Abstract
The Fas/Fas-ligand (FasL) system plays an important role in regulation of apoptosis and the immune response, and is exploited by mycobacteria to evade the immune response. This study was performed to investigate the distribution and levels of FasL and Fas in lymph node granulomas and sera of tuberculous lymphadenitis patients by immunohistochemistry and enzyme-linked immunosorbent assay. The validity of soluble Fas (sFas) or soluble FasL (sFasL) as a diagnostic tool was also examined. Levels of sFasL in serum were elevated among patients. The numbers of FasL stained cells in lymph node granulomas were higher than Fas. Children had significantly higher levels of sFasL as compared to adults. The human immunodeficiency virus (HIV)–tuberculosis (TB)-coinfected patients displayed no differences in the levels of sFasL or sFas compared with HIV-negative patients. The healthy controls from a high endemic tuberculosis country (having latent TB) had significantly higher levels of sFasL than from a country with no TB transmission. The sensitivity and specificity of the FasL and Fas test were low when compared with the culture results as the gold standard. However, by using histology as the gold standard, the sensitivity and specificity of the FasL test were increased to 66·7% and 100%, respectively, but for the Fas test remained low. In conclusion, sFasL and sFas cannot be used as diagnostic tests for tuberculous lymphadenitis. However, its utility in detecting latent TB and childhood tuberculous lymphadenitis remains to be evaluated. FasL seems to play a role in immune modulation and pathogenesis of TB. Modulators of Fas/FasL-mediated apoptosis may therefore be clinically useful.
Keywords: Fas-ligand, Fas, tuberculosis, latent, lymphadenitis
Introduction
The Fas/Fas-ligand (FasL) system has been recognised as a major pathway for the induction of apoptosis in cells and tissues. Initially, Fas was described as a human cell-surface component with associated cell-killing activity similar to the cytolytic activity of tumour necrosis factor.1,2 The corresponding ligand, FasL, is a type II membrane protein, a member of the tumour necrosis factor family.3 Engagement of Fas by either agonistic antibody or its natural ligand, FasL, transmits a death signal to the target cells, potentially triggering apoptosis. Fas is widely expressed in normal and neoplastic cells; in contrast, FasL expression in normal tissues is restricted to activated T cells, natural killer cells, and macrophage lineage.4,5 Fas/FasL system plays an essential role in elimination of autoreactive lymphocytes and the deletion of activated T cells following an immune response.6 FasL is not only expressed in the cells of the lymphoid/myeloid series, but non-lymphoid cells also express it where it contributes to immune privilege inducing apoptosis in the infiltrating proinflammatory immunocytes.7
Both Fas and FasL exist in soluble form. It has been shown that after synthesis FasL protein is maintained as intracellular stores that are released after cellular activation.8 Matrix metalloproteinases (MMPs) cause cleavage of FasL from the cell surface.9,10 Soluble Fas (sFas), generated by alternative mRNA splicing, can antagonize cell-surface Fas function, by blocking FasL on lymphocytes.11
The Fas/FasL system is used by Mycobacterium tuberculosis to contribute to its virulence.12,13 Infection with M. tuberculosis results in an increase in the expression of FasL in the cells in which mycobacteria reside, providing mycobacteria with an immune privileged sanctuary.12,13 A number of studies over the last few years have evaluated the utility of measuring the levels of molecules associated with apoptosis in serum from patients with infectious diseases to assess the disease activity and the rates of apoptosis.14–16 However, data regarding Fas and FasL is sparse.
This study was performed to determine the expression and distribution of FasL and Fas in the serum and lymph nodes from subjects with tuberculous lymphadenitis, and to assess the difference in the levels of sFasL and sFas in relation to patient characteristics and human immunodeficiency virus (HIV) coinfection. In addition the validity of sFas or sFasL as a diagnostic tool was also examined.
Materials and methods
Subjects
The study was performed on the serum and lymph node biopsies of patients diagnosed with mycobacterial lymphadenitis. These patients were recruited in an epidemiological study from the four districts of Arusha region, Tanzania from 1999 through 2001.17,18 These patients were farmers, cattle-keepers and nomads. Diagnosis of mycobacterial lymphadenitis was based on strong clinical evidence, according to the National Tuberculosis and Leprosy Control Programme clinical guidelines19 followed by decision by a clinician to treat with a full course of anti-tuberculosis (TB) chemotherapy. The majority of patients presented with swelling in the neck, and other symptoms like fever, pain, and weight loss were infrequent. Cervical lymph nodes were the main lymph nodes affected, enlarged in about 80% of the cases, while the axillary, inguinal, and mesenteric lymph nodes were involved in a small proportion of cases.17,18 Sera were collected from the patients and open biopsy specimens were taken from patients before starting anti-TB chemotherapy. Laparotomy was indicated for patients presenting with peritonitis. Half of the biopsy specimen was stored in a deep-freezer for culture and the other half for histology was fixed in 10% formalin. Verbal consent was taken from the participants of the study. Ethical clearance was obtained from the Medical Research Co-ordinating committee in Tanzania.
Sera from 33 normal Tanzanian blood donors ages between 18 and 70 years were used as controls. These sera were obtained from the Blood Bank, Muhimbili Medical Centre, Dar es Salaam, Tanzania as part of another study.20 Tanzania is a high endemic country for TB and the majority of the people is assumed to have latent TB, in contrast to Norway where the majority of adult population is assumed to be free of M. tuberculosis. Sera from 13 Norwegian volunteers aged between 25 and 57 years for comparison between the high and low endemic population was used. Skin test for mycobacterial infection was not performed in the study subjects.
Histological examination and immunohistochemistry
The formalin fixed specimens were embedded in paraffin blocks, cut and stained with haematoxylin and eosin (H&E) and the Ziehl–Neelsen stain for the acid-fast bacilli. Histological examination for the presence or absence of granulomas were performed by two independent pathologists.
Immunostaining was automated using the EnVision TM + procedure optimized for Dako automated system. Briefly after microwave antigen retrieval the sections were placed in a Dako autostainer-Universal Staining System (DAKO-USA, Carpinteria, CA). The sections were incubated with 0·03% H2O2 for 10 min, after which primary antibodies, anti-Fas, -FasL (dilution 1 : 50), were applied onto the sections for 60 min. Dextran polymer conjugated with horseradish peroxidase was applied for 30 min. The visualization was with diaminobenzidine (DAB) substrate applied for 10 min. In negative controls primary antibody was substituted with phosphate-buffered saline (PBS). Strong and moderately positive cells as well as negative cells in the granulomas randomly chosen in two to five fields were counted.
Enzyme-linked immunosorbent assay (ELISA) for Fas and FasL and HIV
Sera were tested for Fas using sAPO-1/Fas module set (Bender MedSystems, Norway). ELISA microwell 96-well plates (Maxisorb) were coated with coating antibody (100 µg/ml), 100 µl/well, for overnight incubation at 4°. Non-specific binding sites were blocked by using 0·5% bovine serum albumin (BSA) in PBS containing 5% Tween-20 for overnight at 4°. Plates were washed three times with washing buffer (PBS containing 5% Tween-20) after each incubation period. Standard protein and sera were added in duplicate wells and incubated with the biotin conjugate for 1 hr at 37°. After washing, horseradish peroxidase-conjugated streptavidin (1 : 12 000) was incubated in wells for 1 hr at 37° followed by colour development with 1,2-phenylenediamine dihydrochloride (OPD, 2HCl) (DAKO AS, Denmark). FasL was tested out by an ELISA kit (OncogeneTM research products, Norway) according to the instructions provided by the manufacturer. The samples and the standard proteins were added to the precoated microtitre wells and incubated with biotinylated detector antibody for 3 hr at 37°. After washing, peroxidase conjugate was incubated in wells for 30 min at 37° followed by colour development as described above. Optical density was read at a wavelength of 490 nm in an automated ELISA reader. A standard curve was determined for both proteins each time the assay was performed. The minimum detectable level of both sFasL and sFas was 20 pg/ml.
Sera were tested for HIV infection using single Behring ELISA tests (Dade Behring Marburg GmbH, Emil-von-Behring Marburg/Germany). A repeated test for positive results was performed using Wellcozyme HIV Recombinant (Murex Biotech Limited/UK). The tests were conducted according to manufacture's protocol and results were handled with confidentiality.
Data management and statistical analysis
For ELISA, the 95th percentile value of the Tanzanian healthy control serum levels of sFasL and sFas were taken as the cut-off value to label TB cases as positive and negative for the protein. Statistical analysis was done using SPSS 12.0 for Windows. The data was not normally distributed, thus non-parametric tests were used for two group comparisons. Wilcoxon signed-ranked test was used for matched analysis of FasL with Fas, and Mann–Whitney test was used for two independent groups comparisons. Chi-square test was used for comparison of ratios between two groups. Linear regression was performed to determine the relationship between two variables. A P-value less than 0·05 was taken as significant.
Results
Soluble FasL and Fas in serum
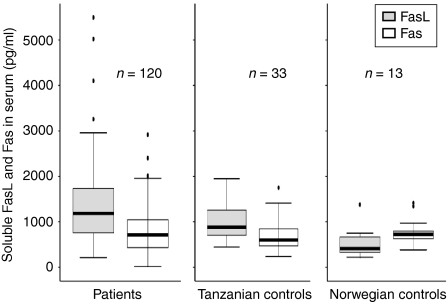
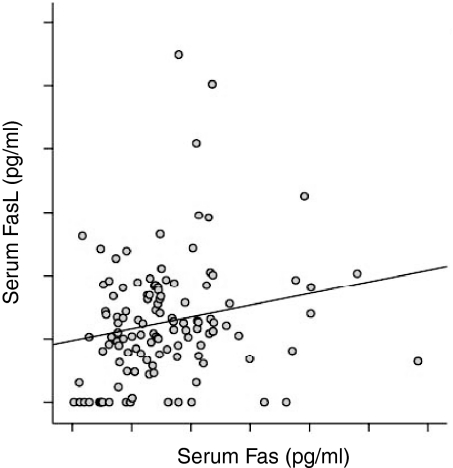
Soluble FasL and sFas were detectable both in patients and controls. The levels of sFasL were significantly higher among the TB patients as compared to the healthy Tanzanian blood donor controls (P < 0·05; Fig. 1). The levels of sFasL were higher than sFas both in TB patients and controls (P < 0·01; Fig. 1). The levels of sFas were not different between the patients and the controls. There was a significant positive correlation between sFasL and sFas in patients (r = 0·22, P < 0·05) (Fig. 2), but not in controls (r = 0·09). When assessed in individual patients, sFasL and sFas levels were above the normal baseline value in only 29 (22%), and 10 (8·6%) cases, respectively. The increase in FasL above normal value did not correlate with an increase in Fas in the same patient except for in three cases.
Figure 1.
Levels of soluble FasL and soluble Fas in the sera of tuberculous (TB) lymphadenitis patients and the healthy controls detected by ELISA. The median, 25th and 75th percentiles and minimum and maximum values are shown. The marks indicate the extreme values. n-values indicate the number of patients in each group. Wilcoxon signed-rank test was used for sFasL and sFas comparison and Mann–Witney test for two independent group comparisons. The levels of sFasL were significantly higher among the TB patients as compared to the Tanzanian blood donor controls (P < 0·05). The levels of sFasL were higher than sFas among both TB patients and Tanzanian controls (P < 0·01). The levels of sFasL among the Tanzanian controls were higher than the Norwegian controls (P < 0·01). The levels of sFasL were lower than Fas in the Norwegian blood donors (P < 0·05).
Figure 2.
Correlation between the soluble FasL and soluble Fas, detected by ELISA in TB patients. There was a significant positive correlation between sFasL and sFas as analysed by anova (r = 0·22, P = 0·03).
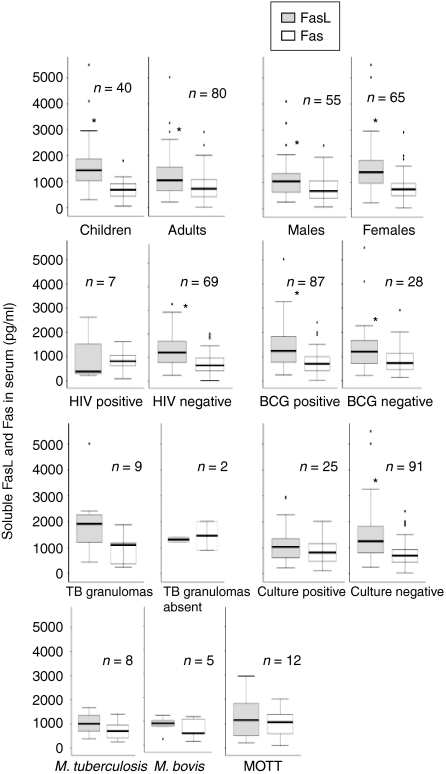
The normal sera from the Tanzanian control group were compared with Norwegian volunteers for comparison between the high and low endemic population. Interestingly, the levels of sFasL in the Tanzanian controls were higher than the Norwegian controls (P < 0·01), whereas there was no difference in the levels of sFas (Fig. 1). The Tanzanian donors had significantly higher levels of sFasL than sFas (P < 0·01), whereas the levels of sFasL were lower than Fas in the Norwegian blood donors (P < 0·05). Figure 3 shows the levels of sFasL and sFas in the various groups of patients. The levels of sFasL were higher than the sFas among all groups except the mycobacterial culture-positive cases, HIV-positive cases and those with or without granulomatous changes, which may be because of small number of cases in these groups. Interestingly children (age 1–15 years) had significantly higher levels of sFasL as compared to the adults, whereas there was no difference in the levels of sFas. Based on these findings the two populations (children and adults) were compared for the distribution of ratios of males and females, HIV coinfected and HIV negatives, bacille Calmette–Guèrin (BCG)-vaccinated to non-vaccinated, culture positive and negative cases and the mycobacterial species, by Chi square test. The two populations were only significantly different for bacterial species (P < 0·05). In the culture positive cases adults had a higher proportion of M. tuberculosis (9/19, 47%) than children (0%), and children had a higher proportion of M. bovis (3/6, 50%) than adults (2/19, 10%; P < 0·05). The proportion of mycobacteria other than tuberculosis (MOTT) was not significantly different between the groups. The values of sFasL and sFas were compared in different groups between the two populations. The levels of sFasL were significantly higher among male children than male adults (P < 0·05), in the children with culture-negative results than adults of the same group (P < 0·01) and in BCG-vaccinated children than vaccinated adults (P < 0·01).
Figure 3.
The levels of soluble FasL and soluble Fas in the various groups of patients detected by ELISA. The median, 25th and 75th percentiles and minimum and maximum values are shown. The marks indicate the extreme values. n-values indicate the number of patients in each group. Wilcoxon signed-rank test was used for sFasL and sFas comparison and Mann–Witney test for two independent group comparisons. Children (age 1–15 years) had significantly higher levels (P < 0·05) of sFasL as compared to the adults (16–80 years). The levels of sFasL were higher than the sFas among various groups as marked by * (P < 0·05). MOTT = Mycobacteria other than tuberculosis.
Table 1 shows the number of patients with positive or negative sFasL and sFas results according to the cut-off criteria based on values in normal serum from Tanzanian donors and the distribution of mycobacterial culture, mycobacterial species, tuberculous granulomas, HIV status, BCG vaccination, and gender. Among the FasL positive cases only three (10%), and among the FasL negative cases 23 (22%) were culture positive. Among Fas-positive cases, four (40%) cases were culture positive, and among Fas negative cases, 21 (20%) were culture positive. The ratios of sFasL positive and negative cases were not different among the various groups.
Table 1.
Distribution of FasL and Fas results according to culture, histology, HIV, BCG, age and gender results
| FasL postive n | FasL negative*n | Fas positive n | Fas negative*n | |
|---|---|---|---|---|
| Culture positive | 3 | 23 | 4 | 21 |
| M. tuberculosis | 0 | 9 | 0 | 8 |
| M. bovis | 0 | 5 | 0 | 5 |
| Atypical | 3 | 9 | 3 | 9 |
| Culture negative | 26 | 78 | 6 | 85 |
| Granulomas present | 6 | 3 | 1 | 8 |
| Granulomas absent | 0 | 2 | 1 | 1 |
| HIV–TB-coinfected | 2 | 7 | 1 | 6 |
| HIV-negative | 17 | 61 | 7 | 62 |
| BCG-positive | 24 | 77 | 6 | 81 |
| BCG negative | 6 | 22 | 5 | 23 |
| Females | 20 | 54 | 6 | 59 |
| Males | 10 | 50 | 5 | 50 |
Negative are the values below the cut-off value based on the 95th percentile values of proteins in the healthy control serum.
n = number of patients in each group. The total number of patients among groups varies because of non-availability of all the information for every patient.
Correlation with histology
The presence of tuberculous granulomas could be correlated with serum Fas and FasL results in the same patients in 11 cases only as shown in Table 1. In these cases acid-fast bacilli were not detected. All the FasL-positive and 50% of the Fas-positive cases had granulomatous responses suggestive of TB.
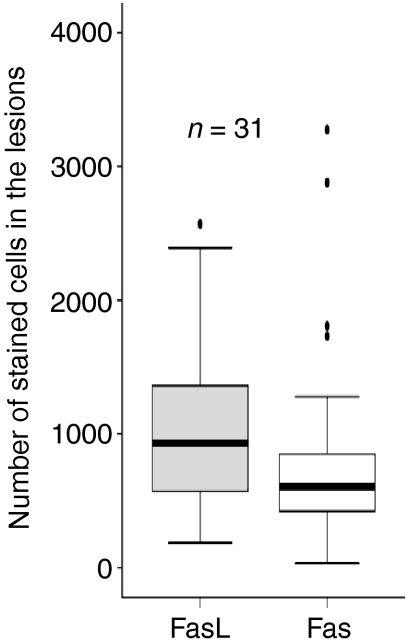
Figure 4 shows the number of stained cells for FasL and Fas in the lymph node granulomas of 31 patients, detected by immunohistochemistry. The numbers of FasL positive cells were higher than Fas-positive cells (P < 0·05). The numbers of FasL-positive cells correlated positively with Fas-positive cells (r = 0·48, P < 0·01). The epithelioid cells, giant cells in the granulomas and the cells surrounding the granulomas expressed both FasL and Fas.
Figure 4.
Number of stained cells for FasL and Fas in the lymph nodes granulomas of 31 patients detected by immunohistochemistry. The median, 25th and 75th percentiles and minimum and maximum values are shown. The marks indicate the extreme values. The absolute numbers of FasL positive cells were higher than Fas positive cells (P < 0·05).
Validation of serum FasL and Fas ELISA as a diagnostic test
The validity of serum FasL and Fas ELISA as diagnostic tests was evaluated. With culture results as the gold standard, the sensitivity and specificity of both sFasL and sFas were too low to be used as diagnostic test. However, by using histology (granulomatous response suggestive of TB) as gold standard, the sensitivity and specificity of the FasL test were increased to 66·7 and 100%, respectively, but the sample size (11 cases) was small for this analysis.
Discussion
In our study we have found an increased level of sFasL in the sera of TB patients. The number of FasL positive cells in the granulomas in lymph nodes were higher than Fas-positive cells that would explain the increase in serum levels of sFasL. The granulomatous immune response in TB is shown to contain the disease by preventing the spread of mycobacteria, but it is also within the granulomas that bacilli may survive and persist.21 These granulomas, by virtue of increased expression of FasL, may provide an immune-privileged site for the bacilli. This is supported by the studies on pulmonary granulomas of mice with slowly progressive primary TB where we have shown that a population of M. tuberculosis infected macrophages express high amounts of FasL and low amounts of Fas.12 These infected macrophages, as a consequence, can be protected from the attack of cytotoxic T cells and could avoid the activation by sensitized lymphocytes, making an intracellular sanctuary for mycobacteria. Furthermore these infected macrophages are shown to express increased levels of antiapoptotic protein Bcl2, thus prolonging their survival.22 In human TB granulomas the absence of lymphocytes among the epithelioid cells might be caused by apoptosis induced by the FasL-expressing epithelioid cells. This hypothesis is indirectly supported by the studies in TB patients where constant apoptosis of T cells has been shown to result in a reduction in T-cell numbers and depressed proliferative responses.23–26 Antigen-presenting cells with increased expression of FasL are shown to induce apoptosis in T cells during antigen presentation, thus inducing antigen-specific T-cell tolerance.27 Because TB progresses slowly, it remains possible that a population of macrophages expressing FasL induces immune suppression by selective deletion of M. tuberculosis-specific lymphocytes. As the activity of cellular FasL can be inhibited by sFasL,28,29 increased levels of sFasL in TB patients can also undermine the cytotoxic activity of cytotoxic cells leading to immune modulation/suppression, thus implicating the role of FasL in the pathogenesis and chronicity of disease.
Although sFasL is said to be a less potent inducer of apoptosis as compared to the membrane-associated FasL28–30 it is shown to be functionally active against cells that are highly sensitive to Fas-mediated apoptosis28,29 and is suggested to be involved in tissue damage.31 The tissue damage causing caseous necrosis and liquefaction of the necrotic centres is the basic process of tuberculous disease in humans, but the mechanism behind it remains largely unknown. In our study the epithelioid cells and the giant cells surrounding the large necrotic areas in the lymph nodes expressed high amounts of FasL. In 54% of the cases the necrotic centres expressed high amounts of FasL, but Fas was absent or weak, implicating a role of FasL in tissue damage. The extent of FasL activity within granulomas may be a critical factor determining the balance between control of infection and tissue destruction. MMPs, which cause cleavage of FasL from the cell surface9,10 have also been shown to be strongly expressed in tuberculous granulomas.32
In our study healthy controls from Tanzania and Norway showed increased level of sFasL (median values were 885 and 409 pg/ml, respectively). This is contrary to other studies where sFasL have been reported to be either absent33 or in amounts lower than 140 pg/ml34,35 About a third of the world's population is infected with M. tuberculosis.36 In areas of high endemicity, the majority of the people is infected with M. tuberculosis, thus constituting a huge reservoir for reactivation to active disease. In countries with low or moderate tuberculosis endemicity, most cases of tuberculosis result from reactivation of latent infection.37–39 However, the mechanism by which the tubercle bacilli persist in host are not understood. In our study the sera from healthy volunteers from Tanzania (high endemic country with latent TB) showed increased level of FasL but not Fas as compared to the Norwegian volunteers (no latent TB). The levels of sFasL were higher than sFas in the Tanzanian donors, whereas the levels of sFasL were lower than sFas in the Norwegian blood donors. The impact of other subclinical infections, however, on the levels of sFasL is not known. During latent infection mycobacteria might be using the FasL- and Bcl-2-expressing cells, as in progressive TB,12,13 to persist in the host cells and this could have caused an increase in the soluble FasL. Whether sFasL can be used to detect latent TB infection remains to be evaluated.
One interesting finding in this study was the high levels of sFasL in the sera of children as compared to adults. Childhood tuberculous lymphadenitis is difficult to diagnose. In all countries, the diagnosis of childhood tuberculosis is often made from clinical history and examination, without laboratory confirmation. Tuberculosis score charts have been widely used to assist in this process, however, increasing numbers of malnourished and HIV-positive children have compromised the value of such scoring systems.40 Tuberculin skin testing gives negative results in up to 40% of children with tuberculosis, and radiographic findings are often nonspecific.41 Whether we can use the levels of sFasL in diagnosis remains to be evaluated as we lack appropriate age-matched controls to make this conclusion in this study.
HIV infection is shown to be associated with an increase in the levels of both sFas and sFasL in serum.42,43 However, in our study the HIV-coinfected patients showed no significant difference in the levels of sFasL or sFas compared with HIV-negative patients. The increase in FasL because of TB in both groups might explain these findings. HIV-1 coinfection impairs cell-mediated immune responses to M. tuberculosis,44–46 which may impair the formation and maintenance of granulomas. As granulomas seem to be responsible for an increase in the sFasL, the finding that the levels of sFasL were higher than sFas in HIV-negative patients, but not so in HIV-coinfected patients, might be because of lack of granulomas in the coinfected patients. This is supported by the similar findings of higher sFasL levels than sFas in the patients with granulomatous response as compared to those who did not mount such a response (Fig. 3). HIV results were available for seven cases with granulomatous response and they were all negative. Unfortunately HIV results were not available for the cases without granulomatous response.
One of the aims of this study was to assess the utility of sFasL and sFas as diagnostic markers for TB. Recent studies have shown an increase in the levels of sFasL in pleural fluid from TB patients, and has been suggested as a differential diagnostic tool.16,47 In our study, by using culture as the gold standard, the sensitivity was too low both for Fas and FasL to be used as a diagnostic test, but only 20% samples were culture positive; this is in line with reports that have shown that culture has low sensitivity especially in paucibacillary conditions like lymphadenitis.48 On a routine surgical pathology report diagnosis is usually made on the basis of histological evidence of chronic granulomatous inflammation, suggestive of tuberculosis. When the presence of TB specific inflammation was used as the gold standard, sFasL turned out to be 100% specific; the sensitivity was 66·7%. But the sample size was too small to derive any conclusion.
In conclusion, sFasL and sFas levels cannot be used as a diagnostic test for tuberculous lymphadenitis because of too low sensitivity and specificity. However, its utility in detecting latent TB and childhood tuberculous lymphadenitis remains to be evaluated. FasL seems to modulate/suppress the immune response and play a role in tissue destruction, which is responsible for morbidity and mortality in TB. Modulation of the Fas/FasL-mediated apoptosis may be clinically useful in limiting the damage and improving the immune response.
Acknowledgments
We thank Dr Said Aboud, at the Muhimbili University College of Health Sciences, Professor Roald Matre at the department of microbiology and Immunology (The Norwegian Council for Higher Education's Programme for Development Research and Education (NUFU) project number: 44003 PRO 42·2.91), University of Bergen and Professor Are Næss at the Department of Infectious Medicine, Haukeland University Hospital for their help in the provision of normal control sera. The ELISA was carried out at the Department of Microbiology and immunology, The Gades Institute. This study is supported by Funds from the University of Bergen and ‘The Gades Legat’, University of Bergen.
References
- 1.Yonehara S, Ishii A, Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169:1747. doi: 10.1084/jem.169.5.1747. 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagata S. Apoptosis by death factor. Cell. 1997;88:355. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 3.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 4.Irmler M, Thome M, Hahne M, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190. doi: 10.1038/40657. 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 5.Glass A, Walsh CM, Lynch DH, Clark WR. Regulation of the Fas lytic pathway in cloned CTL. J Immunol. 1996;156:3638. [PubMed] [Google Scholar]
- 6.Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995;16:39. doi: 10.1016/0167-5699(95)80069-7. 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 7.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 8.Kiener PA, Davis PM, Rankin BM, Klebanoff SJ, Ledbetter JA, Starling GC, Liles WC. Human monocytic cells contain high levels of intracellular Fas ligand: rapid release following cellular activation. J Immunol. 1997;159:1594. [PubMed] [Google Scholar]
- 9.Mariani SM, Matiba B, Baumler C, Krammer PH. Regulation of cell surface APO-1/Fas (CD95) ligand expression by metalloproteases. Eur J Immunol. 1995;25:2303. doi: 10.1002/eji.1830250828. [DOI] [PubMed] [Google Scholar]
- 10.Kayagaki N, Kawasaki A, Ebata T, et al. Metalloproteinase-mediated release of human Fas ligand. J Exp Med. 1995;182:1777. doi: 10.1084/jem.182.6.1777. 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hara T, Tsurumi H, Takemura M, Goto H, Yamada T, Sawada M, Takahashi T, Moriwaki H. Serum-soluble fas level determines clinical symptoms and outcome of patients with aggressive non-Hodgkin's lymphoma. Am J Hematol. 2000;64:257. doi: 10.1002/1096-8652(200008)64:4<257::aid-ajh4>3.0.co;2-2. 10.1002/1096-8652(200008)64:4<257::AID-AJH4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Mustafa T, Phyu S, Nilsen R, Bjune G., RJ Increased expression of Fas ligand on Mycobacterium tuberculosis infected macrophages: a potential novel mechanism of immune evasion by Mycobacterium tuberculosis? Inflammation. 1999;23:507. doi: 10.1023/a:1020286305950. [DOI] [PubMed] [Google Scholar]
- 13.Mustafa T, Bjune TG, Jonsson R, Pando RH, Nilsen R. Increased expression of fas ligand in human tuberculosis and leprosy lesions: a potential novel mechanism of immune evasion in mycobacterial infection. Scand J Immunol. 2001;54:630. doi: 10.1046/j.1365-3083.2001.01020.x. 10.1046/j.1365-3083.2001.01020.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen YM, Whang-Peng J, Chern CH, Kuo BI, Wang SY, Perng RP. The prognostic value of serum cytokine levels in patients with acute infections. Zhonghua Yi Xue Za Zhi. 1995;56:75. [PubMed] [Google Scholar]
- 15.Toyozaki T, Hiroe M, Tanaka M, Nagata S, Ohwada H, Marumo F. Levels of soluble Fas ligand in myocarditis. Am J Cardiol. 1998;82:246. doi: 10.1016/s0002-9149(98)00300-2. 10.1016/S0002-9149(98)00300-2. [DOI] [PubMed] [Google Scholar]
- 16.Wu SH, Chu JJ, Chiang CD. Increased soluble Fas ligand concentration in tuberculous pleural effusion. J Formos Med Assoc. 2001;100:32. [PubMed] [Google Scholar]
- 17.Mfinanga SG, Morkve O, Kazwala RR, Cleaveland S, Sharp JM, Shirima G, Nilsen R. Tribal differences in perception of tuberculosis: a possible role in tuberculosis control in Arusha, Tanzania. Int J Tuberc Lung Dis. 2003;7:933. [PubMed] [Google Scholar]
- 18.Mfinanga SG, Morkve O, Kazwala RR, Cleaveland S, Sharp JM, Shirima G, Nilsen R. The role of livestock keeping in tuberculosis trends in Arusha, Tanzania. Int J Tuberc Lung Dis. 2003;7:695. [PubMed] [Google Scholar]
- 19.Anonymous. Manual of the National Tuberculosis and Leprosy Control programme in Tanzania for the District Tuberculosis and Leprosy Co-ordinators. Dar es Salaam: Ministry of Health. Department of Preventive Medicine Tuberculosis and Leprosy Control Unit; 1987. pp. 1–104. [Google Scholar]
- 20.Aboud S, Lyamuya EF, Kristoffersen EK, Matre R. Immunity to tetanus in male adults in Dar es Salaam, Tanzania. East Afr Med J. 2002;79:73. doi: 10.4314/eamj.v79i2.8904. [DOI] [PubMed] [Google Scholar]
- 21.Dannenberg AM, Rook GAW. Pathogenesis of pulmonary tuberculosis. an interplay of tissue-damaging and macrophage-activating immune responses – dual mechanisms that control bacillary multiplication. In: Bloom BR, editor. Tuberculosis: Pathogenesis, Protection, and Control. Washington DC: ASM Press; 1994. pp. 459–83. [Google Scholar]
- 22.Mogga SJ, Mustafa T, Sviland L, Nilsen R. Increased Bcl-2 and reduced Bax expression in infected macrophages in slowly progressive primary murine Mycobacterium tuberculosis infection. Scand J Immunol. 2002;56:383. doi: 10.1046/j.1365-3083.2002.01140.x. 10.1046/j.1365-3083.2002.01140.x. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch CS, Toossi Z, Vanham G, et al. Apoptosis and T cell hyporesponsiveness in pulmonary tuberculosis. J Infect Dis. 1999;179:945. doi: 10.1086/314667. 10.1086/314667. [DOI] [PubMed] [Google Scholar]
- 24.Boise LH, Thompson CB. Hierarchical control of lymphocyte survival. Science. 1996;274:67. doi: 10.1126/science.274.5284.67. [DOI] [PubMed] [Google Scholar]
- 25.Fraser A, Evan G. A license to kill. Cell. 1996;85:781. doi: 10.1016/s0092-8674(00)81005-3. [DOI] [PubMed] [Google Scholar]
- 26.Manfredi AA, Heltai S, Rovere P, et al. Mycobacterium tuberculosis exploits the CD95/CD95 ligand system of gammadelta T cells to cause apoptosis. Eur J Immunol. 1998;28:1798. doi: 10.1002/(SICI)1521-4141(199806)28:06<1798::AID-IMMU1798>3.0.CO;2-E. 10.1002/(SICI)1521-4141(199806)28:06<1798::AID-IMMU1798>3.3.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Zhang HG, Su X, Liu D, et al. Induction of specific T cell tolerance by Fas ligand-expressing antigen-presenting cells. J Immunol. 1999;162:1423. [PubMed] [Google Scholar]
- 28.Suda T, Hashimoto H, Tanaka M, Ochi T, Nagata S. Membrane Fas ligand kills human peripheral blood T lymphocytes, and soluble Fas ligand blocks the killing. J Exp Med. 1997;186:2045. doi: 10.1084/jem.186.12.2045. 10.1084/jem.186.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nat Med. 1998;4:31. doi: 10.1038/nm0198-031. 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- 30.Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, Tschopp J. Conversion of membrane-bound Fas (CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998;187:1205. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 32.Price NM, Gilman RH, Uddin J, Recavarren S, Friedland JS. Unopposed matrix metalloproteinase-9 expression in human tuberculous granuloma and the role of TNF-alpha-dependent monocyte networks. J Immunol. 2003;171:5579. doi: 10.4049/jimmunol.171.10.5579. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka M, Suda T, Haze K, et al. Fas ligand in human serum. Nat Med. 1996;2:317. doi: 10.1038/nm0396-317. 10.1038/nm0396-317. [DOI] [PubMed] [Google Scholar]
- 34.Redondo P, Solano TBVA, Bauza A, Idoate M. Fas and Fas ligand: expression and soluble circulating levels in cutaneous malignant melanoma. Br J Dermatol. 2002;147:80. doi: 10.1046/j.1365-2133.2002.04745.x. 10.1046/j.1365-2133.2002.04745.x. [DOI] [PubMed] [Google Scholar]
- 35.Hosaka N, Oyaizu N, Kaplan MH, Yagita H, Pahwa S. Membrane and soluble forms of Fas (CD95) and Fas ligand in peripheral blood mononuclear cells and in plasma from human immunodeficiency virus-infected persons. J Infect Dis. 1998;178:1030. doi: 10.1086/515700. [DOI] [PubMed] [Google Scholar]
- 36.Sudre P, ten Dam G, Kochi A. Tuberculosis: a global overview of the situation today. Bull World Health Organ. 1992;70:149. [PMC free article] [PubMed] [Google Scholar]
- 37.Dolin PJ, Raviglione MC, Kochi A. Global tuberculosis incidence and mortality during 1990–2000. Bull World Health Organ. 1994;72:213. [PMC free article] [PubMed] [Google Scholar]
- 38.van Rie A, Warren R, Richardson M, Victor TC, Gie RP, Enarson DA, Beyers N, van Helden PD. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment [see comments] N Engl J Med. 1999;341:1174. doi: 10.1056/NEJM199910143411602. 10.1056/NEJM199910143411602. [DOI] [PubMed] [Google Scholar]
- 39.Fine PE, Small PM. Exogenous reinfection in tuberculosis. N Engl J Med. 1999;341:1226. doi: 10.1056/NEJM199910143411609. 10.1056/NEJM199910143411609. [DOI] [PubMed] [Google Scholar]
- 40.Van Rheenen P. The use of the paediatric tuberculosis score chart in an HIV-endemic area. Trop Med Int Health. 2002;7:435. doi: 10.1046/j.1365-3156.2002.00882.x. 10.1046/j.1365-3156.2002.00882.x. [DOI] [PubMed] [Google Scholar]
- 41.Drobniewski FA, Watt B, Smith EG, Magee JG, Williams R, Holder J, Ostrowski J. A national audit of the laboratory diagnosis of tuberculosis and other mycobacterial diseases within the United Kingdom. J Clin Pathol. 1999;52:334. doi: 10.1136/jcp.52.5.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bahr GM, Capron A, Dewulf J, Nagata S, Tanaka M, Bourez JM, Mouton Y. Elevated serum level of Fas ligand correlates with the asymptomatic stage of human immunodeficiency virus infection. Blood. 1997;90:896. [PubMed] [Google Scholar]
- 43.Fadeel B, Samuelsson A, Hachiya T, Brostrom C, Chiodi F. Elevated serum levels of soluble Fas/APO-1 in human immunodeficiency virus-infected individuals. Blood. 1996;88:4727. [PubMed] [Google Scholar]
- 44.Smith PD, Ohura K, Masur H, Lane HC, Fauci AS, Wahl SM. Monocyte function in the acquired immune deficiency syndrome. Defective chemotaxis. J Clin Invest. 1984;74:2121. doi: 10.1172/JCI111637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trial J, Birdsall HH, Hallum JA, et al. Phenotypic and functional changes in peripheral blood monocytes during progression of human immunodeficiency virus infection. Effects of soluble immune complexes, cytokines, subcellular particulates from apoptotic cells, and HIV-1-encoded proteins on monocytes' phagocytic function, oxidative burst, transendothelial migration, and cell surface phenotype. J Clin Invest. 1995;95:1690. doi: 10.1172/JCI117845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wahl SM, Allen JB, Gartner S, et al. HIV-1 and its envelope glycoprotein down-regulate chemotactic ligand receptors and chemotactic function of peripheral blood monocytes. J Immunol. 1989;142:3553. [PubMed] [Google Scholar]
- 47.Wang PS, Chen YM, Hsieh YL, Yu CF, Tsai CM, Perng RP. Pleural effusion and serum soluble fas-ligand levels are elevated in different clinical conditions. Lung. 2002;180:25. doi: 10.1007/s004080000078. 10.1007/s004080000078. [DOI] [PubMed] [Google Scholar]
- 48.Daniel TM. The rapid diagnosis of tuberculosis: a selective review. J Lab Clin Med. 1990;116:277. [PubMed] [Google Scholar]