Abstract
There is growing interest in the in vitro generation of dendritic cells (DC) from peripheral blood monocytes, but the effect of the method chosen to isolate CD14+ monocytes for subsequent DC generation is poorly documented. The method used to isolate monocytes may have an impact on the subsequent function of DC by affecting their ability to express costimulatory molecules (CD80/86), maturation marker (CD83) and/or to produce important immunomodulatory cytokines. In this study, we show that the positive selection of monocytes by anti-CD14-coated microbeads inhibits the lipopolysaccharide (LPS)-induced production of interleukin (IL)-12, IL-10 and tumour necrosis factor-α (TNF-α) from human DC. However, when DC were grown from monocytes isolated by plastic adherence, LPS induced the production of much higher levels of these cytokines. DC derived from adherence-isolated monocytes induced the development of potent cytotoxic T lymphocytes of the Tc1 subset specific for influenza matrix protein, as confirmed by interferon-γ (IFN-γ) enzyme-linked immunosorbent spot-forming cell assay (ELISPOT), cytotoxicity assay, major histocompatibility complex (MHC)–peptide tetrameric complexes and T helper 1/T helper 2 (Th1/Th2) cytokine production assays.
Keywords: CTL, cytokines, dendritic cells, lipopolysaccharide, monocytes
Introduction
Dendritic cells (DC) are potent antigen-presenting cells that can stimulate both B and T lymphocytes.1–3 There is increasing interest in the use of DC owing to their unique properties in inducing and regulating immune responses. These functions highlight their potential value in vaccines for infectious diseases and cancers and of their role in the induction of T-cell tolerance in other situations.4,5 Typically, immature DC capture and process antigen to peptides which are then presented in the context of major histocompatibility complex (MHC) class II or class I molecules, as shown by cross-priming.6 It is believed that the maturation of DC plays a central role in determining the outcome of immune responses. Maturation can be induced by several stimuli, such as bacterial-derived antigen [e.g. lipopolysaccharide (LPS)], inflammatory cytokines [e.g. tumour necrosis factor-α (TNF-α)], ligation of cell-surface receptor [e.g. CD40 by CD40 ligand (CD40L)] and viral product (e.g. double-stranded RNA).7–10 Upon activation, DC mature into potent immunostimulatory cells that can drive T-cell clonal expansion and, through production of immunomodulatory cytokines such as interleukin (IL)-10 and IL-12, promote the development of T helper 1 (Th1)/Tc1 or T helper 2 (Th2)/Tc2 effectors.11
Human DC can be generated in vitro from peripheral blood CD14+ monocytes, termed monocyte-derived DC (MoDC), or from CD34+ progenitors. Recently, it has been shown that DC can be isolated directly from peripheral blood by using blood dendritic cell antibodies (BDCA).12 Although recent methodological advances in cell purification have provided a number of choices for generating DC from different sources, each method should be carefully characterized for its application in clinical use. CD14+ cells can be isolated either by a positive selection method by using a magnetic activated cell sorting (MACS) method or by plastic adherence. The MACS method is rapid and produces high yields of pure cell populations. The effect of the method chosen to isolate CD14+ cells for subsequent DC generation is poorly defined but may have a significant impact on their future function. In this study, we investigated the effect of the separation method and different activation signals on the ability of DC to express the costimulatory molecules CD80/CD86 and the maturation marker CD83 and to produce immunomodulatory cytokines. Concomitant with this, we studied the difference of immunomodulatory functions, especially of cytokine production, between MACS-isolated monocyte-derived DC (designated MACS/MoDC), and plate-adherence-selected monocyte-derived DC (designated adherence/MoDC). We also investigated the functional capacity of adherence/MoDC to stimulate antigen-specific cytotoxic cells to the influenza matrix protein and their ability to induce cytokines in the responding cell populations.
Materials and methods
Monocyte isolation for subsequent DC generation
Fresh, whole blood was drawn with informed consent from healthy donors into vacutainer tubes (Becton Dickinson Vacutainer; Becton Dickinson, Plymouth, UK) containing EDTA. Peripheral blood mononuclear cells (PBMC) were isolated by using Histopaque-1077 (Sigma Chemicals, Poole, Dorset, UK), as previously described.13 CD14+ monocytes were isolated from PBMC either by positive selection using a MACS system (Miltenyi Biotech, Bergisch Gladbach, Germany), according to the manufacturer's protocol, or by plastic adherence. For monocyte isolation by plastic adherence, 5 × 106 PBMC per well were distributed into 12-well plates (Corning Inc. Costar, NY, USA), and allowed to adhere in a 5% CO2 incubator at 37° for 2 hr in 1 ml of RPMI-1640 containing 0·3 g/L l-glutamine (Sigma) supplemented with 5% (v/v) fetal calf serum (FCS, heat inactivated; Sigma), 100 U/ml penicillin (Sigma) and 0·1 mg/ml streptomycin (referred to as complete medium, 5% CM). Non-adherent cells were removed and the adherent cells were washed carefully, twice, with prewarmed 5% CM.
Generation and maturation of DC
Monocytes isolated by either MACS or adherence were cultured in 12-well flat-bottom plates containing 1 ml of 5% CM per well supplemented with 1000 U/ml recombinant human granulocyte–macrophage colony-stimulating factor (GM-CSF) (R & D Systems, Abingdon, UK) and 1000 U/ml human recombinant (hr)IL-4 (R & D Systems) for the generation of immature DC. Every 2 days 200 µl of the medium was exchanged with 5% CM containing cytokines.
After 6 days of culture, the cells were harvested, pooled together and counted. The supernatants were removed and kept frozen at −80° for assay of cytokine concentrations. Day 6 immature DC were matured by using 0·5 µg/ml of LPS from Escherichia coli 026:B6 (Sigma), 1 µg/ml of hrCD40L (R & D Systems) and 20 ng/ml of TNF-α (R & D Systems). Immature DC grown from MACS or adherence-isolated monocytes were aliquoted at 0·5 × 106 cells in 0·5 ml of supernatant suspension into each well and incubated at 37° with the stimulant for 15–20 min before adding 0·5 ml of fresh 5% CM with cytokines and then incubated further for 3 days.
The expression of CD14, CD1a, human leucocyte antigen (HLA)-DR, CD80, CD86 and CD83 on monocytes, immature DC and mature DC was analysed by flow cytometry (Dako Partec Galaxy flow cytometer; Dako, Münster, Germany). All monoclonal antibodies (mAbs) and their isotype-matched negative-control antibodies were purchased from Serotec (Oxford, UK).
Cytokine measurements
Cytokines in tissue culture supernatants released from different types of DC, or from peptide-specific T cells stimulated by these DC, were measured by using the Bio-Plex protein array system (Bio-Rad Laboratories, Hercules, CA), which was optimized in our laboratory. The Bio-Plex cytokine assay is designed for the multiplexed quantitative measurement of multiple cytokines in a single well using as little as 50 µl of sample.14,15 For cytokine assays, premixed multiplex beads of the Bio-Plex human cytokine Th1/Th2 panel (Bio-Rad, Cat. no. 171-A11081), was used, which included nine cytokines [IL-2, IL-4, IL-5, IL10, IL-12 (P70), IL-13, GM-CSF, interferon-γ (IFN-γ), TNF-α] that can differentiate between Th1/Th2 or Tc1/Tc2 phenotypes according to the pattern of cytokines present.
Generation of peptide-specific CD8+ T cells
Mature DC were pulsed with influenza matrix protein (IMP58–66), a highly immunogenic peptide derived from Influenza Virus M1 peptide,16 at a final concentration of 50 µg/ml for 2 hr at 37°. Non-adherent peripheral blood lymphocytes (PBL) (≈ 2 × 106) and the mature pulsed DC (≈ 1 × 105) were co-cultured at a ratio of 20 : 1 to 30 : 1 in 1 ml of 10% AB (v/v) media in individual wells of a 24-well flat-bottom plate (Corning) at 37°. The cells were cultured in the presence or absence of 25 U/ml of hrIL-2 (R & D Systems). After 4 days, 1 ml of hrIL-2-containing medium was added. Following another 3-day incubation, 1 ml of culture medium was replaced with 1 ml of fresh hrIL-2-containing medium and the cells were incubated for a further 4 days. Eleven days of incubation with pulsed DC and hrIL-2 constituted one round of in vitro stimulation (IVS). After one or two rounds of IVS, CD8+ cells were separated from the bulk cultures by positive selection with anti-CD8-coated microbeads (Miltenyi) and introduced into the IFN-γ enzyme-linked immunosorbent spot-forming cell assay (ELISPOT). After two rounds of IVS, effector cells were harvested without further separation and used as effectors in a flow cytometric cytotoxicity assay.
ELISPOT
The protocol for IFN-γ ELISPOT (Immunodiagnostic Systems, Boldon, Tyne & Wear, UK) was used with some modifications, as described by the manufacturer (Diaclone Research, Besancon cedex, France). Briefly, 96 polyvinylidene difluoride (PVDF)-bottom-well filtration plates were coated overnight at 4° with an anti-IFN-γ capturing antibody. Unpulsed or IMP58–66-pulsed DC were used as stimulators and incubated overnight with different effectors, including IMP-CD8+ clone, naïve CD8+ lymphocytes isolated directly from peripheral blood and in vitro-stimulated CD8+ cells isolated by MACS. The captured IFN-γ was then revealed by a secondary biotinylated anti-IFN-γ, which was, in turn, detected by streptavidin conjugated to alkaline phosphatase, and a blue precipitate was produced by the enzymatic reaction.
Cytotoxcity assay
The ability of clonally expanded effector T cells to kill peptide-pulsed T2A2 cells (purchased from the American Type Culture Collection, LGC Promochem, Middlesex, UK) was assessed in a flow cytometric cytotoxicity assay in which targets were first labelled with lipophilic membrane dye PKH26 (Sigma, St Louis, MO) before pulsing with IMP58–66 peptide and incubation with the effector T cells for 4 hr at 37°. To discriminate dead cells from live by propidium iodide (PI) exclusion, 20 µg/ml of PI dye (Sigma) was added to each tube prior to analysis by flow cytometry.
The level of cytotoxicity was expressed as the percentage of cell death within the PKH26-positive targets and calculated as:
| (1) |
The specific percentage lysis was calculated as:
| (2) |
Tetramer staining
The IMP-CD8+ clone was used as a positive control to optimize the staining conditions, tetramer and antibody concentration used. Briefly, 0·5 × 106 of the clonally expanded T cells were stained first with phycoerythrin (PE)-labelled IMP/tetramer (synthesized by Proimmune Limited, Oxford, UK) for 10 min at 37°, washed twice with buffer and then incubated with anti-CD8 fluorescein isothiocyanate (FITC) mAb (clone LT8, Proimmune Limited) for 45 min on ice before flow cytometric analysis.
Statistical analyses
The unpaired two-tailed Student's t-test was used to compare differences in DC membrane marker expression, and P-values of ≤ 0·05 were considered significant.
Results
Phenotypic differences of monocytes and of immature and mature DC
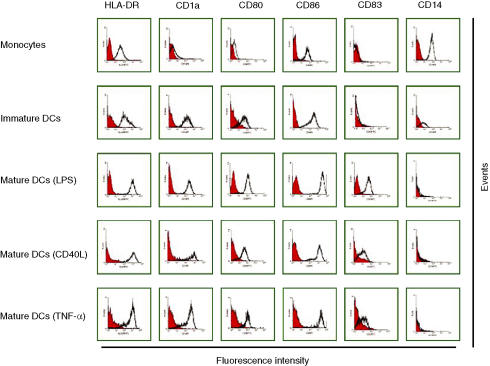
Adherence or MACS-isolated monocytes, and immature and mature DC activated by different maturation signals, were analysed by flow cytometry for the expression of certain surface markers, with the aim of establishing the phenotypic differences between them. Figure 1 shows that CD14+ monocytes express intermediate levels of HLA-DR and CD86 and negligible levels of CD1a, CD80 and CD83. Immature DC are phenotypically different from their monocyte precursors. They express lower levels of CD14 and higher levels of HLA-DR and CD86. Immature DC express CD1a and low levels of CD80, but do not express CD83 (an adhesion molecule expressed on mature DC). It was shown that differentiation into CD83+ DC required the suspension of cells at low numbers per unit volume; therefore, immature DC were redistributed at 0·5 × 106 cells/ml before stimulation.17 Following maturation with LPS, CD40L or TNF-α, DC down-regulate CD14 and up-regulate HLA-DR, CD86 and CD80 expression, and some cells express CD83 (Fig. 1). LPS was shown to be a more potent stimulant for CD83 expression than CD40L or TNF-α (Fig. 1).
Figure 1.
Immunophenotype profile of monocytes, immature dendritic cells (DC) and mature DC. Monocytes were isolated by adherence, and generated immature DC were matured by incubation with 0·5 µg/ml of lipopolysaccharide (LPS), 1 µg/ml of CD40L or 20 ng/ml of tumour necrosis factor-α (TNF-α) for the last 3 days of culture. All cells were stained with fluorochrome-conjugated monoclonal antibodies and analysed by flow cytometry. Data shown are representative of four separate experiments. Black lines represent cells stained with monoclonal antibodies and red lines represent cells stained with isotype-matched control antibodies.
Immature and mature DC derived from adherence or MACS-isolated monocytes express the same level of CD80, CD86 and CD83
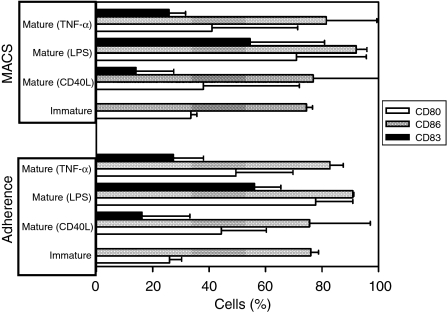
In order to explore the effect of the monocyte isolation method on the expression of costimulatory molecules and DC maturation markers, we investigated the expression of CD80, CD86 and CD83 on the surface of immature DC and mature DC grown from either adherence or MACS-isolated monocytes (Fig. 2). There was no statistically significant difference (all P-values > 0·05) in the percentage of DC expressing CD80, CD86 and CD83, regardless of whether their monocyte precursors were isolated by adherence or by MACS.
Figure 2.
Effect of the monocyte isolation method [adherence or magnetic activated cell sorting (MACS)] on the cell-surface expression of CD80, CD86 and CD83. The expression of these markers was measured, by using flow cytometry, on the surface of immature and mature adherence or MACS/monocyte-derived dendritic cells (MoDC). Data are shown as the percentage of cells that express any of these markers. Data represent the mean values ± standard deviation (SD) of three separate experiments. LPS, lipopolysaccharide; TNF-α, tumour necrosis factor-α.
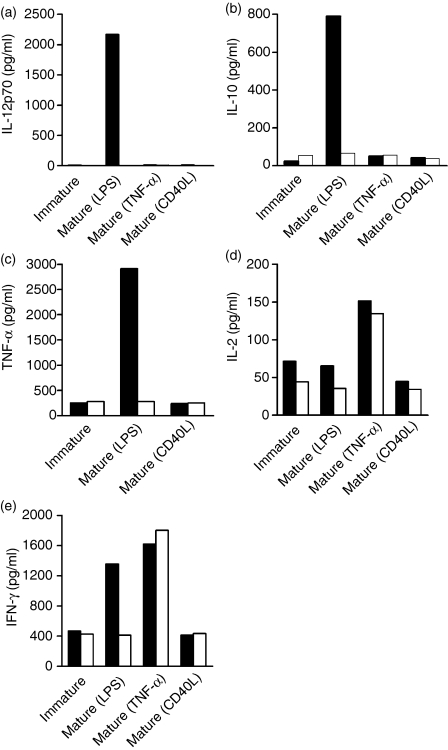
DC derived from adherence-isolated monocytes secrete much higher levels of IL-12, IL-10 and TNF-α than DC grown from MACS-isolated monocytes
DC activated by LPS secreted higher levels of IL-12, IL-10 and TNF-α than unstimulated DC or cells activated by CD40L or TNF-α(Fig. 3a–3c). However, DC activated by TNF-α secreted relatively higher levels of IL-2 when compared to other activators (Fig. 3d). To establish the impact of monocyte separation method on the ability of DC to become mature and release cytokines, the measurement of several cytokines, released from immature or mature DC in culture supernatants taken from adherence/MoDC or from MACS/MoDC was performed. Interestingly, MACS/MoDC did not secrete significant amounts of IL-12, while adherence/MoDC activated by LPS secreted much higher levels (≥ 200-fold) of IL-12 (Fig. 3a). At the same time, the secretion of IL-10 and TNF-α from adherence/MoDC activated by LPS was up to 10-fold higher than from MACS/MoDC (Fig. 3b,3c). No significant difference in IL-2 and IFN-γ secretion was observed between adherence and MACS/MoDC (Fig. 3d,3e).
Figure 3.
Effect of the monocyte isolation method [adherence or magnetic antibody cell sorting (MACS)] on cytokine secretion. Monocytes were isolated by either adherence or MACS, dendritic cell (DC)-generated and matured by lipopolysaccharide (LPS), tumour necrosis factor-α (TNF-α) or CD40L. Then, interleukin (IL)-12p70, IL-10, TNF-α, IL-2 and interferon-γ (IFN-γ), released from unstimulated or stimulated adherence or MACS/monocyte-derived dendritic cells (MoDC), were measured in culture supernatants by using the Bio-Plex protein array system. Cytokines released from adherence/MoDC are shown as black columns, while cytokines from MACS/MoDC are shown as white columns. The graphs shown are representative of experiments performed in quadruplicate from four separate donors.
DC activated by LPS are superior to DC activated by any other stimuli in IFN-γ induction from IMP-CD8+ clone and naïve CD8+ T lymphocytes
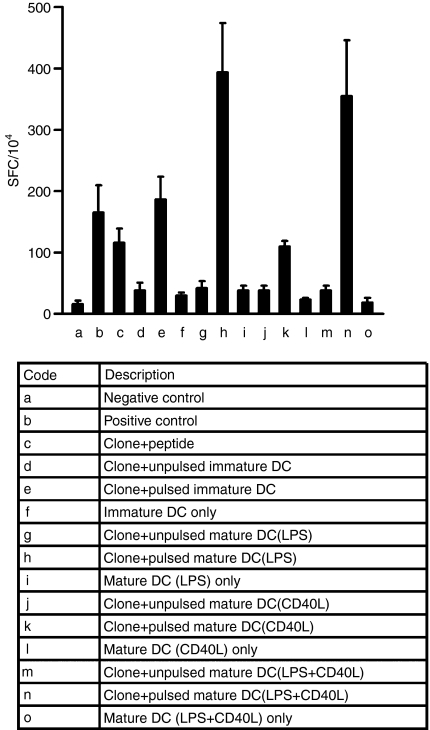
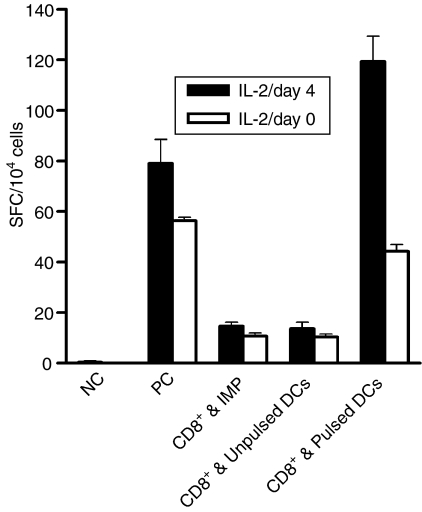
The IFN-γ ELISPOT was performed to enumerate peptide-specific responding CD8+ T cells. In order to determine the DC maturation scheme producing the most potent stimulation of T cells, different types of DC (immature or mature activated by different signals) were pulsed with the IMP58–66 peptide and used as stimulators in an IFN-γ ELISPOT. Cells of the IMP-CD8+ clone were used as effectors. Each spot represents one IFN-γ-secreting CD8+ cell, and the number of spots observed in assays was adjusted to the number of spots per 1 × 104 cells. Among all maturation schemes used, as shown in Fig. 4, DC matured with LPS showed the strongest stimulatory effect on IFN-γ secretion. When CD40L was combined with LPS as the maturation signal, no additional stimulatory effect was observed on IFN-γ secretion.
Figure 4.
Interferon-γ (IFN-γ) enzyme-linked immunosorbent spot-forming cell assay (ELISPOT), performed with the IMP-CD8+ clone as an effector and allogeneic dendritic cells (DC) derived from adherence-isolated monocytes as stimulators. The table shows the description for each column. The negative control was obtained by incubating effectors alone and the positive control by incubating effectors overnight with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) and 500 ng/ml ionomycin. For column (c), clone cells were incubated with peptide in the absence of DC. Results are expressed as the number of spot-forming cells (SFC) per 1 × 104 cells. Data represent the mean value ± standard deviation (SD) of tests repeated three times.
In addition, the ELISPOT was initially performed without IVS, using CD8+ cells isolated from an HLA-A2 normal volunteer as effectors. The purity of the isolated CD8+ cells was > 95%, as determined by flow cytometry staining. Only IFN-γ-secreting CD8+ cells specific for the IMP58–66 peptide (15 per 104) were detected when DC matured by LPS were used as stimulators (data not shown).
Adherence/MoDC activated by LPS are superior inducers of CD8+ T-cell responses specific for IMP58–66
DC grown from adherence-isolated monocytes and activated by LPS expressed high levels of costimulatory molecules and maturation markers. They also secreted the highest level of the immunostimulatory cytokine IL12p70. As these cells might act as superior inducers of antigen-specific CD8+ T-cell responses, we performed in vitro sensitization experiments, using these cells as stimulators, to expand IMP58–66-specific cytotoxic T lymphocytes (CTL). The frequency of IMP58–66-specific IFN-γ-producing CD8+ T cells was used as an indicator of DC stimulatory ability. Following amplification of the precursor cells for one round of IVS, IFN-γ-producing CD8+ cells, specific for the IMP58–66 peptide, were detected in all five HLA-A2-positive donors tested at high frequency, even higher than the positive control [cells stimulated by phorbol 12-myristate 13-acetate (PMA) and ionomycin] in some donors (Fig. 5). Addition of IL-2 after 4 days resulted in larger numbers of IMP58–66-specific IFN-γ-producing CD8+ T cells than addition of IL-2 into culture on day 0 (Fig. 5).
Figure 5.
Interferon-γ (IFN-γ) enzyme-linked immunosorbent spot-forming cell assay (ELISPOT) for IMP58–66-specific CD8+ cells expanded for one round of in vitro stimulation (IVS) by using IMP58–66-pulsed autologous mature dendritic cells (DC) derived from adherence-isolated monocytes and activated by lipopolysaccharide (LPS). The actual number of peptide-specific CD8+ cells can be obtained by subtracting the number of spot-forming cells (SFC) when unpulsed DC are used as stimulators from the number of SFC when pulsed DC are used as stimulators. Data represent the mean value ± standard deviation (SD) of tests repeated three times. One representative experiment of five, performed from five different donors, is shown. IL-2, interleukin-2; NC, negative control; PC, positive control.
Adherence/MoDC induce potent cytotoxic effector T cells specific for IMP58–66
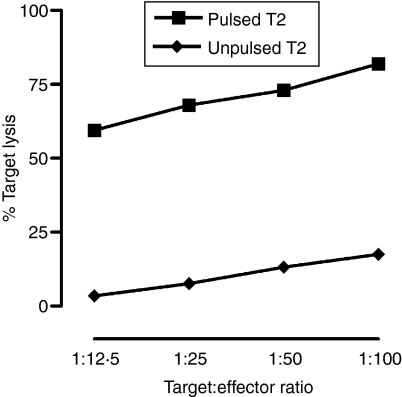
Cytotoxicity assays were performed after two rounds of IVS with IMP58–66-pulsed mature DC (by LPS). IMP58–66-expanded effectors were able to kill a significant percentage of T2A2 targets pulsed with IMP58–66 peptide (> 65%, Fig. 6). The percentage of specific lysis was calculated by subtracting the percentage of killing of unpulsed T2A2 from the killing of pulsed targets. Killing was in a target to effector ratio manner and it was higher when IL-2 was added after 4 days of culture.
Figure 6.
Percentage specific lysis of T2A2 cells by IMP58–66-expanded peripheral blood lymphocyte (PBL) effectors in a 4-hr PKH26-labelling flow cytometric assay. Effectors were expanded for 11 days by using IMP58–66-pulsed autologous dendritic cells (DC) derived from adherence-isolated monocytes and matured by lipopolysaccharide (LPS). In this experiment, interleukin-2 (IL-2) was added on day 4. The expanded effectors were then incubated for 4 hr with unpulsed or peptide-pulsed T2A2 targets, and the percentage of target killing was determined flow cytometrically. The graph shown is representative of experiments performed in triplicate from three separate donors.
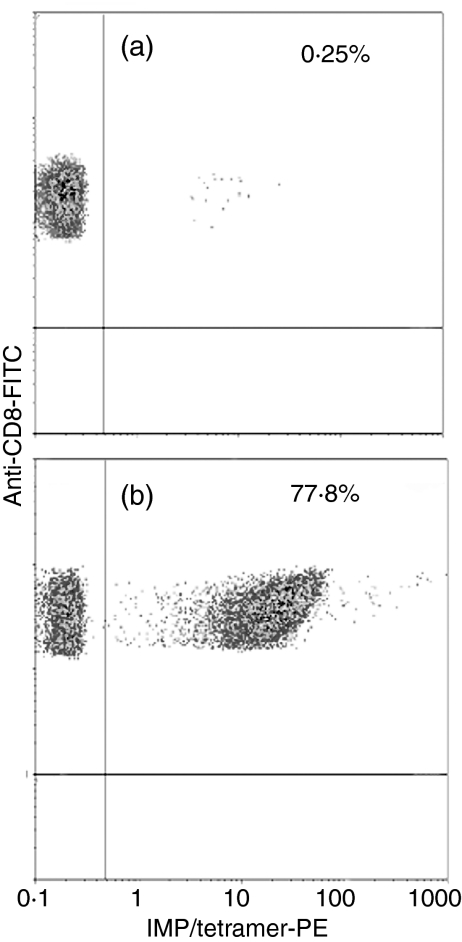
To assess the specificity of the T-cell line generated, naïve or peripheral blood lymphocytes, expanded by IMP58–66-pulsed adherence/MoDC, were stained by PE-labelled IMP/tetramer and anti-CD8 FITC mAb. IMP-specific CD8+ T cells were amplified from 0·25%(Fig. 7a) to ≈ 78% (Fig. 7b) after one round of IVS. More than 90% of CD8+ cells became tetramer positive after two rounds of IVS (data not shown).
Figure 7.
Tetramer staining of unexpanded (a) and expanded (b) CD8+ T lymphocytes. Peripheral blood lymphocytes from HLA-A2-positive normal donors were expanded by using mature adherence/monocyte-derived dendritic cells (MoDC) pulsed with IMP58–66 peptide. Lymphocytes were gated by using their forward- and side-scatter properties, and CD8+ T cells were gated if positive for anti-CD8 monoclonal antibody (mAb). Only 0·25% of CD8+ T cells stained positive for the tetramer before stimulation (a), while 77·8% of these cells became tetramer positive after one round of in vitro stimulation (IVS) (b). PE, phycoerythrin.
Adherence/MoDC induce the development of Tc1 effectors
Finally, to test whether adherence/MoDC stimulators induced the development of Tc1 or Tc2 effectors, expanded CD8+ T cells specific for IMP58–66 were positively selected by MACS and then incubated for 72 hr with or without PMA + ionomycin. Cytokines released in culture supernatants were assayed by using premixed multiplex beads, as described in the Materials and methods. As shown in Table 1, stimulated CD8+ cells secreted large amounts of IFN-γ without producing any IL-4 or IL-5, which indicates that peptide-pulsed adherence/MoDC induced the development of Tc1 effectors specific for the IMP58–66 peptide.
Table 1. T helper (Th)1/Th2 cytokine panel for IMP58–66-specific CD8+ T cells.
| IL-2 | IL-4 | IL-5 | IL-10 | IL-12 | IL-13 | GM-CSF | IFN-γ | TNF-α | |
|---|---|---|---|---|---|---|---|---|---|
| Unstimulated IMP-CD8+ cells | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 109 | 0 |
| Stimulated IMP CD8+ cells | 201 | 0 | 0 | 0 | 0 | 81 | 19 839 | 46 200 | 1664 |
Several cytokines, which can differentiate between Th1/Tc1 and Th2/Tc2 effectors, were measured in the supernatants of unstimulated or stimulated [by phorbol 12-myristate 13-acetate (PMA) and ionomycin] IMP58–66-specific CD8+ T cells.
Cytokines values are expressed in pg/ml.
GM-CSF, granulocyte–macrophage colony-stimulating factor; IFN-γ, interferon-γ; IL, interleukin; TNF-α, tumour necrosis factor-α.
Discussion
The degree of DC differentiation plays an important role in determining the subsequent immune response. Therefore, it is critical to identify the differentiation stage of DC that is most effective at inducing CD8+ cell responses. CD83 is an immunoglobulin superfamily member that has been shown to be up-regulated following the maturation of DC.18–20 It has been used as a marker of mature DC despite its precise role remaining unknown. Interestingly, no inhibitory or neutralizing antibody is currently available. Recently, it has been suggested that CD83 is a functionally important receptor which can regulate the development of cellular immunity by interacting with its ligand.21 Furthermore, it has been shown that the extracellular CD83 domain inhibits DC-mediated T-cell proliferation.22 The appearance of CD83, as a marker of functional maturation, correlates with the ability of the DC to produce increased amounts of immunomodulatory cytokines. During the maturation process, DC secrete cytokines essential for the activation of antigen-specific T lymphocytes. More specifically, mature MoDC are known to release the immunostimulatory cytokine, IL-12, which has been shown to increase IFN-γ secretion from natural killer (NK) and T cells and significantly increases the cytolytic ability of these cells, promoting the development of Th1/Tc1 immune responses.23 However, IL-10 secretion blocks DC maturation by interfering with the up-regulation of costimulatory molecules and IL-12 production, thereby limiting the ability of DC to promote Th1/Tc1 responses.24,25
In this study we have shown that the expression of CD80, CD86 and CD83 molecules, which are important for the stimulatory function of DC, was the same regardless of the method used to isolate the original CD14+ cells. Our data suggest that LPS could be a more potent stimulus for the expression of these molecules on MoDC than other stimuli such as TNF-α and CD40L. We have shown, for the first time, that the process of DC maturation induced by LPS was dramatically affected by the positive selection of monocytes using anti-CD14 microbeads. Interestingly, MACS/MoDC were unable to secrete significant amounts of immunomodulatory cytokines. Indeed, secretion of cytokines such as IL-12p70, IL-10 and TNF-α, which are normally released at high levels by LPS-activated DC, was drastically reduced after the positive selection of monocytes. Membrane CD14 (mCD14), which is a glycosylphosphatidylinositol (GPI)-anchored protein strongly expressed on the surface of monocytes and macrophages, and weakly on neutrophils, is required for the responses of these cells to low concentrations of LPS.26 It has been reported that the absence of mCD14 is a characteristic of DC27 which occurs as a result of the down-regulation of CD14 expression following the down-regulation of CD14 mRNA induced by IL-4 in the DC media.28 However, even under these circumstances, some immature DC still weakly express mCD14.20,29 The inability of MACS/MoDC to secrete significant amounts of cytokines is probably caused by blocking of mCD14 molecules by anti-CD14 microbeads, but the exact mechanism is still unclear. However, LPS stimulated the production of high levels of IL-12p70, TNF-α and IL-10 from adherence/MoDC. This supports the possibility that mCD14 molecules were blocked following positive selection. Activation of DC with TNF-α revealed a lack of MACS and adherence/MoDC to produce IL-12p70 and IL-10. However, they secreted approximately the same levels of IFN-γ and IL-2 (Fig. 4). Adherence is a widely used method of monocyte isolation for in vitro DC generation because it is simple, inexpensive and can be applied to a large number of samples. Most importantly, adherence/MoDC activated by LPS secreted higher levels of biologically active IL-12p70, IL-10 and TNF-α.
IL-12, produced predominantly by antigen-presenting cells, plays an important role in the induction of cell-mediated immunity. This function is promoted by IL-12-induced IFN-γ production from resting and activated NK and T cells.30,31 IL-12 has been shown to augment the cytolytic activity of T cells,32 increase the proliferation of activated NK cells33 and stimulate the induction of a Th1/Tc1 response.23,34–37 All these potent immunomodulatory activities show the importance of IL-12 production from activated DC for the generation of specific immune responses. This emphasizes the importance of generating DC from adequately isolated monocytes that are highly capable of producing IL-12. IL-10 has been recognized as a main factor that can inhibit the differentiation of DC from monocytes and strongly prevents DC maturation by different stimuli.24,25,38,39 Adherence/MoDC activated by LPS induced the development of potent antigen-specific CD8+ T cells, despite the secretion of IL-10 by adherence/MoDC.
We have shown that DC derived from adherence-isolated monocytes and activated by LPS were the strongest stimulators for the induction of IFN-γ from the IMP-CD8+ clone and from naïve CD8+ T cells. These potent DC were used as stimulators to expand antigen-specific CD8+ T cells using the highly immunogeneic HLA-A2-restricted IMP58–66 peptide as a model. By using IFN-γ ELISPOT, cytotoxicity assays, Th1/Th2 cytokine panel assays and MHC–peptide tetrameric complex binding assays, we confirmed that expanded CD8+ T cells are potent cytotoxic effectors of the Tc1 phenotype and specific for IMP58–66 peptide. This work highlights the importance of the method used for monocyte isolation and subsequent generation of DC and of the maturation signal used to activate these DC. Notably, the ability of the generated DC to induce potent and specific immune responses needs to be assessed in functional assays.
Acknowledgments
We thank Dr Stephen Man for his generous gift of the IMP-CD8+ clone and Dr Marcus J. Coffey for critical reading of the manuscript. This work was supported by the Medical Biochemistry Department Fund and the Karim Rida Said Foundation.
References
- 1.Inaba K, Steinman RM, Van Voorhis WC, Muramatsu S. Dendritic cells are critical accessory cells for thymus-dependent antibody responses in mouse and in man. Proc Natl Acad Sci USA. 1983;80:6041–5. doi: 10.1073/pnas.80.19.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanzavecchia A, Sallusto F. The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. Curr Opin Immunol. 2001;13:291–8. doi: 10.1016/s0952-7915(00)00218-1. 10.1016/S0952-7915(00)00218-1. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Gutchinov B, Witmer MD, Nussenzweig MC. Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J Exp Med. 1983;157:613–27. doi: 10.1084/jem.157.2.613. 10.1084/jem.157.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 6.Brossart P, Bevan MJ. Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood. 1997;90:1594–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T–T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med. 1999;189:821–9. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palucka KA, Taquet N, Sanchez-Chapuis F, Gluckman JC. Dendritic cells as the terminal stage of monocyte differentiation. J Immunol. 1998;160:4587–95. [PubMed] [Google Scholar]
- 10.Riva S, Nolli ML, Lutz MB, Citterio S, Girolomoni G, Winzler C, Ricciardi-Castagnoli P. Bacteria and bacterial cell wall constituents induce the production of regulatory cytokines in dendritic cell clones. J Inflamm. 1996;46:98–105. [PubMed] [Google Scholar]
- 11.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 12.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–46. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 13.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest (Suppl) 1968;97:77–89. [PubMed] [Google Scholar]
- 14.Carson RT, Vignali DA. Simultaneous quantitation of 15 cytokines using a multiplexed flow cytometric assay. J Immunol Methods. 1999;227:41–52. doi: 10.1016/s0022-1759(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 15.Fulton RJ, McDade RL, Smith PL, Kienker LJ, Kettman JR., Jr Advanced multiplexed analysis with the FlowMetrix system. Clin Chem. 1997;43:1749–56. [PubMed] [Google Scholar]
- 16.Gotch F, Rothbard J, Howland K, Townsend A, McMichael A. Cytotoxic T lymphocytes recognize a fragment of influenza virus matrix protein in association with HLA-A2. Nature. 1987;326:881–2. doi: 10.1038/326881a0. 10.1038/326881a0. [DOI] [PubMed] [Google Scholar]
- 17.Thurnher M, Papesh C, Ramoner R, Gastl G, Bock G, Radmayr C, Klocker H, Bartsch G. In vitro generation of CD83+ human blood dendritic cells for active tumor immunotherapy. Exp Hematol. 1997;25:232–7. [PubMed] [Google Scholar]
- 18.Kozlow EJ, Wilson GL, Fox CH, Kehrl JH. Subtractive cDNA cloning of a novel member of the Ig gene superfamily expressed at high levels in activated B lymphocytes. Blood. 1993;81:454–61. [PubMed] [Google Scholar]
- 19.Zhou LJ, Schwarting R, Smith HM, Tedder TF. A novel cell-surface molecule expressed by human interdigitating reticulum cells, Langerhans' cells, and activated lymphocytes is a new member of the Ig superfamily. J Immunol. 1992;149:735–42. [PubMed] [Google Scholar]
- 20.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–92. doi: 10.1073/pnas.93.6.2588. 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scholler N, Hayden-Ledbetter M, Dahlin A, Hellstrom I, Hellstrom KE, Ledbetter JA. Cutting edge: CD83 regulates the development of cellular immunity. J Immunol. 2002;168:2599–602. doi: 10.4049/jimmunol.168.6.2599. [DOI] [PubMed] [Google Scholar]
- 22.Lechmann M, Krooshoop DJ, Dudziak D, Kremmer E, Kuhnt C, Figdor CG, Schuler G, Steinkasserer A. The extracellular domain of CD83 inhibits dendritic cell-mediated T cell stimulation and binds to a ligand on dendritic cells. J Exp Med. 2001;194:1813–21. doi: 10.1084/jem.194.12.1813. 10.1084/jem.194.12.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emtage PC, Clarke D, Gonzalo-Daganzo R, Junghans RP, Gonzalo-Dagonzo R. Generating potent Th1/Tc1 T cell adoptive immunotherapy doses using human IL-12. Harnessing the immunomodulatory potential of IL-12 without the in vivo-associated toxicity. J Immunother. 2003;26:97–106. doi: 10.1097/00002371-200303000-00002. 10.1097/00002371-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–8. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 25.De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur J Immunol. 1997;27:1229–35. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 26.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–3. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 27.Freudenthal PS, Steinman RM. The distinct surface of human blood dendritic cells, as observed after an improved isolation method. Proc Natl Acad Sci USA. 1990;87:7698–702. doi: 10.1073/pnas.87.19.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauener RP, Goyert SM, Geha RS, Vercelli D. Interleukin 4 down-regulates the expression of CD14 in normal human monocytes. Eur J Immunol. 1990;20:2375–81. doi: 10.1002/eji.1830201103. [DOI] [PubMed] [Google Scholar]
- 29.Peters JH, Gieseler R, Thiele B, Steinbach F. Dendritic cells: from ontogenetic orphans to myelomonocytic descendants. Immunol Today. 1996;17:273–8. doi: 10.1016/0167-5699(96)80544-5. 10.1016/0167-5699(96)80544-5. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–45. doi: 10.1084/jem.170.3.827. 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stern AS, Magram J, Presky DH. Interleukin-12 an integral cytokine in the immune response. Life Sci. 1996;58:639–54. doi: 10.1016/s0024-3205(96)80003-8. 10.1016/S0024-3205(96)80003-8. [DOI] [PubMed] [Google Scholar]
- 32.Wong HL, Wilson DE, Jenson JC, Familletti PC, Stremlo DL, Gately MK. Characterization of a factor(s) which synergizes with recombinant interleukin 2 in promoting allogeneic human cytolytic T-lymphocyte responses in vitro. Cell Immunol. 1988;111:39–54. doi: 10.1016/0008-8749(88)90049-4. 10.1016/0008-8749(88)90049-4. [DOI] [PubMed] [Google Scholar]
- 33.Stern AS, Podlaski FJ, Hulmes JD, et al. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci USA. 1990;87:6808–12. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gubler U, Chua AO, Schoenhaut DS, et al. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci USA. 1991;88:4143–7. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Garra A, Arai N. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 2000;10:542–50. doi: 10.1016/s0962-8924(00)01856-0. 10.1016/S0962-8924(00)01856-0. [DOI] [PubMed] [Google Scholar]
- 36.Romagnani S, Kapsenberg M, Radbruch A, Adorini L. Th1 and Th2 cells. Res Immunol. 1998;149:871–3. doi: 10.1016/s0923-2494(99)80016-9. 10.1016/S0923-2494(99)80016-9. [DOI] [PubMed] [Google Scholar]
- 37.Trinchieri G, Scott P. Interleukin-12: basic principles and clinical applications. Curr Top Microbiol Immunol. 1999;238:57–78. doi: 10.1007/978-3-662-09709-0_4. [DOI] [PubMed] [Google Scholar]
- 38.Buelens C, Verhasselt V, De Groote D, Thielemans K, Goldman M, Willems F. Interleukin-10 prevents the generation of dendritic cells from human peripheral blood mononuclear cells cultured with interleukin-4 and granulocyte/macrophage-colony-stimulating factor. Eur J Immunol. 1997;27:756–62. doi: 10.1002/eji.1830270326. [DOI] [PubMed] [Google Scholar]
- 39.Enk AH, Angeloni VL, Udey MC, Katz SI. Inhibition of Langerhans' cell antigen-presenting function by IL-10. A role for IL-10 in induction of tolerance. J Immunol. 1993;151:2390–8. [PubMed] [Google Scholar]