Abstract
The role of chemokines and their interactions with extracellular matrix components (ECM) or the capacity of T cells to migrate into and accumulate within three-dimensional (3D) collagen type 1 substrata was studied. We examined the influence of chemokines and fibronectin on the infiltration properties of non-infiltrative (do not migrate into 3D substrata) and spontaneously infiltrative (migrate into 3D substrata) T-cell lines. Infiltrative and non-infiltrative T-acute lymphocytic leukaemic cell lines exhibited no consistent differences with respect to the expression of various chemokine receptors or β1-integrins. Chemokines presented inside the collagen increased the depth of migration of infiltrative T-cell lines, but did not render non-infiltrative T-cell lines infiltrative, although they augmented the attachment of non-infiltrative T-cell lines to the upper surface of the collagen. The presence of fibronectin inside the collagen did not render non-infiltrative T-cell lines infiltrative, but markedly augmented the migration of ‘infiltrative’ T-cell lines into collagen. Both infiltrative and non-infiltrative T-cell lines showed migratory responses to chemokines in Boyden assays (migration detected on 2D substrata). These results indicate that the process of T-cell infiltration/migration into 3D substrata depends on a tissue penetration mechanism distinguishable from migration on 2D substrata and that the basic capacity of T cells to infiltrate is independent of chemokines and ECM components applied as attractants.
Keywords: cell adhesion, cell migration, cell motility, chemokines, extracellular matrix, infiltration, T-leukaemia cells
Introduction
T-cell infiltration may be defined as migration and accumulation in vivo within tissues, or in vitro migration into and within three-dimensional (3D) substrata such as collagen type 1 matrices. T lymphocytes exhibit extensive infiltration of various tissues during diseases of autoimmune and allergic origin. T lymphocytes also infiltrate tissues during rejection of foreign grafts or after neoplastic transformation. The immunosurveillance function of T lymphocytes to infectious agents and neoplastic cells probably depends on the fact that the cells can recirculate and migrate within tissues in an ‘infiltrative’ manner. The mechanisms that mediate and regulate T-cell migration/infiltration into 3D substrata, including differences compared to migration on two-dimensional (2D) substrata, are not understood. This applies particularly to the frequently used 3D collagen invasion assay versus the conventional chemotactic or haptotactic 2D Boyden assay.
Lymphocytes enter tissues by recognition of endothelial ligands via a selection of selectins, integrins and other components.1–4 The penetration of subendothelial basal lamina and extracellular matrix (ECM) is an important step in the extravasation and migration of lymphocytes in tissues.5–7 Although T lymphocytes have been extensively studied with respect to various aspects of motility and adhesive interactions with endothelial cells and ECM components, the understanding of the mechanisms of T-cell infiltration and its regulation is far from complete.8,9 It is therefore important to study, in greater detail, endogenous T-cell factors as well as environmental factors of possible importance for T-lymphocyte infiltration.
ECM-degrading enzymes are important tools facilitating the infiltration of non-lymphoid tumour cells such as carcinomas.10,11 In analogy with the role of matrix metalloproteinases (MMP) that are capable of degrading collagen type IV for the extravasation of metastatic tumour cells, ECM-degrading enzymes are thought to play a role in the infiltration of T lymphocytes.12 Thus, T lymphocytes have been reported to secrete the matrix metalloproteinases MMP-2 and MMP-9 as a consequence of interaction with the integrin ligand vascular cell adhesion molecule-1 (VCAM-1) and exposure to chemokines.13,14 Inhibitors of MMPs have also been reported to interfere with the penetration of T cells through artificial 3D ECM substrata,15,16 but failure to inhibit T-cell migration into such a substrate (Matrigel) with MMP inhibitors has also been reported.8,9
Chemokines constitute a large group of low-molecular-weight (8000–20 000) secreted molecules currently classified into four groups regulating the trafficking of leucocytes to normal and inflamed sites.17–27 Chemokines deliver their activity by interacting with cell surface-expressed chemokine receptors that belong to the family of seven transmembrane domain G-protein-coupled receptors.28,29 The engagement of a chemokine receptor by a chemokine ligand leads to the activation of phospholipase C, which generates inositol triphosphate and diasylglycerol, which in turn generates elevated levels of intracellular Ca2+ and activation of protein kinase C.30 Binding of chemokines to their receptors leads to receptor internalization, receptor desensitization and, in some cases, even cross-desensitization.31,32 Chemokines and their receptors are critical elements for controlling lymphocyte migration and localization, and leucocytes can respond sequentially to chemokines.33
Lymphocyte migration into 3D substrata, such as collagen type I gels, has been proposed to be independent of adhesive interactions.34–36 However, it is reasonable to assume that migration within a complex 3D ECM substratum would be influenced by adhesion to components, such as fibronectin, to which lymphocytes can bind via multiple β1-integrins. Furthermore, integrins are potent triggering receptors for T-lymphocyte motility, and function as mechanical connectors and physiological signal transducers between the cell exterior and the cytoskeleton.37,38 Besides adhesion receptors, chemokines have been studied quite extensively to determine their role in lymphocyte migration, including the migration of T cells.39–41 Although chemokines have been applied to stimulate T-cell migration in numerous studies, only a few studies have examined the influence of chemokines on the basic capacity of lymphocytes to migrate into 3D substrata. Such studies are highly relevant with respect to the understanding of T-cell infiltration of tissues in inflammatory diseases of autoimmune and allergic origin, as well as in graft rejection, and for the possibility of developing new therapeutic methods for these conditions.
Chemokines have been shown to activate MMPs, presumably to create a path for infiltrating T cells.20,42 In the present investigation we analysed the role of chemokines and adhesive interactions with ECM components for the capacity of T cells to infiltrate. We used collagen type 1 gels as model substrata, taking advantage of the fact that T-acute lymphocytic leukaemia cell lines differ markedly with respect to the capacity to infiltrate. Furthermore, we determined chemokine receptor expression in spontaneously infiltrative and non-infiltrative T-cell lines, respectively, as well as in normal T cells. We also studied whether chemokines could render non-infiltrative T-cell lines infiltrative. Collagen type I gels are relatively ‘non-adhesive’ in terms of participation of β1-integrins in migration.36 To analyse the possible influence of adhesive interactions on T-cell migration, we therefore added fibronectin to the collagen gels. Although both chemokines and fibronectin stimulated the migration of ‘infiltrative’ T-cell lines into 3D substrata, they did not render T cells without such competence capable of migrating in a 3D manner.
Materials and methods
Cell preparations and culture conditions
Mononuclear cells [peripheral blood lymphocytes (PBL)] were isolated from healthy blood donors by centrifuging heparinized venous blood on a Ficoll gradient (Lymphoprep, Nycomed, Oslo, Norway). Recovered cells in the interface were carefully washed in phosphate-buffered saline (PBS) and the remaining erythrocytes were lysed in a buffer containing 0·15 m NH4Cl, 0·01 m KHCO3 and 0·1 m EDTA. The remaining mononuclear cells were then treated with carbonyl iron, and phagocytic cells were removed by a magnet. Recovered cells were resuspended in RPMI 1640 (Life Technologies Inc., Paisley, UK) supplemented with 2 mm l-glutamine, 0·16% (w/v) sodium bicarbonate, 100 IU/ml benzyl penicillin, 100 g/ml streptomycin and 10% fetal calf serum (FCS). The cells were cultured in Nunclon Bottles (Nunc, Delta, Denmark) in a humidified CO2 incubator at 37°, and the cell infiltration assays were performed the following day. A total of 91–100% of the PBLs infiltrating collagen type I were CD3 positive. The T-cell lines CCRF HSB2, CCRF CEM, MOLT4, Jurkat, PEER and P30 are derived from patients with acute lymphoblastic leukaemia (ALL), and Hut 78 is a Sézary cell line (American Type Culture Collection, Manassas, VA). The T-leukaemia cell lines were cultured in complete RPMI supplemented with 10% (v/v) FCS, as described above.
Migration assays
Infiltration assays were performed on normal PBL and on T-leukaemia cell lines. Collagen type I was prepared as previously described,43 diluted in serum-free RPMI and PBS (8 : 1 : 1), and 300 µl was applied to a cell culture plate (24 wells, Ø 15·5 mm, Falcon). The gel was allowed to polymerize at 37° for 30–40 min, and 300 µl (1·0 × 106 cells/ml) of cell suspension was added to each well and incubated for 24 hr in a humidified CO2 incubator at 37°. The chemokines macrophage inflammatory protein 1β (MIP-1β), stromal-derived factor-1α (SDF-1α), interferon-γ-inducible 10 000 molecular weight protein (IP-10), and lymphotactin (50 ng/ml) were either co-polymerized in the gel or added to the cells 30 min before the assay, or both. Fibronectin (10 µg/ml) was either co-polymerized in the gels or allowed to bind to the collagen overnight. Plates were routinely fixed with 2·5% (v/v) glutaraldehyde and washed twice with PBS, except when used for immunocytochemistry (see below). Infiltration was evaluated in seven fixed positions in each well by the use of an inverted microscope (Axiovert 100; Zeiss, Wetzlar, Germany) and a digital depth meter (Heidenheim ND221; Dr. Johannes Heidenhaim GmbH, Traunreut, Germany). The cells were counted at every 10 µm throughout the depth of the gel. The results are given as mean number of cells ÷ HPF (200×) ± standard error of the mean (SEM). All experiments were performed in triplicate and repeated three times.
To identify PBL attaching to the collagen, cells were allowed to attach for 15 min and fixed in 2% (v/v) paraformaldehyde in PBS for 20 min. The cells were reacted with anti-CD3 immunoglobulin for 1 hr at room temperature. The reactivity of antibodies to the cells was assessed by using a biotin-avidin detection system (Vectastain ABC kit; Vector Laboratories, Inc., Burlingame, CA). The results were analysed by using light microscopy (Leica DMRB; Leica GmbH, Wetzlar, Germany).
Migration was also studied by using a modified Boyden chamber assay system (Neuro Probe, Cabin John, MA). Poly(vinyl pyrrolidone)-free polycarbonate filters with an 8-µm pore size (Porotics Co., Livermore, CA) were coated on the lower surface, overnight, with fibronectin (10 µg/ml) or collagen type I (40 µg/ml), subsequently washed in distilled water and air dried. The lower wells were filled with RPMI containing 10% (v/v) FCS. The upper wells were filled with 50 µl of cells (2 × 106 cells/ml) in RPMI containing 10% (v/v) FCS. The chambers were incubated for 5 hr in a humidified incubator at 37°. Following incubation, the filters were removed, fixed in methanol and stained with Giemsa (Reidel-de Haën, Seeize, Germany). The filters were subsequently placed onto glass slides and the remaining cells on the upper side of the filter were wiped off. The number of migrating cells was counted by light microscopy at a magnification of × 400. All experiments were repeated three times.
Fluorescence-activated cell sorter (FACS) analysis
The experiments were performed on both PBL and leukaemic T-cell lines. The flow cytometry was performed in microtitre plates with U-shaped wells and with 250 000 cells in 25 µl of FACS buffer [Tris Hank's + 0·2% (v/v) human serum albumin + 0·02% (w/v) NaN3], per well. After blocking with goat gamma globulin (3 µl/106 cells) for 30 min at room temperature and centrifugation at 500 g, the supernatant was removed. The cells were diluted in FACS buffer mixed with 2 µl of RPE-conjugated monoclonal antibodies (mAb) against CXCR-1, -2, -3, -4, -5 and CCR-1, -2, -3, -5, -6 (500 µg/ml) (R & D Systems, Abingdon, UK), and the samples were incubated for 30 min on ice with slow shaking. After this incubation, the cells were washed with 100 µl/well of FACS buffer and with 50 µl of FCS at the bottom of each well. The plates were centrifuged at 800 g for 10 min at 4° and the supernatant was removed. Dead cells were excluded by using propidium iodide. In the experiments with PBL, the T lymphocytes were also mixed with 2 µl of fluorescein isothiocyanate (FITC)-conjugated mAb against CD4 and incubated for 30 min on ice with slow shaking. Subsequently, 100 µl of FACS buffer and 50 µl of FCS were added to the bottom of each well, the plates were centrifuged at 800 g for 10 min at 4° and the supernatant was removed. All samples were then diluted in FACS buffer and the cell-bound fluorescence was determined in a FACScan (Becton Dickinson, Mountain View, CA). All analyses were repeated three times.
Statistical methods
The findings were analysed by using two-way analysis of variance (anova) with repeated measures. Post hoc comparison of means was performed by using the Tukey HSD test.
Results
Capacity of various T-cell lines to migrate into 3D substrata
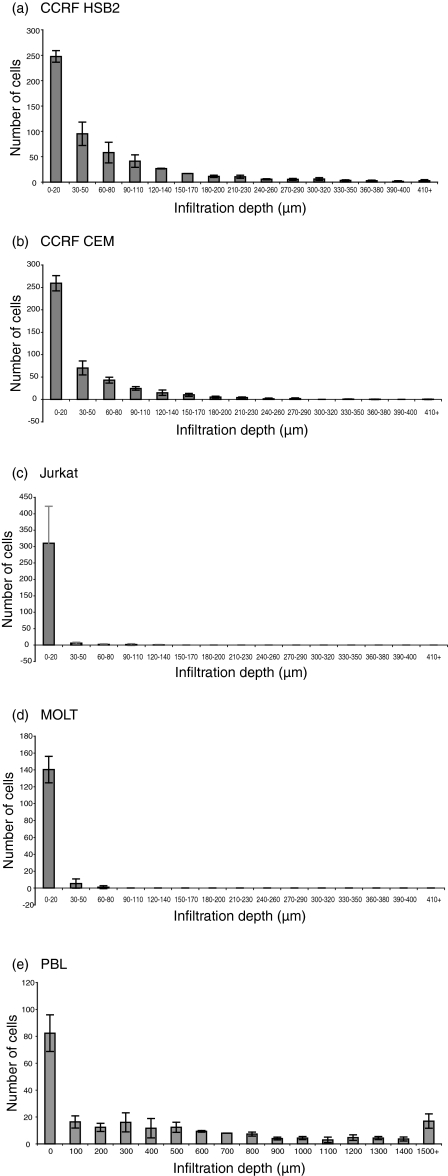
Various T-leukaemia cell lines and blood T cells were compared with respect to their capacity to migrate into/infiltrate a type 1 collagen matrix in the absence of serum or other attractants. The presence of cells on top of the collagen (0–20 µm) is defined as attachment, while the presence of cells below this level is defined as infiltration. The cell lines CCRF HSB2 and CCRF CEM infiltrated > 410 µm (leading front) during a 24-hr time-period (Fig. 1a, 1b) while Jurkat infiltrated to a minor extent, or not at all, during the same period (Fig. 1c). The cell lines MOLT4 (Fig. 1d), Peer (not shown) and P30 (not shown) showed no infiltration into the collagen, although they attached to the upper surface of the collagen to the same extent as the infiltrating cell lines. Furthermore, the number of cells of P30 attaching to the collagen was higher than in all other cell lines, showing that attachment to the collagen and infiltration are distinguishable events. Normal T cells showed a pronounced capacity to infiltrate collagen (Fig. 1e).
Figure 1.
Infiltration capacity distinguishes separate T-cell lines. The figure shows a comparison of the infiltration of the leukaemic T-cell lines CCRF HSB2, CCRF CEM, Jurkat, MOLT4 and normal blood T lymphocytes [peripheral blood lymphocytes (PBL): 92–100% of the PBL cells attaching the collagen were identified as CD3 positive by immunocytochemistry] into collagen type I gels. For each cell line, adhesion and infiltration into the collagen were determined at 10-µm intervals (PBL at 100-µm intervals) by the use of an inverted microscope (magnification × 200) and a digital depth meter. The cell lines CCRF HSB2 and CCRF CEM infiltrated > 410 µm (leading front) over a 24-hr time-period (a) and (b), while Jurkat infiltrated to a minor extent, or not at all, during the same period (c). The cell line MOLT4 (d) showed no infiltration into the collagen, although the cells attached to the upper surface of the collagen to the same extent as the infiltrating cell lines. Normal T cells showed a pronounced capacity to infiltrate collagen (e). The figure shows mean values ± standard error of the mean (SEM) (expressed as mean number of cells per cm2) of seven positions after 24 hr in three single wells.
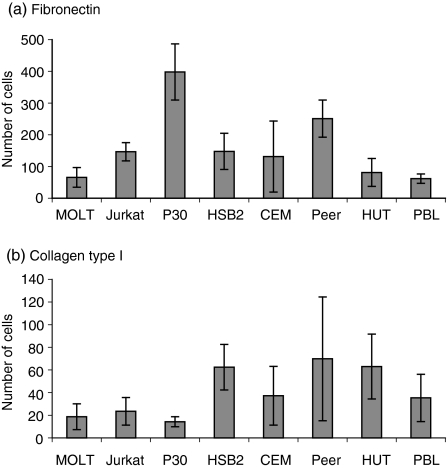
To elucidate the possibility to include ‘adhesive’ ECM components as attractants in the collagen, the adhesion of different T-cell lines to ECM components was studied. As shown in Table 1, all T-cell lines had fibronectin-binding integrins and attached to fibronectin (not shown), with one exception (HUT 78). Therefore, fibronectin was used as an attractant in the collagen gels.
Table 1.
Fluorescein isothiocyanate (FACS) analysis of β1-intregrins in T-leukaemia cell lines*
| T-cell line | CD49a | CD49b | CD49c | CD49d | CD49e | CD49f | CD29 |
|---|---|---|---|---|---|---|---|
| P30 | 0 | 2 | 100 | 100 | 100 | 90 | 100 |
| CCRF-CEM | 7 | 3 | 98 | 98 | 98 | 34 | 99 |
| Molt-4 | 15 | 9 | 84 | 99 | 99 | 31 | 99 |
| Jurkat | 58 | 20 | 97 | 99 | 99 | 34 | 99 |
| CCRF-HSB2 | 5 | 94 | 100 | 92 | 100 | 83 | 100 |
| Peer | 0 | 55 | 99 | 99 | 4 | 86 | 95 |
| HUT-78 | 0 | 0 | 99 | 99 | 99 | 98 | 96 |
Expression of different molecules involved in adhesion, as analysed by FACscan (% positive cells). There was no obvious correlation between the expression of β 1-integrins and cell behaviour in adhesion, migration and infiltration experiments. The figures show representative data of two to three experiments with the different cell lines.
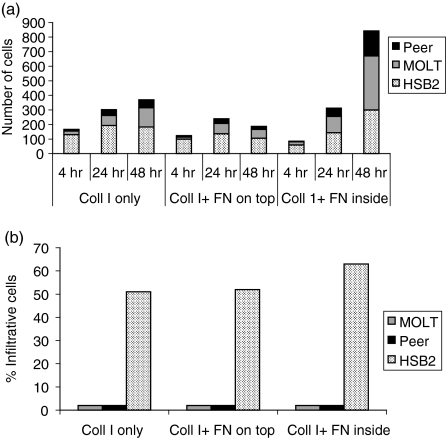
It can be seen in Fig. 2 that the presence of fibronectin in the collagen did not render the non-infiltrative T-cell lines Peer and MOLT infiltrative, although it increased the number of cells on top of the collagen. In contrast, fibronectin present inside the collagen markedly increased the number of cells migrating into the collagen of the infiltrative T-cell line CCRF HSB2, while fibronectin on top of the collagen seemed to interfere with attachment of the same cell line. The results presented in Fig. 2 demonstrate that an adhesive component inside the collagen does not change the infiltrative behaviour of the cells. These results further support the conclusion that the T-cell lines either attach to the upper surface or migrate into the collagen and that there is no direct relationship between cell attachment to the upper surface of the collagen and infiltration.
Figure 2.
The influence of fibronectin (FN), present inside and on top of collagen type I gels (Coll I), on T-cell attachment to the gel (a) and on infiltration into the gel (b). The attachment refers to all cells throughout the collagen per microscope field (× 200) (a), and infiltrative cells refer to the percentage of cells migrating > 20 µm into the collagen gel (b). The presence of fibronectin in the collagen did not render the non-infiltrative T-cell lines Peer and MOLT infiltrative, although it increased the number of cells on top of the collagen. In contrast, fibronectin present inside the collagen markedly increased the number of cells migrating into the collagen in the infiltrative T-cell line, CCRF HSB2. The results shown in this figure demonstrate that an adhesive component inside the collagen does not change the infiltrative behaviour of the cells. The figure shows the results of one representative experiment.
The infiltrative and non-infiltrative T-cell lines were also compared with respect to haptotactic migration to fibronectin and collagen type I in Boyden chambers. The haptotactic Boyden assays detected no consistent differences in migration capacity between infiltrative and non-infiltrative T-cell lines (Fig. 3). Furthermore, the non-infiltrating T-cell line, Peer, migrated on both collagen and fibronectin. Normal PBL, which showed pronounced infiltration into collagen, showed the same or a somewhat lower magnitude of hapotactic migration as the T-cell lines. It follows that the different capacity of the T cells to migrate within a 3D substrate does not correlate with their capacity to migrate in a 2D migration assay.
Figure 3.
Migration of T-cell lines and peripheral blood lymphocytes (PBL) through Boyden filters coated on the lower side with fibronectin (10 µg/ml) (a) or with collagen type I (40 µg/ml) (b). The Boyden assays detected no consistent differences in migration capacity between infiltrative (CCRF HSB2, CCRF CEM) and non-infiltrative (MOLT, Jurkat, P30) T cells. Furthermore, the T-cell line Peer migrated on both collagen and fibronectin, despite its failure to infiltrate. Normal PBL, which showed pronounced infiltration into collagen (Fig. 1e), showed the same, or a somewhat lower, magnitude of hapotactic migration than the T-cell lines. The number of migrating cells represents the mean number ± standard error of the mean (SEM) per field of six microscopic fields (magnification × 400).
It can be seen, in Table 1, that the expression of β1-integrin α-chains, as well as the β1-chain, varies among the separate cell lines. However, it is obvious that differences in the expression of the collagen type I-binding integrins α1 and α2 can not account for the fact that CCRF CEM, CCRF HSB2 and HUT migrated into collagen matrices, while MOLT, Jurkat, Peer and P30 did not.
Chemokine receptor expression of various T-cell lines and of normal T cells
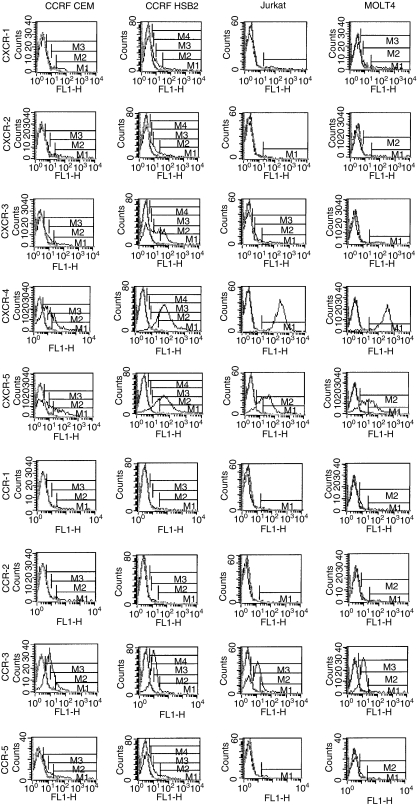
Figure 4 shows chemokine receptor expression of two infiltrative (CCRF CEM and CCRF HSB2), and two non-infiltrative (Jurkat and MOLT) T-cell lines. We also analysed the T-cell lines Peer and P30 with respect to expression of chemokine receptors; the results obtained were similar to those shown in Fig. 4 (data not shown). It can be seen, in Fig. 4, that the T-cell lines expressed a relatively broad repertoire of the chemokine receptors CXCR-1, CXCR-2, CXCR-3, CXCR-4, CXCR-5, CCR-1, CCR-2, CCR-3, CCR-5 and CCR-6, although the number of chemokine receptor-positive cells in each cell line differed markedly between the receptors. Certain receptors were more commonly expressed in all T-cell lines. A high percentage of all T-cell lines were CXCR-4, CCR-3 and CXCR-5 positive. The T-cell lines showed a variable expression of CXCR-1, CXCR-2, CXCR-3 and CCR-5, and a low expression of CCR-1, CCR-2 and CCR-6. Thus, we found no consistent differences in chemokine receptor expression between infiltrating and non-infiltrating T-cell lines.
Figure 4.
Chemokine receptor expression does not differ between infiltrating and non-infiltrating T cells. The figure shows flow cytometry analysis of the expression of chemokine receptors in two infiltrative (CCRF HSB2, CCRF CEM) and two non-infiltrative (MOLT4, Jurkat) T-leukaemia cell lines. A high percentage of the T-cell lines were CXCR-4, CCR-3 and CXCR-5 positive. The T-cell lines showed a variable expression of CXCR-1, CXCR-2, CXCR-3 and CCR-5, and a low expression of CCR-1, CCR-2 and CCR-6 (data not shown). The symbols M1-4 denote different comparisons of the area under the curves of the immunoglobulin G (IgG) control and the monoclonal antibodies to the chemokines, respectively.
The surface expression of chemokine receptors on T cells from 20 healthy blood donors was also analysed (results not shown). CXCR-4 and CCR-3 were present on the majority of blood T cells, similarly to the findings in T-cell lines (90% and 69%, respectively). CXCR-5, CCR-5 and CCR-6 were present on 10–50% of the T cells from different healthy individuals, while CXCR-1, CXCR-2, CXCR-3, CCR-1 and CCR-2 were present on 5–20% of the cells.
Chemokines stimulate migration in a 2D assay, but do not render ‘non-infiltrative’ T cells infiltrative
We have shown previously that the chemokines RANTES (regulated on activation, normal T-cell-expressed and secreted) and MIP-1β stimulate the migration of both infiltrative and non-infiltrative T cells on fibronectin and collagen type IV in chemotactic Boyden assays.44 This means that infiltrative and non-infiltrative T cells can respond to these chemokines under appropriate conditions, which is consistent with the findings above that all cell lines except P30 show a similar expression of various chemokine receptors.
Figure 5(a–d) shows the influence of the chemokine SDF-1α presented in different ways, on the invasion of collagen type I matrices by ‘non-infiltrative’ and ‘infiltrative’ T-cell lines, respectively. The chemokine, when present with the cells or inside the collagen, exerted a significant stimulatory effect on the adhesion of non-infiltrative T-cell lines to the upper surface of the collagen (Fig. 5c, 5d). Notably, there was no obvious stimulatory effect of the chemokine on the infiltration of non-infiltrative T cells into the collagen. In contrast, the chemokine further enhanced the infiltration into collagen of one of the ‘infiltrative’ T-cell lines (Fig. 5b). The chemokine did not stimulate infiltration when present with the cells on top of the collagen and was most effective when presented inside the collagen. The chemokine, when seeded on the opposite side of the collagen layer, only marginally affected the infiltration of infiltrative T-cell lines, and had no stimulatory effect on the infiltration of non-infiltrative T-cell lines (Fig. 6). Experiments with the chemokines MIP-1β, IP-10 and lymphotactin showed similar results (data not shown).
Figure 5.
Chemokines stimulate adhesion and migration, but do not render non-infiltrative T cells infiltrative. The figure shows the influence of stromal-derived factor-1α (SDF-1α) (50 ng/ml) on the adhesion and infiltration on/into collagen type I gels of infiltrative (CCRF HSB2, CCRF CEM) (a) and (b) and non-infiltrative (Jurkat, MOLT4) (c) and (d) T-cell lines. The chemokine was presented to the T cells in different ways: –/–, no chemokines; +/–, chemokines added in the cell culture medium; –/+, chemokines added in the collagen type I gel; and +/+, chemokines added both in the medium and in the gel. Adhesion to and infiltration into collagen was determined in three experiments (in seven positions per experiment) at every 10 µm throughout the gel (magnification × 200), and the figure shows the mean values ± standard error of the mean (SEM). SDF-1α increased the number of cells infiltrating > 60 µm into collagen of ‘infiltrative’ T-cell lines (CCRF HSB2, P < 0·05; CCRF CEM, P < 0·001) when presented in the gel, but the chemokine had no effect on infiltration when present with the cells (a) and (b). In contrast, SDF-1α exerted a significant stimulatory effect on the adhesion of non-infiltrative T-cell lines to the upper surface, comprising > 50 µm of the collagen, when present with the cells (MOLT4, P < 0·05) or in the gel (Jurkat, P < 0·05; MOLT4, P < 0·05), but the infiltrating capacity of the cells was not affected (c) and (d).
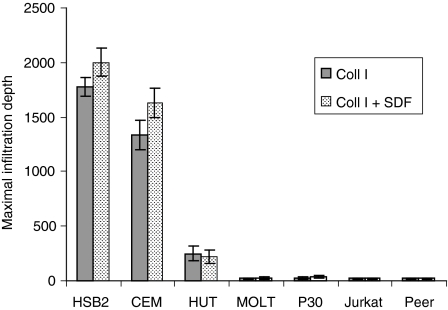
Figure 6.
The influence of stromal-derived factor-1α (SDF-1α) on the maximal infiltration depth of infiltrative and non-infiltrative T-cell lines when the chemokine was presented on the opposite side of the collagen layer at a concentration of 50 ng/ml. SDF-1α only marginally increased the infiltration of infiltrative T cells (CCRF HSB2, CCRF CEM, HUT), and had no stimulatory effect on the infiltration of non-infiltrative T-cell lines (MOLT, P30, Jurkat, Peer). The figure shows the mean maximal infiltration depth ± standard error of the mean (SEM) determined from 10 microscopic fields per cell line (× 100) of cells allowed to migrate for 24 hr.
In conclusion, our results imply that chemokines do not render non-infiltrative T-cell lines infiltrative, but can stimulate migration of the same T-cell lines in a 2D assay. On the other hand, chemokines can stimulate the infiltrative behaviour of T cells that are competent to infiltrate (Fig. 5b). These findings indicate that the basic infiltrative capacity of T cells is independent of chemokines.
Discussion
We have previously examined the T-cell lines, analysed here using 3D collagen, with respect to their capacity to migrate into Matrigel.8 The results of the previous and of the present study, when compared, show that the migration of individual T-cell lines into collagen and Matrigel, respectively, is virtually identical. Therefore, migration into these substrata seems to reflect the same functional property. Our findings show further that while the capacity to migrate on 2D substrata is a general property of T cells, the capacity to migrate within 3D substrata/infiltrate represents a distinct functional property. Consequently, T cells may show excellent migration on 2D substrata, but no infiltration/migration into 3D substrata. Fibronectin or chemokines, present as an attractant on the top of or inside the 3D collagen, did not change the basic behaviour of the T-cell lines as infiltrative or non-infiltrative. The fact that T-cell lines generally can be classified as either infiltrative or non-infiltrative on substrata that differ chemically shows that the capacity to migrate within 3D substrata/infiltrate represents a distinct functional property.
The findings of the present study indicate that the migration/infiltration of T cells into various organs, which is a hallmark of autoimmunity, allergy and graft rejection, is distinguishable from migration on 2D substrata with respect to the effect of chemokines and ECM components. Although chemokines play an important role for T-cell infiltration by stimulating and directing the migration of the T cells, it has not been elucidated how the interaction between chemokines and their receptors influences T-cell infiltration. There are few previous reports concerning chemokine effects on T-cell infiltration in the literature where infiltration can be distinguished from migration in a 2D manner. Chemokines stimulate MMP expression in T cells, which may be consistent with an augmentation of infiltration.20,42 However, the vast majority of published reports of chemokine effects on T-cell motility concerns migration in 2D assay systems, or have been performed in 3D systems by using cells that have an inherent infiltration capacity, even in the absence of chemokines. It is therefore important to define infiltration-specific experimental conditions in order to study endogenous T-cell factors, as well as environmental factors of possible importance for T-lymphocyte infiltration.
To elucidate the role of attractants (such as ECM components or chemokines and chemokine receptors) for the capacity of T lymphocytes to migrate/infiltrate into 3D substrata, we studied the effect of fibronectin and chemokines on the migration of spontaneously infiltrative and non-infiltrative T-cell lines on/within 2D and 3D substrata. The present experimental approach using T-cell lines with different competence to migrate/infiltrate into 3D substrata enables us to define the role of chemokines and ECM attractants for the infiltration of T cells.
None of the T-cell lines used in our investigation produced endogenous chemokines.44 It is therefore highly unlikely that T cells have an autocrine chemokine regulation of migration, although some other autocrine mechanism may play a critical role for their capacity to migrate.
Chemokines and fibronectin stimulated the migration of both infiltrative and non-infiltrative T-cell lines, but did not render non-infiltrative T-cell lines infiltrative. Chemokines and fibronectin stimulated the attachment of both infiltrative and non-infiltrative T cells to the upper surface of the collagen. It follows that attachment to a collagen matrix, migration on 2D collagen and migration/infiltration into the same collagen in 3D form probably represent distinct functions. These results indicate that the basic capacity of T cells to infiltrate is independent of chemokines or adhesive ECM components. Chemokines and adhesive ECM components can, however, stimulate infiltration of T cells that possess the capacity to migrate into a 3D substrate.
One major conclusion from the fact that chemokines do not endow T cells with competence to migrate into 3D substrata/infiltrate is that inhibition of chemokine activity will not prevent cells from infiltrating. However, inhibition of chemokine action may be one way to abrogate the targeting of T cells to certain organs or sites. For example, there is evidence that T cells in synovial tissue show an up-regulation of CXCR-3 and CCR-5.45
The observation that the infiltrative capacity of T-cell lines essentially is independent of chemokines and chemokine receptors, although chemokines can increase the distance of migration of lines that are competent to infiltrate, is interesting and merits further investigation and analysis. Noteworthy is the fact that normal CD4+ T cells, which have a pronounced capacity to infiltrate collagen, show an expression of chemokine receptors which is similar to that of T-leukaemia cell lines. The capacity to infiltrate/migrate into 3D substrata may reflect several interesting, to date undiscovered, properties of the plasma membrane, such as high receptor turnover or expression of matrix-degrading metalloproteinases.16,20 However, the role of matrix-degrading metalloproteinases in 3D T-cell migration has recently been challenged,9 suggesting that other factors determine this function.
The present results demonstrate that chemokines, independent of presentation with the cells or within the collagen, stimulate attachment of non-infiltrative T cells to the upper surface of a collagen matrix. This stimulatory effect of chemokines is consistent with previous findings that various chemokines stimulate T-cell binding to ECM components.17,46
In conclusion, chemokines or adhesive components seem to function as regulators of adhesion and migration of T cells on/to components of the ECM, but they are not of critical importance for the basic capacity of T cells to infiltrate, although they may target infiltrating T cells to specific organs, or sites within organs.
Acknowledgments
This work was supported by grants from the Swedish Cancer Society (project 1940) and the Childrens' Cancer Foundation (project 02/008).
Abbreviations
- ALL
acute lymphocytic leukaemic
- CCR
CC chemokine receptor
- CXCR
CXC chemokine receptor
- 2D
two dimensional
- 3D
three dimensional
- ECM
extracellular matrix
- FACS
fluorescence-activated cell sorter
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- IP-10
interferon-γ-inducible 10 000 molecular weight protein
- mAB
monoclonal antibody
- MIP-1β
macrophage inflammatory protein 1β
- MMP
matrix metalloproteinase
- PBL
peripheral blood lymphocytes
- PBS
phosphate-buffered saline
- RANTES
regulated on activation, normal T-cell-expressed and secreted
- SDF-1α
stromal-derived factor-1α
- VCAM-1
vascular cell adhesion molecule-1
References
- 1.Alon R, Rossiter H, Wang X, Springer TA, Kupper TS. Distinct cell surface ligands mediate T lymphocyte attachment and rolling on P and E selectin under physiological flow. J Cell Biol. 1994;127:1485–95. doi: 10.1083/jcb.127.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hauzenberger D, Klominek J, Bergström SE, Sundqvist KG. T lymphocyte migration: the influence of interactions via adhesion molecules, the T cell receptor and cytokines. Crit Rev Immunol. 1995;15:285–316. doi: 10.1615/critrevimmunol.v15.i3-4.60. [DOI] [PubMed] [Google Scholar]
- 3.Bird IN, Spragg JH, Ager A, Matthews N. Studies of lymphocyte transendothelial migration: analysis of migrated cell phenotypes with regard to CD31 (PECAM-1), CD45RA and CD45RO. Immunology. 1993;80:553–60. [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–73. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 5.Brenner B, Gulbins E, Schlottmann K, et al. l-Selectin activates the Ras pathway via the tyrosine kinase p56lck. Proc Natl Acad Sci USA. 1996;93:15376–81. doi: 10.1073/pnas.93.26.15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu Y, Newman W, Tanaka Y, Shaw S. Lymphocyte interaction with endothelial cells. Immunol Today. 1992;13:106–12. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Frade JM, Vila-Coro AJ, Martin A, et al. Similarities and differences in RANTES- and (AOP)-RANTES-triggered signals: implications for chemotaxis. J Cell Biol. 1999;144:755–65. doi: 10.1083/jcb.144.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ivanoff A, Ivanoff J, Hultenby K, Sundqvist KG. Infiltrative capacity of T leukemia cell lines: a distinct functional property coupled to expression of matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinases-1 (TIMP-1) Clin Exp Metastasis. 1999;17:695–711. doi: 10.1023/a:1006749304315. [DOI] [PubMed] [Google Scholar]
- 9.Wolf K, Muller R, Borgmann S, Brocker E-B, Friedl P. Amoeboid shape change and contact guidance. T-lymphocyte crawling through fibrillar collagen is independent of matrix remodelling by MMPs and other proteases. Blood. 2003;102:3262–9. doi: 10.1182/blood-2002-12-3791. [DOI] [PubMed] [Google Scholar]
- 10.Höyhtyä M, Hujanen E, Turpeenniemi-Hujanen T, Thorgeirsson TU, Liotta LA, Tryggvason K. Modulation of type-IV collagenase activity and invasive behaviour of metastatic human melanoma (A2058) cells in vitro by monoclonal antibodies to type-IV collagenase. Int J Cancer. 1990;46:282–6. doi: 10.1002/ijc.2910460224. [DOI] [PubMed] [Google Scholar]
- 11.MacDougall JR, Matrisian LM. Contributions of tumor and stromal matrix metalloproteinases to tumor progression, invasion and metastasis. Cancer Metastasis Rev. 1995;14:351–62. doi: 10.1007/BF00690603. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery AM, Sabzevari H, Reisfeld RA. Production and regulation of gelatinase B by human T-cells. Biochim Biophys Acta. 1993;1176:265–8. doi: 10.1016/0167-4889(93)90054-s. [DOI] [PubMed] [Google Scholar]
- 13.Romanic AM, Madri JA. The induction of 72-kD gelatinase in T cells upon adhesion to endothelial cells is VCAM-1 dependent. J Cell Biol. 1994;125:1165–78. doi: 10.1083/jcb.125.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia M, Leppert D, Hauser SL, Sreedharan SP, Nelson PJ, Krensky AM, Goetzl EJ. Stimulus specificity of matrix metalloproteinase dependence of human T cell migration through a model basement membrane. J Immunol. 1996;1:160–7. [PubMed] [Google Scholar]
- 15.Leppert D, Hauser S, Kishiyama J, An S, Zeng L, Goetzl E. Stimulation of matrix metalloproteinase-dependent migration of T cells by eicosanoids. FASEB J. 1995;9:1473–81. doi: 10.1096/fasebj.9.14.7589989. [DOI] [PubMed] [Google Scholar]
- 16.Leppert D, Waubant E, Galardy R, Bunnett N, Hauser S. T cell gelatinases mediate basement membrane transmigration in vitro. J Immunol. 1995;154:4379–89. [PubMed] [Google Scholar]
- 17.Schall TJ, Bacon K, Camp RD, Kaspari JW, Goeddel DV. Human macrophage inflammatory protein α (MIP-1α) and MIP-1α chemokines attract distinct populations of lymphocytes. J Exp Med. 1993;177:1821–6. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M Moser. B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:799–802. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taub DD, Conlon K, Lloyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1α and MIP-1α. Science. 1993;260:355–8. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 20.Bacon KB, Szabo MC, Yssel H, Bolen JB, Schall TJ. RANTES induces tyrosine kinase activity of stably complexed p125FAK and ZAP-70 in human T cells. J Exp Med. 1996;184:873–82. doi: 10.1084/jem.184.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelner GS, Kennedy J, Bacon KB, et al. Lymphotactin: a cytokine that represents a new class of chemokine. Science. 1994;266:1395–9. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- 22.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–71. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 23.Pleass R, Camp R. Cytokines induce lymphocyte migration in vitro by direct, receptor-specific mechanisms. Eur J Immunol. 1994;24:273–6. doi: 10.1002/eji.1830240142. [DOI] [PubMed] [Google Scholar]
- 24.Strieter RM, Standiford TJ, Huffnagle GB, Bolen JB, Schall TJ. ‘The good, the bad, and the ugly.’ The role of chemokines in models of human disease. J Immunol. 1996;156:3583–6. [PubMed] [Google Scholar]
- 25.Cai J-P, Hudson S, Ye MW, Chin YH. The intracellular signalling pathways involved in MCP-1-stimulated T cell migration across microvascular endothelium. Cell Immunol. 1996;167:269–75. doi: 10.1006/cimm.1996.0035. [DOI] [PubMed] [Google Scholar]
- 26.Bacon KB, Flores-Romo L, Life PF, et al. IL-8-induced signal transduction in T lymphocytes involves receptor-mediated activation of phospholipases C and D. J Immunol. 1995;154:3654–66. [PubMed] [Google Scholar]
- 27.Amara A, Gall SL, Schwartz O, et al. HIV coreceptor downregulation as antiviral principle: SDF-1α-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–46. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantrell D. G proteins in lymphocyte signalling. Curr Opin Immunol. 1994;6:380–4. doi: 10.1016/0952-7915(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 29.Bohm SK, Grady EF, Bunnett NW. Regulatory mechanisms that modulate signalling by G-protein-coupled receptors. Biochemistry. 1997;322:1–18. doi: 10.1042/bj3220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu D, LaRosa GJ, Simon MI. G protein-coupled signal transduction pathways for interleukin-8. Science. 1993;261:101–3. doi: 10.1126/science.8316840. [DOI] [PubMed] [Google Scholar]
- 31.Aramori I, Zhang J, Ferguson SSG, Bieniasz PD, Cullen BR, Caron MG. Molecular mechanism of desensitization of the chemokine receptor CCR-5: receptor signaling and internalization are dissociable from its role as an HIV-1 co-receptor. EMBO J. 1997;16:4606–16. doi: 10.1093/emboj/16.15.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson RM, Pridgen BC, Haribabu B, Snyderman R. Regulation of the human chemokine receptor CCR1. Cross-regulation by CXCR1 and CXCR2. J Biol Chem. 2000;275:9201–8. doi: 10.1074/jbc.275.13.9201. [DOI] [PubMed] [Google Scholar]
- 33.Foxman EF, Campbell JJ, Butcher EC. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J Cell Biol. 1997;139:1349–60. doi: 10.1083/jcb.139.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haston WS, Shields JM, Wilkinson PC. Lymphocyte locomotion and attachment on two-dimensional surfaces and in three-dimensional matrices. J Cell Biol. 1982;92:747–52. doi: 10.1083/jcb.92.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schor SL, Allen TD, Winn B. Lymphocyte migration into three-dimensional collagen matrices: a quantitative study. J Cell Biol. 1983;96:1089–96. doi: 10.1083/jcb.96.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedl P, Entschladen F, Conrad C, Niggemann B, Zanker KS. CD4+ T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize beta1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur J Immunol. 1998;28:2331–43. doi: 10.1002/(SICI)1521-4141(199808)28:08<2331::AID-IMMU2331>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 37.Volkov Y, Long A, McGrath S, Ni Eidin D, Kelleher D. Crucial importance of PKC-beta (I) in LFA-1-mediated locomotion of activated T cells. Nat Immunol. 2001;2:508–14. doi: 10.1038/88700. [DOI] [PubMed] [Google Scholar]
- 38.Hauzenberger D, Klominek J, Holgersson J, Bergstrom SE, Sundqvist KG. Triggering of motile behavior in T lymphocytes via cross-linking of alpha 4 beta 1 and alpha L beta 2. J Immunol. 1997;158:76–84. [PubMed] [Google Scholar]
- 39.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farber J. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61:246–57. [PubMed] [Google Scholar]
- 41.Biddison WE, Taub DD, Cruikshank WW, Center DM, Connor EW, Honma K. Chemokine and matrix metalloproteinase secretion by myelin proteolipid protein-specific CD8+ T cells: potential roles in inflammation. J Immunol. 1997;158:3046–53. [PubMed] [Google Scholar]
- 42.Robinson SC, Scott KA, Balkwill FR. Chemokine stimulation of monocyte matrix metalloproteinase-9 requires endogenous TNF-α. Eur J Immunol. 2002;32:404–12. doi: 10.1002/1521-4141(200202)32:2<404::AID-IMMU404>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 43.Sundqvist KG, Hauzenberger D, Hultenby K, Bergstrom SE. T lymphocyte infiltration of two- and three-dimensional collagen substrata by an adhesive mechanism. Exp Cell Res. 1993;206:100–10. doi: 10.1006/excr.1993.1125. [DOI] [PubMed] [Google Scholar]
- 44.Ivanoff J, Ivanoff A, Sundqvist KG. Defective chemokine production in T-leukemia cell lines and its possible functional role. Dev Immunol. 2000;7:67–75. doi: 10.1155/2000/28085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buckley CD, Amft N, Bradfield PF, et al. Persistent induction of the chemokine receptor CXCR4 by TGF-α1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J Immunol. 2000;165:3423–9. doi: 10.4049/jimmunol.165.6.3423. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka Y, Adams DH, Hubscher S, Hirano H, Siebenlist U, Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature. 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]