Abstract
Porin of Shigella dysenteriae type 1 increased the mRNA levels for Toll-like receptors TLR2 and TLR6, by 1·8-fold and twofold, respectively, in peritoneal cavity B-2 cells from C57BL/6 mice, implicating that the co-expression of TLR2 and TLR6 occurs as a combinatorial repertoire in response to porin. Among the two key TLRs, TLR2 and TLR4, which are primarily responsible for recognizing the majority of bacterial products, TLR2 alone participates in porin recognition. TLR2 expression was increased on B-2 cells, whereas the expression of TLR4 remained unaffected. Besides TLRs, mRNA for myeloid differentiation factor 88 (MyD88), an effector molecule associated with the TLR-mediated response, was enhanced by twofold, suggesting its involvement in the activity of porin. The B-2 cells showed a 1·8-fold increase in mRNA expression of the signalling molecule, nuclear factor-kappa B (NF-κB), in the presence of porin. Porin treatment of B-2 cells selectively up-regulated the expression of the costimulatory molecule, CD86, by 4·4-fold. Porin induced the cell-surface expression of immunoglobulin (Ig)M, of IgG2a preferentially among the IgG subclasses, and of IgA, on B-2 cells. The porin-mediated inductions of IgG2a and IgA were augmented by interleukin-6 on B-2 cells, by 2·7- and 1·6-fold, respectively.
Keywords: B-2 cell, CD86, IgA, IgG2a, porin, Toll-like receptor
Introduction
Porins are immunogenic without the addition of exogenous adjuvants1 and are known to be able to augment the humoral response to otherwise poorly immunogenic substances, for example, polysaccharides and peptides.2,3 These proteins are of particular interest because they have been characterized as potent adjuvants and have great potential as a novel component of vaccines.4,5 Porin of Shigella dysenteriae type 1 has been found to be surface exposed, strongly immunogenic and antigenically related among Shigella spp.6 These features make the protein attractive as an adjuvant for use in vaccine formulation against shigellosis. In order to study the adjuvanticity of porin, its involvement in the activation and response of mouse peritoneal cavity B-2 cells was examined.
Mice and humans have phenotypically distinct populations of B cells, termed B-1 and B-2, that have been proposed to represent entirely separate B-cell lineages.7,8 The conventional B cells are the B-2 cells on which CD23 (FcεR) are specifically present. These conventional CD23+ B-2 cells are found predominantly in the spleen and are also present in the peritoneal cavity. Thus, in the peritoneal cavity, these markers alone can be used to distinguish conventional B cells from B-1 cells, i.e. B-2 cells are CD23+ and B-1 cells are either CD5+ (B-1a) or CD11b+ (B-1b).9 T-cell-dependent immune responses generally involve conventional B-2 cells. By contrast, B-1 cells appear to produce antibodies in a T-independent manner.10 Besides immunoglobulin M (IgM) and immunoglobulin G (IgG), the expression of immunoglobulin A (IgA) on B-2 cells caused by porin treatment was evaluated with special emphasis because a mucosal immune response predominantly involves the formation of IgA antibody.
Members of the Toll-like receptor (TLR) family have been demonstrated to participate specifically in the process by which antigen-presenting cells (APC) recognize pathogen-associated molecular patterns that distinguish the infectious agents from self.11,12 Each TLR is a type 1 membrane protein, with an extracellular leucine-rich domain that is thought to participate in ligand recognition and an intracellular tail that contains a conserved region, called the Toll/IL-1R homology (TIR) domain13 that, upon activation, results in recruitment of myeloid differentiation factor 88 (MyD88), an adaptor protein that interacts with the TLRs through its own C-terminal TIR domain.14 The study of porin-mediated enhancement of TLR and MyD88 expression of peritoneal cavity B-2 cells would reveal how the adjuvant is distinguished specifically by the mucosal immune system.
Following the recognition by TLR, the mechanism of adjuvanticity of porin correlates with its ability to initiate signalling pathways, such as the up-regulation of CD80 and CD86 on the surface of B cells and other APC, or to activate the proinflammatory nuclear factor-kappa B (NF-κB). A feature of these cells that makes them potential candidates for skewing immune responses is their selective expression of the costimulatory molecules.15 The most fully characterized costimulatory signal is mediated by the binding of B7-1 (CD80) and B7-2 (CD86) on APC to their receptor, CD28, on T cells.16 Whether the costimulatory molecules, CD80 and CD86, play a regulatory role in the mucosal immune response has not yet been investigated in detail. Porin-mediated up-regulation of CD80 and CD86, and the expression of IgG subclasses and IgA on B-2 cells, would reveal whether the immunogen could regulate the conventional B cell for a mucosal immune response. One approach by which the mucosal response may be selectively enhanced is through a combination of a specific subset of cytokines. Interleukin-6 (IL-6) is a multifunctional cytokine which also plays a crucial role in the terminal differentiation of B cells and in the development of secretory IgA responses at the mucosa.17 B-2 cells were treated with IL-6 to establish whether this cytokine could enhance the porin-induced expression of IgG and IgA.
In this study, we describe the enhancement of specific TLRs in association with MyD88 of mouse peritoneal cavity B-2 cells in response to porin of S. dysenteriae type 1. Following the expression of porin-induced TLRs, the involvement of NF-κB, induction of the costimulatory molecules, CD80 and CD86, and expression of specific immunoglobulins on B-2 cells was assessed.
Materials and methods
Bacterial strain
S. dysenteriae type 1 (strain A020332) used in this study was a clinical isolate from the International Center for Diarrhoeal Disease Research, Dacca, Bangladesh. The bacterial strain was plated out, identified and cultured prior to purification of porin, as described previously.18
Immunogen
Porin was purified to homogeneity from S. dysenteriae type 1, and the absence of traces of lipopolysaccharide (LPS) in the purified porin was confirmed as described previously.6,19 Prior to use for the culture of peritoneal cavity B cells, the concentration of sodium dodecyl sulphate (SDS) in porin was reduced from 1% to 0·1% by exhaustive dialysis against 5 mm Tris–HCl (pH 7·5) containing 0·1% SDS.18
Animals
C57BL/6 mice, originally obtained from Jackson Laboratories (Bar Harbor, ME), were bred and reared in the animal care facility of the National Institute of Cholera and Enteric Diseases, Kolkata, India. Six-week-old mice of both sexes were killed for isolation of the peritoneal cavity B cells. The experiments with animals were conducted in accordance with the Animal Ethical Committee guidelines of the Institute.
Culture of peritoneal cavity B cells
C57BL/6 mice were killed by cervical dislocation and thoroughly cleaned with 70% ethyl alcohol. A small incision was made in the abdomen and the peritoneal cavity was rinsed several times through the opening with freshly prepared cold sterile phosphate-buffered saline (PBS; pH 7·2). The peritoneal wash, containing the cells of the monocyte–macrophage lineage, was adhered on the surface of sterile Petri dishes by incubation at 37° in 5% CO2 for 2 hr to form a cell monolayer. The non-adherent unfractionated peritoneal cells were removed by gently washing the Petri dishes. The single-cell suspension of peritoneal cavity B and T cells, devoid of erythrocytes, was found to be 97% viable, as determined by Trypan Blue exclusion. The cells were seeded at 1 × 105 cells/well, in 96-well round-bottomed tissue-culture plates, in 200 µl of RPMI-1640 containing 5 U/ml penicillin, 5 µg/ml streptomycin, 0·1% gentamicin, 2% fetal bovine serum (Life Technologies Inc., Grand Island, NY) and 0·1% insulin–transferrin–selenium (Life Technologies). Porin (1 µg/well) was added to the peritoneal cavity lymphocytes that were then cultured at 37° in 5% CO2 for 72 hr prior to fluorescence-activated cell sorter (FACS) analysis. IL-6 (600 pg/ml) was added, prior to porin, when it was necessary to culture the cells for 72 hr in its presence.
Flow cytometry
Cells, previously treated with complete medium alone, porin, IL-6 or porin + IL-6, were incubated with purified unconjugated anti-mouse CD16/CD32 (Fc Block™; BD Biosciences, San Jose, CA). The cells were washed by suspension in 1 ml of FACSFlow™ (BD Biosciences) and centrifugation at 400 g for 8 min. The cell pellet thus obtained was dispersed in 50 µl of FACSFlow™ and incubated with peridinin chlorophyll protein (PerCP)-conjugated anti-mouse CD19 (B-cell identifying marker) (BD Biosciences) at 4° in the dark for 20 min. The cells were then washed again with FACSFlow™ buffer and incubated with phycoerythrin (PE)-conjugated anti-mouse CD23 (for B-2 cells) at 4° in the dark for 20 min. The CD19 and CD23 double-stained cell aliquots were washed and then incubated with any one of fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD80, CD86, IgM, IgG subclasses or IgA (BD Biosciences) at 4° in the dark for 20 min. Similarly, CD19 and CD23 double-stained cells were incubated with FITC-conjugated anti-mouse TLR2 or TLR4 (eBioscience, San Diego, CA) to detect the cell-surface expression of the TLRs. Parallel sets of cells were incubated with monoclonal immunoglobulin isotypes. The fluorescence intensity of these cells served as a non-specific negative control. After staining, the cells were fixed in 1% paraformaldehyde prior to analysis.
The CD19+ CD23+ double-positive cell population was gated on a dot-plot (CD19/CD23), and the gated cells only were considered for analysis of B-2 cells to study the cell-surface expression of TLRs, costimulatory molecules and immunoglobulins. Flow cytometry was performed on a FACSCalibur using cellquest software (Becton-Dickinson, San Jose, CA).
Sorting of B-2 cells
B cells were seeded at 1 × 105 cells/well and cultured for 72 hr, with and without porin (1 µg/well), at 37° in 5% CO2. The cells were harvested from 96-well round-bottomed tissue-culture plates and incubated, at 4° in the dark for 20 min, with fluorochrome-conjugated anti-mouse CD19 and CD23 to identify B-2 cells. Cells were washed by dilution with FACSFlow™ to be devoid of unbound fluorochrome-conjugated monoclonal antibodies (mAbs), and 1 × 106 double-stained B-2 cells were sorted in single-cell mode on a FACSCalibur using cellquest software (Becton-Dickinson).
Reverse transcription–polymerase chain reaction (RT–PCR) analysis
A total of 1 × 106 B-2 cells were sorted, and total RNA was isolated using an RNAqueous™-4PCR kit (Ambion Inc., Austin, TX). RT was performed with equal amounts of total RNA, obtained from cells treated with and without porin, by using a RETROscript™ kit (Ambion Inc.) and reverse transcribed with AMV reverse transcriptase at 42°. An aliquot of the first strand was amplified by PCR in an automated thermal cycler (Perkin Elmer GeneAmp PCR System 2400; Perkin Elmer, Rotkreuz, Switzerland) using SuperTaq™ polymerase (Ambion Inc.). Specific primers were used for amplification (Table 1). Conditions for PCR were denaturation for 45 seconds at 94°; annealing for 45 seconds at 55° for GAPDH, 56° for TLR1, 57° for TLR6 and NF-κB, and 59° for TLR2, TLR4 and MyD88; and elongation for 45 seconds at 72°. After a hot start at 94° for 3 min, 36 cycles of PCR were carried out with a 5-min final extension at 72° after the last cycle. Appropriate control PCR reactions were performed to ensure that non-specific amplification did not occur. All PCR amplifications were performed within the linear range of amplification of the corresponding mRNA species. The images were captured using a gel documentation system (Bio-Rad, Hercules, CA). The amplified RT–PCR products of TLRs, MyD88 and NF-κB were normalized for the relative quantity of the amplified RT–PCR product of GAPDH using Bio-Rad QUANTITY 1 software of the gel documentation system. Changes in mRNA expression were presented as mean fold induction by porin relative to the untreated control.
Table 1.
Primer sequences
| Name/gene | Primer orientation | Primer sequence | GenBank accession no. |
|---|---|---|---|
| TLR1 | Forward | 5′-ACATCAAGTGTGTGCTTGAA-3′ | AF316985 |
| Reverse | 5′-CCCCATCACTGTACCTTAGA-3′ | ||
| TLR2 | Forward | 5′-ACAGCTACTGTGTGACTCTCCGCC-3′ | AF124741 |
| Reverse | 5′-GGTCTTGGTGTTCATTATCTTGCGC-3′ | ||
| TLR4 | Forward | 5′-GACCTCAGCTTCAATGGTGC-3′ | AF095353 |
| Reverse | 5′-TATCAGAAATGCTACAGTGGATACC-3′ | ||
| TLR6 | Forward | 5′-ACATCTCTTGCTGCCCTATG-3′ | AF314636 |
| Reverse | 5′-GAGGAACACTTGGTTTTTGAC-3′ | ||
| MyD88 | Forward | 5′-CACCTGTGTCTGGTCCATT-3′ | MMU84409 |
| Reverse | 5′-CGCAGGATACTGGGAAAGT-3′ | ||
| NF-κB | Forward | 5′-TGGCTACTATGAGGCTGACC-3′ | AF199371 |
| Reverse | 5′-GTTGATGGTGCTGAGGGAT-3′ | ||
| GAPDH | Forward | 5′-CCATGGAGAAGGCTGGGG-3′ | NM_001001303 |
| Reverse | 5′-CAAAGTTGTCATGGATGACC-3′ |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MyD88, myeloid differentiation factor 88; NF-κB, nuclear factor-kappa B; TLR, Toll-like receptors.
Statistical analysis
Results are expressed as the mean ± standard error of the mean (SEM) where applicable, of at least three independent experiments. The data were analysed by using one-way analysis of variance (anova). A P-value of < 0·05 was considered significant, and a P-value of < 0·005 was considered highly significant.
Results
Porin-induced Toll-like receptors and MyD88 expression of B-2 cells
RT–PCR was conducted by using specific primers of TLRs, MyD88 and GAPDH with total RNA isolated from peritoneal cavity B-2 cells of C57BL/6 mice after incubation for 72 hr with or without porin (Fig. 1). B-2 cells grown in the presence of porin showed a 1·8-fold increase (1·81 ± 0·054 SEM, P < 0·05) in TLR2 mRNA expression compared to the untreated cells. Besides the expression of mRNA for TLR2, B-2 cells expressed TLR6 mRNA at a level that was twofold higher (1·97 ± 0·088 SEM, P < 0·005) than that of the untreated cells.
Figure 1.
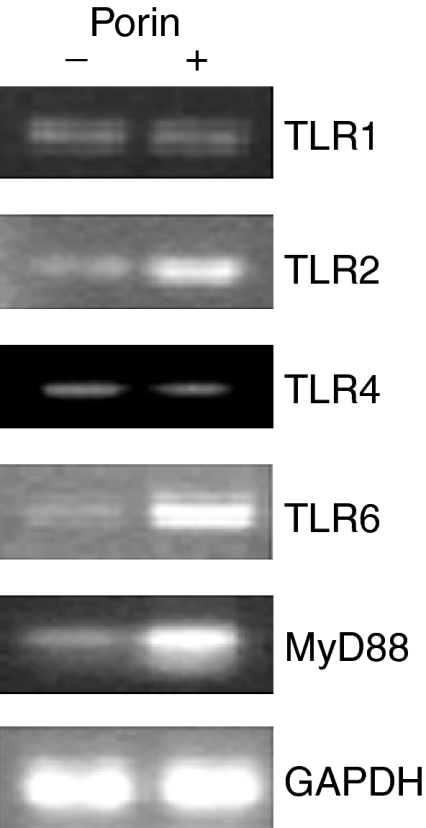
The effect of porin on Toll-like receptor (TLR) and myeloid differentiation factor 88 (MyD88) mRNA expression. Lymphocytes of C57BL/6 mice were treated with and without 1 µg of porin per 1 × 105 cells for 72 hr. Total RNA was extracted from the sorted B-2 cells after 72 hr of culture and subjected to reverse transcription–polymerase chain reaction (RT–PCR). Ethidium bromide-stained PCR products were photographed, and then images were digitized and analysed. PCR products were quantified and expressed as the ratio of each product to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) band density. The data shown are representative of three independent experiments.
Other than TLR2 and TLR6, there was a twofold increase (1·98 ± 0·133 SEM, P < 0·05) of mRNA expression for MyD88 by the B-2 cells. The mRNA expression for TLR1 (known to have homology with TLR6) and TLR4 (known to recognize primarily LPS) remained unaffected with porin treatment. The enhancement of mRNA for TLR2 and TLR6 indicates that B-2 cells co-express TLR2 and TLR6 in response to porin of S. dysenteriae type 1.
Induction of TLR2 and TLR4 on B-2 cells by porin
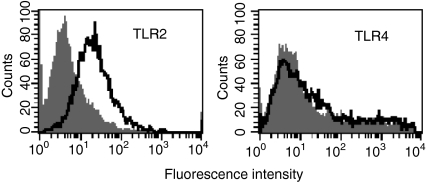
Analysis of relative fluorescence intensity showed that porin induced TLR2 specifically on B-2 cells of C57BL/6 mice (Fig. 2). The increase in expression of TLR2 was 4·2-fold (4·2 ± 0·061 SEM, P < 0·01) compared to the untreated control. The TLR4 expression on the B-2 cells was found to be absent. The response to porin by TLR2 is in combination with TLR6 as indicated by the mRNA expression data of TLR6 (Fig. 1).
Figure 2.
Induction of Toll-like receptor 2 (TLR2) and TLR4 on peritoneal cavity B-2 cells in response to porin. B-2 cells (CD19+ and CD23+) were cultured with complete medium alone (shaded) and porin (open) for 72 hr, and then analysed by flow cytometry for the expression of TLR2 and TLR4. The data shown are representative of three independent experiments.
Up-regulation of CD80 and CD86 on B-2 cells
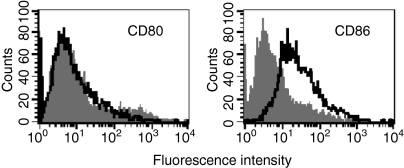
Analysis of relative fluorescence intensity showed that porin induced CD86 on B-2 cells (Fig. 3). The increment in expression of CD86 was 4·4-fold (4·43 ± 0·086 SEM, P < 0·005) on B-2 cells by porin compared to the control. The inability of the protein to induce the expression of CD80 supports porin-mediated selective up-regulation of CD86 on peritoneal cavity B-2 cells.
Figure 3.
Expression of CD80 and CD86 on peritoneal cavity B-2 cells after incubation with porin for 72 hr. B-2 cells (CD19+ and CD23+) were cultured with complete medium alone (shaded) or in the presence of porin (open), then analysed by flow cytometry for the expression of CD80 and CD86. Results shown are representative of three independent experiments.
NF-κB expression by B-2 cells in response to porin
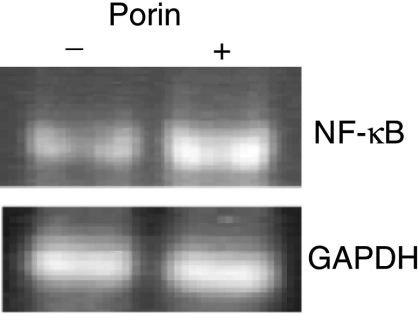
B-2 cells expressed the mRNA for NF-κB in response to porin, at a level that was 1·8-fold higher (1·81 ± 0·109 SEM, P < 0·05) than the untreated control (Fig. 4). The data indicate that enhancement of NF-κB in porin-treated peritoneal cavity B-2 cells may have a role in signalling.
Figure 4.
Effect of porin on nuclear factor-κB (NF-κB) mRNA expression. Lymphocytes were treated with or without porin for 72 hr. Total RNA was extracted from the sorted B-2 cells after 72 hr of culture and subjected to reverse transcription–polymerase chain reaction (RT–PCR). Ethidium bromide-stained PCR products were photographed and then images were digitized and analysed. PCR products were quantified and expressed as the ratio of each product to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) band density. The data shown are representative of three independent experiments.
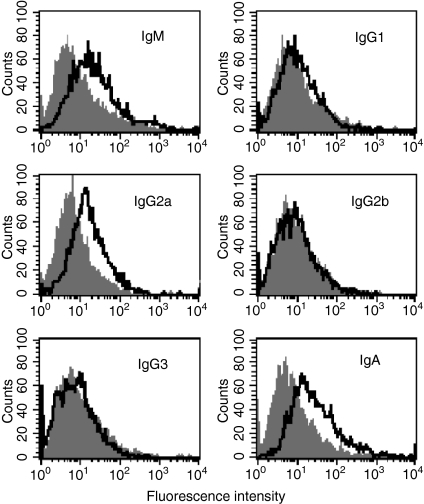
Porin-induced expression of IgM, IgG2a and IgA on B-2 cells
Analysis of relative fluorescence intensity showed that IgM was expressed at a 2·5-fold higher level (2·5 ± 0·06 SEM, P < 0·05) on B-2 cells in response to porin compared to the untreated control (Fig. 5). Among the IgG subclasses, IgG2a was selectively expressed at a level that was 2·6-fold greater (2·61 ± 0·049 SEM, P < 0·005) than the control. The cell-surface expression of IgG1, IgG2b and IgG3 remained unaffected with porin treatment of B-2 cells. The IgA expression was found to be increased by 3·2-fold (3·2 ± 0·052 SEM, P < 0·01) on B-2 cells compared to the control. The data suggest that porin is capable of generating IgA, the key immunoglobulin class that participates in the mucosal immune response, in addition to IgM and IgG2a.
Figure 5.
Induction of immunoglobulin M (IgM), immunoglobulin G (IgG) subclasses and immunoglobulin A (IgA) on peritoneal cavity B-2 cells after treating with porin for 72 hr. B-2 cells were cultured with (open) or without (shaded) porin and analysed by flow cytometry for the expression of IgM, IgG1, IgG2a, IgG2b, IgG3 and IgA. Data shown are representative of three independent experiments.
Effect of IL-6 on porin-induced IgG2a and IgA expression
Treatment of B-2 cells with porin in the presence of mouse IL-6 strongly enhanced the porin-induced IgG2a and moderately the IgA expression. Flow cytometric analysis showed that the cell-surface expression of IgG2a was augmented 2·7-fold (Fig. 6a), and of IgA 1·6-fold (Fig. 6b), on B-2 cells compared to that obtained by treatment with porin alone.
Figure 6.
Effect of interleukin-6 (IL-6) on the porin-induced immunoglobulin G2a (IgG2a) and immunoglobulin A (IgA) expression on B-2 cells. (a) Peritoneal cavity B-2 cells were cultured for 72 hr with complete medium alone (shaded), porin (thick grey line), IL-6 (thin black line) or porin plus IL-6 (thick black line), and analysed by flow cytometry for the expression of IgG2a. (b) Peritoneal cavity B-2 cells were cultured for 72 hr with complete medium alone (shaded), porin (thick grey line), IL-6 (thin black line) or porin plus IL-6 (thick black line), and analysed by flow cytometry for the expression of IgA. Data shown are representative of three independent experiments.
Discussion
Mucosal immunity is likely to have a direct role in conferring resistance to infection by Shigella spp., because the pathogen infects its host by penetration of the colonic mucosa. The activation of conventional CD23+ peritoneal cavity B-2 cells of mouse by porin purified from S. dysenteriae type 1 was studied to assess its ability to enhance the mucosal immune response. The mammalian TLRs play an important role in innate immunity,20 which includes B7 up-regulation.21 It has been postulated that TLRs are involved in regulating the effect of immune adjuvants.22 Our data show that porin of S. dysenteriae, besides enhancing the mRNA of TLR2, induced the expression of the receptor on B-2 cells. Other than TLR2, mRNA for TLR6 was also expressed. This indicates that both TLR2 and TLR6 may be involved in combination to activate the B-2 cells. The primers for TLR6 were designed such that it would differentiate TLR6 from TLR1, which have ≈ 65% homology.23 Besides TLR2 and TLR6, the mRNA for MyD88, an effector molecule associated with the TLR-mediated response in immune cells, was enhanced. This suggests that the presence of the effector molecule, MyD88, is involved in the activity of the porin. Previously, the activation of B cells by Neisserial porin was also found to be TLR2 and MyD88 dependent.21 These facts suggest that TLR2 has a central role in the porin-induced immune response. Moreover, TLR4, known to have specificity for LPS recognition,20 was not up-regulated. These data suggest that TLR2 (in association with TLR6), but not TLR4, can participate in the specific recognition of porin.
TLR2 and TLR4 can recognize a series of ligands, such as bacterial lipopeptide, LPS and other outer membrane bacterial components, thus initiating signalling pathways that affect the immune response, such as up-regulation of B7 molecules on the surface of B cells and dendritic cells, or nuclear translocation of the transcription factor, NF-κB. It was found that B-2 expressed the mRNA for NF-κB, possibly owing to the porin-induced co-expression of TLR2 and TLR6 on B-2 cells. This corresponds to the earlier finding that stimulation of Chinese hamster ovary cells with bacterial lipopeptide resulted in the co-expression of TLR2 with any one of the two TLRs, TLR1 or TLR6. However, this effect was prominent only when TLR2 and TLR6 were present together.20 Our result suggests that NF-κB has a role in the porin-induced mucosal B-2 cell response.
In order to establish porin of S. dysenteriae type 1 as an adjuvant, we evaluated the protein-induced expression of the costimulatory molecules, CD80 and CD86, on B-2 cells, as a good adjuvant acts by up-regulation of these molecules.24 Neisserial porins are known to act as immune adjuvants by up-regulating the surface expression of the costimulatory molecule B7-2 (CD86) on B cells and probably other APCs.25,26 We studied the surface expression of the costimulatory molecules, CD80 and CD86, on B-2 cells in response to porin and found that the B-2 cells selectively expressed CD86. It is known that CD86 is expressed as an early response to antigen, well ahead of CD80 expression, which takes part in a delayed response.15,27 As IgA is a key mediator of mucosal immunity, we studied whether the B-2 cells expressing CD86 under the influence of porin had the capacity to produce IgA. It was found that the B-2 cells expressed IgA efficiently in response to porin. This suggests that porin can effectively trigger the mucosal immune response involving IgA production, besides other immunoglobulins, such as IgM and IgG2a. In order to investigate whether the porin-induced immunoglobulins could be enhanced, B-2 cells were treated with porin and IL-6, known to participate in the terminal differentiation of B cells and development of immunoglobulin classes, particularly the secretory IgA response. IL-6 was found to augment the porin-induced IgG2a and IgA response of B-2 cells, thereby highlighting that IL-6 is a positive regulator of porin. Murine IL-6-responsive B cells have also been found in Peyer's patches. The IgA+ B-2 cells of Peyer's patches could be induced to secrete IgA in vitro by IL-5 and, most importantly, by IL-6.28
In summary, the adjuvanticity of S. dysenteriae type 1 porin involves an increase of mRNA for TLR2 and TLR6, suggesting that a combination of both are required, in association with MyD88, by B-2 cells to respond to porin. This results in the activation of NF-κB, followed by the selective up-regulation of CD86, which may signal for IgM, selectively IgG2a and particularly IgA expression, the signature molecule of the mucosal immune response, which, like IgG2a, could be enhanced by IL-6.
References
- 1.Wetzler LM, Blake MS, Barry K, Gotschlich EC. Neisserial porins induce B lymphocytes to express costimulatory B7-2 molecules and to proliferate. J Infect Dis. 1992;166:551–5. [Google Scholar]
- 2.Livingston PO, Calves MJ, Helling F, Zollinger WD, Blake MS, Lowell GH. GD3/proteosome vaccines induce consistent IgM antibodies against the ganglioside GD3. Vaccine. 1993;11:1199–204. doi: 10.1016/0264-410x(93)90043-w. [DOI] [PubMed] [Google Scholar]
- 3.Donnelly JJ, Deck RR, Liu MA. Immunogenicity of a Haemophilus influenzae polysaccharide–Neisseria meningitidis outer membrane protein complex conjugate vaccine. J Immunol. 1990;145:3071–9. [PubMed] [Google Scholar]
- 4.Lowell GH, Ballou WR, Smith LF, Wirtz RA, Zollinger WD, Hockmeyer WT. Proteosome–lipopeptide vaccines: enhancement of immunogenicity for malaria CS peptides. Science. 1988;240:800–2. doi: 10.1126/science.2452484. [DOI] [PubMed] [Google Scholar]
- 5.Zollinger WD. New and improved vaccines against meningoccal disease. In: Woodrow GC, Levine MM, editors. New Generation Vaccines. New York: Marcel Dekker Inc.; 1990. pp. 325–44. [Google Scholar]
- 6.Roy S, Das AB, Ghosh AN, Biswas T. Purification, pore-forming ability, and antigenic relatedness of the major outer membrane protein of Shigella dysenteriae type 1. Infect Immun. 1994;62:4333–8. doi: 10.1128/iai.62.10.4333-4338.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–8. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 8.Kantor AB, Stall AM, Adams S, Herzenberg LA, Herzenberg L. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci USA. 1992;89:3320–4. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardby E, Lane P, Lycke NY. Requirements for B7-CD28 costimulation in mucosal IgA responses: paradoxes observed in CTLA4-H gamma 1 transgenic mice. J Immunol. 1998;161:49–59. [PubMed] [Google Scholar]
- 10.Fagarasan S, Honjo T. T-independent immune response: new aspects of B-cell biology. Science. 2000;290:89–92. doi: 10.1126/science.290.5489.89. [DOI] [PubMed] [Google Scholar]
- 11.Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–56. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 12.Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–8. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 13.Lien E, Means TK, Heine H, et al. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadler A, Chen C, Ghosh S, Janeway CA., Jr MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–8. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 15.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 16.Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 17.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 18.Ray A, Chatterjee NS, Bhattacharya SK, Biswas T. Porin of Shigella dysenteriae enhances mRNA levels for Toll-like receptor 2 and MyD88, up-regulates CD80 of murine macrophage, and induces the release of interleukin-12. FEMS Immunol Med Microbiol. 2003;39:213–9. doi: 10.1016/S0928-8244(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 19.Roy S, Biswas T. Murine splenocyte proliferation by porin of Shigella dysenteriae type 1 and inhibition of bacterial invasion of HeLa cell by anti-porin antibody. FEMS Microbiol Lett. 1996;141:25–9. doi: 10.1111/j.1574-6968.1996.tb08358.x. [DOI] [PubMed] [Google Scholar]
- 20.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–71. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massari P, Henneke P, Ho Y, Latz E, Golenbock DT, Wetzler LM. Immune stimulation by Neisserial porins is toll-like receptor 2 and MyD88 dependent. J Immunol. 2002;168:1533–7. doi: 10.4049/jimmunol.168.4.1533. [DOI] [PubMed] [Google Scholar]
- 22.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 23.Hajjar AM, Shane O'Mahony D, Ozinsky A, Underhill DM, Aderem A, Klebanoff SJ, Wilson CB. Cutting edge: functional interactions between Toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J Immunol. 2001;166:15–9. doi: 10.4049/jimmunol.166.1.15. [DOI] [PubMed] [Google Scholar]
- 24.Freeman GJ, Borriello F, Hodes RJ, et al. Uncovering of functional alternative CTLA-4 counter-receptor in B7-deficient mice. Science. 1993;262:907–9. doi: 10.1126/science.7694362. [DOI] [PubMed] [Google Scholar]
- 25.Mackinnon FG, Ho Y, Blake MS, Michon F, Chandraker A, Sayegh MH, Wetzler LM. The role of B/T costimulatory signals in the immunopotentiating activity of neisserial porin. J Infect Dis. 1999;180:755–61. doi: 10.1086/314966. [DOI] [PubMed] [Google Scholar]
- 26.Wetzler LM, Ho Y, Reiser H. Neisserial porins induce B lymphocytes to express costimulatory B7-2 molecules and to proliferate. J Exp Med. 1996;183:1151–9. doi: 10.1084/jem.183.3.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ray A, Chattopadhyay K, Banerjee KK, Biswas T. Macrophage distinguishes Vibrio cholerae hemolysin from its protease insensitive oligomer by time dependent and selective expression of CD80–CD86. Immunol Lett. 2003;89:143–7. doi: 10.1016/s0165-2478(03)00135-4. [DOI] [PubMed] [Google Scholar]
- 28.Beagley KW, Eldridge JH, Lee F, et al. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J Exp Med. 1989;169:2133–48. doi: 10.1084/jem.169.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]