Abstract
Swine monocytes constitute a heterogeneous population of cells which can be divided into four subsets based on the expression of SWC3, CD14, CD163 and swine leucocyte antigen (SLA) DR markers. These subsets appear to represent different maturation stages in a pathway along which these cells up-regulate the expression of SLA DR and CD163 antigens and reduce that of CD14. Differences in the expression of adhesion and costimulatory molecules are also patent, with a progressive increase in the expression of CD11a, wCD11R1, CD29, CD49d, CD61, CD1a and CD80/86, and a concomitant decrease in that of wCD11R2. Besides, these subsets differ in their capacity for tumour necrosis factor-α (TNF-α) production in response to lipopolysaccharide + interferon-γ. The CD163+ CD14− SLA DR+ subset produces higher amounts of TNF-α than the CD163− CD14+ SLA DR− subset, whereas CD163+ CD14+ SLA DR+ and CD163− CD14+ SLA DR+ subsets show intermediate values. CD163+ monocytes also display a higher ability to present soluble antigens to T cells than CD163− monocytes.
Keywords: CD163, maturation, monocyte subsets, pig, tumour necrosis factor-α
Introduction
Monocytes play important roles in both innate and adaptive immune responses, killing microbial pathogens and tumour cells, and exerting immunoregulatory functions through cytokine production and processing and presentation of antigens to lymphocytes. These cells arise from haemopoietic precursors in the bone marrow, and after 2–3 days in the blood migrate into different tissues, where they undergo further differentiation to become tissue-resident macrophages.1 Under specific culture conditions in vitro, monocytes can differentiate into macrophages in the presence of serum or macrophage colony-stimulating factor,2,3 or into dendritic cells when cultured with granulocyte–macrophage colony-stimulating factor and interleukin-4 (IL-4).4–6
Although the heterogeneity of monocytes is not as obvious as that displayed by macrophages, in the human several monocyte subpopulations differing in phenotype and functional capabilities have been reported. Ziegler-Heitbrock et al. identified a minor population of CD14+ CD16+ monocytes that represents around 10% of the total monocyte population and exhibits proinflammatory features, producing high amounts of tumour necrosis factor-α (TNF-α) but no or little IL-10, while the major population of CD14+ CD16− monocytes produces both types of cytokines.7–9 Moreover, within the CD14+ CD16+ monocytes, the M-DC8+ marker identifies a subset with capacity to differentiate in vitro into potent dendritic cells.10 Two monocyte subsets have been characterized by Grage-Griebenow et al. based on the expression of CD64.11 Monocytes lacking CD64, comprise less than 10% of the whole monocyte population and display higher capacity for antigen presentation than the predominant CD64+ monocytes which show higher phagocytic activity and produce more reactive oxygen intermediates. Simultaneous analysis of CD16 and CD64 allows a further division into four phenotypically and functionally different subsets. Out of them, CD64+ CD16+ monocytes exhibit dendritic-cell-like high antigen-presenting capacity, while CD64− CD16− monocytes resemble the plasmacytoid dendritic cell blood precursors with high interferon-α (IFN-α) producing capacity.12–14
Two major monocyte subsets have also been identified in the mouse, which differ in the expression of chemokine receptors and share phenotype and homing features with human CD14+ CD16− and CD14+ CD16+ subsets.15
We have previously characterized two subpopulations of porcine monocytes based on the expression of the marker CD163,16 identified as the scavenger receptor of haemoglobin–haptoglobin complexes.17 CD163− and CD163+ monocytes differ in the expression of several surface markers, the pattern of cytokine production, the accessory cell capacity and the permissiveness to African swine fever virus infection.16,18,19 These subsets show further heterogeneity according to the nonuniform expression of swine leucocyte antigen (SLA) II and CD14 antigens. The aim of the present study was to analyse this heterogeneity by using four-colour flow cytometry with a large panel of monoclonal antibodies (mAbs) to leukocyte differentiation antigens, and to test the ability of the different subsets to produce TNF-α and present soluble antigens to T cells.
Materials and methods
Animals and cells
Blood samples were obtained from 6- to 24-month-old Large-White outbred pigs. For lysozyme immunization, two 4-month-old pigs underwent intramuscular immunization three times at 3-week intervals with 1 mg lysozyme from hen egg white (Boehringer Mannheim, Mannheim, Germany) emulsified in complete Freund's adjuvant the first time and in incomplete adjuvant for the following immunizations. Peripheral blood mononuclear cells (PBMC) were isolated on Percoll discontinuous gradients after blood sedimentation in dextran as previously described.20 Cells were resuspended in RPMI-1640 medium containing 10% fetal calf serum, 2 mm l-glutamine, 5 × 10−5 m 2-mercaptoethanol and 30 μg/ml gentamicin (complete medium).
Antibodies and reagents
The mAbs used in this study are shown in Table 1.
Table 1.
Monoclonal antibodies used in this study
| Clone | Specificity | Isotype | Label | Source | Reference |
|---|---|---|---|---|---|
| 76-7-4* | CD1a | IgG2a | S | Dr J. Lunney, ARS, USDA | 34, 35 |
| BB23-8E6* | CD3 | IgG2b | S | Dr M. Pescovitz, Indiana University | 36 |
| 74-12-4* | CD4 | IgG2b | S | Dr M. Pescovitz, Indiana University | 37 |
| 76-2-11* | CD8α | IgG2a | S | Dr J. Lunney, ARS, USDA | 38 |
| BL1H8* | CD11a | IgG2b | S | INIA | 39 |
| MIL-4* | wCD11R1 | IgG1 | S | Dr K. Haverson, University of Bristol | 40, 41 |
| S-Hcl3† | wCD11R2 | IgG2b | PE | Pharmingen BD | 41 |
| BL3F1* | wCD11R3 | IgG1 | S | INIA | 41 |
| Tük4† | CD14 | IgG2a | P, FITC | Dako, Caltag | 42 |
| G7* | CD16 | IgG1 | P | Dr Y. B. Kim, Finch University | 43 |
| BA3H2* | CD18 | IgG1 | S | INIA | 39 |
| UCP1D2* | CD29 | IgG1 | S | Dr F. Zuckermann, University of Illinois | 44, 45 |
| 2A5* | CD45 | IgG1 | S | INIA | 46 |
| 3C3/9* | CD45RA | IgG1 | S | INIA | 46 |
| HP2/1† | CD49d | IgG1 | S | Dr F. Sánchez-Madrid, Hosp. La Princesa | 47 |
| JM2E5* | CD61 | IgG1 | S | Dr D. Llanes, Córdoba University | 48 |
| 2A10/11* | CD163 | IgG1 | S, B | INIA | 16 |
| 76-6-7* | SWC1 | IgM | S | Dr J. Lunney, ARS, USDA | 22 |
| BL1H7* | SWC3 | IgG1 | S, A488 | INIA | 28 |
| PM18-7* | SWC9 | IgG1 | P | Dr Y. B. Kim, Finch University | 23 |
| 1F12* | SLA DR | IgG2b | S, A633 | INIA | 49 |
| Mab11‡ | TNF-α | IgG1 | PE | Pharmingen BD |
Antibodies to swine leucocyte antigens.
Antibodies to human leucocyte antigens cross-reacting with swine antigens.
Antibodies to human cytokines cross-reacting with swine cytokines.
S, hybridoma supernatant; P, purified mAb; B, biotinylated mAb; A488, Alexa 488-labelled mAb; A633, Alexa 633-labelled mAb; PE, phycoerythrin-labelled mAb; FITC, fluorescein isothiocyanate-labelled mAb.
Human CD152 (CTLA-4) murine immunoglobulin fusion protein (immunoglobulin G2a; IgG2a), that binds swine CD80/86, was purchased from Ancell (Byport, MN). The mAbs to CD163, SWC3 and SLA DR, as well as the corresponding isotype-matched control antibodies, were purified by affinity chromatography on protein A–Sepharose CL4B and labelled with biotin, Alexa 488 or Alexa 633 (Molecular Probes, Eugene, OR), respectively. Fluorescein isothiocyanate (FITC) -labelled IgG2a isotype control and PE-labelled IgG1 isotype control were purchased from Caltag (Burlingame, CA) and Pharmingen BD (San Diego, CA), respectively.
Flow cytometry analysis
Phenotypic analyses were performed as previously described.18 PBMC (5 × 105) were incubated with 75 μl of hybridoma supernatant for 30 min at 4°. After two washes in phosphate-buffered saline (PBS) containing 0·1% bovine serum albumin and 0·01% sodium azide (fluorescence buffer), cells were incubated for 30 min at 4° with phycoerythrin (PE) -conjugated rabbit (Fab′)2 anti-mouse immunoglobulin (Dako, Glostrop, Denmark). Cells were washed and free binding sites were blocked with 5% normal mouse serum for 15 min. Then, mAbs labelled with Alexa 488, Alexa 633 and/or biotin were added and incubated for 30 min. After two washes in fluorescence buffer, the biotinylated antibodies were detected using either streptavidin–Cy-Chrome or streptavidin–PercP (Pharmingen BD). Cells were washed twice in fluorescence buffer and fixed in 0·1% formaldehyde prior to analysis on a FACScalibur cytometer (Becton Dickinson, San Diego, CA). At least 60 000 cells were acquired. Irrelevant isotype-matched mAbs, unlabelled and labelled with the different fluorochromes, were used as negative controls. Propidium iodide was used to exclude dead cells.
Sorting of CD163− and CD163+ monocyte subpopulations
Blood monocytes were magnetically isolated by using the VarioMACS cell sorting technique (Miltenyi Biotec, Bergisch-Gladbach, Germany). Briefly, 4 × 108 PBMC were incubated with anti-CD163 hybridoma supernatant for 30 min on ice, washed with PBS containing 5% fetal calf serum and 2 mm ethylenediaminetetraacetic acid [magnetic cell separation system (MACS) buffer] and incubated with 0·2 ml of goat anti-mouse IgG magnetic microbeads for 15 min on ice. After washing with MACS buffer, the cell suspension was passed through a MACS separation column (LS positive selection column) and magnetically labelled cells (CD163+ fraction) were collected. The effluent negative fraction was then incubated with anti-SWC3 mAb and magnetic microbeads as described above. Cells were passed through a MACS LS positive selection column and magnetically labelled cells (CD163− SWC3+ fraction) were collected. Fractions were analysed by flow cytometry and they showed a purity ≥90%
Enrichment of CD163− SLA DR− monocyte subpopulation
PBMC were first depleted of lymphocytes using a cocktail of mAbs to CD3 (T cells), CD8α(T subpopulation and natural killer cells) and CD45RA (B cells and T-cell subset) using the VarioMACS cell-sorting technique described above. The monocyte-enriched fraction was then incubated with a mixture of mAbs to CD163 and SLA DR and passed through a new MACS separation column to deplete monocytes expressing these markers. The effluent negative fraction was analysed by flow cytometry and contained only CD14+ CD163− SLA DR− monocytes.
Detection of intracellular TNF-α by flow cytometric analysis
Sorted CD163− and CD163+ monocytes were stimulated for 4 hr with lipopolysaccharide (LPS; 1 μg/ml) plus IFN-γ (50 U/ml) in the presence of monensin (2 μg/ml). Cells were then incubated for 15 min at 4° with 5% normal mouse serum to block any residual binding sites of the secondary antibodies used in the sorting process, prior to the staining of the cell surface with Alexa 633-conjugated anti-SLA DR and FITC-labelled anti-CD14. Afterwards, cells were permeabilized with the BD FACS Permeabilizing Solution 2 (Becton Dickinson) following the manufacturer's instructions. Cells were then washed in fluorescence buffer and incubated for 30 min with PE-labelled anti-TNF-α mAb (Pharmingen BD). After three washes, 100 000 cells were analysed on a FACScalibur cytometer (Becton Dickinson). Irrelevant isotype-matched mAbs labelled with the different fluorochromes were used as negative controls.
T-cell stimulation assays
To test antigen-specific T-cell proliferation, CD4+ T lymphocytes from pigs immunized with lysozyme were separated by positive selection magnetic cell sorting using the MACS system. Then, 1·5 × 105 of these sorted CD4+ T cells were cocultured in four replicates with graded numbers of irradiated autologous antigen-presenting cells (CD163− and CD163+ monocytes), pulsed with 20 μg of lysozyme, in 96-well flat-bottom plates for 5 days. T-cell proliferation was determined by uptake of [3H]thymidine which was added at a concentration of 1 μCi/well during the last 18 hr of culture and measured using a beta-scintillation counter.
Statistical analysis
Data means from the flow cytometric analyses were compared using the non-parametric Mann–Whitney U-test. Differences were considered significant when P < 0·05.
Results
Phenotypic characterization of monocyte subsets
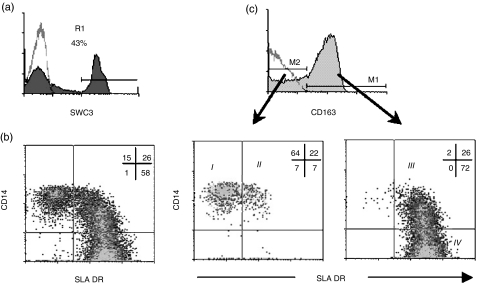
In a previous study we described how CD163− and CD163+ monocytes are heterogeneous in the expression of SLA II and CD14 antigens, respectively.18 To investigate this heterogeneity further we performed four-colour flow cytometry analyses on PBMC using mAbs to SWC3, CD163, CD14 and SLA DR antigens. Monocytes were identified according to their light scatter properties and the expression of the pan-myelomonocytic marker SWC321 (Fig. 1a). Analysis of the expression of CD14 antigen versus that of SLA DR in the SWC3 gated cells distinguished three monocyte populations: CD14+ SLA DR−, CD14+ SLA DR+ and CD14− SLA DR+ (Fig. 1b). When monocytes were divided into CD163− and CD163+ cells, all CD14+ SLA DR− cells and a minor fraction of CD14+ SLA DR+ cells fell into the CD163− subset, whereas all CD14− SLA DR+ cells were found to express CD163. Moreover, within the CD14+ SLA DR+ population, the CD163+ cells expressed higher levels of SLA DR and lower levels of CD14 than the CD163− cells (Fig. 1c). Therefore, this four-colour flow cytometry allows the definition of four monocyte subsets: SWC3+ CD163− CD14+ SLA DR− (I), SWC3+ CD163− CD14+ SLA DR+ (II), SWC3+ CD163+ CD14+ SLA DR+ (III) and SWC3+ CD163+ CD14− SLA DR+ (IV).
Figure 1.
Four-colour immunofluorescence of PBMC with mAbs to SWC3, CD163, CD14 and SLA DR identifies four monocyte subsets in swine. (a) Monocytes were gated (R1) from PBMC as SWC3+ cells. (b) Dot plot of CD14/SLA DR expression in SWC3+ cells of gate R1 defined in (a). (c) CD163 expression in R1-gated cells, and CD14/SLA DR expression in SWC3+ CD163− and SWC3+ CD163+ cells, respectively. Data are representative of seven independent experiments.
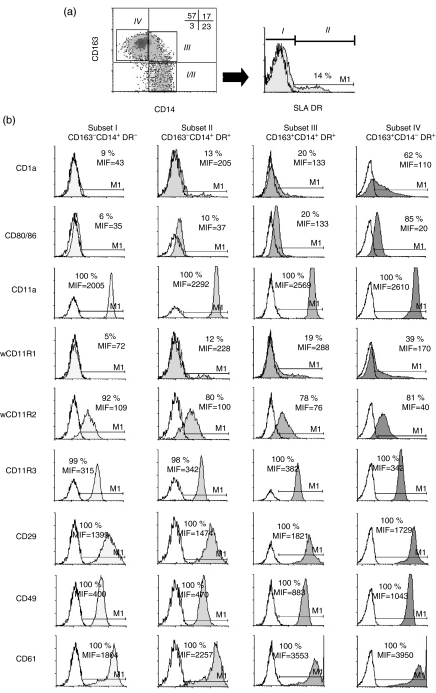
We next compared the expression of various cell-surface antigens on these monocyte subsets using a CD14/CD163/SLA DR combination (Fig. 2). The CD163− CD14+ SLA DR− cells (subset I) were shown to express significantly lower levels of adhesion molecules (CD11a, wCD11R1, CD29, CD49d, CD61), costimulatory molecules (CD80/CD86), and CD1a antigen than CD163+ CD14− SLA DR+ cells (subset IV). The CD163− CD14+ SLA DR+ (subset II) and CD163+ CD14+ SLA DR+ cells (subset III) expressed intermediate levels of these molecules. Conversely, the expression of wCD11R2 tended to decrease from subset I to subset IV, although differences were not statistically significant.
Figure 2.
Phenotypic differences among monocyte subsets assessed by multicolour flow cytometry. (a) Monocyte subsets were gated according to the expression of CD14 and CD163: (I/II) CD163− CD14+ cells (III) CD163+ CD14+ cells and (IV) CD163+ CD14− cells. Percentages of cells in each quadrant are indicated. Subsets I and II were further defined on CD163− CD14+ cells based on SLA DR expression: (I) CD163− CD14+ SLA DR− cells and (II) CD163− CD14+ SLA DR+ cells. (b) Filled histograms represent the expression of the indicated cell-surface markers on the gated subsets. Open histograms correspond to the staining with isotype control mAbs. Percentage of positive cells and mean fluorescence intensity (MFI) are shown. Data are from one representative experiment out of three independently performed.
From these phenotypic analyses, a likely maturation pathway of monocytes can be envisaged along which cells increase the expression of SLA DR, CD163 and of some adhesion and costimulatory molecules, while decreasing the expression of CD14, with CD163− CD14+ SLA DR− cells (subset I) representing the most immature population and CD163+ CD14− SLA DR+ cells (subset IV), representing the most mature.
In vitro serum-induced maturation of subset I
Monocyte maturation into macrophages can be induced in vitro by serum factors. Since CD163− CD14+ SLA DR− cells appeared to represent the most immature population we investigated whether this population can acquire the phenotypic features of the other monocyte subsets after culture in medium supplemented with porcine serum.
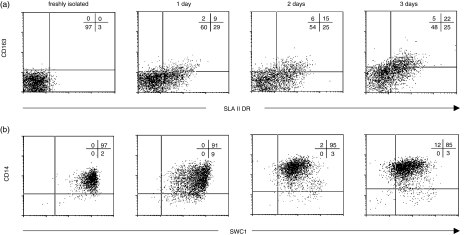
CD163− CD14+ SLA DR− monocytes were obtained from PBMC after two negative sorting steps; the first one with a mixture of mAbs to CD3, CD8α and CD45RA to eliminate lymphocytes, and the second one with mAbs to CD163 and SLA DR to deplete monocytes expressing these markers. Cells were then cultured in 20% (v/v) porcine serum and collected at different times (0, 1, 2 and 3 days) to analyse their phenotype by four-colour flow cytometry (Fig. 3). After 1 day of culture, around 40% of cells became SLA DR positive but only a minor proportion expressed CD163, a phenotype which resembles subset II (CD163− CD14+ SLA DR+). At days 2 and 3, CD163 was up-regulated while SLA DR expression was slightly increased. Therefore, after 3 days in culture a substantial proportion of cells displayed a phenotype similar to that of subset III (CD163+ CD14+ SLA DR+). However, no changes were observed in CD14 expression during these days in culture, although the expression of SWC1 antigen, a marker that has been reported to be down-regulated upon maturation of monocytes in culture,22,23 was reduced.
Figure 3.
In vitro serum-induced maturation of subset I. CD163− CD14+ SLA DR− monocytes were sorted and cultured for 3 days in complete medium supplemented with 20% porcine serum. At different times cells were harvested and analysed by multicolour flow cytometry with mAbs to CD163, CD14, SLA DR and other surface markers. Dot-plot of SLA DR/CD163 and SWC1/CD14 expression are shown in (a) and (b), respectively. Data are from one representative experiment out of five performed.
TNF-α production by monocyte subsets
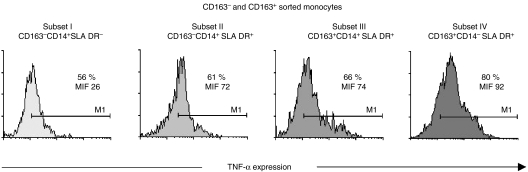
In humans, monocyte subsets have been shown to differ in their ability to produce TNF-α.24 Therefore, we investigated whether porcine monocyte subsets also showed differences in the production of TNF-α, as assessed by flow cytometry. Upon stimulation with LPS (1 μg/ml) and IFN-γ (50 U/ml) around 70–80% of the whole population of monocytes expressed TNF-α, compared to less than 5% of unstimulated monocytes (data not shown). However, as described in human monocytes25,26 LPS + IFN-γ treatment led to the down-modulation of the CD163 molecule preventing an accurate analysis of the four monocyte subsets. To solve this drawback, monocytes were magnetically sorted into CD163− and CD163+ cells, stimulated for 4 hr in the presence of monensin and thereafter stained for SLA DR, CD14 and TNF-α. As shown in Fig. 4, subset IV (CD163+ CD14− SLA DR+) contained the highest percentage of TNF-α-producing cells, followed by subset III (CD163+ CD14+ SLA DR+) and to a lesser extent by subset II (CD163− CD14+ SLA DR+) and subset I.
Figure 4.
TNF-α production by different monocyte subsets. SWC3+ CD163− and SWC3+ CD163+ cells were magnetically sorted from PBMC, and stimulated for 4 hr with LPS (1 μg/ml) plus IFN-γ (50 U/ml) in the presence of monensin (2 μg/ml). Cells were first incubated with Alexa 633-labelled anti-SLA DR and FITC-labelled anti-CD14 mAbs, and then fixed/permeabilized and incubated with PE-labelled anti-TNF-α mAb. The percentage of TNF-α-positive cells in each monocyte subset is shown. Data are representative of two experiments performed.
Capacity of CD163− and CD163+ monocytes to activate T cells against recall antigens
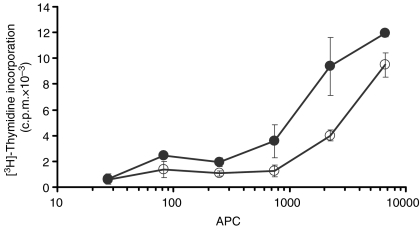
Because of the different phenotype exhibited by CD163− and CD163+ monocytes, particularly regarding SLA II expression, we had previously compared the accessory capacity of these subsets. These analyses showed a higher capacity of CD163+ monocytes to stimulate T cells in an allogeneic mixed leucocyte reaction.16 To examine whether these cells also differ in their capacity to present a soluble antigen to T cells, different numbers of freshly sorted CD163− and CD163+ monocytes were pulsed with lysozyme and cultured with autologous CD4+ T cells from donors immunized with this antigen. As shown in Fig. 5, freshly isolated CD163+ monocytes were more efficient than CD163− monocytes, generating above two-fold levels of T-cell proliferation using the same amount of cells.
Figure 5.
CD163− and CD163+ monocytes differ in their T-cell stimulatory capacity. Graded numbers of irradiated CD163− monocytes (○) or CD163+ monocytes (•) were stimulated with 20 μg of recall antigen lysozyme and cultured with 1·5 × 105 sorted autologous CD4+ T cells. Proliferation was determined by [3H]thymidine uptake. Data represent the mean ± SEM of counts per minute (c.p.m.) of four replicates. Cultures of CD4+ T cells with CD163− and CD163+ monocytes without antigen were carried out as controls obtaining 130 ± 27 and 224 ± 76 c.p.m., respectively. Results shown are representative of three independent experiments.
Discussion
The existence of phenotypically and functionally different monocyte populations in humans and rodents has been clearly established.8,12,27 We had previously characterized in pig two monocyte subsets defined on the basis of the expression of the CD163 marker.16,18 The profile of expression of SLA II and CD14 antigens on CD163− and CD163+ monocytes is indicative of the existence of more heterogeneity within them. To further investigate this heterogeneity, in the present study we have carried out four-colour immunofluorescence assays using mAbs to SWC3, CD163, CD14 and SLA DR antigens. These analyses allow the subdivision of porcine PBMCs into four subsets: (I) SWC3+ CD163− CD14+ SLA DR−, (II) SWC3+ CD163− CD14+ SLA DR+, (III) SWC3+ CD163+ CD14+ SLA DR+, and (IV) SWC3+ CD163+ CD14− SLA DR+. When the expression of other markers was analysed in these subsets, a gradual and significant increase in the expression of CD11a, wCD11R1 (integrin β2), CD29, CD49d (integrins β1), CD61 (integrin β3), CD80/86 and CD1a, together with a reduction in that of wCD11R2, was observed from subset I to IV.
These subsets might therefore correspond to different stages of monocyte development, delineating a hypothetical maturation pathway along which cells increase the expression of SLA DR antigen, CD163, costimulatory molecules and many adhesion molecules, while reducing that of CD14 and wCD11R2. Several findings support this hypothesis. First, sorted CD163− CD14+ SLA DR− monocytes, corresponding to subset I, cultured in media supplemented with porcine serum to induce their maturation, develop into cells phenotypically similar to those belonging to subsets II and III. Second, cells in subset IV display the highest levels of CD163, SLA DR and integrins, and have a phenotype similar to alveolar macrophages. Moreover, CD163+ monocytes share phenotypic and functional features with human CD14+ CD16+ monocytes, that have been considered as mature monocytes.7–9 Indeed, CD14+ CD16+ monocytes express the highest levels of CD163 among all human monocyte subsets.26
While SLA DR expression appears to precede that of CD163 during in vitro maturation, no loss in CD14 expression was observed during this process. This failure to observe a down-regulation in CD14 expression may be explained by a lack of specific soluble factors in the swine serum or a need for additional requirements, such as interactions with other cell types (i.e. endothelial cells).
These different subsets might also have different immuno-regulatory tasks. In this regard, although the majority of LPS + IFN-γ-stimulated monocytes produced TNF-α, as assessed by intracellular staining, differences among these subsets were noticed, detecting a clear increase in TNF-α production from subset I to subset IV. Even though CD163− cells were sorted using a mAb to SWC3, recently identified as one of the signal regulatory proteins (SIRP) that act as negative regulators of signals,28,29 the antibody labelling did not appear to be responsible for the lower TNF-α levels in CD163− monocytes in comparison to CD163+ monocytes, because no significant differences in TNF-α production were observed in CD163+ cells that had been stained or unstained with anti-SWC3 (data not shown).
LFA-1 (CD11a/CD18) and VLA-4 (CD29/CD49d) integrins take part actively in the monocyte adhesion to endothelial cells and further migration to tissues during inflammation.30–32 The higher expression of adhesion molecules displayed by cells of subset IV, and particularly of CD29/CD49d and CD11a, may also endow them with a better ability to migrate to tissues or to be recruited at sites of inflammation.
Altogether, the higher TNF-α production and the higher levels of adhesion molecules displayed by CD163+ monocytes are consistent with a pro-inflammatory function of these cells, as was suggested in a previous report where only transcripts of IL-1α and TNF-α cytokines were detected after LPS stimulation in this subset, whereas CD163− monocytes also produced anti-inflammatory IL-10.16 These results also reinforce the similarity of porcine CD163+ monocytes with human CD14+ CD16+ monocytes, considered proinflammatory cells and the major source of TNF-α in blood.24,33
CD163− and CD163+ monocytes also differ in their antigen-presenting capacity, the latter being more efficient at presenting lysozyme to primed T CD4+ lymphocytes, as was previously reported with allo-antigens.16 Although we cannot rule out differences in antigen capture and presentation abilities between CD163− and CD163+ monocytes, the higher levels of SLA DR antigens and CD80/86 costimulatory molecules found in CD163+ cells may account for their higher antigen-presenting cell function.
In summary, our results show the existence of a marked heterogeneity among porcine monocytes. A better understanding of this heterogeneity and the mechanisms involved in its generation will provide new insights into the pathogenesis of infections caused by viruses with tropism for cells of the monocyte lineage such as African swine fever virus.
Acknowledgments
This work was supported by CICYT grants BIO-2000–1636 CO2-01, AGL2000-1488 and PTR1995-0573-OP.
Abbreviations
- FITC
fluorescein isothiocyanate
- FSC
forward-scatter
- mAb
monoclonal antibody
- MFI
mean fluorescence intensity
- PBMC
peripheral blood mononuclear cells
- PE
phycoerythrin
- SEM
standard error of mean
- SLA
swine leucocyte antigen
- SSC
side-scatter
References
- 1.Gordon S, Keshav S, Chung LP. Mononuclear phagocytes: tissue distribution and functional heterogeneity. Curr Opin Immunol. 1988;1:26–35. doi: 10.1016/0952-7915(88)90047-7. [DOI] [PubMed] [Google Scholar]
- 2.Chapuis F, Rosenzwajg M, Yagello M, Ekman M, Biberfeld P, Gluckman JC. Differentiation of human dendritic cells from monocytes in vitro. Eur J Immunol. 1997;27:431–41. doi: 10.1002/eji.1830270213. [DOI] [PubMed] [Google Scholar]
- 3.Palucka KA, Taquet N, Sanchez-Chapuis F, Gluckman JC. Dendritic cells as the terminal stage of monocyte differentiation. J Immunol. 1998;160:4587–95. [PubMed] [Google Scholar]
- 4.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 7.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–34. [PubMed] [Google Scholar]
- 8.Ziegler-Heitbrock HW, Fingerle G, Strobel M, Schraut W, Stelter F, Schutt C, Passlick B, Pforte A. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. Eur J Immunol. 1993;23:2053–8. doi: 10.1002/eji.1830230902. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler-Heitbrock HW. Heterogeneity of human blood monocytes: the CD14+ CD16+ subpopulation. Immunol Today. 1996;17:424–8. doi: 10.1016/0167-5699(96)10029-3. [DOI] [PubMed] [Google Scholar]
- 10.de Baey A, Mende I, Riethmueller G, Baeuerle PA. Phenotype and function of human dendritic cells derived from M-DC8(+) monocytes. Eur J Immunol. 2001;31:1646–55. doi: 10.1002/1521-4141(200106)31:6<1646::aid-immu1646>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 11.Grage-Griebenow E, Lorenzen D, Fetting R, Flad HD, Ernst M. Phenotypical and functional characterization of Fc gamma receptor I (CD64)-negative monocytes, a minor human monocyte subpopulation with high accessory and antiviral activity. Eur J Immunol. 1993;23:3126–35. doi: 10.1002/eji.1830231213. [DOI] [PubMed] [Google Scholar]
- 12.Grage-Griebenow E, Flad HD, Ernst M, Bzowska M, Skrzeczynska J, Pryjma J. Human MO subsets as defined by expression of CD64 and CD16 differ in phagocytic activity and generation of oxygen intermediates. Immunobiology. 2000;202:42–50. doi: 10.1016/S0171-2985(00)80051-0. [DOI] [PubMed] [Google Scholar]
- 13.Grage-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol. 2001;69:11–20. [PubMed] [Google Scholar]
- 14.Grage-Griebenow E, Zawatzky R, Kahlert H, Brade L, Flad H, Ernst M. Identification of a novel dendritic cell-like subset of CD64(+)/CD16(+) blood monocytes. Eur J Immunol. 2001;31:48–56. doi: 10.1002/1521-4141(200101)31:1<48::aid-immu48>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez C, Domenech N, Vazquez J, Alonso F, Ezquerra A, Dominguez J. The porcine 2A10 antigen is homologous to human CD163 and related to macrophage differentiation. J Immunol. 1999;162:5230–7. [PubMed] [Google Scholar]
- 17.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 18.Chamorro S, Revilla C, Alvarez B, Lopez-Fuertes L, Ezquerra A, Dominguez J. Phenotypic characterization of monocyte subpopulations in the pig. Immunobiology. 2000;202:82–93. doi: 10.1016/S0171-2985(00)80055-8. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Torres C, Gomez-Puertas P, Gomez-Del-Moral M, Alonso F, Escribano JM, Ezquerra A, Dominguez J. Expression of porcine CD163 on monocytes/macrophages correlates with permissiveness to African swine fever infection. Arch Virol. 2003;148:2307–23. doi: 10.1007/s00705-003-0188-4. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez S, Mendoza C, Sanchez-Vizcaino JM, Alonso F. Inhibitory effect of African swine fever virus on lectin-dependent swine lymphocyte proliferation. Vet Immunol Immunopathol. 1990;26:71–80. doi: 10.1016/0165-2427(90)90133-d. [DOI] [PubMed] [Google Scholar]
- 21.Summerfield A, McCullough KC. Porcine bone marrow myeloid cells: phenotype and adhesion molecule expression. J Leukoc Biol. 1997;62:176–85. doi: 10.1002/jlb.62.2.176. [DOI] [PubMed] [Google Scholar]
- 22.Saalmuller A, Aasted B, Canals A, et al. Analysis of mAb reactive with the porcine SWC1. Vet Immunol Immunopathol. 1994;43:255–8. doi: 10.1016/0165-2427(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 23.Dominguez J, Ezquerra A, Alonso F, et al. Workshop studies with monoclonal antibodies identifying a novel porcine differentiation antigen, SWC9. Vet Immunol Immunopathol. 1998;60:343–9. doi: 10.1016/s0165-2427(97)00110-4. [DOI] [PubMed] [Google Scholar]
- 24.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. The proinflammatory CD14(+) CD16(+) DR(++) monocytes are a major source of TNF. J Immunol. 2002;168:3536–42. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 25.Sulahian TH, Hogger P, Wahner AE, et al. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine. 2000;12:1312–21. doi: 10.1006/cyto.2000.0720. [DOI] [PubMed] [Google Scholar]
- 26.Buechler C, Ritter M, Orso E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67:97–103. [PubMed] [Google Scholar]
- 27.Crawford K, Gabuzda D, Pantazopoulos V, Xu J, Clement C, Reinherz E, Alper CA. Circulating CD2+ monocytes are dendritic cells. J Immunol. 1999;163:5920–8. [PubMed] [Google Scholar]
- 28.Alvarez B, Sanchez C, Bullido R, Marina A, Lunney J, Alonso F, Ezquerra A, Dominguez J. A porcine cell surface receptor identified by monoclonal antibodies to SWC3 is a member of the signal regulatory protein family and associates with protein-tyrosine phosphatase SHP-1. Tissue Antigens. 2000;55:342–51. doi: 10.1034/j.1399-0039.2000.550408.x. [DOI] [PubMed] [Google Scholar]
- 29.Tonks NK, Neel BG. From form to function: signaling by protein tyrosine phosphatases. Cell. 1996;87:365–8. doi: 10.1016/s0092-8674(00)81357-4. [DOI] [PubMed] [Google Scholar]
- 30.Chuluyan HE, Issekutz AC. VLA-4 integrin can mediate CD11/CD18-independent transendothelial migration of human monocytes. J Clin Invest. 1993;92:2768–77. doi: 10.1172/JCI116895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.te Velde AA, Keizer GD, Figdor CG. Differential function of LFA-1 family molecules (CD11 and CD18) in adhesion of human monocytes to melanoma and endothelial cells. Immunology. 1987;61:261–7. [PMC free article] [PubMed] [Google Scholar]
- 32.Santamaria Babi LF, Moser R, Perez Soler MT, Picker LJ, Blaser K, Hauser C. Migration of skin-homing T cells across cytokine-activated human endothelial cell layers involves interaction of the cutaneous lymphocyte-associated antigen (CLA), the very late antigen-4 (VLA-4), and the lymphocyte function-associated antigen-1 (LFA-1) J Immunol. 1995;154:1543–50. [PubMed] [Google Scholar]
- 33.Frankenberger M, Sternsdorf T, Pechumer H, Pforte A, Ziegler-Heitbrock HW. Differential cytokine expression in human blood monocyte subpopulations: a polymerase chain reaction analysis. Blood. 1996;87:373–7. [PubMed] [Google Scholar]
- 34.Saalmuller A. Characterization of swine leukocyte differentiation antigens. Immunol Today. 1996;17:352–4. doi: 10.1016/S0167-5699(96)90273-X. [DOI] [PubMed] [Google Scholar]
- 35.Chun T, Wang K, Zuckermann FA, Gaskins HR. Molecular cloning and characterization of a novel CD1 gene from the pig. J Immunol. 1999;162:6562–71. [PubMed] [Google Scholar]
- 36.Pescovitz MD, Book BK, Aasted B, et al. Analyses of monoclonal antibodies reacting with porcine CD3: results from the Second International Swine CD Workshop. Vet Immunol Immunopathol. 1998;60:261–8. doi: 10.1016/s0165-2427(97)00102-5. [DOI] [PubMed] [Google Scholar]
- 37.Pescovitz MD, Aasted B, Canals A, et al. Analysis of monoclonal antibodies reactive with the porcine CD4 antigen. Vet Immunol Immunopathol. 1994;43:233–6. doi: 10.1016/0165-2427(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 38.Saalmuller A, Aasted B, Canals A, et al. Analyses of mAb reactive with porcine CD8. Vet Immunol Immunopathol. 1994;43:249–54. doi: 10.1016/0165-2427(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 39.Alvarez B, Domenech N, Alonso F, Sanchez C, Gomez del Moral M, Ezquerra A, Dominguez J. Molecular and functional characterization of porcine LFA-1 using monoclonal antibodies to CD11a and CD18. Xenotransplantation. 2000;7:258–66. doi: 10.1034/j.1399-3089.2000.00574.x. [DOI] [PubMed] [Google Scholar]
- 40.Haverson K, Bailey M, Higgins VR, Bland PW, Stokes CR. Characterization of monoclonal antibodies specific for monocytes, macrophages and granulocytes from porcine peripheral blood and mucosal tissues. J Immunol Meth. 1994;170:233–45. doi: 10.1016/0022-1759(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 41.Dominguez J, Alvarez B, Alonso F, Thacker E, Haverson K, McCullough K, Summerfield A, Ezquerra A. Workshop studies on monoclonal antibodies in the myeloid panel with CD11 specificity. Vet Immunol Immunopathol. 2001;80:111–19. doi: 10.1016/s0165-2427(01)00286-0. [DOI] [PubMed] [Google Scholar]
- 42.Dominguez J, Ezquerra A, Alonso F, et al. Porcine myelomonocytic markers: summary of the Second International Swine CD Workshop. Vet Immunol Immunopathol. 1998;60:329–41. doi: 10.1016/s0165-2427(97)00109-8. [DOI] [PubMed] [Google Scholar]
- 43.Halloran PJ, Sweeney SE, Kim YB. Biochemical characterization of the porcine Fc gamma RIII alpha homologue G7. Cell Immunol. 1994;158:400–13. doi: 10.1006/cimm.1994.1286. [DOI] [PubMed] [Google Scholar]
- 44.Jimenez-Marin A, Garrido JJ, de Andres-Cara DF, Morera L, Barbancho MJ, Llanes D. Molecular cloning and characterization of the pig homologue to human CD29, the integrin beta1 subunit. Transplantation. 2000;70:649–55. doi: 10.1097/00007890-200008270-00019. [DOI] [PubMed] [Google Scholar]
- 45.Haverson K, Zuckermann F, Saalmuller A, Lipp J, Aasted B, Stokes CR. Summary of workshop findings for porcine adhesion molecule subgroup. Vet Immunol Immunopathol. 1998;60:351–65. doi: 10.1016/s0165-2427(97)00111-6. [DOI] [PubMed] [Google Scholar]
- 46.Bullido R, Gomez del Moral M, Domenech N, Alonso F, Ezquerra A, Dominguez J. Monoclonal antibodies to a high molecular weight isoform of porcine CD45: biochemical and tissue distribution analyses. Vet Immunol Immunopathol. 1997;56:151–62. doi: 10.1016/s0165-2427(96)05728-5. [DOI] [PubMed] [Google Scholar]
- 47.Campanero MR, Arroyo AG, Pulido R, et al. Functional role of alpha 2/beta 1 and alpha 4/beta 1 integrins in leukocyte intercellular adhesion induced through the common beta 1 subunit. Eur J Immunol. 1992;22:3111–19. doi: 10.1002/eji.1830221213. [DOI] [PubMed] [Google Scholar]
- 48.Perez de la Lastra JM, Moreno A, Perez J, Llanes D. Characterization of the porcine homologue to human platelet glycoprotein IIb-IIIa (CD41/CD61) by a monoclonal antibody. Tissue Antigens. 1997;49:588–94. doi: 10.1111/j.1399-0039.1997.tb02806.x. [DOI] [PubMed] [Google Scholar]
- 49.Bullido R, Domenech N, Alvarez B, Alonso F, Babin M, Ezquerra A, Ortuno E, Dominguez J. Characterization of five monoclonal antibodies specific for swine class II major histocompatibility antigens and crossreactivity studies with leukocytes of domestic animals. Dev Comp Immunol. 1997;21:311–22. doi: 10.1016/s0145-305x(97)00008-6. [DOI] [PubMed] [Google Scholar]