Abstract
Macrophages play a crucial role in the perpetuation of inflammation and irreversible cartilage damage during the development of rheumatoid arthritis (RA). LIGHT (TNFSF14) and its receptor TR2 (TNFRSF14) are known to have pro-inflammatory activities in foam cells of atherosclerotic plaques. We tested a hypothesis that LIGHT and TR2 are involved in activation of monocyte/macrophages in RA synovium. Immunohistochemical analysis of RA synovial tissue samples revealed that both LIGHT and TR2 are expressed in CD68 positive macrophages. In contrast, synovial tissue samples from osteoarthritis (OA) patients failed to reveal the expression of LIGHT. Expression of TR2 in RA synovial macrophages was also detected using flow cytometry analysis. To identify the role of LIGHT in the functioning of macrophages in RA, we isolated macrophage enriched cells from RA synovial fluid and stimulated them with LIGHT. LIGHT induced expression of matrix metalloproteinase-9 and pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-8. These data indicate that LIGHT and TR2 expressed in macrophages are involved in the pathogenesis of RA by inducing the expression pro-inflammatory cytokines and matrix degrading enzymes.
Keywords: Arthritis, Cytokines/interleukins, Macrophages/Monocytes
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by synovial inflammation that leads to the destruction of cartilage and bone. Synovial inflammation involves lining layer thickening and infiltration of inflammatory cells into the sublining area.1,2 In normal joints, macrophages are resident cells and cover the synovial layer. The number of macrophages in the joint greatly increases in RA synovium3 and the degree of increase is strongly correlated with the development of severe cartilage destruction.4–6 Furthermore, selective depletion of macrophages from the synovial lining before the induction of experimental arthritis resulted in prevention of both joint inflammation and cartilage destruction.7–9
LIGHT (TNFSF14) is a member of tumor necrosis factor superfamily and is the ligand for TR2 (TNFRSF14/HVEM/HveA/LIGHTR/ATAR) and lymphotoxin (LT)-β.10–12 LIGHT is known to be a costimulatory molecule in T cell activation and the suppressor of in vivo tumor growth through apoptosis induction.13 Targeted disruption of LIGHT in mice caused prolonged cardiac allograft survival.14 LIGHT null mice further revealed that LIGHT cooperates with LT-β for the generation of mesenteric lymph nodes.15,16 LIGHT has roles in mucosal immunity by regulating the interferon (IFN)-γ expression and mediating proinflammatory T–T interactions in the intestinal mucosa.17 LIGHT and TR2 are also involved in inflammatory processes occurring in atherosclerotic plaques.18 LIGHT level was higher in plaques with heavy infiltration of inflammatory cells. The major cell type expressing TR2 was monocyte/foam cells in the atherosclerotic plaques and LIGHT induced pro-inflammatory cytokines and MMPs in macrophage cell line THP-1. The involvement of LIGHT in the pathogenesis of experimental arthritis in mice was demonstrated using LT-β receptor-Ig fusion protein, but the cell type(s) expressing LIGHT in RA synovium and the LIGHT induced cellular responses are not known yet.19
TR2 was initially identified as a cellular coreceptor for herpes simplex virus (HSV) entry, hence the name HVEM (herpes virus entry mediator, later named HveA).20,21 TR2 has a wide tissue distribution, and is prominently expressed on cells in lymphoid tissue such as the spleen and on peripheral blood leukocytes. TR2 mRNA was detected on resting and activated CD4+ and CD8+ T cells, on CD19+ B cells and on monocytes.20,22 Like other TNFRSF members, stimulation of TR2 induces activation of the transcription factors nuclear factor (NF)-κB and AP-1·20,23 Interestingly, LIGHT/TR2 have cross-reactivity with lymphotoxin LTα/(LT)βR, linking LIGHT/TR2 to the lymphotoxin cytokine-receptor system.24
Since the interaction between LIGHT and TR2 in foam cells is implicated to the inflammatory processes in atherosclerosis,18 it is likely that these molecules also have pro-inflmmatory roles in macrophages of RA synovium. Here we describe the expression of LIGHT and TR2 in macrophages in RA synovium and functional consequences of LIGHT mediated macrophage activation in synovial fluid of RA patients.
Materials and Methods
Synovial fluid and synovial tissue samples
Synovial fluid was obtained from RA patients during therapeutic arthrocentesis in sterile tube containing preservative free heparin. Synovial tissue samples were collected from RA patients who were undergoing joint replacement therapy and were snap frozen in OCT compound with storage at −80° until use. The study was approved by an institutional review committee and the subjects gave informed consent. RA was diagnosed according to the criteria of the American College of Rheumatology.
Immunohistochemstry and monoclonal antibodies
For the immunohistochemical analysis, frozen synovial tissues were cut into 5-µm sections and were stained using an LSAB kit (DAKO, Copenhagen, Denmark) according to the manual provided by the manufacturer. For double staining of CD68 and LIGHT or TR2, specimen was sequentially treated with anti-CD68 monoclonal antibody; biotin-linked secondary antibody; streptavidin-alkaline phosphatase; fuchsin for visualization of CD68 staining (red color); anti LIGHT or anti-TR2 monoclonal antibody which was preconjugated with horseradish peroxidase using an Animal Research Kit (DAKO, Copenhagen, Denmark) according to the manual provided by the manufacturer; diaminobenzidine (DAB) for visualization of LIGHT or TR2 (brown color); and finally counterstained with hematoxylin. Monoclonal antibody to LIGHT was purchased from R & D (Minneapolis, MN, USA); monoclonal antibodies to CD68 (KP1) and rabbit polyclonal antibody to von Willebrand factor (vWF) (N1505) from DAKO (Glostrup, Denmark); and monoclonal antibody to TR2 from Immunomics (Ulsan, Korea). To measure the expression levels of LIGHT and TR2 in synovial macrophages (CD68 positive cells), two different researchers analyzed the slide separately and combined the score to get the final value: −, no expression; +, expression in < 30% of macrophage; + +, 30–70%; + + +, > 70%.
Flow cytometric analysis
Flow cytometric analysis was performed on a FACS-vantage (Becton-Dickinson, Mountain View, CA). For the analysis of cells from peripheral blood, whole blood was collected in heparin containing vacutainers and 50 µl blood was used per sample for staining. For the analysis of cells from synovial fluid, 1 × 106 cells were used per sample. For staining, cells were sequentially incubated with either 1 µg of anti-TR2 monoclonal antibody or anti-CD11b monoclonal antibody (omitted for the background staining); 0·5 µg of FITC labeled rat antimouse IgG; and 0·5 µg of PE labeled anti-CD14 antibody (Caltag Laboratories, Burlingame, CA, USA) according to the previously described method.25 The fluorescence profile of 3 × 104 cells was obtained. To obtain mean fluorescence intensity values for TR2 and CD11b, granulocytes were gated using forward and side scatter pattern and macrophages were gated for CD14 positivity.
Cell fractionation from synovial fluid and peripheral blood
Mononuclear cells and granulocytes were isolated from synovial fluid and peripheral blood by density gradient centrifugation using Lymphoprep™ (Nycomed Pharma As, Oslo, Norway). The mononuclear cells were further incubated in culture dishes for one hour and nonadherent cells were removed to get adherent cells which are mainly monocyte/macrophage cells. Cell purity (> 90%) for granulocytes and macrophages were then confirmed using flow cytometry.
Enzyme-linked immunosorbent assay (ELISA)
Cells were seeded in 1 × 105 cells/well in 96 well-plate and stimulated with soluble recombinant human LIGHT (rhsLIGHT, purchased from Alexis, San Diego, CA, USA) in 1, 10, and 100 ng/ml concentrations. Culture supernatants were collected after 24 h and used for ELISA and gelatin zymogram. Cytokines (IL-6, IL-8, MCP-1, and TNF-α) were measured by sandwich ELISA (Endogen Inc., Woburn, MA, USA). The detection limits of ELISA were < 10 pg/ml for all the cytokines. Data are expressed as mean + SD of triplicate measurements. Statistical analysis was performed with two-tailed student's t-test.
Gelatin zymogram
The MMP-9 activity in the culture supernatant was determined by substrate gel electrophoresis, as described previously.26 Briefly, culture supernatants were mixed with running buffer (4% SDS, 20% glycerol, 0·01% bromophenolblue, 0·125 m Tris-Cl, pH 6·8) and separated on SDS-PAGE containing 0·1% gelatin. The gels were then sequentially treated with two changes of 2·5% Triton X-100 for 20 min each for renaturation of proteins, two changes of distilled water for 20 min each for washing; and reaction buffer solution (10 mm Tris, pH 7·5, 10 mm CaCl2, 50 mm NaCl, 2 µm ZnCl2, 0·25% TX-100, 0·002% NaN3) for 24 h at 37°; and the gels were finally Coomassie stained.
Results
The expression pattern of LIGHT in synovial macrophages of arthritis patients
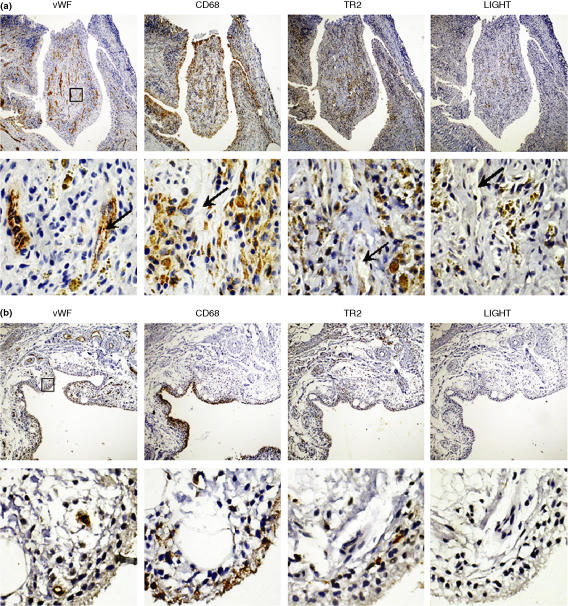
Synovial tissues were obtained from RA and OA patients and the expression patterns of LIGHT, TR2, CD68 (a macrophage marker), and von Willebrand factor (vWF) (an endothelial cell marker) were analyzed using immunohistochemistry. Synovial tissues from RA patients tend to have more infiltration of inflammatory cells and formation of new microvessels in the sublining areas than those from OA patients. Additionally, CD68 staining patterns were different. In RA samples, CD68 positive macrophages were found in the lining layers of RA synovium and also in the sublining areas around newly formed microvessels (Fig. 1a). In OA synovium, CD68 positive cells were found only in the lining layers (Fig. 1b). In RA synovium, expression of TR2 was detected in the CD68 positive macrophage-rich areas (both the lining layer and the sublining area) and the expression levels were higher around the microvessels in the sublining area. High level expression of LIGHT was detected in macrophage rich areas around the microvessels. But expression of LIGHT and TR2 was not detected in endothelial cells in the microvessels (Fig. 1a lower panel). Expression of LIGHT was not detected in OA synovium and expression levels of TR2 in OA synovial macrophages were lower than that of RA synovial macrophages (Fig. 1b). We confirmed the specificity of the staining by staining the tissue sections with isotype matching control antibody, which failed to stain the tissue sections (data not shown). We summarized the expression patterns of LIGHT and TR2 in 5 different RA samples and 4 different OA samples in Table 1. LIGHT tend to be expressed in the sublining area in small number macrophages in RA, but not in OA, synovium. The proportion of TR2 expressing macrophage was higher in RA than OA synovial tissues. Low level expression of LIGHT is expected since LIGHT is secreted from activated macrophages in contrast to TR2 which is mainly a membrane protein.14
Figure 1.
Expression patterns of LIGHT and TR2 in RA synovium. Synovial tissue sections from RA (a) and OA patients (b) were analyzed for the expression of vWF (the marker for endothelial cells), LIGHT, TR2, and CD68 (the marker for macrophages) using immunohistochemical analysis as described in materials and methods. Sublining areas around the newly formed microvessels are magnified in the lower panel. The box in the low magnification picture (40X, upper panel) indicates the area magnified in high magnification pictures (400X, lower panel). Note that microvessels (arrows) are stained with antivWF antibodies but not with other antibodies. The pictures are representatives from analysis using 7 different RA and 5 different OA synovial tissue specimens.
Table 1. Expression levels of LIGHT and TR2 in RA and OA synovial tissue sections.
| Tissue type | LIGHT | TR2 |
|---|---|---|
| RA #1 | + | + + + |
| RA #2 | + + | + + + |
| RA #3 | + | + + + |
| RA #4 | + | + + + |
| RA #5 | + | + + + |
| OA #1 | − | + + |
| OA #2 | − | + |
| OA #3 | − | + + |
| OA #4 | − | + + |
−, no expression; +, expression in < 30% of macrophages; + +, 30–70%; + + +, > 70%
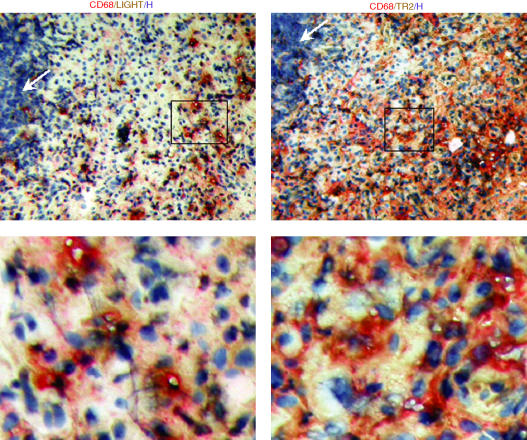
To confirm that macrophages are the cells expressing LIGHT and TR2, we performed double immunohistochemical analysis. As shown in Fig. 2, LIGHT colocalized with CD68 in the sublining area of RA synovium. TR2 also colocalized with CD68 indicating that CD68 positive cells express both LIGHT and TR2. Although LIGHT has been reported to have costimulatory role in T cell activation, expression levels of LIGHT and TR2 were low in areas rich in lymphocytes (Fig. 2).
Figure 2.
Expression of LIGHT and TR2 in macrophages in the RA synovium. Synovial tissue sections were analyzed for the expression of LIGHT, TR2, and CD68 using double immunohistochemical analysis as described in materials and methods. CD68 was stained in red, LIGHT or TR2 in brown, and nuclei were stained with hematoxylin (H) in blue. Box in the low magnification pictures (100X, upper panel) indicate the area magnified in high magnification pictures (400X, lower panel). Note that areas heavily infiltrated with lymphocytes (white arrows) are not stained with anti-LIGHT nor anti-TR2 monoclonal antibodies. The analysis was performed on samples obtained from two different RA patients with essentially the same results.
The expression of TR2 in RA synovial macrophages
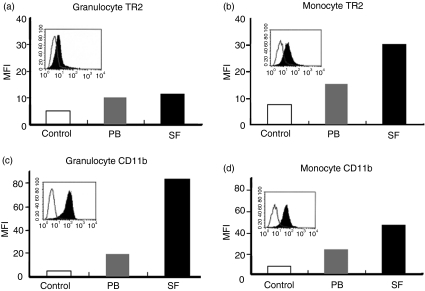
To find out whether the RA synovial macrophages express TR2, peripheral blood monocyte/macrophage cells were compared with synovial macrophages. The expression levels of TR2 in granulocytes were also measured as a control. We also measured the expression levels of adhesion molecule CD11b which is known to be expressed in both activated granulocytes and macrophages.27 As shown in Fig. 3, both peripheral blood granulocytes and monocyte/macrophages expressed low basal levels of TR2 (Fig. 3a and b). TR2 expression was detected in monocyte/macrophage cells from synovial fluid and peripheral blood (Fig. 3b). Low level expression TR2 in granulocytes was also detected (Fig. 3a). When the expression levels of CD11b were compared, both cell types showed increase in the expression levels in cells isolated from synovial fluid and the extent of increase was greater in granulocytes than in macrophages (Figs 3c,d).
Figure 3.
Expression of TR2 is increased in RA synovial macrophages. White blood cells obtained from peripheral blood (PB) or synovial fluid (SF) from RA patients were stained as described in materials and methods. Mean fluorescence intensity (MFI) values of TR2 and CD11b in granulocytes and macrophages were compared. Open, grey, and black bars represent MFI obtained from background staining, specific staining of peripheral blood cells, and specific staining of synovial fluid cells, respectively. In the insets, histograms from specific staining (filled area) and background staining (open area) of synovial fluid cells were compared. The experiments were repeated three times in different RA patients with essentially the same results.
LIGHT induces cytokine/chemokines and MMP-9 in macrophages isolated from RA synovial fluids.
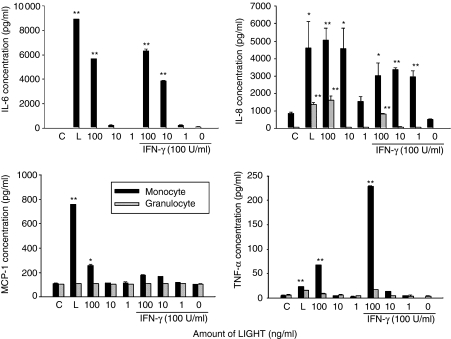
Previous data demonstrated that stimulation of human monocytic cell line THP-1, which express TR2, with either LIGHT or anti-TR2 monoclonal antibody can stimulate expression of pro-inflammatory cytokines such as TNF-α and IL-8.18 To find out whether the macrophages in RA synovial fluids are responsive to LIGHT, we isolated adherent mononuclear cells, which are enriched in macrophages, from RA synovial fluid and stimulated them with recombinant human soluble (rhs)LIGHT. As shown in Fig. 4, rhsLIGHT alone induced high levels of IL-6 and IL-8, which is comparable to the level obtained by treatment with bacterial lipopolysaccharide (LPS). rhsLIGHT also induced low level secretion of MCP-1 and TNF-α. Previous analysis of human monocytic cell line THP-1 revealed that LIGHT synergizes with IFN-γ for the induction of cytokine expression.18 Concomitant treatment of adherent mononuclear cells isolated from RA synovial fluids with rhsLIGHT and IFN-γ caused a synergistic induction of TNF-α but not MCP-1. Granulocytes treated with rhsLIGHT, however, expressed only low levels of IL-8 and additional treatment with IFN-γ failed to enhance the response.
Figure 4.
LIGHT treatment causes induction of pro-inflammatory cytokines in macrophage enriched cells isolated from RA synovium. Adherent mononuclear cells and granulocytes isolated from RA synovial fluids were stimulated with recombinant human LIGHT in the presence or absence of 100 U/ml IFN-γ. Culture supernatants were collected 24 h after activation and cytokine concentrations were measured using ELISA. Error bars represent standard deviation of triplicate measurements. C, no treatment control; L, treatment with 1 µg/ml LPS as a positive control. The experiments were repeated three times with cells isolated from different RA patients with essentially the same results. *P < 0·01 and **P < 0·001 vs. no treatment control.
Since MMPs play important roles in the pathogenesis of RA, it is necessary to find out whether LIGHT can induce MMP expression in RA. Treatment of adherent mononuclear cells isolated from RA synovial fluid with rhsLIGHT also induced matrix metalloproteinase (MMP)-9 and IFN-γ synergized with LIGHT in the induction of MMP-9 (Fig. 5).
Figure 5.
LIGHT treatment causes induction of MMP-9 in macrophage enriched cells isolated from RA synovium. Adherent mononuclear cells isolated from synovial fluid of RA patients were stimulated with recombinant human LIGHT in the presence or absence of 100 U/ml IFN-γ. Culture supernatants were collected 24 h after activation and MMP-9 expression levels were measured using gelatin-zymogram. As a positive control, cells were treated with 1 µg/ml LPS.
Discussion
Immunohistochemistry data revealed that CD68 positive macrophages express both LIGHT and TR2. This indicates that LIGHT is expressed by and stimulates macrophages in autocrine manner. Our FACS data (Fig. 3) further confirm that macrophages can express TR2. When macrophages and granulocytes in RA synovial fluids were compared, macrophages tend to have higher expression levels of TR2 than granulocytes. Furthermore, this expression pattern well correlates with the cellular response to rhsLIGHT. Adherent mononuclear cells, which are enriched in macrophages, responded to rhsLIGHT with the induction of high levels of pro-inflammatory cytokines while granulocytes had no or low responses. The fact that both granulocytes and macrophages expressed increased levels of CD11b demonstrated that both of these cells were in activated status. These data confirm that TR2 expressed on macrophages is the functional receptor for LIGHT and they play specific functions in macrophages. The observation that concentration of soluble TR2 is increased in RA patients further supports our conclusion.28
LIGHT expressed in RA synovial macrophages appears to be involved in the pathogenesis of RA by stimulating the expression of pro-inflammatory cytokines and MMP-9. Macrophage derived pro-inflammatory cytokines such as IL-1, IL-6, IL-8, and TNF-α play a major role in the initiation and perpetuation of the chronic inflammatory process in the synovial membrane.2 Some of the pro-inflammatory cytokines also have the capacity to modulate cartilage and bone metabolism.29 IL-1 and TNF-α are the most well studied cytokines in RA and the importance of these cytokines has been demonstrated by clinical effectiveness of the IL-1 receptor antagonist and neutralizing antibodies directed against TNF-α.30–32 TNF-α triggers both local and systemic inflammation whereas IL-1 is involved at the local cartilage and bone destruction. IL-1 and TNF-α strongly synergize in numerous biological functions as well.33 In addition to these cytokines, IL-6 and IL-8 have also been demonstrated to be involved in RA. Targeted disruption of IL-6 protected an animal model from arthritis.34 In RA patients, IL-8 is the major T-cell chemoattractant in synovial tissues35 and IL-8 levels in synovial fluids are elevated.36 Our data demonstrate that LIGHT has a major role in the development of RA through induction of IL-6, IL-8, and TNF-α from macrophages.
Joint destruction is mediated by enzymes degrading extracellular matrix (ECM) such as serine proteases, MMPs and the cathepsins.1 MMPs are a large group of enzymes responsible for the disruption of the ECM proteins and they contribute to the joint destruction in RA by degrading the cartilage and bone. MMP-9 levels are substantially elevated in the sera and synovial fluid from RA patients.37,38 The induction of MMP-9 by LIGHT treatment in RA synovial macrophages (Fig. 5) further emphasizes the importance of LIGHT in inflammatory processes and the role of macrophages as a source of MMPs in RA synovium. Although it was not tested in RA synovial macrophages, LIGHT can induce other MMPs such as MMP-1 and MMP-13 and MMP inhibitors such as tissue inhibitors of MMP (TIMP)-1 and 2 in human monocytic cell line THP-1.18
It is well known that NF-κB is the main transcription factor for MMP-9 expression.39,40 Our previous analysis with human monocytic cell line THP-1 indicates that LIGHT treatment causes phosphorylation of IκB within 15 min after treatment and subsequent NF-κB nuclear translocation.41 The fact that there is a response within such a short time period indicates that LIGHT directly induces activation of NF-κB. Furthermore, the NF-κB inhibitor pyrrolidine dithiocarbamate completely blocked MMP-9 induction.41 These data indicate that LIGHT directly activates NF-κB which then induce the expression of MMP-9.
LIGHT can synergize with IFN-γ for the induction of TNF-α and MMP-9 (Figs 4 and 5). Activated T cells in synovium secrete IFN-γ and IL-17 which contribute to the enhancement of inflammation and they also interact with macrophages and fibroblasts through cell-to-cell contact mechanisms.42,43 Since both LIGHT and IFN-γ are present in RA synovium, it is highly likely that these cytokines work in concert to activate macrophage to produce TNF-α and MMP-9.
Our data in combination with previous experiments demonstrate that the expression of LIGHT and TR2 in RA synovial macrophages are involved in enhancement of inflammation through production of pro-inflammatory cytokines and destruction of ECM through production of MMP-9.
Acknowledgments
This work was supported by grant No. R01-2003-000-10887-0 from the Basic Research Program of the Korea Science & Engineering Foundation.
Abbreviations
- RA
rheumatoid arthritis
- OA
osteoperosis
- TNFSF
tumour necrosis factor superfamily
- TNFRSF
tumour necrosis factor receptor superfamily
- LT
lymphotoxin
- IL
interleukin
- IFN
interferon
References
- 1.Cunnane G, Hummel KM, Muller-Ladner U, Gay RE, Gay S. Mechanism of joint destruction in rheumatoid arthritis. Arch Immunol Ther Exp (Warsz) 1998;46:1–7. [PubMed] [Google Scholar]
- 2.Vervoordeldonk MJ, Tak PP. Cytokines in rheumatoid arthritis. Curr Rheumatol Rep. 2002;4:208–17. doi: 10.1007/s11926-002-0067-0. [DOI] [PubMed] [Google Scholar]
- 3.Kinne RW, Brauer R, Stuhlmuller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2:189–202. doi: 10.1186/ar86. 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yanni G, Whelan A, Feighery C, Bresnihan B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Ann Rheum Dis. 1994;53:39–44. doi: 10.1136/ard.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39:115–24. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- 6.Bresnihan B. Pathogenesis of joint damage in rheumatoid arthritis. J Rheumatol. 1999;26:717–9. [PubMed] [Google Scholar]
- 7.Van Lent PL, Van den Hoek AE, Van den Bersselaar LA, et al. In vivo role of phagocytic synovial lining cells in onset of experimental arthritis. Am J Pathol. 1993;143:1226–37. [PMC free article] [PubMed] [Google Scholar]
- 8.van Lent PL, Holthuysen AE, van den Bersselaar LA, et al. Phagocytic lining cells determine local expression of inflammation in type II collagen-induced arthritis. Arthritis Rheum. 1996;39:1545–55. doi: 10.1002/art.1780390915. [DOI] [PubMed] [Google Scholar]
- 9.Van Lent PL, Holthuysen AE, Van Rooijen N, Van De Putte LB, Van Den Berg WB. Local removal of phagocytic synovial lining cells by clodronate-liposomes decreases cartilage destruction during collagen type II arthritis. Ann Rheum Dis. 1998;57:408–13. doi: 10.1136/ard.57.7.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamada K, Shimozaki K, Chapoval AI, et al. LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol. 2000;164:4105–10. doi: 10.4049/jimmunol.164.8.4105. [DOI] [PubMed] [Google Scholar]
- 11.Wan X, Zhang J, Luo H, et al. A TNF family member LIGHT transduces costimulatory signals into human T cells. J Immunol. 2002;169:6813–21. doi: 10.4049/jimmunol.169.12.6813. [DOI] [PubMed] [Google Scholar]
- 12.Shi G, Luo H, Wan X, Salcedo TW, Zhang J, Wu J. Mouse T cells receive costimulatory signals from LIGHT, a TNF family member. Blood. 2002;100:3279–86. doi: 10.1182/blood-2002-05-1404. [DOI] [PubMed] [Google Scholar]
- 13.Zhai Y, Guo R, Hsu TL, et al. LIGHT, a novel ligand for lymphotoxin beta receptor and TR2/HVEM induces apoptosis and suppresses in vivo tumor formation via gene transfer. J Clin Invest. 1998;102:1142–51. doi: 10.1172/JCI3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.YeQ Fraser CC, Gao W, et al. Modulation of LIGHT-HVEM costimulation prolongs cardiac allograft survival. J Exp Med. 2002;195:795–800. doi: 10.1084/jem.20012088. 10.1084/jem.20012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheu S, Alferink J, Potzel T, Barchet W, Kalinke U, Pfeffer K. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. J Exp Med. 2002;195:1613–24. doi: 10.1084/jem.20020215. 10.1084/jem.20020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Foster A, Chin R, et al. The complementation of lymphotoxin deficiency with LIGHT, a newly discovered TNF family member, for the restoration of secondary lymphoid structure and function. Eur J Immunol. 2002;32:1969–79. doi: 10.1002/1521-4141(200207)32:7<1969::AID-IMMU1969>3.0.CO;2-M. 10.1002/1521-4141(200207)32:7<1969::AID-IMMU1969>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 17.Cohavy O, Zhou J, Granger SW, Ware CF, Targan SR. LIGHT expression by mucosal T cells may regulate IFN-gamma expression in the intestine. J Immunol. 2004;173:251–8. doi: 10.4049/jimmunol.173.1.251. [DOI] [PubMed] [Google Scholar]
- 18.Lee WH, Kim SH, Lee Y, et al. Tumor necrosis factor receptor superfamily 14 is involved in atherogenesis by inducing proinflammatory cytokines and matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2001;21:2004–10. doi: 10.1161/hq1201.098945. [DOI] [PubMed] [Google Scholar]
- 19.Fava RA, Notidis E, Hunt J, et al. A role for the lymphotoxin/LIGHT axis in the pathogenesis of murine collagen-induced arthritis. J Immunol. 2003;171:115–26. doi: 10.4049/jimmunol.171.1.115. [DOI] [PubMed] [Google Scholar]
- 20.Marsters SA, Ayres TM, Skubatch M, Gray CL, Rothe M, Ashkenazi A. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J Biol Chem. 1997;272:14029–32. doi: 10.1074/jbc.272.22.14029. 10.1074/jbc.272.22.14029. [DOI] [PubMed] [Google Scholar]
- 21.Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–36. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 22.Kwon BS, Tan KB, Ni J, et al. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J Biol Chem. 1997;272:14272–6. doi: 10.1074/jbc.272.22.14272. 10.1074/jbc.272.22.14272. [DOI] [PubMed] [Google Scholar]
- 23.Hsu H, Solovyev I, Colombero A, Elliott R, Kelley M, Boyle WJ. ATAR, a novel tumor necrosis factor receptor family member, signals through TRAF2 and TRAF5. J Biol Chem. 1997;272:13471–4. doi: 10.1074/jbc.272.21.13471. 10.1074/jbc.272.21.13471. [DOI] [PubMed] [Google Scholar]
- 24.Mauri DN, Ebner R, Montgomery RI, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. 10.1016/S1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 25.Lee WH, Lee Y, Kim JR, et al. Activation of monocytes, T-lymphocytes and plasma inflammatory markers in angina patients. Exp Mol Med. 1999;31:159–64. [PubMed] [Google Scholar]
- 26.Birkedal-Hansen H, Taylor RE. Detergent-activation of latent collagenase and resolution of its component molecules. Biochem Biophys Res Commun. 1982;107:1173–8. doi: 10.1016/s0006-291x(82)80120-4. [DOI] [PubMed] [Google Scholar]
- 27.Ross GD, Vetvicka V. CR3 (CD11b, CD18). a phagocyte and NK cell membrane receptor with multiple ligand specificities and functions. Clin Exp Immunol. 1993;92:181–4. doi: 10.1111/j.1365-2249.1993.tb03377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung HW, La SJ, Kim JY, et al. High levels of soluble herpes virus entry mediator in sera of patients with allergic and autoimmune diseases. Exp Mol Med. 2003;35:501–8. doi: 10.1038/emm.2003.65. [DOI] [PubMed] [Google Scholar]
- 29.Romas E, Gillespie MT, Martin TJ. Involvement of receptor activator of NFkappaB ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis. Bone. 2002;30:340–6. doi: 10.1016/s8756-3282(01)00682-2. [DOI] [PubMed] [Google Scholar]
- 30.Carroll G, Bell M, Wang H, Chapman H, Mills J. Antagonism of the IL-6 cytokine subfamily – a potential strategy for more effective therapy in rheumatoid arthritis. Inflamm Res. 1998;47:1–7. doi: 10.1007/s000110050235. [DOI] [PubMed] [Google Scholar]
- 31.Bang LM, Keating GM. Adalimumab: a review of its use in rheumatoid arthritis. Biodrugs. 2004;18:121–39. doi: 10.2165/00063030-200418020-00005. [DOI] [PubMed] [Google Scholar]
- 32.Rubbert-Roth A, Perniok A. [Interleukin-1 receptor antagonist anakinra (Kineret) for treatment of rheumatic arthritis] Z Rheumatol. 2003;62:367–77. doi: 10.1007/s00393-003-0545-4. 10.1007/s00393-003-0545-4. [DOI] [PubMed] [Google Scholar]
- 33.Dayer JM. Interleukin 1 or tumor necrosis factor-alpha: which is the real target in rheumatoid arthritis? J Rheumatol Suppl. 2002;65:10–5. [PubMed] [Google Scholar]
- 34.Alonzi T, Fattori E, Lazzaro D, et al. Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med. 1998;187:461–8. doi: 10.1084/jem.187.4.461. 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishiura H, Tanaka J, Takeya M, Tsukano M, Kambara T, Imamura T. IL-8/NAP-1 is the major T-cell chemoattractant in synovial tissues of rheumatoid arthritis. Clin Immunol Immunopathol. 1996;80:179–84. doi: 10.1006/clin.1996.0112. 10.1006/clin.1996.0112. [DOI] [PubMed] [Google Scholar]
- 36.Endo H, Akahoshi T, Takagishi K, Kashiwazaki S, Matsushima K. Elevation of interleukin-8 (IL-8) levels in joint fluids of patients with rheumatoid arthritis and the induction by IL-8 of leukocyte infiltration and synovitis in rabbit joints. Lymphokine Cytokine Res. 1991;10:245–52. [PubMed] [Google Scholar]
- 37.Gruber BL, Sorbi D, French DL, Marchese MJ, Nuovo GJ, Kew RR, Arbeit LA. Markedly elevated serum MMP-9 (gelatinase B) levels in rheumatoid arthritis: a potentially useful laboratory marker. Clin Immunol Immunopathol. 1996;78:161–71. doi: 10.1006/clin.1996.0025. 10.1006/clin.1996.0025. [DOI] [PubMed] [Google Scholar]
- 38.Giannelli G, Erriquez R, Iannone F, Marinosci F, Lapadula G, Antonaci S. MMP-2, MMP-9, TIMP-1 and TIMP-2 levels in patients with rheumatoid arthritis and psoriatic arthritis. Clin Exp Rheumatol. 2004;22:335–8. [PubMed] [Google Scholar]
- 39.Moon SK, Cha BY, Kim CH. ERK1/2 mediates TNF-alpha-induced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-kappaB and AP-1: Involvement of the ras dependent pathway. J Cell Physiol. 2004;198:417–27. doi: 10.1002/jcp.10435. 10.1002/jcp.10435. [DOI] [PubMed] [Google Scholar]
- 40.Bond M, Fabunmi RP, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kappa B. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- 41.Kim WJ, Lee WH. LIGHT is expressed in foam cells and involved in destabilization of atherosclerotic plaques through induction of matrix metalloproteinase-9 and IL-8. Immune Network. 2004;4:116–22. [Google Scholar]
- 42.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 43.Lubberts E, Joosten LA, Oppers B, et al. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J Immunol. 2001;167:1004–13. doi: 10.4049/jimmunol.167.2.1004. [DOI] [PubMed] [Google Scholar]