are chemoattractive for dendritic cells.66–68 Thus, a more direct approach is to similarly provide appropriate chemokines at the tumour site. Based on chemokine receptor expression data, it is possible to identify chemokines that will be chemoattractive for immature dendritic cells.53,69–74 Immature dendritic cells express the chemokine receptors CCR1, CCR5 and CCR6, which bind, amongst other molecules, the important inflammatory chemokines CCL3, CCL5 and CCL20. Injection of recombinant chemokine has been shown to cause infiltration of cells into the injection site,75,76 while constitutive expression of CCL3 has been shown to abrogate the tumorigenicity of an immunogenic tumour established subcutaneously.77 However, in the less immunogenic B16 melanoma model, the growth of subcutaneous tumours expressing CCL3 was unaffected.77,78 Interestingly, cells injected intravenously (i.v.) to form lung metastasis did not grow where CCL3 was expressed, suggesting that location plays a large role in the efficacy of chemoattraction.78 A slow-release formulation of the chemokine CCL20 using polymer rods was shown to cause accumulation of Langerhans' cells at the subcutaneous implantation site,79 and these cells could subsequently be antigen loaded and activated to enhance antigen-specific immunity.79 As with CCL3, described above, Crittenden et al.77 demonstrated that mice challenged with immunogenic colorectal tumours, which had been genetically modified to secrete CCL20, failed to develop tumours compared with non-chemokine-expressing controls, and that expression of CCL20 was associated with a significant increase in dendritic cells at the site of tumour inoculation. Similarly, intratumoral injection of adenovirus-expressing CCL20 resulted in significant inhibition of tumour growth.57 Intratumoral injection of recombinant CCL21 resulted in the accumulation of dendritic cells, along with T cells,80 and inhibited growth of lung carcinoma and melanoma in a T-cell-dependent manner.59,80 Intratumoral injection of herpes simplex virus (HSV) amplicons expressing CCL21 into established A20 and CT-26 tumours in mice caused significant infiltration of T cells and dendritic cells and resulted in CD8-dependent antitumour immunity.58
Based on these data, we hypothesize that the attraction of dendritic cells to a tumour site can be a vital first step in priming effective antitumour responses in vivo, and that chemokine gene expression can be an effective technique for using to recruit endogenous cells to the tumour without the need for ex vivo manipulations. However, a number of growing human tumours has been characterized to constitutively express chemokines that are chemoattractive to dendritic cells.81–83 Similarly, in less immunogenic tumour models, expression of chemokines in tumours is insufficient to cause tumour rejection.77 It is possible that the cells attracted to tumours by such chemokines do not take up antigen, or remain immature and function as tolerogenic antigen-presenting cells (APC).84–87 The tumour-type dependence of the presence and prognosis of infiltrating dendritic cells probably relates to environmental factors within the tumour that regulate antigen loading and maturation of dendritic cells. For this reason we will directly address strategies to enhance these key features of the initiation of immune responses.
Antigen loading of dendritic cells in tumours
Dendritic cells, as professional APC, must obtain tumour antigen in order to initiate de novo antigen-specific immune responses. An extremely varied range of strategies has been applied to provide antigen to dendritic cells. The major distinction in dendritic cell loading with antigen is between in vitro and in vivo provision of antigen. For those strategies involving adoptive transfer of in vitro-differentiated dendritic cells, it is extremely easy to provide antigen in a suitable form in vitro prior to dendritic cell transfer. Antigen-presentation strategies, such as peptide antigen loading, have only a very short duration of efficacy, with epitopes rapidly lost from MHC on the dendritic cell surface and associated loss of T-cell stimulatory activity.88 Ex vivo loading has been improved by conjugating exogenous protein antigen to molecules that direct it into appropriate cross-presentation pathways, such as heat shock proteins,89 complement90,91 and antibody.92–94 These data translate well to in vivo models, where similar conjugation of antigen prior to in vivo administration dramatically enhances the generation of antigen-specific immune responses.89,91,93,94 Dendritic cells efficiently present antigens coded in tumour-derived RNA,95,96 and more stable expression of tumour antigens via infection with viral vectors generates dendritic cells capable of inducing transgene-encoded antigen-specific immunity.97–99 In addition, dendritic cells actively phagocytose apoptotic tumour cells, and can cross-present apoptotic tumour-derived antigen on MHC class I.100,101 Dendritic cells do not readily take up and present antigen from live cells. However, there is a growing body of literature reporting that dendritic cells can take up and present antigens incorporated in vesicular structures (called exosomes) released by live tumour cells.102–104
Each of these strategies has been applied via in vitro loading to prime immune responses in vivo via vaccination at distant sites. Using the tumour as the site of vaccination ensures that antigen is locally available and that the target antigens need not be defined. Nevertheless, it still remains to transfer tumour-associated antigens, whether antigen-encoding DNA or RNA, or translated proteins or digested peptides, from the tumour cell to the dendritic cell within the tumour in vivo. A very applicable strategy to provide endogenous tumour antigens to dendritic cells is the combination of intratumoral dendritic cells with systemic cytotoxic therapies. A number of recent reports describe the use of systemic chemotherapeutics that cause tumour cell death, combined with intratumoral dendritic cells.105–107 In these models, the combination was significantly more effective than either agent alone105–107 and also caused regression of distant uninjected tumours.106 Similarly, intratumoral injection of dendritic cells was more effective in a breast tumour model when combined with agents that enhance levels of cell death in the tumour.108 For these reasons, we believe that it is unnecessary to prepare and load dendritic cells ex vivo, in circumstances where dendritic cells can be efficiently loaded with relevant antigens within the tumour by combining local attraction of immature dendritic cells with tumour cytotoxicity.
Activating dendritic cells loaded with tumour antigens
Dendritic cells are highly responsive to inflammatory stimuli, such as ligands of the tumour necrosis factor (TNF) family30,109–111 and ligands activating Toll-like receptors on dendritic cells.31–35 However, the uptake of cell-associated antigen can directly influence dendritic cell maturation to full T-cell priming potential. For example, apoptotic cells efficiently load dendritic cells with tumour antigen and do not cause dendritic cell maturation.85,112,113 In contrast, antigen from non-apoptotic cells also loads dendritic cells, but causes dendritic cells to mature and up-regulate costimulatory molecules.35,37 In vivo the immunostimulatory effects of antigen formulation is further confused by the presence of many other cell types. For example, macrophages are commonly present in tumours at higher levels than dendritic cells, and continuing the example from above, macrophages also actively phagocytose apoptotic cells. However, following the phagocytosis of apoptotic cells, macrophages secrete a range of anti-inflammatory cytokines, including interleukin-10 (IL-10) and transforming growth factor-β (TGF-β).114–117 In contrast, macrophages respond to non-apoptotic cells with limited phagocytosis and the secretion of pro-inflammatory cytokines, including TNF-α and interleukin-1β (IL-1β).114–116
Therefore, the data on apoptotic cell vaccination must be interpreted carefully depending on whether the dendritic cells are loaded in vitro alone vs. in vivo in the cellular milieu. Similarly, it is important to distinguish between modes of death that occur in vitro vs. in vivo. Apoptotic cells that have not been efficiently phagocytosed may proceed to secondary necrosis;118 therefore, at increased doses of apoptotic cells, where phagocytes may be overwhelmed, apoptotic cells begin to vaccinate mice against associated antigens.119 In mice bearing mutations resulting in the defective phagocytosis of apoptotic cells, there is an enhanced tendency to develop autoimmunity.120,121 Complement components have recently been described as important opsonins for clearance of apoptotic cells,122,123 and there is a direct correlation between deficiency in certain complement components and the development of autoimmunity.124 Thus, in these models it is likely that apoptotic cells which are not phagocytosed can proceed to secondary necrosis, leading to antigen presentation by, and activation of, dendritic cells. Cells that undergo a physiological non-apoptotic death, i.e. one where cells are fated to die but are not able to activate apoptotic pathways,104,125,126 are highly immunogenic.104,116,125,127,128
Therefore, an interesting strategy to activate dendritic cells concomitantly with uptake of tumour antigens would involve blocking the phagocytosis of apoptotic cells, or blocking the inhibitory effects of cytokines produced following phagocytosis. Thus, it has been shown that apoptotic cells are significantly more immunogenic in mice treated with carageenan to block phagocyte function.119 The in vivo tolerance that can be induced by dendritic cell uptake of apoptotic cells85,129,130 may relate to cytokines secreted by dendritic cells in response to phagocytosis of apoptotic cells,131 particularly IL-10119 and TGF-β.117 TGF-β secreted by tumour cells, or within the tumour microenvironment, has been postulated as one of the mechanisms by which tumours evade immune control, as TGF-β inhibits both dendritic cell maturation and T-cell effector function. Based on these data, we hypothesize that if tumour antigen is provided via conventional intratumoral apoptotic death,105–107 there would be a significant therapeutic advantage generated by blocking the phagocytosis of apoptotic cells, or alternatively blocking IL-10 and/or TGF-β within the tumour environment. Thus, we maintain that in developing intratumoral therapies, dendritic cells attracted to the tumour site should be loaded with antigen via cytotoxic therapies that concomitantly activate the dendritic cell.
One interesting local therapeutic approach that is being actively pursued in the treatment of both basal cell carcinoma and melanoma involves topical application of members of the imidazoquinoline family, particularly Imiquimod. Topical treatment with Imiquimod causes the maturation of local Langerhans' cells,132 and injection of immature dendritic cells into Imiquimod-treated skin results in the maturation of dendritic cells in situ.133 Moreover, Imiquimod-mediated maturation of injected immature dendritic cells generated antitumour immune responses that were superior to similar injection of mature dendritic cells.133 These data suggest that in addition to the phenotypic maturation status of the dendritic cell, the inflammatory status of the local environment plays an important role in the activation of cell-mediated immune responses. Topical treatment with Imiquimod has shown significant therapy in basal cell carcinoma134 and cutaneous melanoma.135 These data demonstrate that such local immunotherapies can provide significant benefit with minimal systemic risk in patients with normal immune status.136 Topical immune adjuvants, such as the imidazoquinolines, could be widely applied to enhance immune responses at accessible sites.
Attraction of primed effector cells to the tumour site
The degree of lymphocyte infiltrate into the tumour is an independent prognostic marker for improved survival in specific classes of melanoma patients.137,138 However, there is much discussion as to the functionality of those lymphocytes found within many tumour types.139,140 Efficient priming of effector cells does not necessarily mean that antigen-specific cells can proceed to clear tumours. In a transgenic T-cell model, where the specific antigen was simultaneously expressed by normal liver cells, endogenous T cells did not cause liver pathology, even following antigen-specific vaccination in vivo.141 In this model, administration of a liver pathogen was required to initiate T-cell-mediated autoimmune liver destruction.141 Similarly, multiple strategies have been demonstrated to generate measurable cytotoxic T-lymphocyte (CTL) responses in patients, without significant correlation to clinical responses.142–144 The presence of large numbers of transgenic or in vitro-expanded tumour antigen-specific T cells does not consistently cause regression of tumours in animals or patients.145–147
One explanation for these data may lie in the trafficking properties of activated T cells, and in the tumour environment. Naïve T cells have ‘central’ trafficking properties.53,148–153 In contrast, subpopulations of activated cells lose expression of CD62L and CCR7, gaining adhesion molecules such as CD44 and the chemokine receptor, CCR5, that enable adherence to the peripheral basement membrane hyaluronate145,154,155 and trafficking towards inflammatory chemokines such as CCL3 and CCL5.156–158 Such effector cells are much more capable of trafficking to peripheral sites that are the source of ongoing infections.157,159,160 These data support the hypothesis that modulation of the tumour site will be critical to enhance the efficacy of tumour immunotherapies, particularly during the effector phase.
The tumour site is not a site of inflammation. Despite expression of a range of chemokines, and even expression of cytokines such as TNF-α,161–163 simultaneous expression of counteractive cytokines, such as IL-10 and TGF-β, means that activated T cells are poorly attracted to the tumour site. T cells that are unresponsive to TGF-β are significantly more effective in adoptive transfer models than T cells that are inhibited by TGF-β expressed by tumours.164 In vivo, radiolabelled antigen-specific T cells showed limited additional tumour-specific trafficking when compared to non-specific cells.165 One innovative solution to this problem has been genetic modification of the T cells to express a receptor for a chemokine expressed by tumours.166 Transfer of this receptor significantly increased T-cell trafficking to tumours and the antitumour efficacy of adoptively transferred T cells. Therefore, it could be possible to design vaccination strategies that generate T cells with a more appropriate trafficking capacity. However, using intratumoral therapies, it may be possible to incorporate features that directly modify the tumour site to increase the efficacy of effector T-cell trafficking.
A number of groups have engineered tumours to express chemokines that are known to attract effector T cells. For example, modification of tumours to express CCL3 and CCL20 has generated effective antitumour immune responses.57–59,77,78,80 However, these chemokines are pleotropic and their receptors are also expressed on other critical cells, in this case immature dendritic cells, which were the intended target of the therapies. Thus, few experiments have assessed the effect of modified tumours specifically on the effector-cell stage of the antitumour immune response. Emigration of effector cells to the tumour site is dependent on the presence of IFN-γ,167 which causes local expression of chemokines capable of attracting further effector cells.40,168 Adoptively transferred effector cells require such chemokine-mediated signals in order to generate antitumour immune responses, as treatment of such cells with pertussis toxin prevented systemic therapy.169 This observation has implications for therapy, as intratumoral injection of adenovirus expressing the chemokine CXCL10, the receptor for which is found on activated T cells, synergistically enhanced the efficacy of adoptive T-cell therapy.170 Similarly, intratumoral injection of adenovirus expressing the T-cell-responsive chemokine XCL1 also synergistically enhanced the efficacy of adoptive T-cell therapy.171 In addition, we have demonstrated that expression of CCL3 in tumours significantly enhances antitumour responses when combined with the adoptive transfer of activated T cells (M. J. Gough et al., submitted). We hypothesize that along with intratumoral therapies that efficiently prime antitumour T-cell responses, strategies should be incorporated to identify the tumour site for effector cell trafficking, and that the combination will greatly improve their efficacy of intratumoral immunotherapy.
Co-ordination of an effective intratumoral immunotherapy
Many of the strategies advocated above to attract immature dendritic cells to the tumour site, and to mature the dendritic cells at the tumour site, would also attract any effector cells generated back to the tumour. Thus, the apparently pleotropic effects of chemokines and the common features of trafficking mechanisms, may underlie organization and cohesions in immune responses. For this reason it is likely that modification of the tumour site is critical for the initiation, execution and promotion of effective immune responses. Examining the mechanics of effective immune responses, and taking into account the results from current immunotherapies applied in animal tumour models and human clinical trials, does provide information for further research. Recruitment or provision of the appropriate APCs, and their loading with tumour antigen, is a critical target of therapies and therapeutic strategies. Comparatively neglected are strategies that target the effector arm of the immune response to the tumour site. Clearly, the generation of sufficient effector cells is a basic requirement for subsequent tumour elimination. Yet, as discussed above, the generation of large quantities of effector cells, as determined by adoptively transferred in vitro-generated cells, or in vivo vaccination procedures, does not necessarily result in tumour elimination. We hypothesize that performing the vaccination procedure within the tumour site will increase the efficacy of the antitumour therapy by simultaneously attracting any effectors generated to the site. Moreover, we hypothesize that designing therapeutic strategies which utilize antigens present endogenously within tumour cells, through the attraction of immature dendritic cells and immunogenic tumour cell death, will provide site-targeted therapeutic immunity.
Nevertheless, difficulties will probably persist. The evolution of immune evasion during tumorigenesis complicates a number of these processes. Cytokines such as TGF-β and IL-10, within the tumour, limit dendritic cell maturation along with effector T-cell generation and function. Moreover, immune responses seem designed to be short-lived. Chronic immune activation seen in diseases such as rheumatoid arthritis is the result of ongoing responses that do not occur in tumour tissues. How these environments differ may again relate to the immunosuppressive cytokine environment, but sustaining a response will be critical to long-term tumour elimination by immunotherapies. Finally, access to the tumour site will be critical. We have provided an example, in this review, of melanoma, a tumour type with typically accessible primary tumours. However, distant metastasis in this tumour type will remain a difficult target for location-dependent therapies. Systemic delivery approaches will be required to reach these distant sites, and may yet be found in the developing field of gene therapy.
We have summarized tumour immunotherapies that target the tumour site and target the key features of optimal immune responses. We hypothesize that co-ordination of an effective immune response within tumours will provide tumour responses superior to those generated via ex vivo or distant vaccination strategies.
References
- 1.Shiku H, Takahashi T, Oettgen HF. Cell surface antigens of human malignant melanoma. II. Serological typing with immune adherence assays and definition of two new surface antigens. J Exp Med. 1976;144:873–81. doi: 10.1084/jem.144.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ueda R, Shiku H, Pfreundschuh M, Takahashi T, Li LT, Whitmore WF, Oettgen HF, Old LJ. Cell surface antigens of human renal cancer defined by autologous typing. J Exp Med. 1979;150:564–79. doi: 10.1084/jem.150.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carey TE, Takahashi T, Resnick LA, Oettgen HF, Old LJ. Cell surface antigens of human malignant melanoma: mixed hemadsorption assays for humoral immunity to cultured autologous melanoma cells. Proc Natl Acad Sci USA. 1976;73:3278–82. doi: 10.1073/pnas.73.9.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfreundschuh M, Shiku H, Takahashi T, Ueda R, Ransohoff J, Oettgen HF, Old LJ. Serological analysis of cell surface antigens of malignant human brain tumors. Proc Natl Acad Sci USA. 1978;75:5122–6. doi: 10.1073/pnas.75.10.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tureci O, Sahin U, Pfreundschuh M. Serological analysis of human tumor antigens: molecular definition and implications. Mol Med Today. 1997;3:342–9. doi: 10.1016/s1357-4310(97)01081-2. [DOI] [PubMed] [Google Scholar]
- 6.Van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van Den Eynde B, Knuth A, Boon T. A gene encoding antigen recognised by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 7.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 8.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–11. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 9.Bodmer WF, Browning MJ, Krausa P, Rowan A, Bicknell DC, Bodmer JG. Tumor escape from immune response by variation in HLA expression and other mechanisms. Ann N Y Acad Sci. 1993;690:42–9. doi: 10.1111/j.1749-6632.1993.tb43994.x. [DOI] [PubMed] [Google Scholar]
- 10.Restifo NP, Esquivel F, Kawakami Y, Yewdell JW, Mule JJ, Rosenberg SA, Bennink JR. Identification human cancers deficient in antigen processing. Journal of Experimental Medicine. 1993;177:265–72. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiedenfeld EA, Fernandez-Vina M, Berzofsky JA, Carbone DP. Evidence for selection against human lung cancers bearing p53 missense mutations which occur within the HLA A*0201 peptide consensus motif. Cancer Res. 1994;54:1175–7. [PubMed] [Google Scholar]
- 12.Geara FB, Ang KK. Radiation therapy for malignant melanoma. Surg Clin North Am. 1996;76:1383–98. doi: 10.1016/s0039-6109(05)70521-1. [DOI] [PubMed] [Google Scholar]
- 13.Storper IS, Lee SP, Abemayor E, Juillard G. The role of radiation therapy in the treatment of head and neck cutaneous melanoma. Am J Otolaryngol. 1993;14:426–31. doi: 10.1016/0196-0709(93)90118-q. [DOI] [PubMed] [Google Scholar]
- 14.Jacquillat C, Khayat D, Banzet P, et al. Final report of the French multicenter phase II study of the nitrosourea fotemustine in 153 evaluable patients with disseminated malignant melanoma including patients with cerebral metastases. Cancer. 1990;66:1873–8. doi: 10.1002/1097-0142(19901101)66:9<1873::aid-cncr2820660904>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Cocconi G, Bella M, Calabresi F, et al. Treatment of metastatic malignant melanoma with dacarbazine plus tamoxifen. N Engl J Med. 1992;327:516–23. doi: 10.1056/NEJM199208203270803. [DOI] [PubMed] [Google Scholar]
- 16.Margolin KA. Liu PY, Flaherty LE, et al. Phase II study of carmustine, dacarbazine, cisplatin, and tamoxifen in advanced melanoma: a Southwest Oncology Group study. J Clin Oncol. 1998;16:664–9. doi: 10.1200/JCO.1998.16.2.664. [DOI] [PubMed] [Google Scholar]
- 17.McClay EF, McClay ME. Systemic chemotherapy for the treatment of metastatic melanoma. Semin Oncol. 1996;23:744–53. [PubMed] [Google Scholar]
- 18.Herben VM, Panday VR, Richel DJ, et al. Phase I and pharmacologic study of the combination of paclitaxel, cisplatin, and topotecan administered intravenously every 21 days as first-line therapy in patients with advanced ovarian cancer. J Clin Oncol. 1999;17:747–55. doi: 10.1200/JCO.1999.17.3.747. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–4. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 20.Cole BF, Gelber RD, Kirkwood JM, Goldhirsch A, Barylak E, Borden E. Quality-of-life-adjusted survival analysis of interferon alfa-2b adjuvant treatment of high-risk resected cutaneous melanoma: an Eastern Cooperative Oncology Group study. J Clin Oncol. 1996;14:2666–73. doi: 10.1200/JCO.1996.14.10.2666. [DOI] [PubMed] [Google Scholar]
- 21.McIllmurray MB, Reeves WG, Langman MJ, Deane M, Embleton MJ. Active immunotherapy in malignant melanoma. Br Med J. 1978;1:579. doi: 10.1136/bmj.1.6112.579-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berd D, Maguire HC, Jr, Mastrangelo MJ. Treatment of human melanoma with a hapten-modified autologous vaccine. Ann N Y Acad Sci. 1993;690:147–52. doi: 10.1111/j.1749-6632.1993.tb44004.x. [DOI] [PubMed] [Google Scholar]
- 23.Sondak VK, Liu PY, Tuthill RJ, et al. Adjuvant immunotherapy of resected, intermediate-thickness, node-negative melanoma with an allogeneic tumor vaccine: overall results of a randomized trial of the Southwest Oncology Group. J Clin Oncol. 2002;20:2058–66. doi: 10.1200/JCO.2002.08.071. [DOI] [PubMed] [Google Scholar]
- 24.Slingluff CL, Jr, Yamshchikov G, Neese P, et al. Phase I trial of a melanoma vaccine with gp100(280–288) peptide and tetanus helper peptide in adjuvant: immunologic and clinical outcomes. Clin Cancer Res. 2001;7:3012–24. [PubMed] [Google Scholar]
- 25.Jager E, Ringhoffer M, Karbach J, Arand M, Oesch F, Knuth A. Inverse relationship of melanocyte differentiation antigen expression in melanoma tissues and CD8+ cytotoxic-T-cell responses: evidence for immunoselection of antigen-loss variants in vivo. Int J Cancer. 1996;66:470–6. doi: 10.1002/(SICI)1097-0215(19960516)66:4<470::AID-IJC10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi K, Kaneda K, Kasama T. Immunopathogenesis of delayed-type hypersensitivity. Microsc Res Tech. 2001;53:241–5. doi: 10.1002/jemt.1090. [DOI] [PubMed] [Google Scholar]
- 27.Reinhardt RL, Bullard DC, Weaver CT, Jenkins MK. Preferential accumulation of antigen-specific effector CD4 T cells at an antigen injection site involves CD62E-dependent migration but not local proliferation. J Exp Med. 2003;197:751–62. doi: 10.1084/jem.20021690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrama D, Pedersen LO, Keikavoussi P, Andersen MH, Straten Pt P, Brocker EB, Kampgen E, Becker JC. Aggregation of antigen-specific T cells at the inoculation site of mature dendritic cells. J Invest Dermatol. 2002;119:1443–8. doi: 10.1046/j.1523-1747.2002.19604.x. [DOI] [PubMed] [Google Scholar]
- 29.Eggermont AM, de Wilt JH, ten Hagen TL. Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol. 2003;4:429–37. doi: 10.1016/s1470-2045(03)01141-0. [DOI] [PubMed] [Google Scholar]
- 30.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 31.Roake JA, Rao AS, Morris PJ, Larsen CP, Hankins DF, Austyn JM. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J Exp Med. 1995;181:2237–47. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacPherson GG, Jenkins CD, Stein MJ, Edwards C. Endotoxin-mediated dendritic cell release from the intestine. Characterization of released dendritic cells and TNF dependence. J Immunol. 1995;154:1317–22. [PubMed] [Google Scholar]
- 33.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 34.Kadowaki N, Antonenko S, Liu YJ. Distinct CpG DNA and polyinosinic-polycytidylic acid double-stranded RNA, respectively, stimulate CD11c- type 2 dendritic cell precursors and CD11c+ dendritic cells to produce type I IFN. J Immunol. 2001;166:2291–5. doi: 10.4049/jimmunol.166.4.2291. [DOI] [PubMed] [Google Scholar]
- 35.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappaB pathway. Int Immunol. 2000;12:1539–46. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 36.Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–70. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–55. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 38.Sinkovics JG, Horvath JC. Vaccination against human cancers (review) Int J Oncol. 2000;16:81–96. doi: 10.3892/ijo.16.1.81. [DOI] [PubMed] [Google Scholar]
- 39.Topham DJ, Castrucci MR, Wingo FS, Belz GT, Doherty PC. The role of antigen in the localization of naive, acutely activated, and memory CD8(+) T cells to the lung during influenza pneumonia. J Immunol. 2001;167:6983–90. doi: 10.4049/jimmunol.167.12.6983. [DOI] [PubMed] [Google Scholar]
- 40.Dobrzanski MJ, Reome JB, Dutton RW. Immunopotentiating role of IFN-gamma in early and late stages of type 1 CD8 effector cell-mediated tumor rejection. Clin Immunol. 2001;98:70–84. doi: 10.1006/clim.2000.4945. [DOI] [PubMed] [Google Scholar]
- 41.Iwamoto M, Shinohara H, Miyamoto A, et al. Prognostic value of tumor-infiltrating dendritic cells expressing CD83 in human breast carcinomas. Int J Cancer. 2003;104:92–7. doi: 10.1002/ijc.10915. [DOI] [PubMed] [Google Scholar]
- 42.Lespagnard L, Gancberg D, Rouas G, et al. Tumor-infiltrating dendritic cells in adenocarcinomas of the breast: a study of 143 neoplasms with a correlation to usual prognostic factors and to clinical outcome. Int J Cancer. 1999;84:309–14. doi: 10.1002/(sici)1097-0215(19990621)84:3<309::aid-ijc19>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 43.Reichert TE, Scheuer C, Day R, Wagner W, Whiteside TL. The number of intratumoral dendritic cells and zeta-chain expression in T cells as prognostic and survival biomarkers in patients with oral carcinoma. Cancer. 2001;91:2136–47. [PubMed] [Google Scholar]
- 44.Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D, Lyerly HK. Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Research. 1999;59:56–8. [PubMed] [Google Scholar]
- 45.Lappin MB, Weiss JM, Delattre V, et al. Analysis of mouse dendritic cell migration in vivo upon subcutaneous and intravenous injection. Immunology. 1999;98:181–8. doi: 10.1046/j.1365-2567.1999.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ehtesham M, Kabos P, Gutierrez MA, Samoto K, Black KL, Yu JS. Intratumoral dendritic cell vaccination elicits potent tumoricidal immunity against malignant glioma in rats. J Immunother. 2003;26:107–16. doi: 10.1097/00002371-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Melcher A, Todryk S, Bateman A, Chong H, Lemoine NR, Vile RG. Adoptive transfer of immature dendritic cells with autologous or allogeneic tumor cells generates systemic antitumor immunity. Cancer Res. 1999;59:2802–5. [PubMed] [Google Scholar]
- 48.Triozzi PL, Khurram R, Aldrich WA, Walker MJ, Kim JA, Jaynes S. Intratumoral injection of dendritic cells derived in vitro in patients with metastatic cancer. Cancer. 2000;89:2646–54. doi: 10.1002/1097-0142(20001215)89:12<2646::aid-cncr18>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 49.Satoh Y, Esche C, Gambotto A, et al. Local administration of IL-12-transfected dendritic cells induces antitumor immune responses to colon adenocarcinoma in the liver in mice. J Exp Therapeutics Oncol. 2002;2:337–49. doi: 10.1046/j.1359-4117.2002.01050.x. [DOI] [PubMed] [Google Scholar]
- 50.Nishioka Y, Hirao M, Robbins PD, Lotze MT, Tahara H. Induction of systemic and therapeutic antitumor immunity using intratumoral injection of dendritic cells genetically modified to express interleukin 12. Cancer Res. 1999;59:4035–41. [PubMed] [Google Scholar]
- 51.Bonham L, Skuk D, Koeberl D, et al. Intratumoral administration of adenoviral interleukin 7 gene-modified dendritic cells augments specific antitumor immunity and achieves tumor eradication. Human Gene Ther. 2000;11:1277–88. doi: 10.1089/10430340050016157. [DOI] [PubMed] [Google Scholar]
- 52.Kleindienst P, Brocker T. Endogenous dendritic cells are required for amplification of T cell responses induced by dendritic cell vaccines in vivo. J Immunol. 2003;170:2817–23. doi: 10.4049/jimmunol.170.6.2817. [DOI] [PubMed] [Google Scholar]
- 53.Sallusto F, Lanzavecchia A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev. 2000;177:134–40. doi: 10.1034/j.1600-065x.2000.17717.x. [DOI] [PubMed] [Google Scholar]
- 54.Cavanagh LL, Von Andrian UH. Travellers in many guises: the origins and destinations of dendritic cells. Immunol Cell Biol. 2002;80:448–62. doi: 10.1046/j.1440-1711.2002.01119.x. [DOI] [PubMed] [Google Scholar]
- 55.Miller G, Pillarisetty VG, Shah AB, Lahrs S, Xing Z, DeMatteo RP. Endogenous granulocyte–macrophage colony-stimulating factor overexpression in vivo results in the long-term recruitment of a distinct dendritic cell population with enhanced immunostimulatory function. J Immunol. 2002;169:2875–85. doi: 10.4049/jimmunol.169.6.2875. [DOI] [PubMed] [Google Scholar]
- 56.Lee CT, Wu S, Ciernik IF, Chen H, Nadaf-Rahrov S, Gabrilovich D, Carbone DP. Genetic immunotherapy of established tumors with adenovirus-murine granulocyte–macrophage colony-stimulating factor. Hum Gene Ther. 1997;8:187–93. doi: 10.1089/hum.1997.8.2-187. [DOI] [PubMed] [Google Scholar]
- 57.Fushimi T, Kojima A, Moore MA, Crystal RG. Macrophage inflammatory protein 3alpha transgene attracts dendritic cells to established murine tumors and suppresses tumor growth. J Clin Invest. 2000;105:1383–93. doi: 10.1172/JCI7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tolba KA, Bowers WJ, Eling DJ, Casey AE, Kipps TJ, Federoff HJ, Rosenblatt JD. HSV amplicon-mediated delivery of LIGHT enhances the antigen-presenting capacity of chronic lymphocytic leukemia. Mol Ther: J Am Soc Gene Therapy. 2002;6:455–63. doi: 10.1006/mthe.2002.0693. [DOI] [PubMed] [Google Scholar]
- 59.Kirk CJ, Hartigan-O'Connor D, Mule JJ. The dynamics of the T-cell antitumor response: chemokine-secreting dendritic cells can prime tumor-reactive T cells extranodally. Cancer Res. 2001;61:8794–802. [PubMed] [Google Scholar]
- 60.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simons JW, Jaffee EM, Weber CE, et al. Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte–macrophage colony-stimulating factor gene transfer. Cancer Res. 1997;57:1537–46. [PMC free article] [PubMed] [Google Scholar]
- 62.Chiodoni C, Paglia P, Stoppacciaro A, Rodolfo M, Parenza M, Colombo MP. Dendritic cells infiltrating tumors cotransduced with granulocyte/macrophage colony-stimulating factor (GM-CSF) CD40 ligand genes take up and present endogenous tumor-associated antigens, and prime naive mice for a cytotoxic T lymphocyte response. Journal of Experimental Medicine. 1999;190:125–34. doi: 10.1084/jem.190.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leong SP, Enders-Zohr P, Zhou YM, et al. Recombinant human granulocyte macrophage-colony stimulating factor (rhGM-CSF) and autologous melanoma vaccine mediate tumor regression in patients with metastatic melanoma. J Immunother. 1999;22:166–74. doi: 10.1097/00002371-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 64.Soiffer R, Lynch T, Mihm M, et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte–macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci USA. 1998;95:13141–6. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte–macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–46. [PubMed] [Google Scholar]
- 66.Shinohara H, Yano S, Bucana CD, Fidler IJ. Induction of chemokine secretion and enhancement of contact-dependent macrophage cytotoxicity by engineered expression of granulocyte–macrophage colony-stimulating factor in human colon cancer cells. J Immunol. 2000;164:2728–37. doi: 10.4049/jimmunol.164.5.2728. [DOI] [PubMed] [Google Scholar]
- 67.Shang XZ, Issekutz AC. Enhancement of monocyte transendothelial migration by granulocyte–macrophage colony-stimulating factor: requirement for chemoattractant and CD11a/CD18 mechanisms. Eur J Immunol. 1999;29:3571–82. doi: 10.1002/(SICI)1521-4141(199911)29:11<3571::AID-IMMU3571>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 68.Kielian T, Nagai E, Ikubo A, Rasmussen CA, Suzuki T. Granulocyte/macrophage–colony-stimulating factor released by adenovirally transduced CT26 cells leads to the local expression of macrophage inflammatory protein 1alpha and accumulation of dendritic cells at vaccination sites in vivo. Cancer Immunol Immunother. 1999;48:123–31. doi: 10.1007/s002620050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dieu M-C, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. Journal of Experimental Medicine. 1998;188:373–86. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foti M, Granucci F, Aggujaro D, et al. Upon dendritic cell (DC) activation chemokines chemokine receptor expression are rapidly regulated for recruitment and maintenance of DC at the inflammatory site. International Immunology. 1999;11:979–86. doi: 10.1093/intimm/11.6.979. [DOI] [PubMed] [Google Scholar]
- 71.Lin CL, Suri RM, Rahndon RA, Austyn JM, Roake JA. Dendritic cell chemotaxis and transendothelial migration are induced by distinct chemokines and are regulated on maturation. European Journal of Immunology. 1998;28:4114–22. doi: 10.1002/(SICI)1521-4141(199812)28:12<4114::AID-IMMU4114>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 72.Sallusto F, Palermo B, Lenig D, et al. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. European Journal of Immunology. 1999;29:1617–25. doi: 10.1002/(SICI)1521-4141(199905)29:05<1617::AID-IMMU1617>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 73.Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, Qin S, Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. European Journal of Immunology. 1998;28:2760–9. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 74.Sozzani S, Allavena P, D'Amico G, et al. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. Journal of Immunology. 1998;161:1083–6. [PubMed] [Google Scholar]
- 75.Didier PJ, Paradis TJ, Gladue RP. The CC chemokine MIP-1alpha induces a selective monocyte infiltration following intradermal injection into nonhuman primates. Inflammation. 1999;23:75–86. doi: 10.1023/a:1020243701890. [DOI] [PubMed] [Google Scholar]
- 76.Lee SC, Brummet ME, Shahabuddin S, Woodworth TG, Georas SN, Leiferman KM. Cutaneous injection of human subjects with macrophage inflammatory protein-1 alpha induces significant recruitment of neutrophils and monocytes. J Immunol. 2000;164:3392–401. doi: 10.4049/jimmunol.164.6.3392. [DOI] [PubMed] [Google Scholar]
- 77.Crittenden M, Gough M, Harrington K, Olivier K, Thompson J, Vile RG. Expression of inflammatory chemokines combined with local tumor destruction enhances tumor regression and long-term immunity. Cancer Res. 2003;63:5505–12. [PubMed] [Google Scholar]
- 78.van Deventer HW, Serody JS, McKinnon KP, Clements C, Brickey WJ, Ting JP. Transfection of macrophage inflammatory protein 1 alpha into B16, F10 melanoma cells inhibits growth of pulmonary metastases but not subcutaneous tumors. J Immunol. 2002;169:1634–9. doi: 10.4049/jimmunol.169.3.1634. [DOI] [PubMed] [Google Scholar]
- 79.Kumamoto T, Huang EK, Paek HJ, Morita A, Matsue H, Valentini RF, Takashima A. Induction of tumor-specific protective immunity by in situ Langerhans' cell vaccine. Nat Biotechnol. 2002;20:64–9. doi: 10.1038/nbt0102-64. [DOI] [PubMed] [Google Scholar]
- 80.Sharma S, Stolina M, Luo J, Strieter RM, Burdick M, Zhu LX, Batra RK, Dubinett SM. Secondary lymphoid tissue chemokine mediates T cell-dependent antitumor responses in vivo. J Immunol. 2000;164:4558–63. doi: 10.4049/jimmunol.164.9.4558. [DOI] [PubMed] [Google Scholar]
- 81.Kleeff J, Kusama T, Rossi DL, et al. Detection and localization of Mip-3alpha/LARC/Exodus, a macrophage proinflammatory chemokine, and its CCR6 receptor in human pancreatic cancer. Int J Cancer. 1999;81:650–7. doi: 10.1002/(sici)1097-0215(19990517)81:4<650::aid-ijc23>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 82.Luboshits G, Shina S, Kaplan O, et al. Elevated expression of the CC chemokine regulated on activation, normal T cell expressed and secreted (RANTES) in advanced breast carcinoma. Cancer Res. 1999;59:4681–7. [PubMed] [Google Scholar]
- 83.Yoong KF, Afford SC, Jones R, Aujla P, Qin S, Price K, Hubscher SG, Adams DH. Expression and function of CXC and CC chemokines in human malignant liver tumors: a role for human monokine induced by gamma-interferon in lymphocyte recruitment to hepatocellular carcinoma. Hepatology. 1999;30:100–11. doi: 10.1002/hep.510300147. [DOI] [PubMed] [Google Scholar]
- 84.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–79. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells [comment] J Exp Med. 2000;191:411–6. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA. 2002;99:351–8. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 88.Ludewig B, McCoy K, Pericin M, et al. Rapid peptide turnover and inefficient presentation of exogenous antigen critically limit the activation of self-reactive CTL by dendritic cells. J Immunol. 2001;166:3678–87. doi: 10.4049/jimmunol.166.6.3678. [DOI] [PubMed] [Google Scholar]
- 89.Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, Udono H, Srivastava PK. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315–22. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jacquier-Sarlin MR, Gabert FM, Villiers MB, Colomb MG. Modulation of antigen processing and presentation by covalently linked complement C3b fragment. Immunology. 1995;84:164–70. [PMC free article] [PubMed] [Google Scholar]
- 91.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–50. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 92.Regnault A, Lankar D, Lacabanne V, et al. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–80. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Akiyama K, Ebihara S, Yada A, et al. Targeting apoptotic tumor cells to Fc gamma R provides efficient and versatile vaccination against tumors by dendritic cells. J Immunol. 2003;170:1641–8. doi: 10.4049/jimmunol.170.4.1641. [DOI] [PubMed] [Google Scholar]
- 94.Schuurhuis DH, Ioan-Facsinay A, Nagelkerken B, et al. Antigen–antibody immune complexes empower dendritic cells to efficiently prime specific CD8+ CTL responses in vivo. J Immunol. 2002;168:2240–6. doi: 10.4049/jimmunol.168.5.2240. [DOI] [PubMed] [Google Scholar]
- 95.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–72. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nair SK, Heiser A, Boczkowski D, Majumdar A, Naoe M, Lebkowski JS, Vieweg J, Gilboa E. Induction of cytotoxic T cell responses and tumor immunity against unrelated tumors using telomerase reverse transcriptase RNA transfected dendritic cells. Nat Med. 2000;6:1011–7. doi: 10.1038/79519. [DOI] [PubMed] [Google Scholar]
- 97.Specht JM, Wang G, Do MT, Lam JS, Royal RE, Reeves ME, Rosenberg SA, Hwu P. Dendritic cells retrovirally transduced with a model antigen gene are therapeutically effective against established pulmonary metastases. J Exp Med. 1997;186:1213–21. doi: 10.1084/jem.186.8.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaplan JM, Yu Q, Piraino ST, Pennington SE, Shankara S, Woodworth LA, Roberts BL. Induction of antitumor immunity with dendritic cells transduced with adenovirus vector-encoding endogenous tumor-associated antigens. J Immunol. 1999;163:699–707. [PubMed] [Google Scholar]
- 99.Zhang Y, Chirmule N, Gao G, Wilson J. CD40 ligand-dependent activation of cytotoxic T lymphocytes by adeno-associated virus vectors in vivo: role of immature dendritic cells. J Virol. 2000;74:8003–10. doi: 10.1128/jvi.74.17.8003-8010.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–68. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–9. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 102.Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wolfers J, Lozier A, Raposo G, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 104.Bateman AR, Harrington KJ, Kottke T, et al. Viral fusogenic membrane glycoproteins kill solid tumor cells by nonapoptotic mechanisms that promote cross presentation of tumor antigens by dendritic cells. Cancer Res. 2002;62:6566–78. [PubMed] [Google Scholar]
- 105.Tong Y, Song W, Crystal RG. Combined intratumoral injection of bone marrow-derived dendritic cells and systemic chemotherapy to treat pre-existing murine tumors. Cancer Res. 2001;61:7530–5. [PubMed] [Google Scholar]
- 106.Tanaka F, Yamaguchi H, Ohta M, Mashino K, Sonoda H, Sadanaga N, Inoue H, Mori M. Intratumoral injection of dendritic cells after treatment of anticancer drugs induces tumor-specific antitumor effect in vivo. Int J Cancer. 2002;101:265–9. doi: 10.1002/ijc.10597. [DOI] [PubMed] [Google Scholar]
- 107.Shin JY, Lee SK, Kang CD, et al. Antitumor effect of intratumoral administration of dendritic cell combination with vincristine chemotherapy in a murine fibrosarcoma model. Histol Histopathol. 2003;18:435–47. doi: 10.14670/HH-18.435. [DOI] [PubMed] [Google Scholar]
- 108.Candido KA, Shimizu K, McLaughlin JC, et al. Local administration of dendritic cells inhibits established breast tumor growth: implications for apoptosis-inducing agents. Cancer Res. 2001;61:228–36. [PubMed] [Google Scholar]
- 109.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 110.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–80. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 111.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 112.Melero I, Vile RG, Colombo MP. Feeding dendritic cells with tumor antigens: self-service buffet or a la carte? Gene Ther. 2000;7:1167–70. doi: 10.1038/sj.gt.3301234. [DOI] [PubMed] [Google Scholar]
- 113.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells [see comments] J Exp Med. 2000;191:423–34. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reiter I, Krammer B, Schwamberger G. Cutting edge: differential effect of apoptotic versus necrotic tumor cells on macrophage antitumor activities. J Immunol. 1999;163:1730–2. [PubMed] [Google Scholar]
- 116.Gough MJ, Melcher AA, Ahmed A, et al. Macrophages orchestrate the immune response to tumor cell death. Cancer Res. 2001;61:7240–7. [PubMed] [Google Scholar]
- 117.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu X, Molinaro C, Johnson N, Casiano CA, et al. Secondary necrosis is a source of proteolytically modified forms of specific intracellular autoantigens: implications for systemic autoimmunity. Arthritis Rheum. 2001;44:2642–52. doi: 10.1002/1529-0131(200111)44:11<2642::aid-art444>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 119.Ronchetti A, Rovere P, Iezzi G, et al. Immunogenicity of apoptotic cells in vivo: role of antigen load, antigen-presenting cells, and cytokines. J Immunol. 1999;163:130–6. [PubMed] [Google Scholar]
- 120.Cohen PL, Caricchio R, Abraham V, et al. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135–40. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–11. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 122.Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J Immunol. 1997;158:4525–8. [PubMed] [Google Scholar]
- 123.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313–20. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Taylor PR, Carugati A, Fadok VA, et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–66. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Melcher A, Todryk S, Hardwick N, Ford M, Jacobson M, Vile RG. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat Med. 1998;4:581–7. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- 126.Higuchi H, Bronk S, Bateman A, Harrington KJ, Vile RG, Gores GJ. Viral fusogenic membrane glycoprotein expression causes syncytia formation with bioenergetic cell death: implications for gene therapy. Cancer Res. 2000;60:6396–402. [PubMed] [Google Scholar]
- 127.Galanis E, Bateman A, Johnson K, Diaz RM, James CD, Vile R, Russell SJ. Use of viral fusogenic membrane glycoproteins as novel therapeutic transgenes in gliomas. Hum Gene Ther. 2001;12:811–21. doi: 10.1089/104303401750148766. [DOI] [PubMed] [Google Scholar]
- 128.Linardakis E, Bateman A, Phan V, et al. Enhancing the efficacy of a weak allogeneic melanoma vaccine by viral fusogenic membrane glycoprotein-mediated tumor cell-tumor cell fusion. Cancer Res. 2002;62:5495–504. [PubMed] [Google Scholar]
- 129.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–1. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 130.Ferguson TA, Herndon J, Elzey B, Griffith TS, Schoenberger S, Green DR. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8(+) T cells produce active immune unresponsiveness. J Immunol. 2002;168:5589–95. doi: 10.4049/jimmunol.168.11.5589. [DOI] [PubMed] [Google Scholar]
- 131.Morelli AE, Larregina AT, Shufesky WJ, et al. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood. 2003;101:611–20. doi: 10.1182/blood-2002-06-1769. [DOI] [PubMed] [Google Scholar]
- 132.Suzuki H, Wang B, Shivji GM, et al. Imiquimod, a topical immune response modifier, induces migration of Langerhans' cells. J Invest Dermatol. 2000;114:135–41. doi: 10.1046/j.1523-1747.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- 133.Nair S, McLaughlin C, Weizer A, Su Z, Boczkowski D, Dannull J, Vieweg J, Gilboa E. Injection of immature dendritic cells into adjuvant-treated skin obviates the need for ex vivo maturation. J Immunol. 2003;171:6275–82. doi: 10.4049/jimmunol.171.11.6275. [DOI] [PubMed] [Google Scholar]
- 134.Marks R, Gebauer K, Shumack S, Amies M, Bryden J, Fox T, Owens M. Imiquimod 5% cream in the treatment of superficial basal cell carcinoma: Results of a multicenter 6-week dose–response trial. J Am Acad Dermatol. 2001;44:807–13. doi: 10.1067/mjd.2001.113689. [DOI] [PubMed] [Google Scholar]
- 135.Bong AB, Bonnekoh B, Franke I, Schon MP, Ulrich J, Gollnick H. Imiquimod, a topical immune response modifier, in the treatment of cutaneous metastases of malignant melanoma. Dermatology. 2002;205:135–8. doi: 10.1159/000063904. [DOI] [PubMed] [Google Scholar]
- 136.Benson E. Imiquimod: potential risk of an immunostimulant. Australas J Dermatol. 2004;45:123–4. doi: 10.1111/j.1440-0960.2004.00060.x. [DOI] [PubMed] [Google Scholar]
- 137.Clark WH, Jr, Elder DE, Guerry Dt, Braitman LE, Trock BJ, Schultz D, Synnestvedt M, Halpern AC. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893–904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 138.Clemente CG, Mihm MC, Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N, et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–10. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 139.Luscher U, Filgueira L, Juretic A, Zuber M, Luscher NJ, Heberer M, Spagnoli GC. The pattern of cytokine gene expression in freshly excised human metastatic melanoma suggests a state of reversible anergy of tumor-infiltrating lymphocytes. Int J Cancer. 1994;57:612–9. doi: 10.1002/ijc.2910570428. [DOI] [PubMed] [Google Scholar]
- 140.Whiteside TL. Signaling defects in T lymphocytes of patients with malignancy. Cancer Immunol Immunother. 1999;48:346–52. doi: 10.1007/s002620050585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Limmer A, Sacher T, Alferink J, Kretschmar M, Schonrich G, Nichterlein T, Arnold B, Hammerling GJ. Failure to induce organ-specific autoimmunity by breaking of tolerance: importance of the microenvironment. Eur J Immunol. 1998;28:2395–406. doi: 10.1002/(SICI)1521-4141(199808)28:08<2395::AID-IMMU2395>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 142.Lee KH, Wang E, Nielsen MB, et al. Increased vaccine-specific T cell frequency after peptide-based vaccination correlates with increased susceptibility to in vitro stimulation but does not lead to tumor regression. J Immunol. 1999;163:6292–300. [PubMed] [Google Scholar]
- 143.Osanto S, Schiphorst PP, Weijl NI, et al. Vaccination of melanoma patients with an allogeneic, genetically modified interleukin 2-producing melanoma cell line. Hum Gene Ther. 2000;11:739–50. doi: 10.1089/10430340050015635. [DOI] [PubMed] [Google Scholar]
- 144.Rodolfo M, Zilocchi C, Cappetti B, Parmiani G, Melani C, Colombo MP. Cytotoxic T lymphocyte response against non-immunoselected tumor antigens predicts the outcome of gene therapy with IL-12-transduced tumor cell vaccine. Gene Ther. 1999;6:865–72. doi: 10.1038/sj.gt.3300874. [DOI] [PubMed] [Google Scholar]
- 145.Hermans IF, Daish A, Yang J, Ritchie DS, Ronchese F. Antigen expressed on tumor cells fails to elicit an immune response, even in the presence of increased numbers of tumor-specific cytotoxic T lymphocyte precursors. Cancer Res. 1998;58:3909–17. [PubMed] [Google Scholar]
- 146.Melief CJ. Tumor eradication by adoptive transfer of cytotoxic T lymphocytes. Adv Cancer Res. 1992;58:143–75. doi: 10.1016/s0065-230x(08)60294-8. [DOI] [PubMed] [Google Scholar]
- 147.Speiser DE, Miranda R, Zakarian A, et al. Self antigens expressed by solid tumors do not efficiently stimulate naive or activated T cells. Implications for immunotherapy. J Exp Med. 1997;186:645–53. doi: 10.1084/jem.186.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kjaergaard J, Shu S. Tumor infiltration by adoptively transferred T cells is independent of immunologic specificity but requires down-regulation of 1-selectin expression. J Immunol. 1999;163:751–9. [PubMed] [Google Scholar]
- 149.Warnock RA, Askari S, Butcher EC, von Andrian UH. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J Exp Med. 1998;187:205–16. doi: 10.1084/jem.187.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cyster JG. Leukocyte migration: scent of the T zone. Curr Biol. 2000;10:R30–3. doi: 10.1016/s0960-9822(99)00253-5. [DOI] [PubMed] [Google Scholar]
- 151.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 152.Mackay CR, Marston W, Dudler L. Altered patterns of T cell migration through lymph nodes and skin following antigen challenge. Eur J Immunol. 1992;22:2205–10. doi: 10.1002/eji.1830220904. [DOI] [PubMed] [Google Scholar]
- 153.Mackay CR, Marston WL, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990;171:801–17. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aruffo A, Stamenkovic E, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–13. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 155.Koopman G, van Kooyk Y, deGraaff M, Meyer CJLM, Figdor CG, Pals ST. Triggering of the CD44 antigen on T lymphocytes promotes T cell adhesion through the LFA-1 pathway. Journal of Biological Chemistry. 1992;145:3589–93. [PubMed] [Google Scholar]
- 156.Murai M, Yoneyama H, Harada A, et al. Active participation of CCR5(+) CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J Clin Invest. 1999;104:49–57. doi: 10.1172/JCI6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nansen A, Marker O, Bartholdy C, Thomsen AR. CCR2+ and CCR5+ CD8+ T cells increase during viral infection and migrate to sites of infection. Eur J Immunol. 2000;30:1797–806. doi: 10.1002/1521-4141(200007)30:7<1797::AID-IMMU1797>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 158.Tomiyama H, Matsuda T, Takiguchi M. Differentiation of human CD8(+) T cells from a memory to memory/effector phenotype. J Immunol. 2002;168:5538–50. doi: 10.4049/jimmunol.168.11.5538. [DOI] [PubMed] [Google Scholar]
- 159.Trifilo MJ, Bergmann CC, Kuziel WA, Lane TE. CC chemokine ligand 3 (CCL3) regulates CD8(+) T-cell effector function and migration following viral infection. J Virol. 2003;77:4004–14. doi: 10.1128/JVI.77.7.4004-4014.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 161.Scott KA, Moore RJ, Arnott CH, East N, Thompson RG, Scallon BJ, Shealy DJ, Balkwill FR. An anti-tumor necrosis factor-alpha antibody inhibits the development of experimental skin tumors. Mol Cancer Ther. 2003;2:445–51. [PubMed] [Google Scholar]
- 162.Moore RJ, Owens DM, Stamp G, et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5:828–31. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 163.Balkwill F, Osborne R, Burke F, Naylor S, Talbot D, Durbin H, Tavernier J, Fiers W. Evidence for tumour necrosis factor/cachectin production in cancer. Lancet. 1987;2:1229–32. doi: 10.1016/s0140-6736(87)91850-2. [DOI] [PubMed] [Google Scholar]
- 164.Shah AH, Tabayoyong WB, Kundu SD, et al. Suppression of tumor metastasis by blockade of transforming growth factor beta signaling in bone marrow cells through a retroviral-mediated gene therapy in mice. Cancer Res. 2002;62:7135–8. [PubMed] [Google Scholar]
- 165.Griffith KD, Read EJ, Carrasquillo JA, et al. In vivo distribution of adoptively transferred indium-111-labeled tumor infiltrating lymphocytes and peripheral blood lymphocytes in patients with metastatic melanoma. J Natl Cancer Inst. 1989;81:1709–17. doi: 10.1093/jnci/81.22.1709. [DOI] [PubMed] [Google Scholar]
- 166.Kershaw MH, Wang G, Westwood JA, et al. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum Gene Ther. 2002;13:1971–80. doi: 10.1089/10430340260355374. [DOI] [PubMed] [Google Scholar]
- 167.Nakajima C, Uekusa Y, Iwasaki M, et al. A role of interferon-gamma (IFN-gamma) in tumor immunity: T cells with the capacity to reject tumor cells are generated but fail to migrate to tumor sites in IFN-gamma-deficient mice. Cancer Res. 2001;61:3399–405. [PubMed] [Google Scholar]
- 168.Dorner BG, Scheffold A, Rolph MS, Huser MB, Kaufmann SH, Radbruch A, Flesch IE, Kroczek RA. MIP-1alpha, MIP-1beta, RANTES, and ATAC/lymphotactin function together with IFN-gamma as type 1 cytokines. Proc Natl Acad Sci USA. 2002;99:6181–6. doi: 10.1073/pnas.092141999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mukai S, Kjaergaard J, Shu S, Plautz GE. Infiltration of tumors by systemically transferred tumor-reactive T lymphocytes is required for antitumor efficacy. Cancer Res. 1999;59:5245–9. [PubMed] [Google Scholar]
- 170.Huang H, Liu Y, Xiang J. Synergistic effect of adoptive T-cell therapy and intratumoral interferon gamma-inducible protein-10 transgene expression in treatment of established tumors. Cell Immunol. 2002;217:12–22. doi: 10.1016/s0008-8749(02)00508-7. [DOI] [PubMed] [Google Scholar]
- 171.Huang H, Li F, Gordon JR, Xiang J, et al. Synergistic enhancement of antitumor immunity with adoptively transferred tumor-specific CD4+ and CD8+ T cells and intratumoral lymphotactin transgene expression. Cancer Res. 2002;62:2043–51. [PubMed] [Google Scholar]