Abstract
We report that haptoglobin, an acute-phase protein produced by liver cells in response to interleukin-6 (IL-6), can modulate the inflammatory response induced by endotoxins. We provide evidence that haptoglobin has the ability to selectively antagonize lipopolysaccharide (LPS) effects in vitro by suppressing monocyte production of tumour necrosis factor-α, IL-10 and IL-12, while it fails to inhibit the production of IL-6, IL-8 and IL-1 receptor antagonist. In two animal models of LPS-induced bronchopulmonary hyperreactivity and endotoxic shock, haptoglobin knockout mice were more sensitive to LPS effects compared to their wild-type counterparts. The present data suggest that haptoglobin regulates monocyte activation following LPS stimulation. The increase in haptoglobin levels during an acute-phase reaction may generate a feedback effect which dampens the severity of cytokine release and protects against endotoxin-induced effects.
Keywords: haptoglobin, lipopolysaccharide, proinflammatory cytokines, septic shock
Introduction
The host response to Gram-negative bacterial infection is driven mainly by lipopolysaccharides (LPS) from bacterial cell walls. LPS interact with the CD14 and Toll-like receptors (TLR)1–3 on monocytes and macrophages. LPS are also able bind to and signal through alternative receptors such as CD11/CD18 integrins4,5 and moesin (the membrane-organizing extension spike protein).6 On the cell surface, CD11b/CD18 can form molecular complexes with other leucocyte receptors, such as FcγRI, FcγRII, FcγRIIIB, uPAR (CD87), and CD14,7 and act in concert with CD14 and TLR-4 to elicit full LPS-induced gene expression.8 Upon LPS triggering, monocytes and macrophages produce a panel of proinflammatory cytokines such as interleukin-6 (IL-6), IL-8 and tumour necrosis factor-α (TNF-α). Although the inflammatory response is mediated by a variety of secreted factors, the harmful effects of LPS have mainly been ascribed to TNF-α activity.9–11 IL-6. on the other hand. induces acute-phase protein (APP) synthesis by the liver.12
For many of the APP, no specific biological functions have yet been identified. However, some of them exhibit a variety of immunomodulatory effects.13–19 Among those, haptoglobin (Hp) has been reported to be a potent anti-inflammatory agent.18,20–25 Hp has been shown to inhibit the respiratory burst and rise in intracellular calcium in neutrophils following their stimulation with N-formyl-methionyl-leucyl-phenylalanine.15 It also suppresses lectin- and LPS-induced proliferation of T lymphocytes and B cells13,14,16,26–28 and it modulates several macrophage functions.13 It was further demonstrated that Hp is taken up and stored in monocytes and neutrophils within a cytoplasmic granular compartment and subsequently secreted following exposure to Candida albicans29 or TNF-α.30 However, the mechanism of action of Hp on the immune and inflammatory system remains largely unresolved. Our group has previously identified the promiscuous Mac-1 leucocyte integrin β2 (CD11b/CD18) as a receptor for Hp on monocytes, macrophages, granulocytes, natural killer cells, and a small subset of B and CD8+ T lymphocytes.31 Additionally, we have demonstrated non-CD11b/CD18-mediated binding of Hp to mast cells32 and T lymphocytes27 through an as yet unidentified receptor. Another Hp receptor is the CD22 molecule expressed on B cells,33 and Hp blocks binding of CD22 to TNF-α-activated endothelial cells33 thereby potentially reducing B-lymphocyte trafficking. This wide receptor distribution might reflect the multifaceted suppressive effects of Hp on different cell types of the immune system.
The ability of Hp to antagonize endotoxin effects was first addressed by Baseler and Burrell on rabbit B-lymphocyte mitogenesis and alveolar macrophage prostaglandin E release using Hp purified from rabbits in the acute phase.13
We now studied the effects of Hp on the release of pro- and anti-inflammatory cytokines following endotoxin stimulation and cytokine priming of human monocytes, as well as on the expression of one of the LPS receptors, the CD14 antigen. We further investigated the contribution of serum factors and the Mac-1 receptor to both cytokine release and Hp effects. In vivo evidence of anti-inflammatory effects came from murine models of LPS-induced bronchopulmonary hyperreactivity (BHR) and endotoxic shock. Our results suggest that Hp is a selective suppressor of certain monocyte functions and may thus be considered as a model protein for studies on the anti-inflammatory potential of APP.
Materials and methods
Cytokines and reagents
Recombinant interferon-γ (IFN-γ) was from Roche Diagnostics (Mannheim, Germany). Lipopolysaccharide (LPS), from Escherichia coli, serotype 0111:B4, was from Difco Laboratories (Detroit, MI). Apyrogen X-Vivo15 serum-free culture medium, a medium shown to support monocyte growth and survival, and phosphate-buffered saline (PBS) were from BioWhitaker (Walkersville, MD).
Polyclonal and monoclonal antibodies
Rabbit polyclonal anti-human Hp antibodies were purchased from Dako (Glostrup, Denmark). Fluorescein isothiocyanate (FITC)-labelled anti-CD14 monoclonal antibody [mAb; immunoglobulin G2b (IgG2b)] was from Becton Dickinson (San Diego, CA), anti-CD11b mAb clone 44 (IgG1) was a gift from L.A. Mayo-Bond (University of Michigan).
Purification of Hp from human serum
Hp from healthy individuals with an Hp1–1 phenotype was purified by affinity chromatography using a rabbit anti-human Hp-coated Sepharose 4B column (Pharmacia Biotech, Uppsala, Sweden). Serum was applied to the column and eluted with 0·1 m PBS, pH 7·4. The column was washed overnight with the same buffer. Then Hp was eluted with 0·1 m glycine–HCl, pH 2·3. The eluted Hp fractions were collected in 1 m Tris–HCl buffer to neutralize the acidic pH. The Hp-containing fractions were dialysed overnight against a 50-fold excess of 0·1 m PBS, pH 7·4. The Hp content was quantified by measuring the absorbance at 280 nm using a molar extinction coefficient of 10·2 × 104.34 Then it was concentrated by ultrafiltration using a filter with a cut-off of 30 000 molecular weight (Amicon, Danvers, MA), and sterilized by filtration through a 0·22-μm pore size filter (Gelman Sciences Inc., Ann Arbor, MI).
Isolation of peripheral blood mononuclear cells (PBMC)
PBMC from the blood of healthy individuals were isolated on Lymphoprep™, Nycomed Pharma AS (Oslo, Norway). After three washings in PBS, cells were resuspended in X-VIVO 15 serum-free medium, or in X-Vivo15 supplemented with 10% affinity chromatography-obtained Hp-free serum (HFS). Cell viability was always > 96% as assessed by a trypan blue dye exclusion method.
Cell culture for induction of cytokine production and CD14 expression
One million PBMCs were incubated in either X-Vivo15 apyrogene serum-free medium or in 10% HFS-supplemented X-Vivo15 medium, both supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml). Cells were triggered with either LPS (1 μg/ml) alone or in combination with IFN-γ (250 U/ml) for either 24 hr for CD14 receptor expression studies or for 48 or 72 hr for induction of cytokine release.
Assessment of cytokine production
The production of TNF-α, IL-1 receptor antagonist (IL-1ra), IL-6, IL-8, IL-10 and IL-12 p70 by stimulated PBMC was measured in cell-free supernatants by quantitative enzyme-linked immunosorbent assays (ELISA).
For TNF-α measurement, capture mAb clones 68B2B3 (IgG2) and 68B6A3 (IgG1) and detection mAb clone 68B3C5 (IgG1) were obtained from BioSource Europe (Nivelles, Belgium). Anti-IL-10 capture mAb clone JES10-5A2 and detection mAb clone JES3-12G8 were from Pharmingen (San Diego, CA). The anti-IL-12p70 capture mAb, clone 24945.11 (IgG1) and detection anti-human IL-12 (anti-hIL-12) mAb were from R & D Systems (Minneapolis, MN). Detection mAbs were used in biotinylated form.
Streptavidin–peroxidase conjugate (Lucron, Brussels, Belgium) and 3,3′,5,5′-tetramethylbenzidine (TMB) (Acros Organics, Pittsburgh, PA) were used for detection.
Release of IL-6, IL-8 and IL-1ra was evaluated using Human Flexia™ kits from Biosource Europe (Nivelles, Belgium). Mouse anti-hIL-6 mAb clone 677B6A2 (IgG1), anti-hIL-8 mAb clone 893A6G8 (IgG1) and anti-hIL-1ra mAb clone 998A2A2 (IgG1) were used for coating; mouse anti-hIL-6 mAb clone 505E23C7 (IgG1), anti-hIL-8 mAb clone 790A28G2 (IgG1) and anti-hIL-1ra mAb clone A71B6D11 (IgG1) were used for detection as peroxidase conjugates.
As standards, recombinant human cytokines were used: rIL-6, rIL-8, rIL-12, rIL-1ra and rTNF-α were from BioSource Europe and rIL-10 was from PeproTech Inc. (Rocky Hill, NJ). The sensitivity was < 10 pg/ml for all assays.
Flow cytometric analysis of monocyte CD14 expression
To study CD14 expression on peripheral blood monocytes, PBMC were depleted of CD4+ and CD8+ T lymphocytes by magnetic immunoselection using anti-CD4 and anti-CD8 Dynabeads from Dynal (Oslo, Norway). The percentage of monocytes in the remaining population exceeded 50%. CD14 receptor expression on monocytes was detected by flow cytometry. Fresh or cultured cells were washed in PBS and incubated with 1% human serum albumin and 0·1% human IgG for 30 min at room temperature to prevent non-specific binding and block Fc-γ receptors. After a second wash, cells were stained with FITC-labelled anti-CD14 mAb (IgG2b) for 30 min at 4°, followed by two washes with PBS and fixation in 1% paraformaldehyde in PBS. The mean fluorescence intensity (MFI) of gated monocytes was determined using a FACSort and cellquest software (Becton Dickinson, Mountain View, CA).
Mice
C57BL/6 Hp knockout mice (Hp−/−) were generated by homologous recombination35 and provided by Dr F. Berger (University of South Carolina, Columbia, SC). Control wild-type mice (Hp+/+) of the same strain were obtained from Harlan Netherlands (Horst, the Netherlands). Mice were housed in the animal facility at Gasthuisberg University Hospital (Leuven, Belgium) in specific pathogen-free conditions, allowing free access to food and water. All procedures involving animals were performed according to the guidelines of the Animal Ethical Committee of the Catholic University of Leuven.
Induction and measurement of bronchopulmonary hyperresponsiveness
Both Hp+/+ and Hp−/− male mice, 7–8 weeks old, were intraperitoneally (i.p.) administered a 10-μg dose of LPS to induce the acute-phase response. To induce BHR, a second dose of LPS (25 μg/mouse) was given i.p. 36 hr later. After 180 min36 airway responsiveness was assessed by methacholine-induced airflow obstruction in conscious mice placed in a whole body plethysmograph (Buxco®, EMKA Technologies, Paris, France). Airway responsiveness was evaluated by the enhanced pause (Penh), a calculated value which correlates with measurement of airway resistance.37 The Penh was calculated for each mouse at resting state, 3 min after saline nebulization, and after increasing doses of methacholine (10, 25 and 50 mg/ml) for 1 min.
Induction of endotoxic shock
Male Hp+/+ and Hp−/− mice (∼8 weeks old, ∼25 g of body weight) were subjected to a high-dose endotoxic shock. Each mouse received 500 μg of LPS i.p., previously determined as being the lowest lethal dose in wild-type mice. Mouse survival was monitored every 12 hr for 2 days and daily afterwards.
Statistical analysis
Comparisons between the effects generated in control cultures (without Hp) and in the presence of different Hp concentrations were performed using the one-way analysis of variance (anova) test followed by a Dunett's multiple comparisons test using the instat program, graphpad Software (San Diego, CA). Comparison between Penh values in different groups of mice was performed using a Student's t-test. Differences in survival were determined by χ2 analysis. Kaplan–Meier curves were used to show survival over time, and differences between curves were analysed using a log-rank test. Statistical significance was set at P ≤ 0·05.
Results
Dose-dependent suppression of LPS-induced TNF-α, IL-10 and IL-12 p70 production by Hp
In healthy individuals, Hp occurs at serum concentrations varying from 0·3 to 2 mg/ml and these concentrations increase further during inflammatory conditions. We first investigated the production of TNF-α, IL-10 and IL-12p70 by LPS-challenged human PBMCs and their modulation by increasing physiological doses of Hp. No cytokine production could be detected after 3 days of culture in the absence of exogenous LPS. As shown in Fig. 1(a), TNF-α and IL-10 were produced in relatively high amounts upon LPS stimulation. The release was progressively suppressed by adding increasing doses of Hp. The minimal dose of Hp which had inhibitory effects on the release of the two cytokines was 250 μg/ml. IL-12p70 was also produced in low but detectable amounts following LPS stimulation, and its production was also strongly dampened by Hp. A dose of Hp as low as 50 μg/ml inhibited 60% of the IL-12 production, whereas doses ≥ 250 μg/ml resulted in about 80% inhibition.
Figure 1.
Dose-dependent effect of haptoglobin on LPS-induced cytokine release by PBMC. (a) PBMC (1 × 106/ml) were incubated in serum-free culture medium. LPS (1 μg) was added at the beginning of the culture, Hp was added simultaneously resulting in final concentrations of 0, 50, 250, 500, or 1000 μg/ml. Cytokine production was assessed by ELISA after 72 hr of culture. Results are reported as mean ± SEM of four (TNF-α and IL-10) and three (IL-12p70) independent experiments. (b) Results for IL-1ra, IL-6 and IL-8 are reported as mean ± SEM from six experiments. (c) PBMC (1 × 106/ml) were cultured in X-Vivo15 medium in the presence of 1 μg LPS, 250 U rIFN-γ, and increasing Hp concentrations. TNF-α and IL-12p70 concentration was assessed after 3 days of culture using ELISA. Results are reported as mean ± SEM of four independent experiments. *P < 0·05, **P < 0·01 vs. control.
Hp effects were selective for certain cytokines, as Hp was not able to inhibit the LPS-induced production of IL-6, IL-8 and IL-1ra (Fig. 1b).
LPS and IFN-γ have synergistic effects for induction of TNF-α and IL-1238 and surface expression of intercellular adhesion molecule type 1.39 Very small doses of LPS can be extremely stimulating in the presence of IFN-γ.38 Under these experimental conditions, the addition of IFN-γ and LPS simultaneously at the beginning of the culture indeed led to a 3·9-fold and 8·8-fold enhancement of the production of TNF-α and IL-12p70, respectively, as compared to cultures from the same donors with LPS alone (control data not shown). Still, Hp significantly inhibited cytokine production: Hp at doses of 50, 250, 500 and 1000 μg/ml resulted in inhibitions of 22, 68, 82 and 88% for TNF-α, and 49, 57, 60 and 69% for IL-12p70 release, respectively (Fig. 1c).
The data thus show that Hp has anti-inflammatory effects by suppressing the release of LPS-induced cytokine production.
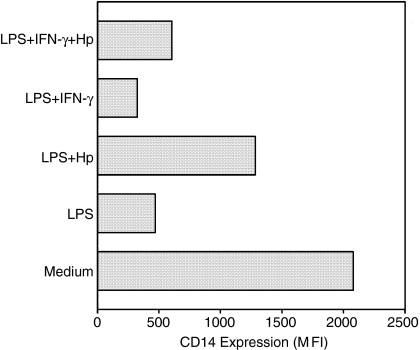
Hp maintains CD14 expression on monocytes following LPS stimulation
Down-regulation of CD14 expression on monocytes following in vitro stimulation with LPS has been reported.40 Under our experimental conditions, using cultures of enriched monocytes, monocytes showed a high spontaneous CD14 expression. Stimulation overnight with 1 μg of LPS led to 76% inhibition of CD14 expression, whereas the presence of Hp reduced this effect to 44% (Fig. 2). Priming with IFN-γ reinforced the suppressive effect of LPS (84% inhibition of CD14 expression), and in the presence of Hp, inhibition was 73% (Fig. 2).
Figure 2.
Effect of Hp on the expression of surface CD14 by LPS-stimulated monocytes. One million monocyte-enriched PBMCs were cultured overnight in the presence of LPS (1 μg/ml) with or without Hp (1 mg/ml) and IFN-γ (250 U/ml). Cells were labelled for CD14, and CD14 expression on the gated monocyte population was expressed as the MFI. Results are reported from one representative out of two experiments.
CD11b/CD18 receptor is involved in LPS activation but is not required for Hp effects
We have previously reported that Hp is one of the ligands of the CD11b/CD18 receptor,31 which suggests that at least a part of the anti-inflammatory effects of Hp could be mediated through triggering or blocking of this receptor. In addition, some alternative CD11b/CD18 ligands, such as C3bi and heparin, were shown to act as inhibitors of IL-1241 and TNF-α42 secretion, respectively.
Anti-CD11b mAb clone 44, known to markedly inhibit the binding of Hp to its receptor on monocytes31 and to induce IL-1β mRNA synthesis in monocytes,43 inhibited the production of IL-10 by LPS-stimulated monocytes. However, the addition to the LPS-driven cell cultures of this anti-CD11b mAb did not modify the inhibitory effect of Hp on TNF-α and IL-10 release (Fig. 3). CD11b thus is not the main conductor of Hp inhibitory effects.
Figure 3.
Effect of anti-CD11b mAb and/or Hp on LPS-induced cytokine production by monocytes. PBMCs (1 × 106/ml) were incubated with or without 10 μg of the anti-CD11b mAb (clone 44) for 30 min at room temperature. Then 1 μg of LPS and/or 500 μg of Hp were added. TNF-α and IL-10 production were evaluated after 2 days of culture. The results shown are from three different donors.
The effect of Hp is influenced by serum factors
The role of LPS-binding protein (LBP) in LPS-induced proinflammatory cytokine production by monocytes is well established.1,10 LBP increases the affinity of CD14 for LPS and strengthens the intracellular signal resulting from binding to the CD14/TLR-4 receptor complex. Therefore, we cultured the cells either in serum-free medium, or in X-Vivo15 medium supplemented with HFS as a source of LBP. As expected, LPS at a concentration of 1 μg/ml induced more TNF-α production in the presence than in the absence of serum, 593 ± 65 pg/ml versus 328 ± 38 pg/ml (mean ± SEM, n = 4). Importantly, in the presence of LBP, Hp failed to suppress TNF-α production at doses ranging from 50 to 500 μg/ml and only concentrations above 500 μg/ml resulted in a significant decrease of TNF-α release (Fig. 4).
Figure 4.
PBMCs (1 × 106/ml) were incubated in either serum-free culture medium (SFM) or 10% Hp-free serum (HFS)-supplemented medium. At the beginning of the culture, 1 μg of LPS and Hp was added, the latter at final concentrations of 0, 50, 250, 500, or 1000 μg/ml. TNF-α production was assessed using ELISA after 72 hr of culture. Results are reported as mean ± SEM of four independent experiments. *P < 0·05, **P < 0·01 versus control.
Hp-knockout mice are more susceptible to LPS effects
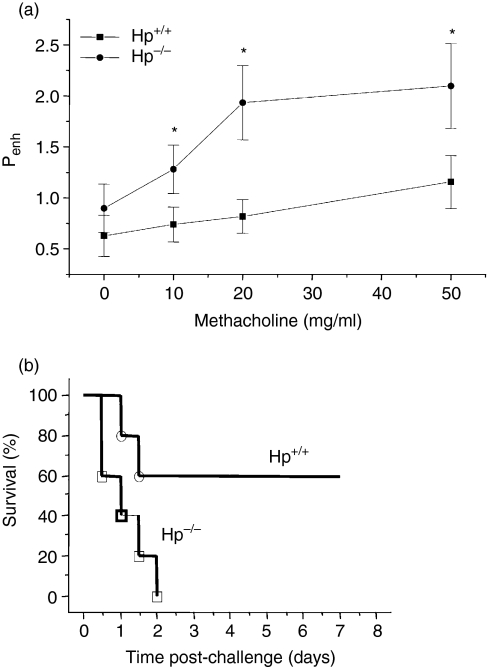
To examine the potential protective anti-endotoxin effects of Hp in an in vivo system, we have selected two mouse models of endotoxin challenge. In the first model, we have induced bronchopulmonary hyper-reactivity in both Hp+/+ and Hp−/− mice by intraperitoneal administration of 25 μg of E. coli LPS.36 Although the mechanisms involved in this model remain unclear, there is evidence discarding the involvement of TNF-α and neutrophil recruitment into the lungs in the induction of BHR.36 Since the basal level of Hp in wild-type mice is low, we first administered 10 μg of LPS 36 hr prior to LPS challenge to both groups of mice to induce an acute-phase reaction. Mice were tested for airway reactivity to increasing concentrations of inhaled methacholine 3 hr after administration of LPS.
As shown in Fig. 5(a), Penh increased with increasing doses of methacholine in both Hp+/+ and Hp−/− mice. However, Hp−/− mice exhibited higher Penh than did the Hp+/+ mice when methacholine was given at concentrations of 10, 20 and 50 mg/ml. There were no significant differences in basal (PBS inhalation) Penh between the two groups of mice.
Figure 5.
Hp-deficient mice are more susceptible to LPS-induced bronchopulmonary hyperresponsiveness and endotoxic shock. (a) Hp+/+ and Hp−/− mice were injected i.p. with 10 μg of LPS. Thirty-six hours later, they received a second i.p. injection of 25 μg LPS. Penh was measured in response to nebulized saline and to different doses of methacholine using the Buxco® body plethysmograph. Results are reported as the mean ± SEM of 10 mice per group. *P < 0·05. (b) Mice (10 per group) were given an i.p. injection of 500 μg E. coli LPS and survival was monitored daily for 1 week.
In the second model, we induced endotoxic shock in mice using a high dose of LPS. Endotoxic shock is a pathophysiological condition resulting from a cascade of events that frequently have a fatal outcome. The high-dose endotoxin model involves lethal amounts of LPS without presensitization of the animals, and the mechanisms involved in toxicity are mainly driven by a massive infiltration of neutrophils into the liver.44
When given a lethal dose of endotoxins, Hp-deficient mice showed a more pronounced vulnerability to the shock as they all succumbed during the first 2 days after the LPS injection. Wild-type mice showed resistance; 60% of wild-type mice survived over a 1-week period (P = 0·0035) (Fig. 5b).
Discussion
In this manuscript, we report that Hp reduces the effects of LPS on monocytes in vitro and protects the host against the deleterious effect of LPS in vivo. In the first place, we studied Hp effects on LPS-induced proinflammatory cytokine production by monocytes/macrophages for the pivotal role they play in the onset and progression of the inflammatory reaction. Our results revealed a very clear suppressive effect of Hp on the LPS-induced release of TNF-α, IL-10 and IL-12p70 from human monocytes. In the presence of IFN-γ, LPS was more effective at inducing these cytokines, especially IL-12. While IFN-γ renders monocytes more sensitive to LPS, it also enhances their resistance to the inhibitory potential of Hp as far as IL-12 production is concerned. Interestingly, Hp failed to exert any effect on IL-6, IL-8, or IL-1ra release by LPS-stimulated PBMCs. The effect of Hp is thus selective. Hp effects do not discriminate between pro- and anti-inflammatory cytokines, as effects on IL-10 and IL-1ra, two anti-inflammatory cytokines, were discordant, similar to the discordant effects on TNF-α and IL-8, two proinflammatory products. The Hp inducer, IL-6, was not affected by Hp. One could hypothesize that the discrimination of Hp between different monocytic products might be the result of the disparities in gene structure and expression mechanisms, but a clear explanation is still lacking.
The in vivo relevance of Hp effects was further elaborated in animal models of endotoxic shock and endotoxin-induced bronchial hyperreactivity. Our data with the Hp+/+ and Hp−/− mice in this model of LPS-induced BHR revealed an evident role of endogenous Hp in inhibiting airway hyperreactivity as measured by airway obstruction in response to methacholine. Although the mechanism underlying the development of BHR in response to systemic endotoxins is still unknown, the only cellular targets of endotoxins are the monocytes/macrophages and the neutrophils, which both also exhibit specific binding of Hp. It is likely that the dampening effect of Hp on BHR in Hp+/+ mice is a consequence of the Hp effect on those cell types.
The mechanisms responsible for the effects of Hp on endotoxin-induced cytokine release are not yet clear.
A variety of human plasma components have been shown to counteract the effects of LPS on monocytes, namely different types of lipoproteins,45 lactoferrin,46 iC3b,41 C5a,47 IL-4, IL-10, IL-13 and transforming growth factor-β48 and anticoagulant activated protein C.21 The antagonistic capacity of these factors is mediated either by their ability to interfere with LPS-CD14 binding, or by transmission of negative signals through their respective receptors on monocytes. Which of both mechanisms is responsible for the interference of Hp with LPS effects is still to be defined. An intracellular mechanism is certainly not excluded because it is known that Hp enters monocytes and neutrophils29 which could interrupt cellular functions triggered by intracellular LPS. It might also be hypothesized that the observed Hp inhibitory effects on monocyte function could be the result of the release by monocytes of monocyte-deactivating mediators such as IL-10, prostaglandin E2 (PGE2) or transforming growth factor-β. However, IL-10 production was markedly suppressed by Hp. PGE2 release was not measured but it is unlikely that it could contribute to the observed effects because Hp is known to inhibit prostaglandin synthase and PGE2 synthesis.13,18,24,49
The fact that mAb 44, which interferes with Hp binding to CD11b,31 did not prevent Hp effects on cytokine release makes it unlikely that functional Hp effects are mediated only through CD11b binding. This strongly argues against the expression of only one Hp-binding receptor on peripheral blood monocytes, as was shown for the THP-1 cell line.31 A similar finding suggested the presence of a second, unidentified receptor on T cells.27
LBP is known to increase the affinity of CD14 to LPS.1 When cultured in the presence of Hp-free serum as a source of LBP, monocytes show more resistance to the suppressive effects of Hp. This could be explained by the presence in the serum of factors that may interfere with Hp binding to its receptor (i.e. potential ligands sharing the same receptor as Hp), or with LPS–CD14 interaction such as LBP. The latter hypothesis remains however, unlikely because LBP is not required when LPS is used at high doses.50 The inability of Hp to act at low doses may reflect its neutral role in the immune system under normal physiological conditions. However, the initiation of an inflammatory process will increase Hp synthesis by the liver, and Hp might then exhibit its anti-inflammatory potential to slow down the inflammation.
One possibility for explaining the effects of Hp is the interference with the LPS-binding/signalling molecular cluster, namely with LPS binding to CD14 or with signal transduction through TLR-4. Our experiments showed however, that FITC-labelled LPS binds to human peripheral blood monocytes similarly in the presence and absence of Hp (unpublished data). Additionally, in our hands, Hp did not influence TLR-4 expression on monocytes (unpublished data). In this view, we may hypothesize that Hp may act through TLR-4 by preventing the transfer of LPS from CD14 to TLR-4. This requires, however, more investigation.
To corroborate our in vitro findings, we have used two murine models of endotoxin challenge. Despite the divergences in the molecular and cellular mechanisms underlying our models, the macrophage remains the primary sensor of endotoxins, and the predominant trigger of the inflammatory response and its physiological consequences. Hp knockout mice react with more pronounced responses to the devastating effects of endotoxins, and this is presumably because of the absence of Hp and its suppressive effects on macrophages.
In summary, we have shown that Hp suppresses LPS-induced TNF-α, IL-10 and IL-12p70 release in PBMC cultures, and this effect was accompanied by a preservation of CD14 receptor expression. In vivo evidence for the anti-endotoxic effects of Hp is also provided. Our observations suggest that Hp, through binding to monocytes, may act as a selective endogenous modulator of inflammation, by preventing the potentially harmful proinflammatory cytokine overproduction. Hp might also favour a Th2 cytokine environment by dampening IL-12 production. The interference of Hp with various cellular events in monocytes may be pointed to as an important model for studies related to the role of APP in the immune system. Hp is a tempting candidate for future investigations that aim to develop new therapeutic anti-inflammatory agents.
Acknowledgments
The authors are grateful to Dr L.A. Mayo-Bond (University of Michigan, Ann Arbor, MI) for providing the anti-CD11b mAb clone 44, to Dr M. Langlois and Dr J. Delanghe (Central Laboratory, Ghent University Hospital, Belgium) for determining haptoglobin phenotypes in sera, and to Ms Martine Adé for excellent technical assistance. This work was supported by a grant from the Research Council of the Catholic University of Leuven (onderzoekstoelage) and by SCAP (Study Center for Allergy Projects), Haarlem, the Netherlands.
Abbreviations
- APP
acute-phase protein
- HFS
haptoglobin-free serum
- Hp
haptoglobin
- IL-1ra
interleukin-1 receptor antagonist
- LBP
lipopolysaccharide-binding protein
- LPS
lipopolysaccharide
- MFI
mean fluorescence intensity
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate-buffered saline
- Penh
enhanced pause
- SFM
serum-free medium
References
- 1.Fenton MJ, Golenbock DT. LPS-binding proteins and receptors. J Leukoc Biol. 1998;64:25–32. doi: 10.1002/jlb.64.1.25. [DOI] [PubMed] [Google Scholar]
- 2.Means TK, Lien E, Yoshimura A, Wang S, Golenbock DT, Fenton MJ. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J Immunol. 1999;163:6748–55. [PubMed] [Google Scholar]
- 3.Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12:20–6. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty SF, Golenbock DT, Milham FH, Ingalls RR. CD11/CD18 leukocyte integrins: new signaling receptors for bacterial endotoxin. J Surg Res. 1997;73:85–9. doi: 10.1006/jsre.1997.5195. 10.1006/jsre.1997.5195. [DOI] [PubMed] [Google Scholar]
- 5.Medvedev AE, Flo T, Ingalls RR, Golenbock DT, Teti G, Vogel SN, Espevik T. Involvement of CD14 and complement receptors CR3 and CR4 in nuclear factor-kappaB activation and TNF production induced by lipopolysaccharide and group B streptococcal cell walls. J Immunol. 1998;160:4535–42. [PubMed] [Google Scholar]
- 6.Tohme ZN, Amar S, Van Dyke TE. Moesin functions as a lipopolysaccharide receptor on human monocytes. Infect Immun. 1999;67:3215–20. doi: 10.1128/iai.67.7.3215-3220.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todd RF, 3rd, Petty HR. Beta 2 (CD11/CD18) integrins can serve as signaling partners for other leukocyte receptors. J Lab Clin Med. 1997;129:492–8. doi: 10.1016/s0022-2143(97)90003-2. 10.1016/S0022-2143(97)90003-2. [DOI] [PubMed] [Google Scholar]
- 8.Perera PY, Mayadas TN, Takeuchi O, Akira S, Zaks-Zilberman M, Goyert SM, Vogel SN. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J Immunol. 2001;166:574–81. doi: 10.4049/jimmunol.166.1.574. [DOI] [PubMed] [Google Scholar]
- 9.Tracey KJ, Beutler B, Lowry SF, et al. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–4. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 10.Karima R, Matsumoto S, Higashi H, Matsushima K. The molecular pathogenesis of endotoxic shock and organ failure. Mol Med Today. 1999;5:123–32. doi: 10.1016/s1357-4310(98)01430-0. 10.1016/S1357-4310(98)01430-0. [DOI] [PubMed] [Google Scholar]
- 11.Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–71. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 12.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 13.Baseler MW, Burrell R. Purification of haptoglobin and its effects on lymphocyte and alveolar macrophage responses. Inflammation. 1983;7:387–400. doi: 10.1007/BF00916303. [DOI] [PubMed] [Google Scholar]
- 14.Oh SK, Kim SH, Walker JE. Interference with immune response at the level of generating effector cells by tumor-associated haptoglobin. J Natl Cancer Inst. 1990;82:934–40. doi: 10.1093/jnci/82.11.934. [DOI] [PubMed] [Google Scholar]
- 15.Oh SK, Pavlotsky N, Tauber AI. Specific binding of haptoglobin to human neutrophils and its functional consequences. J Leukoc Biol. 1990;47:142–8. doi: 10.1002/jlb.47.2.142. [DOI] [PubMed] [Google Scholar]
- 16.Oh SK, Ross S, Walker J, Zeisel S. Role of a SER immune suppressor in immune surveillance. Immunology. 1988;64:73–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Jeannin P, Lecoanet-Henchoz S, Delneste Y, Gauchat JF, Bonnefoy JY. Alpha-1 antitrypsin up-regulates human B cell differentiation selectively into IgE- and IgG4-secreting cells. Eur J Immunol. 1998;28:1815–22. doi: 10.1002/(SICI)1521-4141(199806)28:06<1815::AID-IMMU1815>3.0.CO;2-5. 10.1002/(SICI)1521-4141(199806)28:06<1815::AID-IMMU1815>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Kendall PA, Saeed SA, Collier HO. Identification of endogenous inhibitor of prostaglandin synthetase with haptoglobin and albumin [proceedings] Biochem Soc Trans. 1979;7:543–5. doi: 10.1042/bst0070543. [DOI] [PubMed] [Google Scholar]
- 19.Bennett M, Schmid K. Immunosuppression by human plasma alpha 1-acid glycoprotein: importance of the carbohydrate moiety. Proc Natl Acad Sci USA. 1980;77:6109–13. doi: 10.1073/pnas.77.10.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hennet T, Chui D, Paulson JC, Marth JD. Immune regulation by the ST6Gal sialyltransferase. Proc Natl Acad Sci USA. 1998;95:4504–9. doi: 10.1073/pnas.95.8.4504. 10.1073/pnas.95.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grey ST, Tsuchida A, Hau H, Orthner CL, Salem HH, Hancock WW. Selective inhibitory effects of the anticoagulant activated protein C on the responses of human mononuclear phagocytes to LPS, IFN-gamma, or phorbol ester. J Immunol. 1994;153:3664–72. [PubMed] [Google Scholar]
- 22.Buckley CD, Pilling D, Henriquez NV, et al. RGD peptides induce apoptosis by direct caspase-3 activation. Nature. 1999;397:534–9. doi: 10.1038/17409. 10.1038/17409. [DOI] [PubMed] [Google Scholar]
- 23.Pagano M, Nicola MA, Engler R. Inhibition of cathepsin L and B by haptoglobin, the haptoglobin-hemoglobin complex, and asialohaptoglobin. ‘In vitro’ studies in the rat. Can J Biochem. 1982;60:631–7. doi: 10.1139/o82-078. [DOI] [PubMed] [Google Scholar]
- 24.Saeed SA, Mahmood F, Shah BH, Gilani AH. The inhibition of prostaglandin biosynthesis by human haptoglobin and its relationship with haemoglobin binding. Biochem Soc Trans. 1997;25:S618. doi: 10.1042/bst025s618. [DOI] [PubMed] [Google Scholar]
- 25.Arredouani M, Ceuppens JL. Haptoglobin – a new link between innate and adaptive immunity. Curr Trends Immunol. 2002;4:73–83. [Google Scholar]
- 26.Samak R, Edelstein R, Israel L. Immunosuppressive effect of acute-phase reactant proteins in vitro and its relevance to cancer. Cancer Immunol Immunother. 1982;13:38–43. doi: 10.1007/BF00200198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arredouani M, Matthijs P, Van Hoeyveld E, Kasran A, Baumann H, Ceuppens JL, Stevens E. Haptoglobin directly affects T cells and suppresses T helper cell type 2 cytokine release. Immunology. 2003;108:144–51. doi: 10.1046/j.1365-2567.2003.01569.x. 10.1046/j.1365-2567.2003.01569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arredouani M, Matthys P, Kasran A, Baumann H, Ceuppen JL. Haptoglobin and the Th1/Th2 balance. hints from in vitro and in vivo studies. Redox Rep. 2001;6:369–71. doi: 10.1179/135100001101536481. [DOI] [PubMed] [Google Scholar]
- 29.Wagner L, Gessl A, Parzer SB, Base W, Waldhausl W, Pasternack MS. Haptoglobin phenotyping by newly developed monoclonal antibodies. Demonstration of haptoglobin uptake into peripheral blood neutrophils and monocytes. J Immunol. 1996;156:1989–96. [PubMed] [Google Scholar]
- 30.Berkova N, Gilbert C, Goupil S, Yan J, Korobko V, Naccache PH. TNF-induced haptoglobin release from human neutrophils. Pivotal role of the TNF p55 receptor. J Immunol. 1999;162:6226–32. [PubMed] [Google Scholar]
- 31.El Ghmati SM, Van Hoeyveld EM, Van Strijp JG, Ceuppens JL, Stevens EA. Identification of haptoglobin as an alternative ligand for CD11b/CD18. J Immunol. 1996;156:2542–52. [PubMed] [Google Scholar]
- 32.El-Ghmati SM, Arredouani M, Van Hoeyveld EM, Ceuppens JL, Stevens EA. Haptoglobin interacts with the human mast cell line HMC-1 and inhibits its spontaneous proliferation. Scand J Immunol. 2002;55:352–8. doi: 10.1046/j.1365-3083.2002.01067.x. 10.1046/j.1365-3083.2002.01067.x. [DOI] [PubMed] [Google Scholar]
- 33.Hanasaki K, Powell LD, Varki A. Binding of human plasma sialoglycoproteins by the B cell-specific lectin CD22. Selective recognition of immunoglobulin M and haptoglobin. J Biol Chem. 1995;270:7543–50. doi: 10.1074/jbc.270.13.7543. 10.1074/jbc.270.13.7543. [DOI] [PubMed] [Google Scholar]
- 34.Elson EC. Quantitative determination of serum haptoglobin. A simple and rapid method. Am J Clin Pathol. 1974;62:655–63. doi: 10.1093/ajcp/62.5.655. [DOI] [PubMed] [Google Scholar]
- 35.Lim SK, Kim H, bin Ali A, Lim YK, Wang Y, Chong SM, Costantini F, Baumman H. Increased susceptibility in Hp knockout mice during acute hemolysis. Blood. 1998;92:1870–7. [PubMed] [Google Scholar]
- 36.Lefort J, Singer M, Leduc D, et al. Systemic administration of endotoxin induces bronchopulmonary hyperreactivity dissociated from TNF-alpha formation and neutrophil sequestration into the murine lungs. J Immunol. 1998;161:474–80. [PubMed] [Google Scholar]
- 37.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156:766–75. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 38.Held TK, Weihua X, Yuan L, Kalvakolanu DV, Cross AS. Gamma interferon augments macrophage activation by lipopolysaccharide by two distinct mechanisms, at the signal transduction level and via an autocrine mechanism involving tumor necrosis factor alpha and interleukin-1. Infect Immun. 1999;67:206–12. doi: 10.1128/iai.67.1.206-212.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Most J, Schwaeble W, Drach J, Sommerauer A, Dierich MP. Regulation of the expression of ICAM-1 on human monocytes and monocytic tumor cell lines. J Immunol. 1992;148:1635–42. [PubMed] [Google Scholar]
- 40.Bazil V, Strominger JL. Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. J Immunol. 1991;147:1567–74. [PubMed] [Google Scholar]
- 41.Marth T, Kelsall BL. Regulation of interleukin-12 by complement receptor 3 signaling. J Exp Med. 1997;185:1987–95. doi: 10.1084/jem.185.11.1987. 10.1084/jem.185.11.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Attanasio M, Gori AM, Giusti B, et al. Cytokine gene expression in human LPS- and IFNgamma-stimulated mononuclear cells is inhibited by heparin. Thromb Haemost. 1998;79:959–62. [PubMed] [Google Scholar]
- 43.Rezzonico R, Chicheportiche R, Imbert V, Dayer JM. Engagement of CD11b and CD11c beta2 integrin by antibodies or soluble CD23 induces IL-1beta production on primary human monocytes through mitogen-activated protein kinase-dependent pathways. Blood. 2000;95:3868–77. [PubMed] [Google Scholar]
- 44.Gutierrez-Ramos JC, Bluethmann H. Molecules and mechanisms operating in septic shock: lessons from knockout mice. Immunol Today. 1997;18:329–34. doi: 10.1016/s0167-5699(97)01085-2. 10.1016/S0167-5699(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 45.Netea MG, de Bont N, Demacker PN, Kullberg BJ, Jacobs LE, Verver-Jansen TJ, Stalenhoef AF, Van der Meer JW. Lipoprotein(a) inhibits lipopolysaccharide-induced tumor necrosis factor alpha production by human mononuclear cells. Infect Immun. 1998;66:2365–7. doi: 10.1128/iai.66.5.2365-2367.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baveye S, Elass E, Mazurier J, Spik G, Legrand D. Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin Chem Lab Med. 1999;37:281–6. doi: 10.1515/CCLM.1999.049. [DOI] [PubMed] [Google Scholar]
- 47.Wittmann M, Zwirner J, Larsson VA, Kirchhoff K, Begemann G, Kapp A, Gotze O, Werfel T. C5a suppresses the production of IL-12 by IFN-gamma-primed and lipopolysaccharide-challenged human monocytes. J Immunol. 1999;162:6763–9. [PubMed] [Google Scholar]
- 48.D'Andrea A, Ma X, Aste-Amezaga M, Paganin C, Trinchieri G. Stimulatory and inhibitory effects of interleukin (IL)-4 and IL-13 on the production of cytokines by human peripheral blood mononuclear cells: priming for IL-12 and tumor necrosis factor alpha production. J Exp Med. 1995;181:537–46. doi: 10.1084/jem.181.2.537. 10.1084/jem.181.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jue DM, Shim BS, Kang YS. Inhibition of prostaglandin synthase activity of sheep seminal vesicular gland by human serum haptoglobin. Mol Cell Biochem. 1983;51:141–7. doi: 10.1007/BF00230400. [DOI] [PubMed] [Google Scholar]
- 50.Troelstra A, Antal-Szalmas P, de Graaf-Miltenburg LA, Weersink AJ, Verhoef J, Van Kessel KP, Van Strijp JA. Saturable CD14-dependent binding of fluorescein-labeled lipopolysaccharide to human monocytes. Infect Immun. 1997;65:2272–7. doi: 10.1128/iai.65.6.2272-2277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]