Abstract
Previous studies have shown that chlamydial infection is accompanied by significant infiltration of neutrophils at the site of infection. However, the role of neutrophils in host defence against chlamydial infection is not clearly understood. Using genetically different inbred mouse strains and CXCR-2 gene knockout (KO) mice, we examined the mechanism for neutrophil recruitment and the role of neutrophils during chlamydial lung infection. Our data showed that C3H mice exhibited significantly higher and more persistent neutrophil infiltration in the lung than did C57BL/6 mice following Chlamydia trachomatis mouse pneumonitis infection. The massive neutrophil infiltration in C3H mice was paralleled by high-level expression of CXCR-2 and its ligands, CXC chemokines (macrophage inflammatory protein 2, cytokine-induced neutrophil attractant (KC) and lipopolysaccharide-induced CXC chemokine), and proinflammatory cytokines (tumour necrosis factor-α, interleukin-1 and interleukin-6) in the lung. Although much greater infiltration of neutrophils was observed in C3H mice than in C57BL/6 mice, the former mice had more severe disease and higher in vivo chlamydial growth than the latter. Moreover, CXCR-2 KO mice, which revealed a dramatic reduction in neutrophil activity, showed comparable chlamydial infection to wild-type mice. These results suggest that neutrophils are not efficient for controlling chlamydial lung infection.
Keywords: chemokine, Chlamydia trachomatis, CXCR-2, gene knockout, neutrophil
Introduction
Chlamydia trachomatis is an obligate intracellular bacterium that causes a variety of human and animal diseases, including trachoma, pelvic inflammatory disease, infertility and pneumonia. Although T-cell-mediated immune responses, especially T helper type 1 cytokines, have been demonstrated to be critical in the host defence against C. trachomatis infection,1–3 the role of innate immune responses in host defence against chlamydial infection remains largely unknown. Neutrophils are professional phagocytes which contribute to host defence against numerous infections caused by extracellular and intracellular pathogens.4–9 Previous studies have shown that chlamydial infection is accompanied by significant infiltration of neutrophils at the site of infection.4,10–12 However, the role of neutrophil infiltration in host defence against chlamydial infection is not clearly understood.
The molecular mechanisms for recruitment of neutrophils to the lung probably involve the local release of neutrophil chemoattractants and a cascade of regulated expression of neutrophil adhesion molecules.13 Chemokines play a critical role in regulating cellular migration from the circulation into inflammatory tissues.14,15 The murine Gln-Len-Arg (ELR)+ CXC family of chemokines, including macrophage inflammatory protein 2 (MIP-2), cytokine-induced neutrophil attractant (KC) and lipopolysaccharide-induced CXC chemokine (LIX), have been shown to be particularly important in neutrophil chemotaxis and neutrophil activation in pulmonary and extrapulmonary inflammatory responses.16–19 Moreover, mouse neutrophils express a receptor homologous to human CXCR-2 that mediates neutrophil chemotaxis in response to human interleukin-8 (IL-8) and murine ELR+ CXC chemokines.20 In addition, proinflammatory cytokines, such as IL-1, IL-6 and tumour necrosis factor-α (TNF-α) also participate in the up-regulation of adhesion molecules needed for leucocyte extravasation into tissues.21
To further examine the mechanism for neutrophil recruitment and the role of neutrophils in chlamydial infection, we investigated, in the present study, the relationship between chemokine/cytokine expression and the infiltration of neutrophils to the site of chlamydial infection and the relationship between neutrophil infiltration and the clearance of the infection from the lung using two genetically different inbred mouse strains and one type of chemokine receptor gene knockout (KO) mouse. Our data showed that neutrophil infiltration was paralleled by expression of CXCR-2 and its ligands, CXC chemokines (MIP-2, KC and LIX) and proinflammatory cytokines in the lung. Although much greater infiltration of neutrophils was observed in C3H mice than in C57BL/6 mice, the former mice showed much more severe disease and higher in vivo chlamydial growth than the latter. Moreover, CXCR-2 KO mice, which revealed dramatic reduction in neutrophil infiltration, showed levels of chlamydial infection comparable to those of wild-type mice. These results suggest that neutrophils are not efficient for controlling chlamydial infection.
Materials and methods
Mice
Female C57BL/6 and C3H/HeN mice (6–8 weeks old) were purchased from Charles River Canada (St Constant, Quebec, Canada). Mice were kept at the University of Manitoba central animal-care facility (Winnipeg, Canada). Mice were maintained and used in accordance with the guidelines of the Animal Council of Canada. Female homozygous CXCR-2 gene KO mice (6–8 weeks old) with BALB/c background (Il8rbtm1Mwm) were purchased from Jackson Laboratories (Bar Harbor, ME). Wild-type BALB/c mice were purchased from Charles River Canada. Mice were kept in a specific pathogen-free facility for animals at the University of Manitoba with filtered air flow and autoclaved cage, food and water.
Infection of mice
The mouse pneumonitis biovar of C. trachomatis (MoPn) was grown in HeLa 229 cells and purified by discontinuous density gradient centrifugation as described previously.22 Mice were inoculated intranasally with various dosages (100–100 × 102 inclusion-forming units; IFUs) of MoPn in a volume of 40 μl after anaesthesia with isoflurane using a anaesthetic vaporizer. To determine the in vivo growth of the organism, the lungs were aseptically isolated from mice on selected days after infection and homogenized in 4 ml of sucrose phosphate glutamic acid buffer. Tissue homogenates were spun down at 500 g for 10 min at 4° and the supernatants were divided into aliquots (1 ml/vial) and kept at −86° until tested for infectivity. Repeating tests always used a fresh aliquot of the lung sample. Infectivity of the samples was titrated by infection of Hela cell monolayers for 48 hr followed by methanol fixation of cells and enumeration of inclusions that were stained by a genus-specific monoclonal antibody (mAb) conjugated with horseradish peroxidase as described elsewhere.22
Lung inflammatory cell preparation
Lung tissues (50 mg/mouse) were harvested from MoPn-infected mice on days 7 and 14 postinfection. Inflammatory cells in the lung tissues were isolated as previously described.23 Briefly, lung tissue was minced with scissors to a fine slurry in 5 ml lung digestion buffer [RPMI-1640 with 10% fetal calf serum, 1 mg/ml collagenase A (Gibco, Grand Island, NY), 30 μg/ml DNAse (Gibco)]. After incubation in the above medium for 30–45 min, undigested fragments were further dispersed by drawing the solution up and down through the bore of a 10-ml syringe. The total lung cell suspensions were centrifuged at 400 g for 40 min and washed once with 10 ml of cold phosphate-buffered saline. Cell differentials were determined by Wright–Giemsa staining. The amount of each cell type was determined by multiplying the percentage of each type by the total number of cells.
Lung myeloperoxidase (MPO) assay
Lung MPO, as an indirect measurement of neutrophil infiltration, was quantified as described previously.24 Briefly, fresh lung samples were weighed (50 mg) and homogenized in 1 ml buffer A (3·4 mm KH2PO4, 16 mm Na2HPO4, pH 7·4). After centrifugation for 30 min at 10 000 g, the pellet was resuspended in 1 ml buffer B (43·2 mm KH2PO4, 6·5 mm KH2PO4, 10 mm ethylenediaminetetraacetic acid, 0·5% hexadecyltrimethylammonium, pH 6·0) and sonicated for 10 seconds. Following heating for 2 hr at 60° and spinning down at 10 000 g for 10 min, the supernatant was reacted with 3,3′,5,5′-tetramethylbenzidine (Sigma Chemical Co., St Louis, MO) and read at 650 nm using human MPO as a standard. Results are expressed as per unit weight of lung.
Histopathological and immunohistofluorescence analysis
Lungs for histological assessment were removed and fixed in 10% buffered formalin, embedded in paraffin. Sections were stained with haematoxylin & eosin, and the histological changes and cellular infiltration were determined by light microscopy. To analyse CXCR-2 expression in the lung following MoPn infection, lung tissues were snap-frozen in liquid nitrogen and 10-μm sections were fixed on slides in purified acetone. After blocking using blocking buffer, the slides were incubated at room temperature for 2 hr with phycoerythrin-conjugated rat anti-mouse CXCR2 (R & D Systems Inc. Minneapolis, MN) followed by a wash with washing buffer. The slides were then stained for epithelial cells using mouse anti-pan-cytokeratin antibody (Calbiochem Inc. San Diego, CA) for 1 hr. After washing, the slides were incubated with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G antibody (Sigma Inc. Oakville, ON, Canada) for 40 min. The slides were examined using a fluorescence microscope.
Analysis of cytokine and chemokine production
For analysis of mRNA by reverse transcriptase–polymerase chain reaction (RT-PCR), lungs were harvested from mice, frozen immediately in liquid nitrogen, and stored at −86°. Total cellular RNA from frozen lungs was isolated using TRIzol Reagent (Gibco) according to the manufacturer's protocol. cDNA was synthesized by reverse transcription at 37° for 1 hr from 1·2 μg total cellular RNA with random hexamers to prime the RT. The cDNA was then amplified using specific primers for MIP-2, KC, LIX, CXCR-2, IL-1β, TNF-αand IL-6, with a housekeeping gene (β-actin) as a control. Ten microlitres of each RT-PCR reaction was electrophoresed in a 1% agarose gel containing ethidium bromide (0·003%). The bands were visualized and photographed using ultraviolet transillumination and were analysed for density on scion image software. The expression of chemokine and cytokine mRNA is presented as a percentage of β-actin. The primers used in the PCR analysis are as follows: MIP-2 sense 5′-ACCCTGCCAAGGGTTGACTTC-3′, antisense 5′-GGCACATCAGGTACGATCCAG-3′, to give a 285-base-pair (bp) product; KC sense 5′-AACGGAGAAAGAAGACAGACTG-3′, antisense 5′-GACGAGACCAGGAGAAACAG-3′ to give a 530-bp product; LIX sense 5′-AGCTCGCCATTCATGCGGATG-3′, antisense 5′-CTATTGAACACTGGCCGTTCT-3′, to give a 344-bp product; CXCR-2 sense 5′-CTCCTTGGTGATGCTGGTCA-3′, antisense 5′-AGAATTAAGATGGGCAGGGC-3′ to give a 331-bp product; IL-1β sense 5′-GCAACTGTTCCTGAACTCA-3′, antisense 5′-CTCGGAGCCTGTAGTGCAG-3′ to give a 363-bp product; TNF-α sense 5′-AACTAGTGGTGCCAGCCGAT-3′, antisense 5′-CTTCACAGAGCAATGACTCC-3′ to give a 314-bp product; IL-6 sense 5′-TTCCTCTCTGCAAGAGACT-3′, antisense 5′-TGTATCTCTCTGAAGGACT-3′ to give a 413-bp product; and β-actin sense 5′-GTGGGGCGCCCCAGGCACCA-3′, antisense 5′-CTCCTTAATGTCACGCACGATTTC-3′ to give a 550-bp product. For analysis of IL-6 in the lung homogenates, enzyme-linked immunosorbent assays (ELISA) using paired mAbs purchased from BD PharMingen were performed as described elsewhere.25
Statistical analysis
Differences in cytokine and chemokine expression were analysed using Student's t-test. Chlamydial IFUs in lung tissue homogenates were first normalized by transformation to log10 and then analysed with the Student's t-test.
Results
Influx of neutrophils into the lungs following intranasal MoPn infection
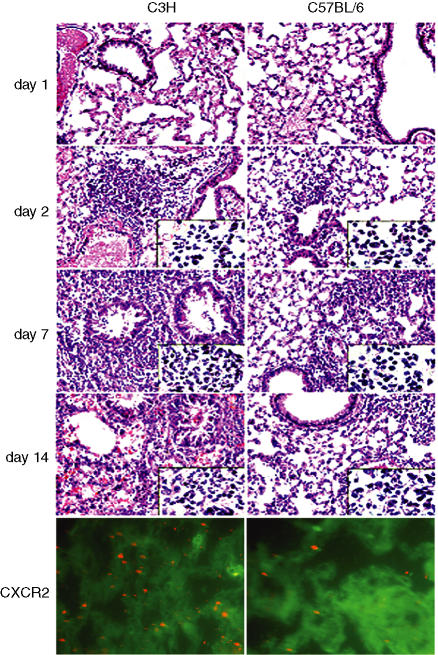
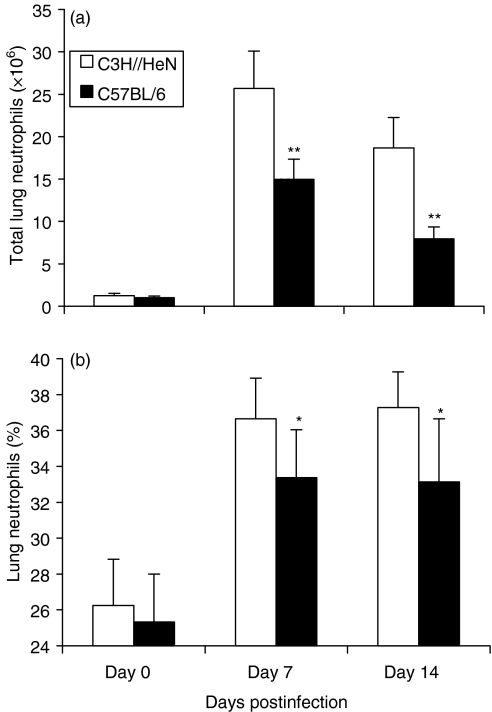
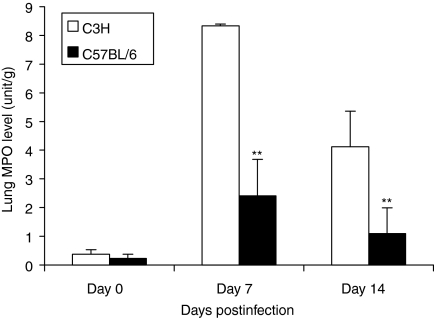
Our previous studies have shown that intranasal inoculation of mice with MoPn readily causes lung infection and inflammation, demonstrated by significant cellular infiltration in the lung and the recovery of chlamydial organisms from the local tissues.22,25,26 In the present study, we examined cellular infiltration in the lung using histological and cytological approaches. As shown in Fig. 1, C3H mice began to show more neutrophil infiltration than C57BL/6 mice at day 2 postinfection and kept showing higher levels up to day 14, when the experiments terminated. A more precise quantification of the number and percentage of neutrophils infiltrating into the lung after MoPn infection in C57BL/6 and C3H mice is shown in Fig. 2. It was found that neutrophil infiltration was obvious in both strains of mice following MoPn infection but that the absolute number and the percentage of neutrophils in the lung were significantly greater in C3H than C57BL/6 mice. Using an alternative approach for the measurement of neutrophil activity, the MPO assay, we found a sharp increase in lung MPO levels in both strains of mice following chlamydial infection, confirming the significant accumulation of neutrophils at the site of MoPn infection (Fig. 3). Similar to the finding of greater infiltration of neutrophils in C3H mice (Figs 1 and 2), the MPO levels in C3H mice were significantly higher than in C57BL/6 mice (P < 0·01). The results suggest that acute chlamydial lung infection can induce significant neutrophil infiltration and that genetically different strains of mice mount differential patterns of this cellular recruitment following the infection.
Figure 1.
C3H mice show higher neutrophil infiltration, CXCR-2 expression and more severe pathological reactions than C57BL/6 mice following MoPn lung infection. Top four panels, mice were intranasally infected with 3 × 103 IFU of MoPn and killed 1, 2, 7, or 14 days following infection. Lungs were removed and fixed in 10% buffered formalin, embedded in paraffin. Sections were stained with haematoxylin & eosin and the histological changes and cellular infiltration were analysed by light microscopy. Magnification, ×200; inserts, ×400. Bottom panels, mice were killed at day 7 postinfection. Frozen lung sections were stained for CXCR-2 (red) and epithelial cells (green) using methods described in the Materials and methods section; magnification, ×400.
Figure 2.
C3H mice show significantly higher neutrophil infiltration than C57BL/6 mice following MoPn lung infection. C3H and C57BL/6 mice (14 mice per group) were infected with MoPn as described in Figure 1 and killed 7 or 14 days following infection. The lungs were digested with collagenase and the recovered cells were stained using Wright–Giemsa staining. The number (a) and percentages (b) of neutrophils in the recovered lung cells are presented as mean ± SEM. *P < 0·05; **P < 0·01, comparison between C3H and C57BL/6 mice.
Figure 3.
Higher local myeloperoxidase (MPO) activity in C3H than C57BL/6 mice following MoPn lung infection. C3H and C57BL/6 mice were infected as described in Figure 1. Fresh lung tissues were collected on 7 and 14 days postinfection (five mice per group at each time-point). MPO activity in these tissues was determined as described in the Materials and methods section. The data are presented as mean ± SEM. **P < 0·01, comparison between C3H and C57BL/6 mice.
MoPn infection induces CXC chemokine and chemokine receptor mRNA expression in the lung
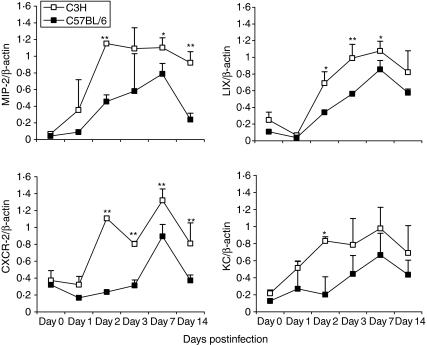
To address the mechanism of neutrophil influx into the lungs in response to C. trachomatis infection, we next examined the expression of several CXC chemokines which have been shown to be particularly important for neutrophil chemotaxis. As shown in Fig. 4, the amounts of mRNA of these chemokines, including, MIP-2, LIX and KC, were very low in the lungs of both strains of mice prior to infection. However, their expression was significantly up-regulated by MoPn infection from day 2 postinfection; expression was higher in C3H mice than C57BL/6 mice for all of the chemokines tested. In particular, the MIP-2 response remained significantly greater in C3H than C57BL/6 mice even at the late stages (day 14) postinfection (P < 0·01) while the difference in other chemokines gradually diminished starting from day 7. In general, the pattern of these chemokines' expression correlated well with the pattern of neutrophil infiltration in the local tissues in both the extent and the duration in the two groups of mice. In a control experiment, no significant increase of neutrophil infiltration or of chemokine expression was found at day 7 postinoculation in the lungs of the mice that were treated by intranasal inoculation with a preparation of uninfected HeLa229 cells that underwent the same procedure for preparing purified MoPn elementary bodies.22 The results suggest that CXC chemokines contribute to the recruitment of neutrophils into the lung of Chlamydia-infected mice.
Figure 4.
Higher and persistent chemokine/chemokine receptor expression in the lung of C3H mice following MoPn infection. C3H and C57BL/6 mice were infected as described in Figure 1. Fresh lung tissues were collected on 0, 1, 2, 3, 7 and 14 days (from three to seven mice per group in each time-point) postinfection and snap frozen by liquid nitrogen. Total cellular RNA from frozen lungs was isolated using TRIzol Reagent (Gibco). RT-PCR analysis of chemokine/chemokine receptor mRNA was performed as described in the Materials and methods section. The data are presented as mean ±SEM of the density of the bands of chemokine/chemokine receptor as a percentage of β-actin. *P < 0·05; **P < 0·01, comparison between C3H and C57BL/6 mice.
We next measured mRNA expression in the lung for CXCR-2, a receptor for CXC chemokines related to neutrophil chemotaxis. Before infection, CXCR-2 mRNA was very low in the lung tissues of C3H and C57BL/6 mice. Following infection, significant up-regulation of the mRNA of this chemokine receptor was observed in the lung tissues of both strains of mice (Fig. 4). Similar to the expression pattern of the tested chemokines, especially MIP-2, CXCR-2 expression was significantly greater in C3H mice than C57BL/6 mice, at both early and late stages of infection. The higher expression of CXCR-2 protein in the lungs from C3H mice than in those from C57BL/6 mice following MoPn infection was confirmed by immunohistofluorescence staining (Fig. 1, bottom panel). It is clearly shown that CXCR-2 was expressed by infiltrating cells (neutrophils). The results further confirm the higher infiltration of neutrophils in C3H mice and the correlation between expression of chemokines such as MIP-2 and the recruitment of CXCR-2-expressing cells.
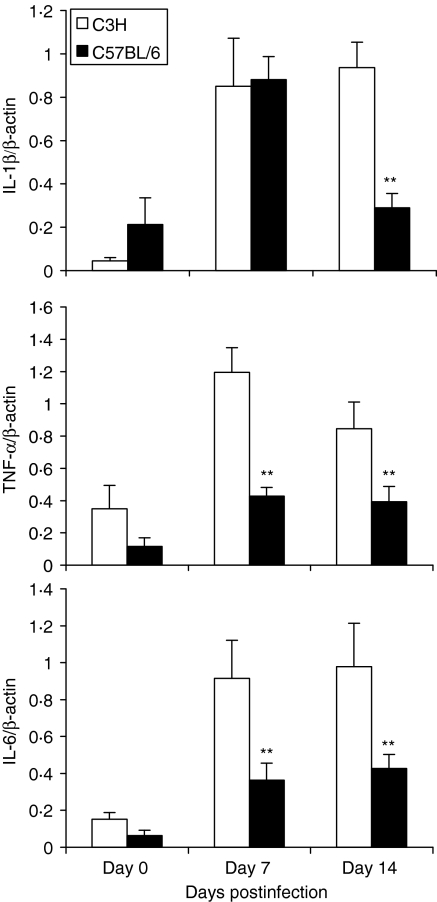
MoPn lung infection induced proinflammatory cytokine production in local tissues
TNF-α, IL-1 and IL-6 have been reported to be potent proinflammatory cytokines that induce expression of adhesion molecules and chemokines, thus triggering inflammatory cell recruitment. We therefore compared the production of these cytokines in the lungs of the two strains of mice. As shown in Fig. 5, IL-1β mRNA expression was significantly increased in both C57BL/6 and C3H mice on day 7 postinfection, but remained high only in C3H mice on day 14 postinfection. For TNF-α and IL-6, the expression of these cytokines was increased following MoPn infection in both strains of mice, but C3H mice showed significantly higher expression of these cytokines than C57BL/6 mice at both early and late stages of infection. The pattern of TNF-α and IL-6 expression was consistent with the pattern of CXC chemokine expression in the two strains of mice (Fig. 4). ELISA for IL-6 in the lung homogenates showed that C3H mice produced significantly more IL-6 than C57BL/6 mice at both day 7 (1800 ± 500 μg/ml in C3H mice versus 760 ± 450 μg/ml in C57BL/6 mice, P < 0·01) and day 14 (2600 ± 650 μg/ml in C3H mice versus 410 ± 250 μg/ml in C57BL/6 mice, P < 0·001). The data reveal an association between the expression of chemokines/chemokine receptors and proinflammatory cytokines in the lung following chlamydial infection.
Figure 5.
Higher proinflammatory cytokine production in the lung in C3H mice than C57BL/6 mice following MoPn infection. C3H and C57BL/6 mice were infected as described in Figure 1. Fresh lung tissues were collected 7 and 14 days postinfection (five mice per group at each time-point) and snap frozen in liquid nitrogen. Total cellular RNA from frozen lungs was isolated using TRIzol Reagent (Gibco). RT-PCR analysis of cytokine mRNA was performed as described in the Materials and methods section. The data are presented as mean ± SEM of the density of the bands of cytokines as a percentage of β-actin. *P < 0·05; **P < 0·01, comparison between C3H and C57BL/6 mice.
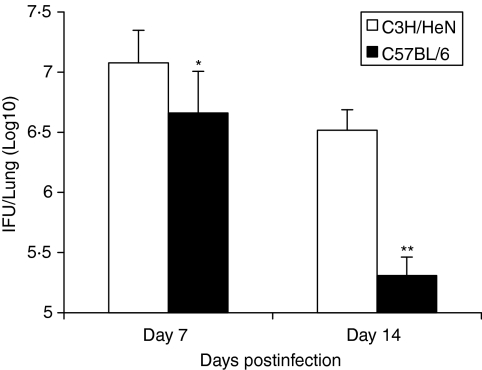
Greater neutrophil infiltration is not associated with better protection against chlamydial infection
To elucidate the relationship between neutrophil infiltration and the clearance of chlamydial infection, we compared the chlamydial growth in the lung in the different strains of mice following intranasal MoPn infection. As shown in Fig. 6, although both C3H mice and C57BL/6 mice showed chlamydial growth in the lung on 7 and 14 days postinfection, the level was significantly higher in the former, especially at the late stage of infection (day 14, P < 0·01). Analysis of chlamydial major outer membrane (MOMP) genes at very early stages of infection using RT-PCR showed higher chlamydial gene expression in C3H mice starting at day 3 (data not shown). In addition, the body weight loss in C57BL/6 mice was significantly less than in C3H mice and their body weights had partially recovered by day 14 postinfection, while C3H mice showed continuous decline of their body weights throughout the study (data not shown). Moreover, histological analysis showed that the inflammation in C3H mice was significantly greater and more persistent than in C57BL/6 mice (Fig. 1). In particular, at day 14 postinfection, the inflammation in C57BL/6 mice began to resolve, with evidence of tissue healing (presence of fibroblasts), while the pathological reaction in the lungs of C3H mice became more serious, characterized by increased production of viscous mucus, inflammatory cell infiltration, bronchial obstruction, expansion and destruction of alveoli, and leakage of capillaries and bleeding (Fig. 1). In addition, we also examined chlamydial growth in vivo in the two strains of mice following a wide range of dosages (100–100 × 102 IFUs) of infection. The results showed that C3H mice always had eight- to ten-fold higher organism growth in the lung than C57BL/6 mice following the various doses of infection. The results suggest that the higher neutrophil infiltration is not associated with better protection and, in contrast, it is correlated with more severe pathological reactions and diseases, especially when massive neutrophil infiltration is generated.
Figure 6.
Slower clearance of chlamydial infection in the lung by C3H mice compared with C57BL/6 mice. Mice (10 mice per group) were infected as described in Figure 1 and were killed on days 7 or 14 postinfection. The levels of Chlamydia in the lung homogenates were analysed as described in the Materials and methods section. The data are presented as mean ± SEM. *P < 0·05; **P < 0·01, comparison between C3H and C57BL/6 mice.
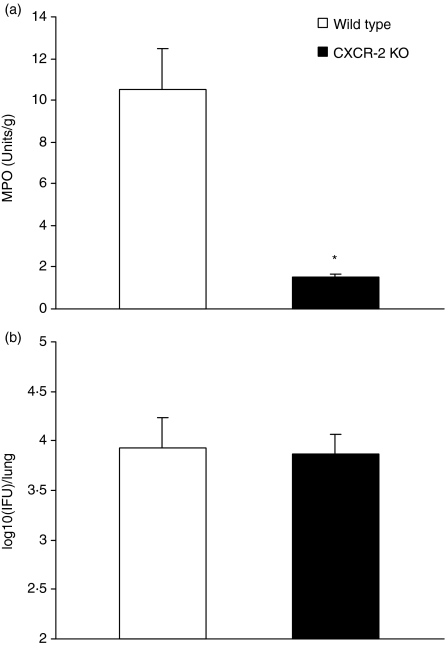
CXCR-2 KO mice show comparable levels of chlamydial growth in vivo to wild-type mice
To further elucidate the role of neutrophils in chlamydial infection, we examined neutrophil activity and chlamydial infection in CXCR-2 KO mice (Fig. 7). The results showed that CXCR-2 KO mice exhibited a sevenfold reduction in neutrophil activity by MPO assay (1·5 units/g lung tissue in CXCR-2 KO mice versus 10·5 units/g lung tissue in wild-type mice, P < 0·05). However, the mice showed comparable levels of chlamydial growth in the lung following MoPn infection [3·87 IFU (log10) in CXCR-2 KO mice versus 3·93 IFU (log10) in wild-type mice at day 7 postinfection]. Similarly, the degree of body weight loss following infection was comparable between the KO and wild-type mice. The finding that the mice with dramatic differences in neutrophil recruitment had similar organism growth in vivo and a comparable course of disease suggests that neutrophils do not play a significant role in controlling chlamydial infection.
Figure 7.
CXCR-2 gene knockout (KO) mice which show dramatic reduction of neutrophils exhibit comparable levels of infection to wild-type mice. CXCR-2 KO mice (BALB/c background) and control wild-type BALB/c mice (four mice per group) were infected intranasally with 1 × 103 IFUs of MoPn and killed on day 7 postinfection. The levels of MPO (a) and of chlamydial infection (b) in the lungs were analysed as described in the Materials and methods. The data are presented as mean ± SD. *P < 0·01, comparison between CXCR-2 KO and wild-type mice.
Discussion
We focused, in the present study, on the mechanism of neutrophil recruitment to the site of infection and the role of this cell type in host defence against Chlamydia. Our results showed that although infection with C. trachomatis led to the infiltration of neutrophils in both strains of mice, the absolute number and the percentage of neutrophils in the lung were significantly greater in C3H than C57BL/6 mice. The extent and duration of infiltration of neutrophils in the different strains of mice were paralleled with the production of MPO in local tissues. Interestingly, the C3H mice which showed higher and more persistent neutrophil infiltration, failed to control the infection and demonstrated a more serious course of disease. Moreover, CXCR-2 KO mice, which were deficient in neutrophil recruitment, showed a comparable course of infection to that in wild-type mice. The results suggest that neutrophils are not efficient in controlling chlamydial infection in this lung infection model. It should be noted that there is a previous report which showed that depletion of neutrophils in vivo or blockage of neutrophil migration using mAbs increased chlamydial growth in mice at early stages of infection following intraperitoneal or intravaginal inoculation, suggesting a protective role of neutrophils in chlamydial infection.4 The reason for the difference between the report and our data from KO mice and genetically different strains is unclear. One apparent difference between the studies is the model systems used. The report used intraperitoneal and intravaginal infection models while our data are from a lung infection model. Notably, the work of Darvil et al. comparing C3H and C57BL/6 mice using a genital infection model also showed that C3H mice exhibit higher neutrophil infiltration and suffer more serious diseases than C57BL/6 mice.
The data in the present study showed a close association between neutrophil infiltration and the expression of chemokines, MIP-2, KC and LIX, and their receptor, CXCR-2, following chlamydial infection. MIP-2 and LIX are the best-studied murine ELR+ CXC chemokines and are functional homologues of the human chemokines IL-8 and GRO-α/β, respectively.27 Two human receptors for ELR+ CXC chemokines have been identified, CXCR1 and CXCR2.28 Ligands binding to either receptor activate neutrophil chemotaxis and exocytosis. Whereas CXCR1 is selective for IL-8, CXCR2 is activated by all ELR+ CXC chemokines. In mice, no homologues of human IL-8 and CXCR1 have been identified. Therefore, CXCR2 is the major receptor for murine ELR+ CXC chemokines which mediate neutrophil chemotaxis. The increase of MIP-2 mRNA following chlamydial infection has been reported in previous studies. Darville et al. observed a correlation between the kinetics of neutrophil infiltration and MIP-2 expression in BALB/c and C3H mice following genital tract chlamydial infection.21 Huang et al.29 found that administration of exogenous IL-12 during C. psittaci lung infection in mice reduced the severity of and mortality from the chlamydial pneumonia, which was associated with reduced MIP-2 levels and neutrophil infiltration into the lung. In the present study, we found that the MIP-2 response was significantly greater in C3H than C57BL/6 mice at both early and late stages of infection. Moreover, we found that CXCR-2, the receptor for MIP-2, was significantly higher in C3H mice than C57BL/6 mice. Therefore, the higher expression of MIP-2 and its receptor in C3H mice may be the reason for the higher infiltration of neutrophils in the lung. The dramatic reduction in neutrophil activity in CXCR-2 KO mice compared to wild-type mice following chlamydial infection also confirmed the extreme importance of CXCR-2 for neutrophil recruitment.30 LIX and KC responses following chlamydial infection have not been reported in previous studies. We found in the present study that LIX and KC were significantly up-regulated by MoPn infection and that C3H mice showed significantly higher expression of LIX and KC than C57BL/6 mice at the early, but not the late, stages of infection. The results suggest that not only MIP-2 but also other chemokines related to neutrophil chemotaxis are involved in the recruitment of neutrophils following chlamydial infection. Moreover, the data suggest that the persistence of neutrophils in C3H mice may be more related to CXCR-2 and MIP-2 expression than to KC and LIX expression.
In conclusion, the present data show that neutrophils are not efficient for the clearance of chlamydial infection. The over-expression and the persistence of chemokines (MIP-2, KC and LIX) and their receptor (CXCR-2) may be the basis for the massive infiltration of neutrophils in C3H mice that fail to control chlamydial lung infection.
Acknowledgments
This work was supported by operating grants from the Canadian Institutes of Health Research (CIHR) and the Manitoba Health Research Council (MHRC) to X.Y. H.B. is a registered PhD student at Peking University and was a visiting scholar at the University of Manitoba. X.Y. is a Canada Research Chair in Infection and Immunity granted by the federal government of Canada.
Abbreviations
- EB
elementary body
- ELR
Gln-Len-Arg
- IFU
inclusion forming unit
- KC
cytokine-induced neutrophil attractant
- MoPn
Chlamydia trachomatis mouse pneumonitis
- MPO
myeloperoxidase
References
- 1.Cain TK, Rank RG. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis (MoPn) biovar of Chlamydia trachomatis. Infect Immun. 1995;63:1784–9. doi: 10.1128/iai.63.5.1784-1789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol. 1997;158:3344–52. [PubMed] [Google Scholar]
- 3.Yang X. Role of cytokines in Chlamydia trachomatis protective immunity and immunopathology. Curr Pharm Des. 2003;9:67–73. doi: 10.2174/1381612033392486. [DOI] [PubMed] [Google Scholar]
- 4.Barteneva N, Theodor I, Peterson EM, de la Maza LM. Role of neutrophils in controlling early stages of a Chlamydia trachomatis infection. Infect Immun. 1996;64:4830–3. doi: 10.1128/iai.64.11.4830-4833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conlan JW. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect Immun. 1997;65:630–5. doi: 10.1128/iai.65.2.630-635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czuprynski CJ, Brown JF, Wagner RD, Steinberg H. Administration of antigranulocyte monoclonal antibody RB6-8C5 prevents expression of acquired resistance to Listeria monocytogenes infection in previously immunized mice. Infect Immun. 1994;62:5161–3. doi: 10.1128/iai.62.11.5161-5163.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sjostedt A, Conlan JW, North RJ. Neutrophils are critical for host defense against primary infection with the facultative intracellular bacterium Francisella tularensis in mice and participate in defense against reinfection. Infect Immun. 1994;62:2779–83. doi: 10.1128/iai.62.7.2779-2783.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appelberg R, Castro AG, Gomes S, Pedrosa J, Silva MT. Susceptibility of beige mice to Mycobacterium avium: role of neutrophils. Infect Immun. 1995;63:3381–7. doi: 10.1128/iai.63.9.3381-3387.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saunders BM, Cheers C. Intranasal infection of beige mice with Mycobacterium avium complex: role of neutrophils and natural killer cells. Infect Immun. 1996;64:4236–41. doi: 10.1128/iai.64.10.4236-4241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arya OP, Mallinson H, Andrews BE, Sillis M. Diagnosis of urethritis: role of polymorphonuclear leukocyte counts in gram-stained urethral smears. Sex Transm Dis. 1984;11:10–17. [PubMed] [Google Scholar]
- 11.Brunham RC, Paavonen J, Stevens CE, Kiviat N, Kuo CC, Critchlow CW, Holmes KK. Mucopurulent cervicitis – the ignored counterpart in women of urethritis in men. N Engl J Med. 1984;311:1–6. doi: 10.1056/NEJM198407053110101. [DOI] [PubMed] [Google Scholar]
- 12.Yoneda C, Dawson CR, Daghfous T, Hoshiwara I, Jones P, Messadi M, Schachter J. Cytology as a guide to the presence of chlamydial inclusions in Giemsa-stained conjunctival smears in severe endemic trachoma. Br J Ophthalmol. 1975;59:116–24. doi: 10.1136/bjo.59.3.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta S, Feng L, Yoshimura T, Redick J, Fu SM, Rose CE., Jr Intra-alveolar macrophage-inflammatory peptide 2 induces rapid neutrophil localization in the lung. Am J Respir Cell Mol Biol. 1996;15:656–63. doi: 10.1165/ajrcmb.15.5.8918372. [DOI] [PubMed] [Google Scholar]
- 14.Proost P, Wuyts A, van Damme J. The role of chemokines in inflammation. Int J Clin Lab Res. 1996;7:355–8. doi: 10.1007/BF02602952. [DOI] [PubMed] [Google Scholar]
- 15.Wells TN, Proudfoot AE, Power CA. Chemokine receptors and their role in leukocyte activation. Immunol Lett. 1999;65:35–41. doi: 10.1016/s0165-2478(98)00121-7. 10.1016/S0165-2478(98)00121-7. [DOI] [PubMed] [Google Scholar]
- 16.Antony VB, Godbey SW, Kunkel SL, Hott JW, Hartman DL, Burkick MD, Strieter RM. Recruitment of inflammatory cells to the pleural space. Chemotactic cytokines, IL-8, and monocyte chemotactic peptide-1 in human pleural fluods. J Immunol. 1993;151:7216–23. [PubMed] [Google Scholar]
- 17.Donnelly SC, Strieter RM, Kunkwl SL, et al. Interleukin-8 and development of adult respiratoru distress syndrome in at-risk patient groups. Lancet. 1993;341:643–7. doi: 10.1016/0140-6736(93)90416-e. 10.1016/0140-6736(93)90416-E. [DOI] [PubMed] [Google Scholar]
- 18.Goodman RB, Wood RG, Martin TR, Hanson-Painton O, Kinasewitz GT. Cytokine-stimulated human mesothelial cells produce chemotactic activity for neutrophils including NAP-1/IL-8. J Immunol. 1992;148:457–65. [PubMed] [Google Scholar]
- 19.Miller EJ, Cohen AB, Nagao S, et al. Elevated levels of NAP-1/interleukin-8 are present in the airspaces of patients with the adult respiratory distress syndrome and are associated with increased mortality. Am Rev Respir Dis. 1992;146:427–32. doi: 10.1164/ajrccm/146.2.427. [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Cacalano G, Camerato T, Toy K, Moore MW, Wood WI. Chemokine binding and activities mediated by the mouse IL-8 receptor. J Immunol. 1995;155:2158–64. [PubMed] [Google Scholar]
- 21.Darville T, Andrews CW, Sikes JD, Jr, Fraley PL, Rank RG. Early local cytokine profiles in strains of mice with different outcomes from chlamydial genital tract infection. Infect Immun. 2001;69:3556–61. doi: 10.1128/IAI.69.6.3556-3561.2001. 10.1128/IAI.69.6.3556-3561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X, Hayglass KT, Brunham RC. Genetically determined differences in IL-10 and IFN-γresponses correlate with clearance of Chlamydia trachomatis mouse pneumonitis infection. J Immunol. 1996;156:4338–44. [PubMed] [Google Scholar]
- 23.Birnboim HC, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucl Acids Res. 1979;7:1513–23. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schierwagen C, Bylund-Fellenius AC, Lundberg C. Improved method for quantification of tissue PMN accumulation measured by myeloperoxidase activity. J Pharmacol Meth. 1990;23:179–86. doi: 10.1016/0160-5402(90)90061-o. 10.1016/0160-5402(90)90061-O. [DOI] [PubMed] [Google Scholar]
- 25.Yang X, Gartner J, Zhu L, Wang S, Brunham RC. IL-10 gene knockout mice show enhanced Th-1-like protective immunity and absent granuloma formation following Chlamydia trachomatis lung infection. J Immunol. 1999;162:1010–17. [PubMed] [Google Scholar]
- 26.Wang S, Fan Y, Brunham RC, Yang X. IFN-gamma knockout mice show Th2-associated delayed-type hypersensitivity and the inflammatory cells fail to localize and control chlamydial infection. Eur J Immunol. 1999;29:3782–92. doi: 10.1002/(SICI)1521-4141(199911)29:11<3782::AID-IMMU3782>3.0.CO;2-B. 10.1002/(SICI)1521-4141(199911)29:11<3782::AID-IMMU3782>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 27.Roval LE, Herschman HR, Smith JB. The murine neutrophil-chemoattractant chemokines LIX, KC, and MIP-2 have distinct induction kinetics, tissue distributions, and tissue-specific sensitivities to glucocorticoid regulation in endotoxemia. J Leukoc Biol. 1998;64:494–502. doi: 10.1002/jlb.64.4.494. [DOI] [PubMed] [Google Scholar]
- 28.Tsai WC, Strieter RM, Mehrad B, Newstead MW, Zeng X, Standiford TJ. CXC chemokine receptor CXCR-2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infec Immun. 2000;68:4289–96. doi: 10.1128/iai.68.7.4289-4296.2000. 10.1128/IAI.68.7.4289-4296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang J, Wang MD, Lenz S, Gao D, Kaltenboeck B. IL-12 administered during Chlamydia pssittaci lung infection in mice confers immediate and long-term protection and reduces macrophage inflammatory protein-2 level and neutrophil infiltration in lung tissue. J Immunol. 1999;162:2217–26. [PubMed] [Google Scholar]
- 30.Terkeltaub R, Baird S, Sears P, Santiago R, Boisvert W. The murine homolog of the interleukin-8 receptor CXCR-2 is essential for the occurrence of neutrophilic inflammation in the air pouch model of acute urate crystal-induced gouty synovitis. Arthritis Rheum. 1998;41:900–9. doi: 10.1002/1529-0131(199805)41:5<900::AID-ART18>3.0.CO;2-K. 10.1002/1529-0131(199805)41:5<900::AID-ART18>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]