Abstract
Studies of tuberculosis have suggested a shift in dominance from a T helper type 1 (Th1) towards a Th2 immune response that is associated with suppressed cell-mediated immune (CMI) responses and increased humoral responses as the disease progresses. In this study a natural host disease model was used to investigate the balance of the evolving immune response towards Mycobacterium bovis infection in cattle with respect to pathogenesis. Cytokine analysis of CD4 T-cell clones derived from M. bovis-infected animals gave some indication that there was a possible relationship between enhanced pathogenesis and an increased ratio of Th0 [interleukin-4-positive/interferon-γ-positive (IL-4+/IFN-γ+)] clones to Th1 (IFN-γ+) clones. All animals developed strong antimycobacterial CMI responses, but depressed cellular responses were evident as the disease progressed, with the IFN-γ test failing to give consistently positive results in the latter stages. Furthermore, a stronger Th0 immune bias, depressed in vitro CMI responses, elevated levels of IL-10 expression and enhanced humoral responses were also associated with increased pathology. In minimal disease, however, a strong Th1 immune bias was maintained and an anti-M. bovis humoral response failed to develop. It was also seen that the level of the anti-M. bovis immunoglobulin G1 (IgG1) isotype antibody responses correlated with the pathology scores, whereas CMI responses did not have as strong a relationship with the development of pathology. Therefore, the development and maintenance of a Th1 IFN-γ response is associated with a greater control of M. bovis infection. Animals progressing from a Th1-biased to a Th0-biased immune response developed more extensive pathology and performed less well in CMI-based diagnostic tests but developed strong IgG1 humoral responses.
Keywords: antibody responses; interferon-γ; Mycobacterium bovis; proliferation; T cells (Th0, Th1)
Introduction
It is generally accepted that cell-mediated immunity (CMI) is of greatest importance in the control of intracellular pathogens, such as tuberculosis.1 As part of the antimycobacterial immunity, the development of a T helper type 1 (Th1)-biased CMI response, through the production of cytokines such as tumour necrosis factor-α, interleukin-12 (IL-12) and interferon-γ (IFN-γ), is considered essential to activate macrophage microbicidal pathways.2 IFN-γ is particularly important in tuberculosis infections because disruption of the IFN-γ gene leads to an inability to control a normally sublethal challenge with Mycobacterium tuberculosis in mice.3 Although this Th1 CMI response has the potential to control mycobacterial infections, the mycobacterium–macrophage interaction and the resulting balance of chemokines and cytokines produced can lead to several different outcomes in the infection. The bacterium can be killed and eliminated from the host, lie dormant in the host, lead to the development of active tuberculosis, or reactivate from dormancy at some stage in the future. It is likely that disease progression will be related to the balance of the evolving antimycobacterial immune response.
Cytokines will have a major role in determining the nature of immune responses to infection. Indeed, the Th1/Th2 paradigm was originally reported in terms of different cytokine profiles leading to the induction of different aspects of the immune system.4 It has been shown that during the early stages of mycobacterial infections dominant CMI Th1 IFN-γ responses develop, but as disease progresses, there can be a shift in the dominance from Th1 to Th2, with an associated anergy of cellular responses and the development of humoral responses.5–7
Immune responses are utilized in the diagnosis of tuberculosis. Diagnosis is usually based on tuberculin skin testing and the measurement of a delayed-type hypersensitivity (DTH) response, which is mediated by Th1 immunity.8 More recently, the IFN-γ test has been assessed and used for tuberculosis diagnosis in humans and cattle.9,10 Both of these tests rely on the development of a CMI response, and therefore any change in the immune balance could have profound effects on diagnostic performance. In bovine tuberculosis, because of the reciprocal relationship between the early CMI responses and the later development of humoral responses,7,11 early detection of infection has depended upon the measurement of CMI responses. However, assays to measure humoral responses have been shown to have a role in detecting M. bovis-infected animals at a later stage in the disease, and are being evaluated.12–14 Therefore, further understanding of the Th1/Th2 balance of the immune response will aid in the development of improved disease control strategies.
The aims of this study were to use the natural host disease model of tuberculosis in cattle to determine the Th1/Th2 immune bias in the developing antimycobacterial immune response. Correlations between immune responses and the development of pathology were also investigated and an assessment was made of how immune responses related to the performance of diagnostic tests. Cellular immune responses towards M. bovis antigens were assessed by measuring skin-test DTH responses, release of IFN-γ, lymphocyte proliferation, cytokine messenger RNA (mRNA) production (IL-2, IL-4, IL-10 and IFN-γ) and the antimycobacterial immunoglobulin G (IgG) isotype-specific humoral response.
Materials and methods
Experimental infections
Friesian-cross, castrated male calves (6 months of age), from herds with no history of M. bovis infection for at least 5 years, were screened using both a lymphocyte proliferation assay (LPA) and IFN-γ assay against M. bovis and M. avium tuberculins (PPD-B and PPD-A) to confirm their disease-free status. The animals were housed in secure isolation, under negative pressure, with expelled air filtered through a high-efficiency particulate air (HEPA) filter. A group of three animals (group 1; A1, A2 and A3) was used as a source of peripheral blood mononuclear cells (PBMC) to generate M. bovis-specific bovine T-cell clones. These animals were infected intranasally with M. bovis[(106 colony-forming units], strain T/91/1378 as described previously15 and CMI responses were measured using LPA and IFN-γ assays. Subsequently another group of six animals (group 2; B1, B2, B3, B4, B5 and B6) were also inoculated as above. Blood samples from this group of animals were collected at regular intervals from preinfection to 26 weeks postinfection (p.i.). Following collection of the last blood samples, animals were skin-tested (neck, mid-cervical) by intradermal inoculation of 0·1 ml PPD-B (1 mg/ml). Development of the DTH reaction, indicated by an increase in skin thickness, was measured after 72 hr using callipers. All animals were confirmed as having tuberculous lesions at post-mortem (PM) (9 and 6 months p.i. for animal groups 1 and 2, respectively) and by M. bovis culture.16 A similar group of uninfected animals was used as a source of PBMC to determine the level of background responses in assays. All work was undertaken in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986 under the auspices of the Veterinary Sciences Division Ethics Review Committee.
Antigens and mitogens
Mycobacterium bovis sonic extract (MBSE) was prepared as previously described.17 Briefly, M. bovis (T/91/1378) was grown in Middlebrook 7H9 medium, harvested, washed, and then ultrasonicated. MBSE was then clarified by centrifugation and filtered (0·22 μm). PPD-A and PPD-B were obtained from the Veterinary Laboratory Agency (Weybridge, UK). Pokeweed mitogen (PWM) was obtained from Sigma (Sigma-Aldrich Company, Poole, Dorset, England, UK).
Lymphocyte proliferation assay
PBMC were separated from heparinized blood samples over Ficoll–Paque and resuspended in complete T-cell medium [RPMI-1640 supplemented with 10 mm HEPES buffer, 2 mm l-glutamine, 5% fetal calf serum (Gibco, Paisley, UK) and 25 μg/ml gentamycin sulphate (Sigma)] as described previously.17 PBMC prepared at 1 × 106 cells/ml (200 μl/well) were cultured in triplicate in 96-well microtitre plates (Costar UK Ltd, High Wycombe, Bucks, UK). Antigens or mitogen was added (25 μl/well) to a final concentration of 4 μg/ml (PPD-A, PPD-B, MBSE, or PWM as indicated), and 25 μl phosphate-buffered saline (PBS) was added to the control wells. PBMC LPA cultures were incubated at 37° in 6% CO2 for 5 days and pulsed with 0·25 μCi [3H]thymidine (Amersham International, Amersham, UK) for the last 18 hr of culture. Incorporated radiolabel was measured by liquid scintillation17 and results were recorded as counts per minute (c.p.m.) and reported as net c.p.m. (mean test c.p.m. − mean control c.p.m.).
IFN-γ enzyme-linked immunosorbent assay (ELISA)
Whole blood cultures (200 μl/well in duplicate) were set up as described for proliferation assays using PPD-B, PWM, or PBS as control. Following overnight incubation at 37° and 6% CO2, 100 μl of supernatant was aspirated from each well and assayed for IFN-γ using the Bovigam enzyme immunoassay (CSL Ltd, Victoria, Australia). Results were recorded as optical density at 450 nm (OD450) and are expressed as an optical density index (ODI), which was calculated as test antigen OD450/control OD450.
Generation and analysis of T-cell clones
T-cell lines were established from group 1 animals (A1, A2 and A3) at 17 weeks p.i. for the generation of T-cell clones using the previously described protocols.18 Briefly, PBMC were cultured for 14 days at 37° in 6% CO2 in 75-cm2 flasks (20 ml at 1 × 106 cells/ml) in the presence of MBSE (4 μg/ml). Subsequently, viable cells were resuspended at 2 × 105 cells/ml with MBSE, 10 U/ml recombinant human IL-2 (rHuIL-2; Sigma) and antigen-presenting cells (APC). The APC were prepared by incubating freshly isolated autologous PBMC (1 × 107 cells/ml) with mitomycin-C (50 μg/ml) (Sigma) at 37° for 30 min. The APC were washed three times with PBS by centrifugation and resuspended in medium before being added to T-cell lines at 5 × 105 cells/ml. The T-cell lines were maintained by the addition of fresh APC, MBSE and rHuIL-2 at 7-day intervals. T-cell clones were produced by limiting dilution after the lines had become established. Proliferating clones were expanded into 24-well plates (Costar) for functional assays and clones were phenotyped by flow cytometry as detailed below.
Prior to the establishment of T-cell clone LPA and mRNA cytokine cultures, maintained clones were washed in PBS, resuspended in complete medium and rested for 24 hr in the absence of antigen or rHuIL-2. T-cell clone proliferation assays were performed using suspensions of 5 × 104 T cells/ml with 5 × 105 APC/ml dispensed into 96-well microtitre plates (200 μl/well) and stimulated with MBSE antigen as described above for PBMC LPA. For mRNA cytokine analysis (IL-4 and IFN-γ) 10 ml of T-cell clones (5 × 104 cells/ml) with APC (5 × 105 cells/ml) were dispensed into 25-cm2 tissue culture flasks (Costar) and incubated with MBSE in the same way as the LPA cultures. Cells were harvested from cultures, and washed twice in complete T-cell medium. RNA was extracted from 1 × 106 viable T cells from each clone. Synthesis of cDNA, polymerase chain reaction (PCR) and gel analysis were performed as described below for PBMC cytokine mRNA analysis.
PBMC cytokine mRNA analysis
For cytokine analysis PBMC (2·2 × 106 cells) were cultured in 24-well plates (Costar) with PPD-B (4 μg/ml) and incubated (96 hr) as for LPA above. Cells were harvested, pelleted and washed once in PBS (Eppendorf tubes; 3000 g/2 min). Cell pellets were resuspended in 100 μl RNA Later (Qiagen, Crawley, West Sussex, UK) and stored at −20°. Total RNA was extracted (RNeasy Mini Kit; Qiagen), treated with DNase (DNAse treatment kit; Invitrogen, Paisley, UK) and stored at −20°. The cDNA was synthesized using a Superscript First-strand synthesis system (Invitrogen) using 11 μl RNA, then treated with 1 μl Escherichia coli RNAse H (2 units/μl) (Invitrogen). The final product was stored at −20°.
Primers (Table 1), with a predicted product size between 200 and 500 bases, were designed using bovine sequences for β-actin (S.P. Suchyta, M.D. Elftman and P.M. Coussens, direct submission to the National Center for Biotechnology Information), IL-2,19 IFN-γ,20 IL-421 and IL-1022 published in the GenBank database of the National Center for Biotechnology Information (Bethesda, MD) and the European Molecular Biology Laboratory database (EMBL; Hiedelberg, Germany). PCR used the following components: diethylpyrocarbonate-treated water (35·5 μl), × 10 PCR buffer (5 μl), 10 mm dNTP mix (1 μl) and Hot Start Taq DNA polymerase (0·5 μl) (Qiagen). To this was added forward primer (2 μl) and reverse primer (2 μl). All primer solutions were at 10 pmol/μl. The PCR program used was as follows: 94° for 15 min followed by 35 cycles (94° for 1 min; 58° for 1 min; 72° for 1 min) and finally 72° for 7 min plus 4° indefinitely. Gel analysis of PCR products was performed on 2% E-gels (Invitrogen) using 20 μl of PCR samples or standard (50-base-pair step ladder 25 ng/μl; Sigma) per well. Gels were visualized by ultraviolet illumination and the image was captured digitally. PCR results of cytokine mRNA expression were scored, independently on two separate occasions, relative to β-actin (3/+ + +) as positive control (strong positive 3/+ + +, medium 2/+ +, weak 1/+, very weak 0·5/+ and negative 0/–).
Table 1.
PCR primer sequences for bovine cytokine gene expression
| Forward primer | Reverse primer | |
|---|---|---|
| β-actin | 5′-GGAGAAGATCTGGCACC-3′ | 5′-CCATCTCCTGCTCGAAGTCC-3′ |
| IFN-γ | 5′-GCAAGTAGCCCAGATGTAGC-3′ | 5′-GTCCTCCAGTTTCTCAGAGC-3′ |
| IL-2 | 5′-CCTCAAGCTCTCCAGGATGC-3′ | 5′-ATGAGAGGCACTTAGTGATC-3′ |
| IL-4 | 5′-GGGTCTCACCTACCAGCTGA-3′ | 5′-GCTTGCCAAGCTGTTGAGAT-3′ |
| IL-10 | 5′-GTTGCCTGGTCTTCCTGGCTG-3′ | 5′-GACATCTTCATCAACTACATA-5′ |
Isotype-specific antimycobacterial antibody response
Serum, separated from clotted blood, collected at various time-points throughout the experiments, was used to determine the nature of the antibody response towards MBSE using an IgG isotype-specific ELISA as described previously.12,23 Briefly, MBSE at 0·5 μg/ml in 0·05 m bicarbonate buffer pH 9·5, was used to coat plates overnight at 4°. Antigen-coated plates were washed six times with PBS containing 0·02% v/v Tween-20. Serum, antibodies and conjugates were diluted in PTN (PBS/0·02% Tween-80/0·2%NaCl) for the ELISA. Test serum (1/100 in PTN) together with M. bovis-positive and -negative sera was added to MBSE-coated plates. Isotype-specific mouse anti-bovine IgG1 or IgG2 (1/500) (Serotec, Oxford, UK) was added to wells for 1 hr at 37°. Following washing, goat anti-mouse IgG–horseradish peroxidase (1/5000) (Sigma) was added, then the plates were incubated and washed as before. Substrate tetramethylbenzidine (TMB) was added to the wells and they were then incubated for 15 min at 37°. The substrate reaction was stopped by the addition of 2·4 m H2SO4 and results were reported as OD450.
Flow cytometric analysis
T-cell clones were labelled with anti-bovine monoclonal antibodies CC8 (anti-CD4), CC63 (anti-CD8) and CC15 (anti-γδ-WC1) (ECACC; Porton Down, Wiltshire, UK) to determine cell phenotype and activation status (anti-bovine CD25; VMRD, Pullman, WA) by flow cytometry as described previously.24
Post-mortem examination
At the end of the experiment a detailed PM examination was performed. Following euthanasia, group 1 animals were examined to confirm infection and determine the degree of spread of the infection, while group 2 animals were examined in more detail to assign a PM score to each animal. Following gross examination of all the major organs, in the group 2 animals, those lymph nodes closely associated with the upper and lower respiratory tract were excised along with the lungs. Lungs were examined by serial transection (at 2-cm intervals) for the presence of tuberculous lesions. Each node or tissue was then sliced at 1-cm intervals and examined for the presence of tuberculous or abnormal lesions. Each node or tissue was allocated a PM score, using a semiquantitative scoring system, similar to that described by Vordermeier et al. 200225 on the basis of the presence or absence of lesions, which if present, were enumerated and measured. Tissue samples were selected for examination by histopathology and bacteriology.
Statistical analysis
Statistical analyses were performed as follows. Specific time-points for LPA and IFN-γ responses were analysed using the Student's t-test. LPA data were log10-transformed prior to statistical analysis, and IFN-γ data were analysed as raw OD450 data. The Spearman Rank Test was used for analysis of pathology data versus immunological responses (one-tailed hypothesis). The Wilcoxon Signed Rank Test was used for the analysis of cytokine PCR scores. Figures are presented as the group mean response ± the standard error of the mean (SEM), or median and range as indicated.
Results
Proliferation and mRNA cytokine analysis of T-cell clones
The group 1 animals (A1, A2, and A3) developed strong antimycobacterial CMI responses following M. bovis infection, as determined by LPA and IFN-γ assay. Preinfection CMI responses towards PPD-B were negative (group mean net LPA c.p.m. 258 ± 76, and ODI 0·97 ± 0·1 for PPD-B). Highly significant (P < 0·001) CMI responses towards PPD-B were evident prior to T-cell cloning (16 weeks p.i.: group mean net LPA c.p.m. 74 408 ± 10 840, and ODI 10·2 ± 2·36). Later in the infection (25–34 weeks p.i.) there was an increasing tendency for individual animals to give a negative result on the PPD-B test (ODI < 2) in the IFN-γ assay (animal A1, week 32 p.i. ODI = 1·01; animal A2, week 32–34 p.i. ODI < 1·44; animal A3 week 26–32 p.i. ODI < 1·47). In total, 30 CD4+ T-cell clones with MBSE specificity were derived from these animals. Proliferative responses and cytokine profiles of the T-cell clones are shown in Table 2. Out of a total of 30 T-cell clones, eight displayed particularly strong proliferation to MBSE (net c.p.m. > 10 000). Of the three T-cell clone animals, animal A1 had the largest proportion of strongly proliferating T-cell clones.
Table 2.
Proliferation and cytokine profiles of Mycobacterium bovis-specific CD4 T-cell clones generated from group 1 M. bovis-infected cattle at 17 weeks postinfection
| % Clones expressing | ||||||||
|---|---|---|---|---|---|---|---|---|
| Animal no. | Clone no. | LPA (c.p.m.)* | IFN-γ mRNA† | IL-4 mRNA | Clone profile | IFN-γ | IL-4 | IFN-γ/IL-4 |
| A1 | 12IIIB3 | 1085 | 3 | 0 | Th1 | |||
| 12IIIE12 | 15 916 | 3 | 0 | Th1 | ||||
| 12IIID12 | 17 927 | 3 | 0·5 | Th0 | ||||
| 12IIID8 | 33 893 | 3 | 0·5 | Th0 | 28·6 | 0 | 71·4 | |
| 12IIIC7 | 13 617 | 3 | 1 | Th0 | ||||
| 12IIIG4 | 16 430 | 3 | 1 | Th0 | ||||
| 12IIIF3 | 28 807 | 3 | 1 | Th0 | ||||
| A2 | 23IIID12 | 0 | 3 | 0 | Th1 | |||
| 23IIIF5 | 292 | 3 | 0 | Th1 | ||||
| 23IIIF10 | 0 | 3 | 0 | Th1 | ||||
| 23IIID2 | 1056 | 3 | 0 | Th1 | ||||
| 23IIID6 | 1223 | 3 | 0 | Th1 | ||||
| 23IIIE2 | 1472 | 3 | 0 | Th1 | 83·3 | 0 | 16·7 | |
| 23IIID8 | 1883 | 3 | 0 | Th1 | ||||
| 23IIID10 | 1897 | 3 | 0 | Th1 | ||||
| 23IIIB3 | 2066 | 1 | 0 | Th1 | ||||
| 23IIIC6 | 1530 | 3 | 0 | Th1 | ||||
| 23IIIF4 | 4710 | 3 | 0·5 | Th0 | ||||
| 23IIIB12 | 42 325 | 3 | 2 | Th0 | ||||
| A3 | 32IIIF10 | 0 | 2 | 0 | Th1 | |||
| 32IIIH5 | 0 | 3 | 0 | Th1 | ||||
| 32IIIA8 | 17 | 2 | 0 | Th1 | ||||
| 32IIIC10 | 0 | 0·5 | 0 | Th1 | ||||
| 32IIIC9 | 0 | 2 | 0 | Th1 | ||||
| 32IIID3 | 901 | 1 | 0 | Th1 | 81·8 | 0 | 18·2 | |
| 32IIIE3 | 1313 | 2 | 0 | Th1 | ||||
| 32IIIB10 | 2713 | 3 | 0 | Th1 | ||||
| 32IIIG10 | 50 171 | 3 | 0 | Th1 | ||||
| 32IIIB9 | 239 | 3 | 0·5 | Th0 | ||||
| 32IIIF3 | 1689 | 2 | 0·5 | Th0 | ||||
Proliferation by [3H]thymidine uptake. Results reported as net c.p.m. (MBSE c.p.m. − PBS c.p.m.).
PCR results were scored from 0 to 3 for IFN-γ and IL-4 (0 = no detectable PCR product; 3 = strong detection of PCR product).
All of the clones derived from these animals were positive for IFN-γ mRNA, and of these 70% had a Th1 profile (IFN-γ+) and 30% had a Th0 profile (IFN-γ+/IL-4+) (Table 2). No clones were identified that expressed IL-4 alone. Individual animals presented different cytokine profiles of their T-cell clones. As seen in Table 2, the majority of T-cell clones (71·4%) derived from A1 had a Th0 cytokine profile. The majority of the T-cell clones (> 80%) derived from animals A2 and A3 expressed a Th1 cytokine profile.
PBMC proliferation and whole blood IFN-γ responses towards PPD-B
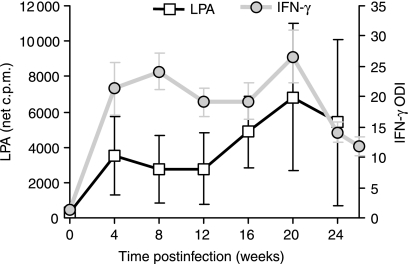
Following intranasal inoculation with M. bovis, all of the group 2 animals developed significant CMI responses towards PPD-B (LPA, P < 0·01 and IFN-γ, P < 0·001) antigen by 28 days p.i. (group mean responses are shown in Fig. 1). This group of animals developed progressively stronger proliferation responses towards PPD-B, however, following week 20 p.i. a number of changes were observed in their individual ability to respond to antigenic and mitogenic stimulation. Compared to animal B1, animals B2, B3, B4, B5 and B6 had much weaker proliferative responses towards PPD-B and PWM at the later stages of the infection as summarized in Table 3, along with the pathology scores. Strong PPD-B-specific IFN-γ secretion from whole blood cultures was evident by 4 weeks p.i., with initial levels continuing to rise until 8 weeks p.i. (Fig. 1). Towards the end of the experiment waning IFN-γ responses were evident in the group 2 animals. However, compared to the group 1 animals, the IFN-γ-test remained positive (PPD-B ODI > 2) throughout the later stages of the disease in all animals (Table 3). PBMC collected from uninfected animals (n = 3) did not display positive CMI responses towards PPD-B in LPA and IFN-γ assays (group mean net LPA c.p.m. 112 ± 8·2 SEM, and ODI 1·05 ± 0·04 for PPD-B).
Figure 1.
Lymphocyte proliferation and IFN-γ secretion following in vitro PPD-B stimulation of PBMC isolated from Mycobacterium bovis-infected cattle. Results are presented as the group mean (± SEM) responses for the animals in group 2 (n = 6, B1–B6). Proliferation was measured by incorporation of [3H]thymidine and the data are presented as the net c.p.m., where net c.p.m. = test antigen c.p.m. − control PBS c.p.m. Secreted IFN-γ was measured by ELISA, and the data are presented as ODI (test antigen OD/control PBS OD).
Table 3.
Summary of the antimycobacterial cellular and humoral immunological responses by Mycobacterium bovis-infected cattle (group 2 animals) in the week prior to PM (arranged by increasing PM score)
| Cellular assays† | Cytokineβ mRNA PCR | Antibody OD450 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal | PM score | DTH* PPD-B Increase (mm) | LPA (net cpm) PPD-B | PWM | IFN-γ ODI | IFN-γ | IL-2 | IL-4 | IL-10 | IgG1 | IgG2 |
| B1 | 12 | 28·5 | 31 644 | 213 172 | 11·6 | + + + | + + | − | + | 0·09 | 0·16 |
| B5 | 17 | 11·5 | − | 189 585 | 12·4 | + + | + + | + − | + + + | 0·09 | 0·12 |
| B4 | 20 | 17 | 5240 | 2072 | 14·3 | + | + + | − | + + + | 0·37 | 0·28 |
| B2 | 22 | 15 | 2232 | 80 862 | 11·9 | + + + | + + | − | + + + | 0·38 | 0·16 |
| B6 | 25 | 12·5 | − | 49 493 | 4·9 | + + + | + + | − | + + | 0·76 | 0·77 |
| B3 | 47 | 8·5 | 999 | 3176 | 16·0 | + + | − | − | + + + | 0·87 | 0·16 |
Following collection of the last blood samples, animals were skin-tested. The DTH response was measured after 72 hr using callipers and reported as increase in skin thickness.
LPA and IFN-γ data are presented as follows. Net c.p.m. (test antigen c.p.m. − control c.p.m.), a net c.p.m. reported as negative = no net positive proliferation towards PPD-B. ELISA ODI (test antigen OD/control OD). All animals retained an IFN-γ response to PWM (ODI > 7).
Cytokine mRNA PCR results are presented as scores relative to β-actin.
Isotype-specific antibody responses to MBSE
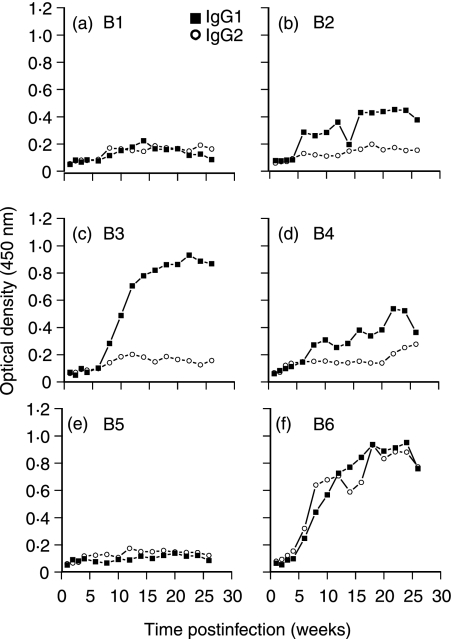
Testing of antibody reactivity against MBSE identified differences in the immune bias of the humoral response developing in each animal as a result of the infection with M. bovis (Fig. 2). Four animals (B2, B3, B4 and B6) developed IgG1 antibody responses towards MBSE by 6–8 weeks p.i. From weeks 8 to 20 p.i. the anti-MBSE IgG1 titre continued to rise rapidly in animals B3 and B6 (Fig. 2c,f). Only one animal (B6) developed a strong IgG1 and IgG2 antibody response (Fig. 2f), while an antimycobacterial humoral response failed to develop in animals B1 and B5 (Fig. 2a,e).
Figure 2.
Bovine IgG1 and IgG2 isotype-specific antimycobacterial (MBSE antigen) antibody responses in six animals following intranasal (106 colony-forming units) infection with M. bovis. MBSE antigen absorbed onto ELISA microtitre plates was used to screen for the presence of anti-MBSE-reactive antibodies in serum samples. Bound bovine IgG antibodies were detected using mouse anti-bovine isotype-specific monoclonal antibodies followed by goat anti-mouse–horseradish peroxidase conjugate.
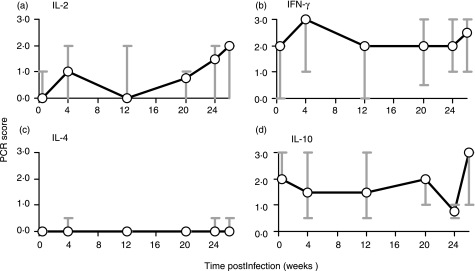
PBMC cytokine mRNA analysis
Examples of the mRNA cytokine analysis PCR products are shown in Fig. 3, while Fig. 4 displays a summary of the median results of mRNA cytokine analysis for IL-2, IL-4, IL-10 and IFN-γ in PPD-B-stimulated PBMC cultures (group 2 animals). By 4 weeks p.i. there were increases in the production of IL-2 and IFN-γ mRNA in PPD-B-stimulated PBMC cultures, as was also observed for PBMC proliferation and IFN-γ secretion (Figs 1, 4a,b). Late in the infection the expression of IL-2 and IFN-γ mRNA did not correspond directly with the levels of cellular responses (Fig. 1). It is possible that the cellular response may have been influenced by other cytokines, such as IL-10 expression, particularly in some animals (Fig. 4d) (Table 3).
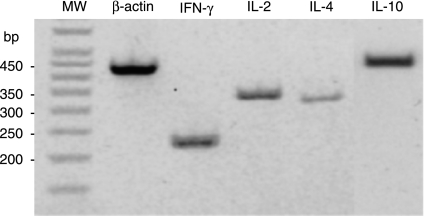
Figure 3.
Agarose gel (2%) showing the size of PCR product amplified by each primer set. Total RNA, extracted from PPD-B-stimulated PBMC, was reverse transcribed to cDNA and defined sequences were amplified using specific primers for bovine β-actin, IFN-γ, IL-2, IL-4 and IL-10. MW (molecular weight) markers are shown in a 50-bp step ladder. PCR results were scored relative to β-actin (+ + +) as positive control. For example, the above bands for IFN-γ, IL-2 and IL-10 were scored (+ +) and IL-4 was scored (+).
Figure 4.
Cytokine mRNA expression, as determined by PCR, in PBMC isolated from Mycobacterium bovis-infected cattle and stimulated in vitro with PPD-B. Results are presented as the median and range response of the group 2 animals (n = 6, B1–B6).
IL-4 did not have an extensive role in these animals and was only detectable at low levels (Fig. 4c), in B4 and B6 (week 4 p.i.), and B5 (weeks 24 and 26 p.i.) PPD-B-stimulated cultures. As the overall level and frequency of IL-4 mRNA expression was low, it was not possible to correlate IL-4 expression with other immunological parameters.
High levels of IL-10 mRNA were evident in PPD-B-stimulated PBMC cultures prior to infection. However, these levels gradually declined following infection, as strong M. bovis-specific IFN-γ responses became apparent (Figs 1 and 4d). By week 26 p.i. the level of IL-10 mRNA expression in the group 2 animals was greater than preinfection levels of expression in PPD-B-stimulated PBMC cultures (Fig. 4d). Based on the available data, the statistical comparison of the level of IL-10 expression in the group 2 animals from preinfection to postinfection, there was no reason to reject the null hypothesis.
Experimental infection and PM findings
The presence of tuberculous lesions was confirmed in the group 1 animals at PM examination, as described previously,16 9 months p.i. Upon PM, animal A1 had the most widely disseminated disease pattern, with tissues of the upper respiratory tract (nasal mucosa, turbinates, nasal septum, nasopharynx, pharyngeal tonsils and the proximal area of the trachea) bearing lesions. Those lymph nodes associated with the head (retropharyngeal, parotid and submandibular) were enlarged and carried lesions, and in the lower respiratory tract the apical, middle and diaphragmatic lung lobes, and the bronchial–mediastinal lymph nodes also had lesions. Animals A2 and A3 both showed a more restricted dissemination of disease, with evidence of infection observed in the turbinates, nasopharynx and upper trachea of animal A2, while only the nasal mucosa and nasopharynx were affected in animal A3. In both of these animals the retropharyngeal, parotid and submandibular lymph nodes bore lesions, while in the lower respiratory tract, only the right diaphragmatic lung lobe had lesions in calf A3, although both cervical, bronchial and mediastinal lymph nodes had lesions. Selected tissues were taken for histopathological confirmation of tuberculous lesions (data not shown). Comparison of the PM results and the T-cell clone data (Table 2) indicated a possible association between the proportion of Th0 clones derived from an animal, and the degree of pathology that developed within the animal.
The group 2 animals were also all confirmed as having tuberculous lesions following an extensive pathology examination, and each animal was given a PM score as described above. One animal (B3) had a pathological pattern of disease that indicated spread of infection from the upper respiratory tract to the lungs. In this calf, the cranial, caudal and middle lungs were all found to bear lesions in addition to the retropharyngeal, submandibular and parotid lymph nodes, giving this animal the highest PM score (47). As well as having the highest PM score, animal B3 had the most prominent Th0 immune profile, as indicated by an IgG1 antibody and IFN-γ response, together with a depressed cellular proliferation and elevated IL-10 mRNA expression (Table 3). This may also compare with animal A1, described above, which also had indications of a stronger Th0 response and the greatest extent of disseminated M. bovis infection. The other animals had varying degrees of pathology, mainly confined to the upper respiratory tract apart from two animals (B4, PM score 20 and B5, PM score 17) in which there were lesions on both the bronchial and mediastinal lymph nodes. Animal B1 had the lowest PM score of 12 (Table 3).
Correlation of pathology and immunological responses
Analysis of the ratio of Th1 : Th0 clones generated from individual group 1 animals infected with M. bovis indicated that there may be some relationship with the development of pathology recorded at PM. Animal A1 had the greatest proportion of Th0 T-cell clones and at PM this animal showed a greater dissemination of the infection, including extensive lung involvement. Animals A2 and A3 had a greater frequency of Th1 T-cell clones along with a much lower degree of pathology, with the infection mainly restricted to the upper respiratory tract.
Immune responses (at the last sample point) for the individual animals in group 2 were compared with their PM results and these data are presented in Table 3. Proliferation and IFN-γ production by PBMC in responses to PPD-B did not have a clear relationship with the extent of pathology (P > 0·05), although earlier in the infection (12 week p.i.) IFN-γ responses did show a significant correlation to PM results (R = 0·83, P = 0·0416). However, there was a correlation between the PM score and the level of the antimycobacterial IgG1 antibody response. Anti-MBSE IgG1 antibody responses correlated positively with the extent of pathology at the termination of the experiment (week 26 p.i.; R = 0·9429, P = 0·0024). Analysis of earlier antibody responses, for the group 2 animals (Fig. 2), indicated that the level of the IgG1 antibody response from 8 weeks p.i. through to the termination of the experiment showed a correlation with the subsequent development of pathology (R = 0·8286–0·9429; P = 0·0208–0·0024). There was no significant correlation in the group 2 animals between the skin test DTH response and the PM score (P > 0·05), although the least diseased animal (B1) had the largest skin test response (28·5 mm). Although significant correlations were not apparent between the PM scores and the expression of cytokine mRNA, interesting results were observed. In particular, the least diseased animal (B1) had the lowest ratio of IL-10 mRNA expression to IFN-γ production (message and protein) in PPD-B-stimulated PBMC cultures from 20 weeks p.i. compared to group 2 animals with more advanced disease (Table 3).
Discussion
Alterations in the immune balance associated with disease progression in tuberculosis infection have implications for the outcome of the disease and may have a significant impact on diagnosis and control of the infection. Disease models (e.g. the bovine and BALB/c mouse models) are very important in providing a better understanding of the antimycobacterial immune response, and this knowledge will aid in the development of improved disease control strategies.
In this study, analysis of M. bovis-antigen-reactive T-cell clones indicated a weak but possibly important link between the proportion of clones expressing a Th0 profile (IFN-γ and IL-4) and the pathogenesis of the disease. The largest proportion of Th0 clones was derived from the most diseased animal, and animals with a more restricted pattern of infection had a predominantly Th1 (IFN-γ) clone bias. As infection progressed in the T-cell clone group, all animals displayed reduced CMI responses and such changes are associated with an altered immune bias.7,26 In particular the IFN-γ test failed to give consistently positive PPD-B results when regular samples were collected during the period 7–8 months p.i. (ODI < 2).
To further investigate any relationship between immune balance and the development of pathology a more detailed investigation to assess CMI and humoral responses was performed. All of the group 2 animals developed early CMI responses, which were followed shortly by the development of humoral responses in some of the animals. Analysis of these results also indicated an association of a more prominent Th0 immune profile (IgG1 antibody and IFN-γ response) with an enhanced disease load, as shown by PM scores. Disease progression was also accompanied by reduced T-cell responsiveness (less well-developed PPD-B skin test DTH, impaired PBMC proliferation and reduced levels of IFN-γ secretion). Compared to PM scores, there was a correlation between the levels of PPD-B-stimulated IFN-γ secretion (week 12 p.i.), as has been reported previously for ESAT-6 IFN-γ secretion.25 With respect to proliferation responses, only the least diseased of the group 2 animals maintained a strong PPD-B proliferation response late in the infection, whereas all animals remained PPD-B IFN-γ test positive.
Analysis of the mRNA cytokine expression in PPD-B-stimulated PBMC cultures demonstrated that expression of the Th1 cytokines (IL-2 and IFN-γ) represented cellular activity for proliferation and IFN-γ secretion (respectively) through to week 20 p.i. Later the expression of these cytokines did not follow cellular activity, possibly because of changes in the immune balance. IL-4 and IL-10 cytokine expression was also measured, and these cytokines potentially have a role in the pathogenesis of mycobacterial infections.27–29 IL-4 is not always readily detected in M. tuberculosis infections30 and similarly in this study occasional expression of IL-4 mRNA was detected in some group 2 animals p.i., as similarly found for the measurement of secreted IL-4.31 However, Rhodes et al.32 have reported the detection of IL-4 (secreted) in a larger number of M. bovis-infected cattle, and in humans IL-4 is associated with advanced immunopathology33 although overall the presence or absence of IL-4 does not correlate with clinical outcome.34
IL-10 is generally considered to be primarily anti-inflammatory and its absence or inhibition encourages stronger Th1 immune responses, whereas elevated expression increases susceptibility to mycobacterial infection.35–37 In this set of experiments, elevated levels of IL-10 mRNA expression in the later stages of the infection were more closely associated with the most diseased animals, which also displayed waning CMI (particularly antigen-specific proliferation) and increasing humoral responses. Levels of IL-10 mRNA expression were high preinfection and waned as the antimycobacterial IFN-γ response increased, possibly as a result of regulation by IFN-γ or IL-12.38,39 Elevated levels of IL-10 expression in the group 2 animals may partially explain the suppression of CMI responses, as has been observed in humans.6,40
In M. bovis-infected deer, higher antibody responses were associated with disseminated disease.41 For the group 2 animals it was found that increased IgG1 antibody levels were also associated with a more widespread pattern of disease, and from 2 to 3 months p.i. IgG1 antibody levels correlated positively with pathology. The most diseased animal (B3) developed the strongest IgG1 antibody response and also had a high level of IL-10 mRNA expression, a cytokine that is involved in B-cell immunoglobulin class switching to IgG1.42 In human tuberculosis the switching of IgM antibody responses to antigen-specific IgG1 has been considered a critical event in disease progression43 and raised IgG1 levels may be indicative of an immune response progressing towards a Th0/Th2 immune bias.44,45
In human tuberculous granulomas, heterogeneity is observed within individual patients, with granulomas having a Th1 (IFN-γ+) or Th0 (IFN-γ+/IL-4+) cytokine profile33 as has similarly been observed in PPD-B skin test biopsies from M. bovis-infected cattle.46 In the present set of experiments, all animals developed strong Th1-biased responses as infection became established, and later a number of animals developed more prominent Th0 responses. It was the overall balance in an animal that was associated with a pathology outcome.
It is not known what triggers the Th1 to Th2 shift in the immunodominance associated with disease progression in tuberculosis infections.47 From the data presented here, maintenance of a strong Th1-biased CMI response is clearly associated with a greater capacity to contain the infection, whereas progression towards a Th0 immune profile (IgG1 antibody response in the presence of an IFN-γ response) was associated with increased pathogenesis. It has been recognized that those animals with a greater extent of pathology are associated with an increased frequency and duration of M. bovis shedding in their nasal mucus (Dr T. McCorry et al. unpublished data).
In the course of the antimycobacterial immune response there is a fine line between containing the infection and immunopathology caused by a strong and sustained inflammatory response.48 It is not known if the Th1 to Th0 shift represents a dampening down of the host inflammatory response to limit tissue damage49 or alternatively a stimulation by the mycobacterial antigens of the immunosuppressive cytokines.50 In particular, IL-10 and transforming growth factor-β play a prominent role in the down-regulation of potentially protective host effector mechanisms (T-cell proliferation and IFN-γ secretion).40,51,52
In conclusion, the development and maintenance of a Th1-biased CMI response following M. bovis infection in cattle results in a greater capacity to control the infection. Conversely, increased pathogenesis is observed in animals with a more prominent Th0 profile (IFN-γ and IgG1 humoral response). Elevated levels of antigen-driven production of IL-10 in the more diseased animals may have some influence on antigen-specific CMI responses such as the tuberculin skin test DTH responses, IFN-γ secretion and PBMC proliferation.
Clearly, these findings have significant implications for the control of bovine tuberculosis. In the field, the presence of seropositive animals may suggest the presence of active disease. Therefore, diagnostically the most accurate system for the detection of M. bovis-infected animals may be to employ cellular and humoral testing. Further analysis of other cytokine responses in M. bovis can only expand this area of knowledge to further improve the disease control strategies of M. bovis.
Acknowledgments
This work was supported by the Department of Agriculture and Rural Development (DARD) and Dr R. T. Cunningham was supported by the Department for Environment, Food and Rural Affairs (DEFRA). Thanks are given to B. Gangadharan (Queen's University Belfast) for assistance in post-mortem evaluations, and A. Gordon (Biometrics Division, DARD) for the statistical correlation analysis.
References
- 1.Boom WH. The role of T-cell subsets in Mycobacterium tuberculosis infection. Infect Agents Dis. 1996;5:73–81. [PubMed] [Google Scholar]
- 2.Orme IM, Cooper AM. Cytokine chemokine cascades in immunity to tuberculosis. Immunol Today. 1999;20:307–12. doi: 10.1016/s0167-5699(98)01438-8. [DOI] [PubMed] [Google Scholar]
- 3.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon-γ gene-depleted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 5.Dlugovitzky D, Bay ML, Rateni L, Fiorenza G, Vietti L, Farroni MA, Bottasso OA. Influence of disease severity on nitrite and cytokine production by peripheral blood mononuclear cells (PBMC) from patients with pulmonary tuberculosis (TB) Clin Exp Immunol. 2000;122:343–9. doi: 10.1046/j.1365-2249.2000.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boussiotis VA, Tsai EY, Yunis EJ, et al. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J Clin Invest. 2000;105:1317–25. doi: 10.1172/JCI9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ritacco V, Lopez B, Dekantor IN, Barrera L, Errico F, Nader A. Reciprocal cellular and humoral immune-responses in bovine tuberculosis. Res Vet Sci. 1991;50:365–7. doi: 10.1016/0034-5288(91)90143-c. [DOI] [PubMed] [Google Scholar]
- 8.Cher DJ, Mosman TR. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by Th1 clones. J Immunol. 1987;138:3688–94. [PubMed] [Google Scholar]
- 9.Katial RK, Hershey J, Purohit-Seth T, Belisle JT, Brennan PJ, Spencer JS, Engler RJM. Cell-mediated immune response to tuberculosis antigens: comparison of skin testing and measurement of in vitro gamma interferon production in whole-blood culture. Clin Diagn Lab Immunol. 2001;8:339–45. doi: 10.1128/CDLI.8.2.339-345.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood PR, Rothel JS. In vitro immunodiagnosis assays for bovine tuberculosis. Vet Micro. 1994;40:125–35. doi: 10.1016/0378-1135(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 11.Fifis T, Corner LA, Rothel JS, Wood PR. Cellular and humoral immune-responses of cattle to purified Mycobacterium bovis antigens. Scand J Immunol. 1994;39:267–74. doi: 10.1111/j.1365-3083.1994.tb03370.x. [DOI] [PubMed] [Google Scholar]
- 12.Lightbody KA, Skuce RA, Neill SD, Pollock JM. Mycobacterial antigen-specific antibody responses in bovine tuberculosis: an ELISA with potential to confirm disease status. Vet Rec. 1998;142:295–300. doi: 10.1136/vr.142.12.295. [DOI] [PubMed] [Google Scholar]
- 13.McNair J, Corbett DM, Girvin RM, Mackie DP, Pollock JM. Characterization of the early antibody response in bovine tuberculosis: MPB83 is an early target with diagnostic potential. Scand J Immunol. 2001;53:365–71. doi: 10.1046/j.1365-3083.2001.00874.x. [DOI] [PubMed] [Google Scholar]
- 14.Wiker HG, Lyashchenko KP, Aksoy AM, et al. Immunochemical characterization of the MPB70/80 and MPB83 proteins of Mycobacterium bovis. Infect Immun. 1998;66:1445–52. doi: 10.1128/iai.66.4.1445-1452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neill SD, Hanna J, Obrien JJ, McCracken RM. Excretion of Mycobacterium bovis by experimentally infected cattle. Vet Rec. 1988;123:340–3. doi: 10.1136/vr.123.13.340. [DOI] [PubMed] [Google Scholar]
- 16.Cassidy JP, Bryson DG, Pollock JM, Evans RT, Forster F, Neill SD. Early lesion formation in cattle experimentally infected with Mycobacterium bovis. J Comp Pathol. 1998;119:27–44. doi: 10.1016/s0021-9975(98)80069-8. [DOI] [PubMed] [Google Scholar]
- 17.Pollock JM, Douglas AJ, Mackie DP, Neill SD. Identification of bovine T-cell epitopes for 3 Mycobacterium bovis antigens MPB70, 19,000-MW and MPB57. Immunology. 1994;82:9–15. [PMC free article] [PubMed] [Google Scholar]
- 18.Pollock JM, Pollock DA, Campbell DG, Girvin RM, Crockard AD, Neill SD, Mackie DP. Dynamic changes in circulating and antigen-responsive T-cell subpopulations post-Mycobacterium bovis infection in cattle. Immunology. 1996;87:236–41. doi: 10.1046/j.1365-2567.1996.457538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerretti DP, McKereghan K, Larsen A, Cantrell MA, Anderson D, Gillis S, Cosman D, Baker PE. Cloning, sequence and expression of bovine interleukin-2. Proc Natl Acad Sci USA. 1986;83:3223–7. doi: 10.1073/pnas.83.10.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerretti DP, McKereghan K, Larsen A, Cosman D, Gillis S, Baker PE. Cloning, sequence and expression of bovine interferon-γ. J Immunol. 1986;136:4561–4. [PubMed] [Google Scholar]
- 21.Heussler VT, Eichorn M, Dobbelaere DA. Cloning of a full-length cDNA encoding bovine interleukin 4 by the polymerase chain reaction. Gene. 1992;114:273–8. doi: 10.1016/0378-1119(92)90587-f. [DOI] [PubMed] [Google Scholar]
- 22.Hash SM, Brown WC, Rice-Ficht AC. Characterization of a cDNA-encoding bovine interleukin-10: kinetics of expression in bovine lymphocytes. Gene. 1994;139:257–61. doi: 10.1016/0378-1119(94)90766-8. [DOI] [PubMed] [Google Scholar]
- 23.Lightbody KA, McNair J, Neill SD, Pollock JM. IgG isotype antibody responses to epitopes of the Mycobacterium bovis protein MPB70 in immunised and in tuberculin skin test reactor cattle. Vet Microbiol. 2000;75:177–88. doi: 10.1016/s0378-1135(00)00215-7. [DOI] [PubMed] [Google Scholar]
- 24.Smyth AJ, Welsh MD, Girvin RM, Pollock JM. In vitro responsiveness of γδ T cells from Mycobacterium bovis-infected cattle to mycobacterial antigens. Predominant involvement of WC1(+) cells. Infect Immun. 2001;69:89–96. doi: 10.1128/IAI.69.1.89-96.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vordermeier HM, Chambers MA, Cockle PJ, Whelan AO, Simmons J, Hewinson RG. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect Immun. 2002;70:3026–32. doi: 10.1128/IAI.70.6.3026-3032.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dlugovitzky D, TorresMorales A, Rateni L, Farroni MA, Largacha C, Molteni O, Bottasso O. Circulating profile of Th1 and Th2 cytokines in tuberculosis patients with different degrees of pulmonary involvement. Fems Immunol Med Microbiol. 1997;18:203–7. doi: 10.1111/j.1574-695X.1997.tb01046.x. [DOI] [PubMed] [Google Scholar]
- 27.Goff WL, Johnson WC, Parish SM, Barrington GM, Elsasser TH, Davis WC, Valdez RA. IL-4 and IL-10 inhibition of IFN-γ- and TNF-α-dependent nitric oxide production from bovine mononuclear phagocytes exposed to Babesia bovis merozoites. Vet Immunol Immunopathol. 2002;84:237–51. doi: 10.1016/s0165-2427(01)00413-5. [DOI] [PubMed] [Google Scholar]
- 28.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rojas RE, Balaji KN, Subramanian A, Boom WH. Regulation of human CD4(+) αβ T-cell-receptor-positive (TCR+) and γδ TCR+ T-cell responses to Mycobacterium tuberculosis by interleukin-10 and transforming growth factor β. Infect Immun. 1999;67:6461–72. doi: 10.1128/iai.67.12.6461-6472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Y, Zhang M, Hofman FM, Gong J, Barnes PF. Absence of a prominent Th2 cytokine response in human tuberculosis. Infect Immun. 1996;64:1351–6. doi: 10.1128/iai.64.4.1351-1356.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy HE, Welsh MD, Bryson D, Cassidy J, Forster FI, Howard CJ, Collins RA, Pollock JM. Modulation of immune responses to Mycobacterium bovis in cattle depleted of WC1+γδ T cells. Infect Immun. 2002;70:1488–500. doi: 10.1128/IAI.70.3.1488-1500.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhodes SG, Palmer N, Graham SP, Bianco AE, Hewinson RG, Vordermeier HM. Distinct response kinetics of gamma interferon and interleukin-4 in bovine tuberculosis. Infect Immun. 2000;68:5393–400. doi: 10.1128/iai.68.9.5393-5400.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fenhalls G, Stevens L, Bezuidenhout J, Amphlett GE, Duncan K, Bardin P, Lukey PT. Distribution of IFN-γ, IL-4 and TNF-α protein and CD8 T cells producing IL-12p40 mRNA in human lung tuberculous granulomas. Immunology. 2002;105:325–35. doi: 10.1046/j.1365-2567.2002.01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fenhalls G, Wong A, Bezuidenhout J, van Helden P, Bardin P, Lukey PT. In situ production of gamma interferon, interleukin-4, and tumor necrosis factor alpha mRNA in human lung tuberculous granulomas. Infect Immun. 2000;68:2827–36. doi: 10.1128/iai.68.5.2827-2836.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–18. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs M, Fick L, Allie N, Brown N, Ryffel B. Enhanced immune response in Mycobacterium bovis Bacille Calmette Guerin (BCG) -infected IL-10-deficient mice. Clin Chem Lab Med. 2002;40:893–902. doi: 10.1515/CCLM.2002.158. [DOI] [PubMed] [Google Scholar]
- 37.Feng CG, Kullberg MC, Jankovic D, Cheever AW, Caspar P, Coffman RL, Sher A. Transgenic mice expressing human interleukin-10 in the antigen-presenting cell compartment show increased susceptibility to infection with Mycobacterium avium associated with decreased macrophage effector function and apoptosis. Infect Immun. 2002;70:6672–9. doi: 10.1128/IAI.70.12.6672-6679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuo WB, Estes DM, Brown WC. Comparative effects of interleukin-12 and interleukin-4 on cytokine responses by antigen-stimulated memory CD4(+) T cells of cattle: IL-12 enhances IFN-gamma production, whereas IL-4 has marginal effects on cytokine expression. J Interferon Cytokine Res. 1999;19:741–9. doi: 10.1089/107999099313587. [DOI] [PubMed] [Google Scholar]
- 39.Giambartolomei GH, Dennis VA, Lasater BL, Murthy PK, Philipp MT. Autocrine and exocrine regulation of interleukin-10 production in THP-1 cells stimulated with Borrelia burgdorferi lipoproteins. Infect Immun. 2002;70:1881–8. doi: 10.1128/IAI.70.4.1881-1888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirsch CS, Toossi Z, Othieno C, et al. Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis. 1999;180:2069–73. doi: 10.1086/315114. [DOI] [PubMed] [Google Scholar]
- 41.Palmer MV, Whipple DL, Olsen SC, Jacobson RH. Cell mediated and humoral immune responses of white-tailed deer experimentally infected with Mycobacterium bovis. Res Vet Sci. 2000;68:95–8. doi: 10.1053/rvsc.1999.0324. [DOI] [PubMed] [Google Scholar]
- 42.Garraud O, Nutman TB. The role of cytokines in human B-cell differentiation into immunoglobulin-secreting cells. Bull Inst Pasteur. 1996;94:285–309. [Google Scholar]
- 43.Hussain R, Dawood G, Abrar N, Toossi Z, Minai A, Dojki M, Ellner JJ. Selective increases in antibody isotypes and immunoglobulin-G subclass responses to secreted antigens in tuberculosis patients and healthy household contacts of the patients. Clin Diagn Laboratory Immunol. 1995;2:726–32. doi: 10.1128/cdli.2.6.726-732.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erb KJ, Kirman J, Delahunt B, Moll H, Le Gros G. Infection of mice with Mycobacterium bovis-BCG induces both Th1 and Th2 immune responses in the absence of interferon-gamma signalling. Euro Cytokine Netw. 1999;10:147–53. [PubMed] [Google Scholar]
- 45.Hussain R, Shiratsuchi H, Ellner JJ, Wallis RS. PPD-specific IgG1 antibody subclass upregulate tumour necrosis factor expression in PPD-stimulated monocytes: possible link with disease pathogenesis in tuberculosis. Clin Exp Immunol. 2000;119:449–55. doi: 10.1046/j.1365-2249.2000.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ng KH, Watson JD, Prestidge R, Buddle BM. Cytokine mRNA expressed in tuberculin skin test biopsies from BCG-vaccinated and Mycobacterium bovis inoculated cattle. Immunol Cell Biol. 1995;73:362–8. doi: 10.1038/icb.1995.55. [DOI] [PubMed] [Google Scholar]
- 47.Jiao XN, Lo-Man R, Winter N, Deriaud E, Gicquel B, Leclerc C. The shift of Th1 to Th2 immunodominance associated with the chronicity of Mycobacterium bovis bacille Calmette-Guerin infection does not affect the memory response. J Immunol. 2003;170:1392–8. doi: 10.4049/jimmunol.170.3.1392. [DOI] [PubMed] [Google Scholar]
- 48.Collins HL, Kaufmann SHE. Acquired immunity against bacteria. In: Kaufmann SHE, Sher A, Ahmed R, editors. Immunology of Infectious Diseases. Washington, D.C.: ASM Press; 2002. pp. 207–21. [Google Scholar]
- 49.Stenger S, Modlin R. Pathology and pathogenesis of bacterial infections. In: Kaufmann SHE, Sher A, Ahmed R, editors. Immunology of Infectious Diseases. Washington, D.C.: ASM Press; 2002. pp. 291–92. [Google Scholar]
- 50.Dahl KE, Shiratsuchi H, Hamilton BD, Ellner JJ, Toossi Z. Selective induction of transforming growth factor beta in human monocytes by lipoarabinomannan of Mycobacterium tuberculosis. Infect Immun. 1996;64:399–405. doi: 10.1128/iai.64.2.399-405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toossi Z, Gogate P, Shiratsuchi H, Young T, Ellner JJ. Enhanced production of TGF-β by blood monocytes from patients with active tuberculosis and presence of TGF-β in tuberculous granulomatous lung lesions. J Immunol. 1995;154:465–73. [PubMed] [Google Scholar]
- 52.Hirsch CS, Hussain R, Toossi Z, Dawood G, Shahid F, Ellner JJ. Cross-modulation by transforming growth factor beta in human tuberculosis: suppression of antigen-driven blastogenesis and interferon gamma production. Proc Natl Acad Sci USA. 1996;93:3193–8. doi: 10.1073/pnas.93.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]