Abstract
Haematophagous arthropod vectors such as mosquitoes, tsetse flies, sandflies and ticks have evolved salivary immunomodulatory factors that prevent the vertebrate host from rejecting them meanwhile enhancing pathogen transmission. As dendritic cells (DC) play a major role in host immune responses, we studied the effects of Rhipicephalus sanguineus tick saliva on DC differentiation and maturation. Flow cytometry analysis revealed that the addition of saliva to bone marrow cells inhibits the differentiation of DC and decreased the population of differentiated immature DC, increasing the levels of major histocompatibility complex (MHC) class II while not altering the expression of costimulatory (CD40, CD80 and CD86) and adhesion (CD54) molecules. Furthermore, maturation of DC stimulated by lipopolysaccharide (LPS) in the presence of saliva resulted in a lower expression of costimulatory molecules, but did not alter the up-regulation of MHC class II and CD54. The lipopolysaccharide (LPS)-matured DC cultured with saliva also presented reduced production of interleukin-12, whereas interleukin-10 production was unaltered. Assessment of the function of DC cultured with tick saliva revealed them to be poor stimulators of cytokine production by antigen-specific T cells. Our data indicate a novel modulatory role for the saliva of arthropod vectors at an initial step of the immune response through the inhibition of differentiation and maturation of DC into functional antigen-presenting cells.
Keywords: costimulation, dendritic cells, immunomodulation, Rhipicephalus sanguineus, tick
Introduction
Pathogens that cause many of the world's most common infectious diseases, ranging from malaria, filariasis, trypanosomiasis and leishmaniasis to Lyme disease, are transmitted by haematophagous arthropod vectors such as mosquitoes, tsetse flies, sandflies and ticks.1 Immunomodulatory factors prevent the vertebrate host from rejecting and/or becoming sensitized to the vaso-modulatory proteins/factors of saliva that facilitate blood-feeding and enhance pathogen transmission.2–4
In the case of ticks, feeding requires them to attach on a host for several days or weeks until they complete their meal. In order to cope with potentially harmful host responses, they inoculate saliva containing anti-haemostatic, anti-inflammatory and immunosuppressive components.5–9 Indeed, saliva of different tick species inhibits neutrophil function, hampers the complement system and natural killer (NK) cell and macrophage activity, diminishes the production of cytokines, such as interleukin (IL)-12 and interferon-γ (IFN-γ), and decreases T-cell proliferation.10–16 Determining the mechanism of this inhibitory effect may have important ramifications regarding the inability of the host to mount effective immune responses to ticks and pathogen transmission.
The ‘brown dog tick’, Rhipicephalus sanguineus, the most widely distributed species of tick in the world, has been linked to many diseases, such as ehrlichiosis, babesiosis and haemobartonellosis, which are primarily canine, but may occur sporadically in other mammals.17–20 It has been shown that tick-infested mice do not develop resistance to further infestations with R. sanguineus, and that the immune response in infested mice is of a T helper 2 (Th2) pattern.21 However, knowledge of the effect of tick saliva on dendritic cells (DC), the most potent antigen-presenting cell (APC), and the initiators of the immune response, is lacking.
In the last few years, a number of new procedures have been described in order to obtain DC, starting from haemopoietic progenitor cells of peripheral blood or bone marrow (BM),22 by culturing them with tumour necrosis factor-α (TNF-α) + granulocyte–macrophage colony-stimulating factor (GM-CSF) or IL-4 + GM-CSF.23–25 These cultured DC present functional and phenotypic features of an immature stage of differentiation (i.e. a high capacity of antigen uptake and processing, and a low capacity to stimulate T-cell proliferation) and can be further differentiated in vitro into mature DC if stimulated with TNF-α, lipopolysaccharide (LPS), IL-1 or CD40L.23,26
During tick feeding, salivary antigens are introduced into the host skin and may be captured and processed by epidermal APC, such as DC (Langerhans' cells) and macrophages, which can subsequently migrate to the draining lymph nodes and present them to antigen-specific T lymphocytes. In effect, the skin and draining lymph nodes of tick-infested animals may contain cells that stain for tick salivary antigens.27 Moreover, ultraviolet radiation prior to an initial tick infestation significantly reduces the acquisition of tick resistance and, in a resistant animal, treatment with radiation before a challenge infestation reduces the expression of anti-tick immunity.28,29 These findings indicate a role for DC in the immune response to ticks. Furthermore, the immunosuppression seen in tick infestations could be rendered through an effect of the parasite saliva upon DC. The consequences of tick saliva on phenotypically well-defined DC have not been studied.
The identification of factors that might affect the differentiation and maturation of DC is crucial, as these cells, rather than macrophages and monocytes, are likely to be responsible for the initiation of T-cell-mediated immunity. Given that tick saliva can disperse, or even be transported to and reach distant organs,30,31 saliva-induced disturbance of DC differentiation could result in reduced numbers of these cells in peripheral tissues during tick infestation. Decreased numbers of DC in the skin can greatly impair the development of an appropriate anti-tick acquired immune response by the host. Moreover, understanding the saliva-induced modulation of DC phenotype and function could yield support for the control of other arthropod vectors.
Here we report that exposure of murine BM-derived cells to GM-CSF and IL-4 in the presence of tick saliva inhibits the process of differentiation of DC and reduces the population of DC in an immature stage. Moreover, saliva significantly blocks the terminal maturation of DC, reduces the production of IL-12 and impairs the production of cytokines by keyhole limpet haemocyanin (KLH)-specific T cells upon in vivo adoptive transfer of DC presenting the antigen KLH.
Materials and methods
Experimental animals
C57BL/6 mice (6–8 weeks of age) and mongrel dogs (1–3 years old) were bred and maintained under standard conditions in the animal facilities of the School of Medicine, University of São Paulo (FMRP-USP) (Ribeirão Preto-SP, Brazil).
Reagents and chemicals
Recombinant mouse GM-CSF and IL-4 were obtained from R & D Systems (Minneapolis, MN). LPS (Escherichia coli 0127:B8) was purchased from Difco Laboratories (Detroit, MI).
Saliva collection
R. sanguineus ticks were laboratory-reared, as previously described.32 All ticks used for infestations were 1–3-month-old adults. To obtain engorged ticks for saliva collection, dogs (n = 20) were infested with 60 pairs of adult R. sanguineus ticks contained in plastic feeding chambers fixed to their backs. The saliva-collection procedure was performed using fully engorged female ticks (after ≈ 7 days of feeding) by inoculation of 10–15 µl of a 0·2% (v/v) solution of dopamine in phosphate-buffered saline (PBS), pH 7·4, using a 12·7 × 0·33-mm needle (Becton-Dickinson, Franklyn Lakes, NJ). Saliva was harvested by using a micropipette, reserved on an ice bath, pooled, centrifuged onto a 0·22-µm pore filter (Costar-Corning Inc., Cambridge, MA) and stored at − 20° for further use. Each saliva pool consisted of material harvested from 100 to 200 female ticks. The protein concentration was determined by using a bicinchoninic acid solution (Procedure TPRO-562; Sigma Chemical Co., St Louis, MO).
Dopamine contamination in the induced saliva was quantified by high performance liquid chromatography (HPLC) as previously described.33
The prostaglandin E2 (PGE2) concentration in the tick saliva was measured by radioimmunoassay, as previously described.34
Generation of BM-derived DC
BM-derived DC were generated, as previously described, by Lutz et al.,35 with some modifications. Briefly, BM cells were removed from femurs and tibias of mice and cultured in 24-well-culture plates, at a concentration of 2·5 × 106 cells per well, in 800 µl of RPMI-1640 (Gibco-BRL Life Technologies, Grand Island, NY) supplemented with heat-inactivated 10% fetal calf serum (FCS), 100 µg/ml of penicillin, 100 µg/ml of streptomycin, 5 × 10−5m 2-mercaptoethanol (all from Sigma) plus GM-CSF (50 ng/ml) and IL-4 (10 ng/ml). On days 3, 6 and 9 the supernatant was gently removed and replaced with the same volume of the supplemented medium. On day 9 of culture, ≈ 80% of the cells were CD11c+ DC.
For the differentiation assays, BM-derived DC were cultured as described above, in the presence or absence of the indicated concentrations of tick saliva. The differentiated cells were harvested on days 3, 6 and 9 of culture, labelled with the specific antibodies and analysed by two-colour fluorescence-activated cell sorting (FACS). FACS was performed exclusively in the CD11c+ subset (DC-restricted marker for mice), and the CD11c+ cells expressing CD86, CD80, CD40 and major histocompatibility complex (MHC) class II molecules were analysed. For the maturation assays, the cells were differentiated, as previously described, but in the absence of saliva. On day 9, the cells were activated by the addition of LPS (1 µg/ml), with or without tick saliva, or cultured only with saliva. Cells cultured with supplemented medium plus the recombinant cytokines were considered immature DC (controls). After 48 hr of incubation, the cells were recovered and labelled with specific monoclonal antibodies (mAbs). The mean fluorescence intensity (MFI) value for the CD11c+ cells expressing CD80, CD86, CD40, CD54 and MHC class II molecules was analysed by FACS. Supernatants from all cell cultures (differentiation and maturation assays) were also collected and stored at −20° until assayed for IL-10 and IL-12.
FACS analysis
Cell staining was performed by using the following mAbs: anti-CD11c fluorescein isothiocyanate (FITC)/phycoerythrin (PE) (HL3), anti-CD40 FITC (HM40-3), anti-Ly-6G (Gr-1) PE (B6-8C5), anti-CD54 PE (3E2), anti-CD19 FITC (1D3), all obtained from PharMingen (San Diego, CA); anti-CD11b FITC (M1/70.15) from Caltag (Burlingame, CA); or anti-CD4 FITC (GK1.5), anti-CD8α FITC (53-6.71), anti-CD80 PE (G10), anti-CD86 FITC (GL1), anti-MHC class II PE (NIMR-4) and isotype-matched control mAbs from Southern Biotechnology Associates, Inc. (Birmingham, AL). For apoptosis detection, DC were previously stained with anti-CD11c PE and then labelled with annexin V FITC (R & D Systems). Data acquisition was performed by using a FACscan flow cytometer with cellquest software (both Becton-Dickinson Immunocytometry Systems Inc., San Jose, CA). Appropriate isotype-matched irrelevant mAbs served as negative controls for each molecule and for each stimulus analysed.
Adoptive transfer
DC were differentiated with GM-CSF and IL-4 (as described above) and on day 9 of culture, the cells were treated with saliva (64 µg/ml). After 24 hr of incubation, the cells were pulsed for 2 hr with KLH (10 µg/ml, Sigma), washed three times and injected into mice hind footpads (2 × 105 cells/footpad). Six days later, the popliteal lymph nodes were collected and the cell suspensions prepared. Cells were seeded (5 × 105 cells/well) in 96-well culture plates (Corning Inc.) and incubated in RPMI-1640 containing 10% FCS plus KLH (at 1, 5 or 10 µg/ml). For the proliferation assays, each group was performed in triplicate. Thymidine incorporation was measured on day 3 by a 16-hr pulse with methyl-[3H]thymidine (0·5 µCi/well; Amersham Life Science, Bucks., UK). Levels of cytokines (IFN-γ, IL-10 and IL-4) were determined in the supernatants of lymph node cells (2 × 106 cells/well) cultured in the presence of KLH (1, 5 and 10 µg/ml) for a period of 48 hr in 24-well culture plates (Corning Inc.). As unrelated antigen, lymph node cells were also incubated with ovalbumin (OVA) (10 µg/ml, Sigma).
Cytokine assays
Measurements of IL-4, IL-10, IL-12 (p40 and p70) and IFN-γ in the supernatants were performed by using specific solid-phase sandwich enzyme-linked immunosorbent assay (ELISA). Capture and detection mAb pairs and recombinant cytokines used were purchased from PharMingen, using the procedure recommended by the manufacturer. The mAb pairs used in these studies were as follows (capture and detection clones, respectively): IL-12 p40, C17.15.10 and C17.8; IL-12 p70, 9A5 and C17.8; IL-4, 11B11 and BVD6-24G2; IL-10, JES5-2A5 and SXC-1; IFN-γ, R4-6A2 and XMG1.2. The absorbances were measured at 490 nm by use of a microplate ELISA reader. The detection limits for IL-12 p40, IL-12 p70, IL-4, IL-10 and IFN-γ were 31·3, 19·5, 39·0, 7·8 and 39·1 pg/ml, respectively.
Statistical analysis
Data for the differentiation assay were analysed by analysis of variance (anova) and the Mann–Whitney method for comparisons between groups. The levels of cytokines and cellular proliferation, as well as the fluorescence intensity of the mature DC, were also analysed by anova followed by the Newman–Keuls test to determine multiple comparisons. Values were considered significant when different at P < 0·05.
Results
Tick saliva impairs the differentiation of BM-derived DC
The effects of tick saliva on the differentiation of DC from BM precursors were investigated by culturing BM-derived cells in the presence of GM-CSF, IL-4 and different concentrations of tick saliva (16, 32, 64 and 128 µg/ml) for 9 days. Saliva significantly inhibited the differentiation of BM cells into CD11c+ CD11b+ DC in a dose-dependent manner, reaching a suppression of 65·4 ± 6·9% (mean ± SEM of duplicate wells from two independent experiments, P < 0·05 compared to the control group), at a concentration of 64 µg/ml of saliva (Fig. 1a). As little as 16 µg/ml of protein, which is equivalent to a dilution of saliva of ≈ 80-fold, inhibited 29·9% of the DC differentiation. Higher concentrations of tick saliva (i.e. 128 µg/ml) did not exhibit any further increase in the inhibition than the effect of the dose of 64 µg/ml of saliva protein (Fig. 1a). Accordingly, we chose to employ the concentration of 64 µg/ml of saliva protein in all further experiments.
Figure 1.
Tick saliva inhibits differentiation of bone marrow (BM)-derived dendritic cells (DC). BM-derived cells from C57BL/6 mice were cultured in 24-well plates (2·5 × 106 cells per well) with granulocyte–macrophage colony-stimulating factor (GM-CSF) (50 ng/ml) and interleukin-4 (IL-4) (10 ng/ml) in the presence or absence of tick saliva, as indicated. (a) Cell culture was performed in the presence of different concentrations of tick saliva protein, cells were harvested on day 9, labelled with the designated monoclonal antibodies (mAbs) and analysed by two-colour flow cytometry. Results are shown as the mean percentage ± standard error of the mean (SEM) of CD11c+ CD11b+ cells from duplicate wells. *P < 0·05 and **P < 0·01 compared to cells cultured only with GM-CSF + IL-4 (control group). Data shown are representative of two experiments. (b) The cell culture employed 64 µg/ml of saliva and cells were harvested on the days indicated. Results are shown as dot-plots and are representative of four independent experiments.
In order to verify if the saliva-inhibitory effect was directed against an early or a late phase of DC differentiation, we collected the DC on days 3, 6 and 9 and determined the CD11c+ CD11b+ cell population by two-colour FACS. Our data showed that, despite a small inhibition of differentiation on day 6, tick saliva only significantly reduced DC (CD11c+ CD11b+) differentiation on day 9 of BM cell culture [53·4 ± 6·7%, mean ± SEM from four independent experiments, P < 0·05 compared to the control group, Fig. 1(b)]. Results presented in Fig. 1(b) are representative of four independent experiments.
We next sought to examine the mechanisms by which saliva affects the differentiation of DC. Using a Trypan Blue exclusion assay, we observed no significant losses in cell viability between control and treated cells throughout the culture period (on day 9 of culture the viable cell numbers were 1·1 × 106 and 1·3 × 106 cells/ml for BM cells cultured with or without saliva, respectively). Additionally, in order to verify whether the inhibition was caused by apoptosis, we analysed the population of CD11c+ DC staining for annexin V on days 3, 6 and 9 of culture. The percentage of annexin V+ cells cultured with tick saliva varied from 1·25 to 9·89%, while in the control group it varied from 3·23 to 11·44%, suggesting that the cells differentiated in the presence of tick saliva are not being killed (data not shown).
We also examined whether the effect of saliva could be caused by dopamine, because this mediator was used to induce tick salivation and is detected in pooled samples of R. sanguineus tick saliva.16 Dopamine at 0·05, 1·5 or 15 µg/ml had no effect on the differentiation of DC (data not shown). The highest concentration, of 15 µg/ml of dopamine, is 30 times higher than that present in 1 ml of tick saliva.
In addition to the effect of saliva in reducing the differentiated, CD11c+ CD11b+ DC population, by day 9 of culture saliva also significantly decreased the CD11c+ DC population expressing CD80 and CD86 surface molecules (mean ± SEM = 62·5 ± 0·6 and 87·0 ± 1·0% of reduction, respectively, P < 0·05 compared to control cells), whereas the population of DC expressing CD40 and MHC class II was unaffected. No statistically significant difference was observed in the CD11c+ DC population cultured with saliva for only 3 and 6 days (Fig. 2).
Figure 2.
Tick saliva reduces the differentiation of bone marrow (BM)-derived dendritic cells (DC) that express costimulatory molecules CD86 and CD80. BM-derived cells from C57BL/6 mice were cultured in 24-well plates (2·5 × 106 cells per well) with granulocyte–macrophage colony-stimulating factor (GM-CSF) (50 ng/ml) and interleukin-4 (IL-4) (10 ng/ml) in the absence (control group) or presence of tick saliva (64 µg/ml) (saliva group), as described in the Materials and methods. On days 3, 6 and 9 of culture the cells were harvested, labelled with the designated monoclonal antibodies (mAbs) and analysed by two-colour flow cytometry. Cells were gated on CD11c+ cells. Data are presented as the mean percentage of CD11c+ cells expressing CD86, CD80, CD40 or major histocompatibility complex (MHC) class II ± standard error of the mean (SEM) (a, b, c and d, respectively) from four independent experiments. *P < 0·05 compared with cells cultured with GM-CSF and IL-4 alone (control group).
The supernatants of BM-DC-differentiated cell cultures in the presence or absence of tick saliva were analysed (days 3, 6 and 9) for IL-10 and IL-12, and negligible cytokine production was detected throughout the culture period (data not shown).
Effects of tick saliva on differentiated DC
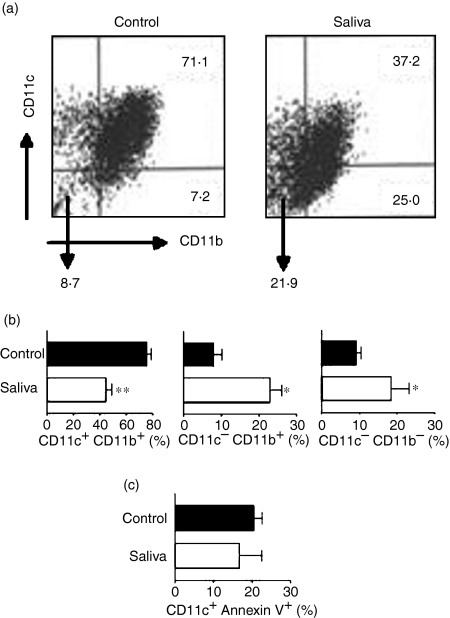
Next we evaluated whether tick saliva alters the population of DC that have already differentiated into CD11c+ CD11b+ cells. This in vitro situation could, in part, mimic the natural situation where immature DC, present at the host skin, interact directly with saliva inoculated by the tick. Exposure of previously differentiated DC to tick saliva for 48 hr (70–80% of cells were CD11c+) yielded a reduction from 49·2 to 50·5% in the CD11c+ CD11b+ DC population compared to DC cultured without saliva (P < 0·01, Fig. 3a). This reduction was accompanied by a 3·5- and a 2·5-fold increase in the CD11c− CD11b+ and CD11c− CD11b− cell populations, respectively, as compared to control cell cultures (P < 0·05) (Fig. 3b, mean ± SEM from five independent experiments). The absolute number of cells harvested after 9 days of culture plus 48 hr of incubation, with or without tick saliva, was 13·8 × 105 ± 1·8 and 11·0 × 105 ± 1·4, respectively, suggesting that the effect of tick saliva on DC is caused by changes on the cell phenotype and not by absolute cell numbers.
Figure 3.
Differentiated dendritic cells (DC) are also affected by tick saliva. Bone marrow (BM)-derived cells from C57BL/6 mice were cultured in 24-well plates (2·5 × 106 cells per well) with granulocyte–macrophage colony-stimulating factor (GM-CSF) (50 ng/ml) and interleukin-4 (IL-4) (10 ng/ml) for 9 days in order to obtain immature differentiated DC (CD11c+ CD11b+). On day 9, when ≈ 70–80% of the cells were CD11c+, tick saliva (64 µg/ml) was added to the indicated groups. After 48 hr the cells were stained with designated antibodies or annexin V, and then analysed by two-colour flow cytometry. Data shown as dot-plots are representative of five experiments (a). Data in bar graphs are presented as the mean ± standard error of the mean (SEM) of the percentage of cells expressing the CD11c+ CD11b+, CD11c− CD11b+ and CD11c− CD11b− phenotypes from five independent experiments (b) or as the mean ± SEM of the percentage of cells CD11c+ annexin V+ from two independent experiments (c). **P < 0·01 and *P < 0·05 compared with control cells.
In an attempt to determine whether the decline of the previously differentiated DC population was caused by the induction of apoptosis, we cultured immature DC in the presence of saliva for 48 hr, and examined the population of CD11c+ DC staining for annexin V. Our results demonstrate that tick saliva does not induce apoptosis in DC (Fig. 3c) (mean ± SEM from two independent experiments).
Modulation of DC maturation by tick saliva
In order to carry out their main role of initiating a primary immune response, immature DC must be stimulated for maturation. A widely used stimulus that induces DC maturation is bacterial LPS.36 We investigated the influence of saliva on the LPS-induced maturation of DC. Immature DC received LPS in the presence or absence of tick saliva for 48 hr. Consistent with previously published data, LPS-induced CD11c+ maturation of DC was accompanied by a significant up-regulation of MHC class II, adhesion (CD54) and costimulatory molecules (CD80, CD86 and CD40) (Fig. 4). However, when tick saliva was added to the cell cultures, LPS-induced maturation was impaired because the level of expression of CD80, CD86 and CD40 was significantly diminished. On the other hand, the levels of expression of CD54 and MHC class II molecules were similar to those seen in mature DC (Fig. 4). Interestingly, immature DCs cultured in the presence of saliva alone presented a 2·8-fold up-regulation of MHC class II molecules, but did not influence the expression of CD54, CD80 and CD86 molecules when compared to DC incubated without saliva (Fig. 4).
Figure 4.
Tick saliva inhibits dendritic cell (DC) maturation. Bone maarow (BM)-derived cells (2·5 × 106/well) from C57BL/6 mice were differentiated for 9 days in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) (50 ng/ml) and interleukin-4 (IL-4) (10 ng/ml). On day 9, when ≈ 80% of the cells were CD11c+, DC maturation was induced with the addition of lipopolysaccharide (LPS) (1 µg/ml) for 48 hr in the presence or absence of saliva (64 µg/ml) [rows (d) and (c) respectively]. In addition, these cells were cultured only with saliva (64 µg/ml) or with GM-CSF + IL-4 only [rows (e) and (b) respectively]. Cells were then harvested, labelled with the designated monoclonal antibodies (mAbs) and analysed by two-colour flow cytometry for the expression of surface molecules gated on CD11c. Histograms in row (a) represent the controls of DC labelled with isotype-matched, irrelevant mAbs. Results are expressed as the mean fluorescence intensity (MFI) of CD11c+ cells expressing the molecule of interest, which is indicated within each histogram. Data are representative of four independent experiments.
Considering that saliva of several tick species contains PGE24,8,31 and some studies suggest that PGE2 can inhibit costimulatory molecule expression and cytokine production by DC,37 we measured the level of this component in R. sanguineus saliva. The results show that the saliva of R. sanguineus ticks contained 3·59 ng/ml of PGE2. As the inhibitory effect of tick saliva was observed when saliva protein was added at 64 µg/ml, we can estimate that the amount of PGE2 in saliva which was added to the DC cultures achieved a concentration of only 10−9m, a concentration 100 times lower than that demonstrated to have an inhibitory effect on the expression of DC costimulatory molecules.37 Additionally, we performed an assay where DC were stimulated with LPS in the presence of PGE2 (10−7m) or tick saliva. The data showed that while saliva inhibited LPS-induced CD40 and CD86 expression (59·6 and 32·4%, respectively), PGE2 had no effect. Moreover, the presence of PGE2 did not alter IL-12 secretion mediated by LPS (data not shown).
Tick saliva reduces the production of IL-12 by DC triggered by LPS stimulation
To determine the effect of tick saliva on LPS-induced DC IL-12 production, we exposed previously differentiated DC to LPS for 48 hr in the presence or absence of tick saliva, and measured the production of IL-12 p40 and p70 by ELISA. As shown in Fig. 5, saliva led to a decrease of 46·3% and 64% in the production of IL-12 p40 and p70, respectively, by these cells upon stimulation with LPS (P < 0·01 compared to control cells). Unexpectedly, DC cultured in the presence of saliva alone presented a significant increase in the production of IL-12 p40 and p70 (P < 0·001 and P < 0·05, respectively) when compared to immature DC cultured with medium only (Fig. 5). To eliminate the possibility of contaminating LPS affecting IL-12 production, we quantified the level of LPS in saliva by using the Limulus assay and performed a cell culture, adding polymixin B sulphate (30 µg/ml; Sigma) to the culture medium. The Limulus assay demonstrated that the amount of tick saliva (64 µg/ml) used in the cell culture assays contained only 0·009 ng/ml of LPS. Furthermore, the data from the cell culture demonstrated that while polymixin B sulphate inhibited LPS-induced IL-12 p70 synthesis, it did not impair its enhancement by tick saliva (1·87 and 2·16 ng/ml, with or without polymixin B sulphate, respectively). These results do not, however, preclude the presence of other microbial contaminants in the saliva.
Figure 5.
Lipopolysaccharide (LPS)-induced dendritic cell (DC) interleukin-12 (IL-12)p40 and p70 production is impaired in the presence of tick saliva. Bone marrow (BM)-derived cells (2·5 × 106/well) from C57BL/6 mice were differentiated for 9 days in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) (50 ng/ml) and IL-4 (10 ng/ml). On day 9, when ≈ 80% of the cells were CD11c+, maturation was induced by the addition of LPS (1 µg/ml) in the presence (black bar) or absence (white bar) of saliva (64 µg/ml). In addition, these cells were cultured with saliva alone (64 µg/ml) (hatched bar) or with GM-CSF + IL-4 alone (grey bar). After 48 hr of incubation, the supernatants were collected and assayed by specific enzyme-linked immunosorbent assay (ELISA) for IL-12p40 and p70. **P < 0·01 compared to cells stimulated with LPS, or +++P < 0·001 and +P < 0·05 compared to cells cultured with GM-CSF and IL-4 alone. Results are presented as the mean of duplicate cultures ± standard deviation (SD). The figure is representative of three independent experiments.
DC cultured with saliva possess diminished capacity to stimulate antigen-specific T-cell IFN-γ and IL-10 production
To determine whether the saliva-induced DC co-receptor impairment could influence its antigen presentation ability or favour a pattern of T-helper differentiation, we cultured 9-day-differentiated DC in the presence or absence of tick saliva for 24 hr, and subsequently pulsed them with KLH (10 µg/ml, 2 hr), washed and injected them into mice footpads. Six days later, the cells were collected from the draining popliteal lymph nodes and cultured with or without KLH (1, 5 and 10 µg/ml) or an unrelated antigen (OVA; 10 µg/ml). The antigen-specific T-cell proliferative response and the production of IFN-γ, IL-10 and IL-4 were evaluated. As depicted in Fig. 6, although exposure of DC to saliva before antigen loading did not alter the induction of specific-T cell proliferation (insets in Fig. 6), it reduced significantly the synthesis of KLH-specific IFN-γ and IL-10. The cytokine IL-4 was undetectable in both experimental and control groups. No cell proliferation was observed following the addition of KLH (1, 5 and 10 µg/ml) in assays performed with naïve mice.
Figure 6.
Interferon-γ (IFN-γ) and interleukin (IL)-10 production is decreased when keyhole limpet haemocyanin (KLH)-specific T cells are stimulated with KLH-pulsed dendritic cells (DC) in the presence of saliva. Nine-day cultured murine bone marrow (BM)-derived DC were incubated with or without saliva for 24 hr and subsequently pulsed with KLH (10 µg/ml) for 2 hr, washed and injected into the footpads of mice. Six days later, the mice were killed and their popliteal lymph node cells were cultured with or without KLH (1, 5 and 10 µg/ml) or unrelated antigen [ovalbumin (OVA), 10 µg/ml]. After 48 hr of incubation, the supernatants were collected and assayed by specific enzyme-linked immunosorbent assay (ELISA) for IFN-γ (a and b) and IL-10 (c and d) or assayed for cell proliferation by methyl-[3H]thymidine incorporation [counts per minute (c.p.m.)] (inset box; the legends for bars are equivalent to the main figure). Results are presented as the mean of duplicate (cytokine production) or triplicate (cell proliferation) cultures ± standard deviation (SD). *P < 0·05, **P < 0·01 and ***P < 0·001 compared to cells cultured with medium only (M). The figure is representative of three independent experiments.
Discussion
The present study demonstrates that saliva from R. sanguineus ticks inhibits the differentiation and terminal maturation of DC, and subsequently modulates their immunostimulatory functions. This can be the mechanism by which blood-feeding arthropods lead to immunosuppression and to the impaired microbicidal activity observed in parasitized hosts,38 and could also explain how tick saliva can modulate the production of cytokines by the host.5,13–16,21
We found that tick saliva inhibited GM-CSF/IL-4-driven differentiation of BM precursors into DC. This effect was dose dependent, reaching the highest suppression on day 9 of culture, with 64 µg/ml of saliva protein. The concentration of saliva protein employed in the assays was not in excess because it correlates with ≈ 40 µl of saliva/2 × 106 cells in a final volume of 800 µl/well (≈ 1 : 20 v/v) and ticks can regurgitate ≈ 125 µl of saliva back into the host every 24 hr.39
As the addition of IL-10 to peripheral blood human mononuclear cells blocks their differentiation,40,41 we hypothesized that the inhibitory effect by tick saliva could be caused by the production of IL-10 by DC. However, we did not find augmented levels of IL-10 in the BM-DC differentiated cell cultures supernatants in any of the days when the samples were analysed (days 3, 6 and 9). Previous studies described IL-10-independent mechanisms for inhibition of DC differentiation,42 suggesting that saliva may act through these mechanisms.
The culture system using GM-CSF/IL-4 to differentiate BM precursors into DC favours the expression of genes that are related to the development of these cells to the detriment of other haemopoietic populations, such as granulocytes and macrophages. The diminished percentage of DC found when saliva was present in the culture was not caused by cell death and was not compensated by B cells (CD19) or granulocytes (Gr-1+), similarly to previous published results using other agents.40–42 Nevertheless, an expansion of some other type of cell population cannot be excluded.
Interestingly, we observed that on day 9 of culture, the population of CD11c+ DC that was differentiated in the presence of tick saliva was similar to BM-derived DC cultured without GM-CSF/IL-4, suggesting that saliva could be sequestrating IL-4 and/or GM-CSF, thus resulting in the differentiation of CD11c− cells. In effect, recent studies have shown that ticks can produce and secrete neutralizing cytokine-binding proteins that can be inoculated into hosts through tick saliva.43,44 This possibility is currently under investigation.
Our data shows that when saliva is added at the beginning of the cell culture, it induces some paradoxical modifications. Thus, saliva simultaneously reduced the population of DC-expressing molecules involved in T-cell costimulation (CD86 and CD80), but did not affect the population of DC expressing CD40 and MHC class II molecules. Reduced expression of costimulatory molecules can be correlated with impaired antigen presentation, as costimulation driven by CD80/CD86 is essential for effective T-cell activation and avoids the induction of T-cell anergy.45 This host condition could probably help blood-feeding arthropods to complete their meal, and moreover, could facilitate the transmission of vector-borne pathogens.
Besides impairing the differentiation of BM cells into immature DC, saliva could also affect immature DC present at the tick-feeding site. Indeed, we show that the effects of saliva on differentiated, but still immature DC, appear to be similar to the effects of saliva included at the beginning of cell culture to generate DC. The observed reduction of the population of immature DC was not caused by cell death, suggesting that saliva may be causing the reversal of DC into CD11c− CD11b− and CD11c− CD11b+ cells (Fig. 3). These results contrast with those from other studies, in which different pathogens, such as viruses or bacteria, control DC by infecting and killing cells by apoptosis.46 Thus, during tick infestations, saliva could be reducing the pool of immature DC present at the tick-feeding site.
Upon exposure to inflammatory or microbial stimuli, such as LPS or TNF-α, immature DC up-regulate the surface expression of MHC class II, and adhesion and costimulatory molecules.36 After stimulation, these newly matured DC reduce their antigen-uptake capacity and migrate to the regional lymph nodes, where they exert their function as potent APC. Results presented herein demonstrate that saliva inhibits the augmented surface expression of CD80, CD86 and CD40 induced by LPS on DC; however, the expression of MHC class II and CD54 molecules was not altered. Fewer CD40 molecules on the DC can result in diminished presentation of antigen, reduced production of cytokines and, as a consequence, lack of CD80 and CD86 up-regulation.47 Our results indicate that saliva may impair the competence of DC for T-cell priming, owing to a lack of appropriate costimulation. Unexpectedly, our findings show that saliva per se augments the expression of MHC class II, leaving the costimulatory and adhesion molecules unchanged. It seems that saliva-treated immature DC are induced to remain in an intermediate maturation stage. Recently we have obtained data showing that tick saliva probably also has an effect on DC migration because it reduced the expression of CCR5 (data not published).
Taking into account that ticks salivate continuously into their hosts,30,31 and that saliva reduces the number of costimulatory molecules of DC, it is possible that when these cells migrate to the lymph nodes they cannot efficiently activate T cells, impairing the host immune response, as we have observed in vivo48 and as demonstrated for herpes simplex virus, cytomegalovirus and Plasmodium falciparum.49–51
Our results show that saliva inhibited the production of IL-12 by LPS-stimulated DC, in accordance with the effect of R. sanguineus tick saliva on Trypanosoma cruzi-infected macrophages.16 This saliva-induced modulation of cytokine production might have profound consequences for any interacting T cells, as IL-12 drives T helper 1 (Th1) responses by inducing secretion of IFN-γ by T cells and NK cells.52 In support of this, a large body of data have demonstrated that tick-infested mice respond with polarization of the immune response towards the development of Th2 lymphocytes.21,53,54
The mechanism by which saliva operates is still unclear. It may induce the production of a down-regulatory cytokine that decreases the synthesis of IL-12, such as transforming growth factor-β (TGF-β).55 A further possibility might be that saliva impairs the interaction of LPS with toll-like receptors (TLR) present on the DC, reducing the production of IL-12. The unexpected effect of saliva alone in increasing the synthesis of IL-12 by the DC could be explained by the presence of some component in tick saliva that binds to other TLR (different from the LPS ligand), which could also trigger the intracellular cascade for IL-12 production. This hypothesis is supported by data showing that the production of IL-12 induced by tick saliva was similar to that obtained in the presence of polymixin B sulphate (data not shown). Additionally, diverse pathogens, or their products, can act through different TLR, for example, LPS triggers TLR4,56 and CpG-containing oligonucleotides activates via TLR9.57 Both pathways lead to the induction of nuclear factor-κB signalling and result in the production of cytokines.56–59 An additional mechanism by which saliva could be regulating DC may be through the inhibition of some intracellular factor, similarly to that reported for anthrax lethal toxin which severely impairs the function of DC by disrupting the mitogen-activated protein (MAP) kinase intracellular signalling network.60
It has been proposed that the nature of T-cell responses in vivo, generated after cognate interactions with DC, is influenced by the stage of maturation of the DC. Recent experiments have provided evidence that antigen-loaded immature DC silence T cells either by deleting them or by expanding regulatory T cells.61 As our results showed that saliva, in spite of increasing the production of IL-12, did not alter the expression of costimulatory molecules, remaining in an intermediate maturation stage, we investigated the capacity of saliva-treated DC to induce an immune response in vivo. The adoptive transfer experiments showed that KLH-specific T cells were able to proliferate but did not produce IFN-γ, IL-10 or IL-4 when they had been primed in vivo with KLH-pulsed DC exposed to saliva. These findings suggest that saliva-modulated DC can induce a non-polarized T-cell response.
Taken together, these results highlight a novel immunomodulatory property of tick saliva. Thus, not only does saliva exert its immunosuppressive effects by inhibiting T-cell proliferation13–16 but also by impairing APC function, as assessed using BM-derived DC as an exemplary APC type. Therefore, the decreased immune response generated in the presence of saliva is caused by the inhibition of differentiation and maturation of DC, preventing the polarization of naïve T cells. Elucidation of the effects of tick saliva on DC may lead to the discovery of novel immunosuppressive agents of broad therapeutic interest.
Acknowledgments
This work was supported by grants from Fundação de Amparo e Pesquisa de São Paulo (FAPESP); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação e Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We thank Giuliana Bertozi, Cristiane M. Milanezi, Elder Tambellini, João Sérgio Epifânio, Antônio F. de Souza, Maria M. Rossi and Wander C.R. da Silva for excellent technical assistance.
References
- 1.Moore J. Parasites and the behavior of biting flies. J Parasitol. 1993;79:1–16. 10.1007/BF00931210. [PubMed] [Google Scholar]
- 2.Jones LD, Hodgson E, Nuttall PA. Enhancement of virus transmission. By tick salivary glands. J Gen Virol. 1989;70:1895–8. doi: 10.1099/0022-1317-70-7-1895. [DOI] [PubMed] [Google Scholar]
- 3.Nuttall PA. Displaced tick–parasite interactions at the host interface. Parasitology. 1998;116:S65–72. doi: 10.1017/s003118200008495x. [DOI] [PubMed] [Google Scholar]
- 4.Gillespie RD, Mbow ML, Titus RG. The immunomodulatory factors of blood-feeding arthropod saliva. Parasite Immunol. 2000;22:319–31. doi: 10.1046/j.1365-3024.2000.00309.x. 10.1046/j.1365-3024.2000.00309.x. [DOI] [PubMed] [Google Scholar]
- 5.Ribeiro JMC. Role of saliva blood-feeding by arthropods. Annu Rev Entomol. 1987;32:463–78. doi: 10.1146/annurev.en.32.010187.002335. 10.1146/annurev.en.32.010187.002335. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro JMC. Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect Agents Dis. 1995;4:143–52. [PubMed] [Google Scholar]
- 7.Champagne DE. The role of salivary vasodilators in blood-feeding and parasite transmission. Parasitol Today. 1994;10:430–5. doi: 10.1016/0169-4758(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 8.Bowman AS, Coons LB, Needham GR, Sauer JR. Tick saliva: recent advances and implications for vector competence. Med Vet Entomol. 1997;11:277–85. doi: 10.1111/j.1365-2915.1997.tb00407.x. [DOI] [PubMed] [Google Scholar]
- 9.Francischetti IMB, Valenzuela JG, Andersen JF, Mather TN, Ribeiro JMC. Ixolaris, a novel recombinant tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick, Ixodes scapularis: identification of factor X and factor Xa as scaffolds for the inhibition of factor VIIa/tissue factor complex. Blood. 2002;99:3602–12. doi: 10.1182/blood-2001-12-0237. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro JMC, Weis JJ, Telford SR. Saliva of the tick Ixodes dammini inhibits neutrophil function. Exp Parasitol. 1990;70:382–8. doi: 10.1016/0014-4894(90)90121-r. 10.1016/0014-4894(90)90121-R. [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro JMC. Ixodes dammini: salivary anti-complement activity. Exp Parasitol. 1987;64:347–53. doi: 10.1016/0014-4894(87)90046-4. 10.1016/0014-4894(87)90046-4. [DOI] [PubMed] [Google Scholar]
- 12.Kubes M, Fuchsberger N, Labuda M, Zuffova E, Nuttal PA. Salivary gland extracts of partially fed Dermacentor reticulatus ticks decrease natural killer cell activity in vitro. Immunology. 1994;82:113–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Ramachandra R, Wikel SK. Modulation of host-immune responses by ticks (Acari: Ixodidae): Effect of salivary gland extracts on host macrophages and lymphocyte cytokine production. J Med Entomol. 1992;29:818–26. doi: 10.1093/jmedent/29.5.818. [DOI] [PubMed] [Google Scholar]
- 14.Ramachandra RN, Wikel SK. Effects of Dermacentor andersoni (Acari: Ixodidae) salivary gland extracts on Bos indicus and B. taurus lymphocytes and macrophages: in vitro cytokine elaboration and lymphocyte blastogenesis. J Med Entomol. 1995;32:338–45. doi: 10.1093/jmedent/32.3.338. [DOI] [PubMed] [Google Scholar]
- 15.Urioste S, Hall LR, Telford SR, III, Titus RG. Saliva of the Lyme disease vector, Ixodes dammini, blocks cell activation by a nonprostaglandin E2-dependent mechanism. J Exp Med. 1994;180:1077–85. doi: 10.1084/jem.180.3.1077. 10.1084/jem.180.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira BR, Silva JS. Saliva of Rhipicephalus sanguineus tick impairs T cell proliferation and IFN-γ-induced macrophage microbicidal activity. Vet Immunol Immunopathol. 1998;64:267–93. doi: 10.1016/s0165-2427(98)00135-4. 10.1016/S0165-2427(98)00137-8. [DOI] [PubMed] [Google Scholar]
- 17.Pegram RG, Clifford CM, Walker JB, Keirans JE. Clarification of the Rhipicephalus sanguineus group (Acari; Ixodoidea, Ixodidae). I R. sulcatus (Neuman 1908) R. turanicus (Pomerantsev 1936) Sys Parasitol. 1987;10:3–26. [Google Scholar]
- 18.Cupp EW. Vet Clin North Am: Small Anim Pract. Vol. 21. 1991. Biology of ticks; pp. 1–26. [DOI] [PubMed] [Google Scholar]
- 19.Goddard J. Focus of human parasitism by the brown dog tick, Rhipicephalus sanguineus (Acari: Ixodidae) J Med Entomol. 1989;26:628–9. doi: 10.1093/jmedent/26.6.628. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter TL, McMeans MC, McHugh CP. Additional instances of human parasitism by the brown dog tick (Acari: Ixodidae) J Med Entomol. 1990;27:1065–6. doi: 10.1093/jmedent/27.6.1065. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira BR, Silva JS. Successive tick infestations selectively promote a T helper 2 cytokine profile in mice. Immunology. 1999;96:434–9. doi: 10.1046/j.1365-2567.1999.00683.x. 10.1046/j.1365-2567.1999.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernhard H, Disis ML, Heimfeld S, Hand S, Gralow JR, Cheever MA. Generation of immunostimulatory dendritic cells from human CD34+ hematopoietic progenitor cells of the bone marrow and peripheral blood. Cancer Res. 1995;55:1099–104. [PubMed] [Google Scholar]
- 23.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans' cells. Nature. 1992;360:258–61. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 24.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapuis F, Rosenzwajg M, Yagello M, Ekman M, Biberfeld P, Gluckman JC. Differentiation of human dendritic cells from monocytes in vitro. Eur J Immunol. 1997;27:431–41. doi: 10.1002/eji.1830270213. [DOI] [PubMed] [Google Scholar]
- 27.Allen JR, Khalil HM, Wikel SK. Langerhans' cells trap tick salivary gland antigens in tick-resistant guinea pigs. J Immunol. 1979;55:157–63. [PubMed] [Google Scholar]
- 28.Nithiuthai S, Allen JR. Effects of ultraviolet irradiation on epidermal Langerhans' cells in guinea-pigs. Immunology. 1984;51:143–51. [PMC free article] [PubMed] [Google Scholar]
- 29.Nithiuthai S, Allen JR. Langerhans' cells present tick antigens to lymph node cells from tick-sensitized guinea-pigs. Immunology. 1985;55:157–63. [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufman SHE. Tick–host interaction: a synthesis of current concepts. Parasitol Today. 1989;5:47–56. doi: 10.1016/0169-4758(89)90191-9. 10.1016/0169-4758(89)90191-9. [DOI] [PubMed] [Google Scholar]
- 31.Sauer JR, McSwain JL, Bowman AS, Essenberg RC. Tick salivary gland physiology. Annu Rev Entomol. 1995;40:245–67. doi: 10.1146/annurev.en.40.010195.001333. 10.1146/annurev.en.40.010195.001333. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira BR, Machado RZ, Bechara GH. Western blot analysis of tick antigens from a Rhipicephalus sanguineus unfed larval extract and identification of antigenic sites in tick section using immunohistochemistry. A comparative study between resistant and susceptible host species. Vet Parasitol. 1996;62:161–74. doi: 10.1016/0304-4017(95)00838-1. 10.1016/0304-4017(95)00838-1. [DOI] [PubMed] [Google Scholar]
- 33.Krstulovic AM. Investigations of catecholamine metabolism using high-performance liquid chromatography: analytical methodology and clinical applications. J Chromatogr. 1982;229:1–34. doi: 10.1016/s0378-4347(00)86033-8. [DOI] [PubMed] [Google Scholar]
- 34.Ciabattoni G, Pugliese F, Spaldi M, Cinotti GA, Patrono C. Radioimmunoassay measurement of prostaglandins E2 and F2alpha in human urine. J Endocrinol Invest. 1979;2:173–82. doi: 10.1007/BF03349310. [DOI] [PubMed] [Google Scholar]
- 35.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. 10.1016/S0022-1759(98)00204-X. [DOI] [PubMed] [Google Scholar]
- 36.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting funtion of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 37.Vassiliou E, Jing H, Ganea D. Prostaglandin E2 inhibits TNF production in murine bone marrow-derived dendritic cells. Cell Immunol. 2003;223:120–32. doi: 10.1016/s0008-8749(03)00158-8. 10.1016/S0008-8749(03)00158-8. [DOI] [PubMed] [Google Scholar]
- 38.Schoeler GB, Wikel SK. Modulation of host immunity by haematophagous arthropods. Ann Trop Med Parasitol. 2001;95:755–71. doi: 10.1080/0003498012011118. 10.1080/0003498012011118. [DOI] [PubMed] [Google Scholar]
- 39.Balashov YS. Bloodsucking ticks (Ixodidae): vectors of diseases in man and animals. Mis Publications Entomol Soc Am. 1972;8:161–6. [Google Scholar]
- 40.Buelens C, Verhasselt V, De Groote D, Thielemans K, Goldman M, Willems F. Interleukin-10 prevents the generation of dendritic cells from human peripheral blood mononuclear cells cultured with interleukin-4 and granulocyte/macrophage-colony-stimulating factor. Eur J Immunol. 1997;27:756–62. doi: 10.1002/eji.1830270326. [DOI] [PubMed] [Google Scholar]
- 41.Allavena P, Piemonti L, Longoni D, Bernasconi S, Stoppacciaro A, Ruco L, Mantovani A. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur J Immunol. 1998;28:359–69. doi: 10.1002/(SICI)1521-4141(199801)28:01<359::AID-IMMU359>3.0.CO;2-4. 10.1002/(SICI)1521-4141(199801)28:01<359::AID-IMMU359>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, Allavena P, Di Carlo V. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–51. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 43.Gillespie RD, Dolan MC, Piesman J, Titus RG. Identification of an IL-2 binding protein in the saliva of the Lyme disease vector tick, Ixodes scapularis. J Immunol. 2001;166:4319–26. doi: 10.4049/jimmunol.166.7.4319. [DOI] [PubMed] [Google Scholar]
- 44.Hajnicka V, Kocakova P, Slavikova M, Slovak M, Gasperik J, Fuchsberger N, Nuttall PA. Anti-interleukin-8 activity of tick salivary gland extracts. Parasite Immunol. 2001;23:483–9. doi: 10.1046/j.1365-3024.2001.00403.x. 10.1046/j.1365-3024.2001.00403.x. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–56. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 46.Rescigno M, Borrow P. The host–pathogen interaction: new themes from dendritic cell biology. Cell. 2001;106:267–70. doi: 10.1016/s0092-8674(01)00454-8. [DOI] [PubMed] [Google Scholar]
- 47.Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T-cell activation. Immunol Rev. 1996;153:85–106. doi: 10.1111/j.1600-065x.1996.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 48.Ferreira BR, Szabó MJP, Cavassani KA, Bechara GH, Silva JS. Antigens from Rhipicephalus sanguineus ticks elicit potent cell-mediated immune responses in resistant but not in susceptible animals. Vet Parasitol. 2003;115:35–48. doi: 10.1016/s0304-4017(03)00190-0. 10.1016/S0304-4017(03)00190-0. [DOI] [PubMed] [Google Scholar]
- 49.Salio M, Cella M, Suter M, Lanzavecchia A. Inhibition of dendritic cell maturation by herpes simplex virus. Eur J Immunol. 1999;29:3245–3. doi: 10.1002/(SICI)1521-4141(199910)29:10<3245::AID-IMMU3245>3.0.CO;2-X. 10.1002/(SICI)1521-4141(199910)29:10<3245::AID-IMMU3245>3.3.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 50.Andrews DM, Andoniou CE, Granucci F, Ricciardi-Castagnoli P, Degli-Esposti MA. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat Immunol. 2001;2:1077–84. doi: 10.1038/ni724. 10.1038/ni724. [DOI] [PubMed] [Google Scholar]
- 51.Urban BC, Ferguson DJ, Pain A, Willcox N, Plebanski M, Austyn JM, Roberts DJ. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400:73–7. doi: 10.1038/21900. 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 52.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 53.Ganapamo F, Rutti B, Brossard M. In vitro production of interleukin-4 and interferon-γ by lymph node cells from BALB/c mice infested with nymphal Ixodes ricinus ticks. Immunology. 1995;85:120–4. [PMC free article] [PubMed] [Google Scholar]
- 54.Zeidner N, Mbow ML, Dolan M, Massung R, Baca E, Piesman J. Effects of Ixodes scapularis and Borrelia burgdorferi on modulation of the host immune response: induction of a Th2 cytokine response in Lyme disease-susceptible (C3H/HeJ) mice but not in disease-resistant (BALB/c) mice. Infect Immun. 1997;65:3100–6. doi: 10.1128/iai.65.8.3100-3106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamaguchi Y, Tsumura H, Miwa M, Inaba K. Contrasting effects of TGF-beta 1 and TNF-alpha on the development of dendritic cells from progenitors in mouse bone marrow. Stem Cells. 1997;15:144–53. doi: 10.1002/stem.150144. [DOI] [PubMed] [Google Scholar]
- 56.Hoshino K, Takeuchi O, Kawai T, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the LPS gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 57.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 58.Yang RB, Mark MR, Gray A, et al. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–8. doi: 10.1038/26239. 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 59.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 60.Agrawal A, Lingappa J, Leppla SH, Agrawal S, Jabbar A, Quinn C, Pulendran B. Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature. 2003;424:329–34. doi: 10.1038/nature01794. 10.1038/nature01794. [DOI] [PubMed] [Google Scholar]
- 61.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: The importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA. 2002;99:351–8. doi: 10.1073/pnas.231606698. 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]