Abstract
Interleukin-10 (IL-10) is known to inhibit IL-12 production in macrophages primarily at the transcriptional level with the involvement of p50 and p65 nuclear factor-κB (NF-κB). We demonstrate that the c-rel transcription factor also plays a major role in IL-10-mediated IL-12 suppression. Treatment of macrophages with recombinant IL-10 inhibited nuclear c-rel levels, whereas addition of neutralizing anti-IL-10 antibody up-regulated both nuclear c-rel levels and IL-12 production by macrophages. Decreased nuclear c-rel was associated with a reduction in phosphorylation of inhibitory kappa B alpha (IκBα) in the cytoplasm, indicating that IL-10 prevents degradation of IκBα and the subsequent translocation of c-rel into the nucleus. Treatment with leptomycin B, a known inhibitor of c-rel at a concentration of 10 nm, when used with anti-IL-10 antibody, resulted in reduced expression of IL-12. In a complementary experiment, in vitro transient expression of p65 NF-κB could not rescue the inhibitory effect of IL-10 on IL-12 production, suggesting that NF-κB alone was not sufficient to restore IL-12 production during IL-10 treatment. However, over-expression of c-rel resulted in IL-12 restoration upon stimulation with lipopolysaccharide plus interferon-γ during IL-10 treatment. Our studies highlight the involvement of c-rel in IL-10-mediated IL-12 regulation.
Keywords: cytokines, macrophages, transcription factors/gene regulation
Introduction
The interleukin-12 (IL-12), produced primarily by antigen-presenting cells, 1 is a major player in differentiating T-helper type 1 (Th1) responses.2, 3 IL-12 is a heterodimeric cytokine made up of a p40 subunit and a p35 subunit. The IL-12 p35 chain is constitutively produced at a low level by a variety of cell types, whereas the p40 chain is only produced by cells making biologically active IL-12.4
IL-10, a product of macrophages, lymphocytes and other cells, is the physiologically most relevant inhibitor of IL-12 production and the Th1 immune responses.5 Nuclear run-on assays revealed that the inhibition of IL-12 by IL-10 was at the gene transcription level.6 The p50/p65 nuclear factor-κB (NF-κB) complex is known to play an important role in IL-12 transactivation7 and it is reported that the IL-10 inhibits IL-12 through the suppression of both IκB kinase (IKK) activity and the p50/p65 NF-κB-DNA binding activity.8 Earlier, we reported that macrophages from Bruton's tyrosine kinase-deficient mice can produce higher amounts of IL-12 despite having poorer NF-κB expression, 9 indicating that the p50/p65 NF-κB might not be the major regulator of the IL-12 gene. It has also been reported that C57BL/6 mice showed higher NF-κB expression compared to the BALB/c mice when stimulated with Setaria cervi microfilariae. However, the IL-12 induction was poorer in C57BL/6 compared to the BALB/c mice.10 Interestingly, the C57BL/6 macrophages produced higher amounts of IL-10 in response to S. cervi microfilariae compared to the BALB/c macrophages.10 Recent findings have documented that the IL-12 p40 gene is under the direct regulation of c-rel rather than of the p65 NF-κB11, 12 and that inhibition of the c-rel transcription factor leads to down-regulation of IL-12. We therefore, examined whether IL-10 suppresses IL-12 by inhibiting the c-rel transcription factor in addition to the p50/p65 NF-κB. Our results point to the involvement of c-rel transcription factor in IL-10-mediated suppression of IL-12 production.
Materials and methods
Macrophage activation assay
The RAW 264.7 macrophages were obtained from NCCS, Pune, India and maintained in Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Grand Island, NY) containing 10% fetal calf serum (Invitrogen) and antibiotics (DMEM-10). Cells were cultured in 96-well plates at 3 × 105−4 × 105 cells per well in the presence of a combination of 1 μg/ml lipopolysaccharide (LPS; Sigma-Aldrich, St Louis, MO) and 3 ng/ml interferon-γ (IFN-γ; R & D Systems, Minneapolis, MN). The recombinant IL-10 (rIL-10; BD Biosciences Pharmingen, San Diego, CA) or antibodies to IL-10 (anti-IL-10 antibodies; BD Biosciences Pharmingen) or leptomycin B (Sigma-Aldrich) were added 60 min prior to stimulation. IL-12 and IL-10 secretion in the culture supernatants was measured after 48 hr of incubation.
Enzyme immunoassay (EIA) to measure cytokine
Murine IL-10 and IL-12 p40/p70 concentrations were assayed by two-site sandwich EIA as described previously.13 Standard curves for the cytokines were generated using the recombinant standard proteins provided by the manufacturer (BD Biosciences Pharmingen). The results are expressed as mean ± SD.
Western blot analysis of p50 NF-κB, p65 NF-κB and c-rel in the nuclear extracts and total or phosphorylated IκBα in the cytoplasmic extracts
Western blot analysis was carried out to detect IκBα or various transcription factors as described earlier.14 The cells were either left unstimulated or were stimulated with LPS + IFN-γ for 1 hr in the absence or presence of 10 μg/ml of anti-IL-10 or increasing concentrations (0·5 ng/ml, 5 ng/ml and 10 ng/ml) of rIL-10. Wherever applicable, cells were pretreated with 10 nm leptomycin B. Cytosolic and nuclear extracts from these macrophage cultures were prepared from nonidet P-40-lysed cells as described.15 Equal quantities of cytosolic or nuclear proteins were separated by 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis, performed under reducing conditions, and electroblotted onto nitrocellulose membranes (Amersham Biosciences, Little Chalfont, UK). The membranes were then incubated with affinity-purified rabbit antibodies to total IκBα (Santa Cruz Biotechnology, Santa Cruz, CA) or phosphorylated IκBα (Cell Signaling Technology, Beverly, MA) or p50 NF-κB or p65 NF-κB or c-rel (Santa Cruz Biotechnology) followed by anti-rabbit immunoglobulin–horseradish peroxidase conjugate (Sigma-Aldrich). Bound enzyme was detected by chemiluminescence following the manufacturer's protocols (Amersham Biosciences). The gel intensity was measured by scanning the blot with a densitometer and subsequent analysis was performed on a Macintosh computer using the public domain nih image program (developed at the US National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/) and the relative quantities were expressed as arbitrary units.
Immunofluorescence microscopy
The RAW 264.7 cells were either left untreated or were pretreated for 1 hr with rIL-10 or anti-IL-10 antibody or leptomycin B or with both leptomycin B and anti-IL-10 antibody and stimulated further with LPS + IFN-γ for 4 hr followed by another 4 hr incubation with 20 μg/ml brefeldin A (Sigma-Aldrich) that retained the cytokine within the cell. The cells were fixed with 3% paraformaldehyde for 30 min, washed and permeabilized with 0·1% Triton X for 15 min. After blocking with 2% bovine serum albumin (Sigma) cells were incubated with rat anti-IL-12 p40 antibody and probed with anti-rat–fluorescein isothiocyanate (Sigma). Cells were washed and embedded in Vectashield mounting medium (Vector Laboratories, Burlingame, CA). Microscopy was performed on a Nikon fluorescence microscope (Nikon DX1, Japan).
Transfection with p65 NF-κB or c-rel plasmid construct
The p65 NF-κB and c-rel plasmid constructs were kind gifts from Jürgen Heesemann, Max von Pettenkofer-Institut für Hygiene und Medizinische Mikrobiologie, München, Germany. Transfections were conducted with 10 μg of the p65 NF-κB/c-rel plasmid as described earlier.16 Expression vector without any insert was used as control. The plasmid constructs were transfected into RAW 264.7 macrophage cells using the cationic lipid suspension lipofectin (Invitrogen). Eighteen hours after transfection, the cells were stimulated with LPS + IFN-γ in the absence or presence of rIL10 for either 1 hr to detect the p65 and the c-rel levels in the nucleus or for 48 hr to estimate the amounts of IL-12 secreted in the culture supernatants by EIA.
Immunoprecipitation of p50 or c-rel transcription factor
The p50 or c-rel in the nuclear extracts was immunoprecipitated overnight at 4° with anti-c-rel or anti-p50 antibody (Santa Cruz Biotechnology) at a final concentration of 1 : 100 and then incubated with protein A/G beads for 3 hr at 4°. Beads were washed extensively with the nuclear extraction buffer as described earlier.14 The co-immunoprecipitated p50 or c-rel was detected by Western blot analysis using the specific antibody to p50 or c-rel as described above.
Statistical analysis
All the data were analysed using Student's t-test wherever applicable. P < 0·05 was considered to be significant.
Results
Anti-IL-10 antibody up-regulates both c-rel and p65 NF-κB and enhances IL-12 production by macrophages
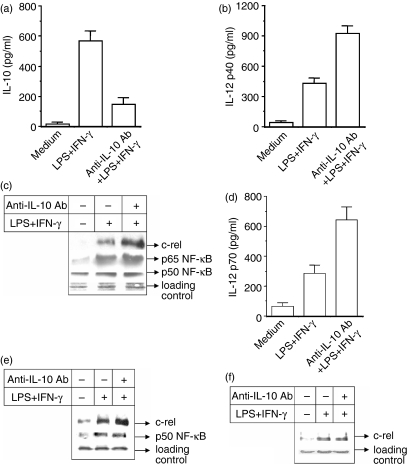
In the present study, we examined whether the negative effect of IL-10 on IL-12 induction is mediated also by the inhibition of c-rel in addition to p65 NF-κB. Induction of IL-10 in macrophages commonly occurs in response to the same stimuli that lead to macrophage activation for the production of IL-12. Therefore, we studied whether neutralization of the endogenously produced IL-10 by anti-IL-10 antibody could improve the IL-12 induction. The macrophages were stimulated with LPS + IFN-γ in the presence of 10 μg/ml anti-IL-10 neutralizing antibody (anti-IL-10 Ab) and the levels of both IL-10 and IL-12 p40/p70 cytokines secreted in the cultures were estimated by EIA. Treatment of macrophages with anti-IL-10 Ab, although it diminishes IL-10 production (Fig. 1a), it significantly up-regulates IL-12 p40 production (Fig. 1b; P < 0·01) by the LPS + IFN-γ activated macrophages. Next we examined the relative involvement of the nuclear p50, p65 and c-rel transcription factors in promoting IL-12 induction during anti-IL-10 Ab treatment. The expression of p50, p65 and c-rel transcription factors was examined by Western blot analysis of the nuclear extracts prepared from macrophages stimulated with LPS + IFN-γ in the absence or presence of 10 μg/ml of anti-IL-10 Ab. It is evident that anti-IL-10 Ab increases nuclear levels of both p65 NF-κB and c-rel (Fig. 1c). As expected, 17 anti-IL-10 did not show much effect on the nuclear p50 NF-κB (Fig. 1c). These data indicate that c-rel may be involved in IL-10-mediated down-regulation of IL-12 in addition to p65 NF-κB. The anti-IL-10 Ab also up-regulates the expression of biologically active IL-12 p70 in RAW 264.7 macrophages (Fig. 1d; P < 0·01).
Figure 1.
Inhibition of endogenous IL-10 by treating macrophages with anti-IL-10 antibody (anti-IL-10 Ab) significantly increases nuclear c-rel level in addition to p65 NF-κB and causes up-regulation of IL-12 production. The RAW 264.7 macrophages were pretreated with 10 μg/ml anti-IL-10 Ab for 1 hr and further stimulated with LPS + IFN-γ for 48 hr. The production of IL-10 (a) and IL-12 p40 (b) was measured by EIA (mean ± SD). In another experiment, the macrophages were stimulated with LPS + IFN-γ for 1 hr in the absence or presence of anti-IL-10 Ab and the expression of c-rel and p65 and p50 NF-κB in the nuclear extracts was examined by immunoblot analysis of identical amounts of total proteins loaded in (c). IL-12 p70 level was measured by EIA in the IL-10-treated macrophages (d). In a separate experiment, the nuclear extracts prepared from RAW 264.7 macrophages activated with LPS + IFN-γ in the absence or presence of 10 μg/ml of anti-IL-10 Ab. The nuclear extracts were incubated first with anti-c-rel Ab overnight at 4° and then protein A/G–Sepharose was added to the mixture and it was incubated for a further 3 hr. Co-immunoprecipitated p50 was detected by Western blot analysis using the anti-p50 Ab(e, middle panel). The same preparation was also used to detect c-rel using anti-c-rel Ab, respectively (e; upper panel). In a complementary experiment, c-rel was coimmunoprecipitated using anti-p50 Ab and subjected to Western blotting for c-rel using anti-c-rel Ab (f). Results shown are representative of four independent experiments.
To understand whether c-rel acts as a regulator of p40 during IL-10 treatment following the formation of a dimer with p50 NF-κB, 11, 18 the p50 was co-immunoprecipitated as p50–c-rel complex, using anti-c-Rel antibody, from the nuclear extracts prepared from macrophages treated either with LPS + IFN-γ alone (IL-10–) or co-treated with anti-IL-10 Ab and LPS + IFN-γ (IL-10+). Western blot assay using an antibody specific for p50 revealed that the amount of p50 that was co-immunoprecipitated in the IL-10– group was similar to that of the IL-10+ group (Fig. 1e; middle panel). However, when the same extracts were used to detect the c-rel in Western blot using anti-c-rel antibody, it could be seen that more c-rel could be co-immunoprecipitated in the IL-10+ as compared to the IL-10– group (Fig. 1e; upper panel). To further confirm this, we used anti-p50 antibody to co-immunoprecipitate c-rel. Western blot analysis of c-rel that was co-immunoprecipitated along with p50 revealed that the level was not appreciably different between the two groups (Fig. 1f). These studies (Fig. 1e,f) reveal an up-regulation of the c-rel–p50 dimer during LPS + IFN-γ stimulation which however, remained unaltered during IL-12 p40 enhancement by anti-IL-10 Ab, thereby suggesting that the c-rel–p50 dimer may not be responsible for modulating p40 during anti-IL-10 treatment.
Exogenous IL-10 inhibits c-rel level in nucleus and also IL-12 production by macrophages
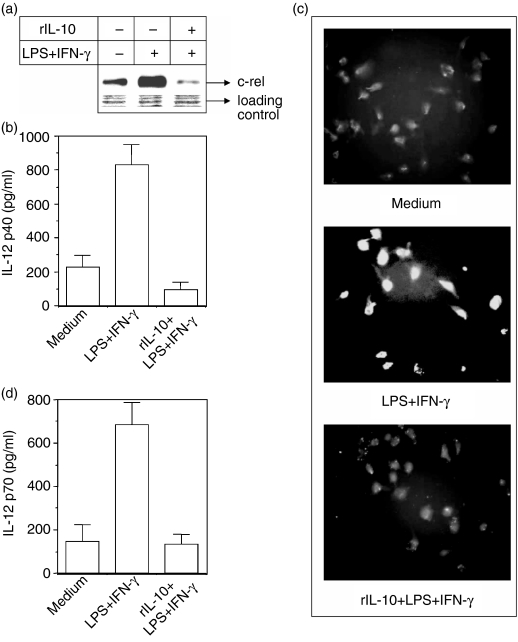
Having demonstrated, using antibody neutralization experiments, that IL-10 could down-regulate the nuclear c-rel level, we further investigated whether exogenous rIL-10 could affect the status of c-rel levels. Macrophages were pretreated with 5 ng/ml of rIL-10 and further stimulated with LPS + IFN-γ either for 1 hr, after which the nuclear accumulation of c-rel was analysed by enhanced chemiluminescence (ECL) immunoblot, or for another 48 hr, after which the amounts of IL-12 p40 and IL-12 p70 secreted in these cultures were quantified by EIA. The results reveal that the addition of rIL-10 results in poorer accumulation of c-rel in the nucleus (Fig. 2a) and a reduction in IL-12 p40 production by macrophages activated with LPS + IFN-γ as measured by either EIA (Fig. 2b; P < 0·001) or immunofluorescence microscopy (Fig. 2c). The decrease in IL-12 p40 correlated well with a decrease in IL-12 p70 production (Fig. 2d). Therefore, it was evident that exogenous IL-10 treatment resulted in reduced c-rel expression as well as IL-12 production.
Figure 2.
Exogenous IL-10 inhibits nuclear c-rel levels and also IL-12 production. The RAW 264.7 macrophages were cultured with LPS + IFN-γ in the absence or presence of 5 ng/ml of rIL-10. The nuclear accumulation of c-rel was examined by immunoblot analysis of identical amounts of total proteins loaded in the blot (a). The IL-12 p40 production was determined either by EIA (b) or by immunofluorescence microscopy (c) as described in the Materials and methods. The IL-12 p70 level was determined by EIA (d). Data are representative of four different experiments.
IL-10 prevents phosphorylation and degradation of IκBα
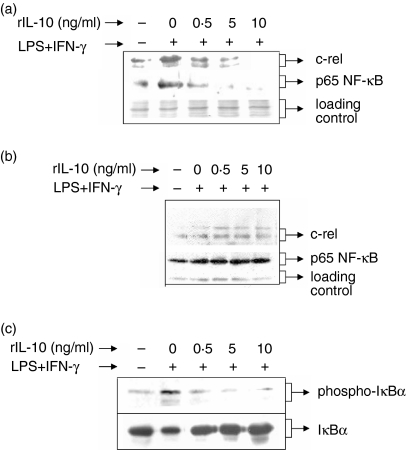
The Rel/NF-κB family member proteins form a complex with the inhibitory IκB protein19 in the cytoplasm and phosphorylation of IκB by IKK triggers rapid dissociation of IκB, 20 resulting in translocation of the Rel/NF-κB proteins to the nucleus. Therefore, in the next experiment, we examined whether IL-10 affected LPS + IFN-γ-induced IκBα phosphorylation to prevent p65/c-rel translocation to the nucleus. The RAW 264.7 macrophages were stimulated with LPS + IFN-γ in the presence of increasing concentrations (0·5 ng/ml, 5 ng/ml and 10 ng/ml) of rIL-10. Nuclear and cytosolic p65 NF-κB and c-rel were detected by Western blotting using specific antibodies. The LPS + IFN-γ-induced phosphorylated form of IκBα was examined by Western blot analysis using an antibody that detects only the serine-phosphorylated form of IκBα. It could be seen that the nuclear (Fig. 3a) but not the cytosolic p65 NF-κB (Fig. 3b) was inhibited by IL-10. Similar to p65, the nuclear but not the cytosolic c-rel level was reduced by IL-10, suggesting that IL-10 did not inhibit the induction of p65 NF-κB and c-rel transcription factors. Further studies reveal that LPS + IFN-γ induced IκBα phosphorylation while rIL-10 inhibited IκBα phosphorylation (Fig. 3c, upper panel) and the degradation of IκBα (Fig. 3c, lower panel) in a dose-dependent manner. These results indicate that IL-10 mainly inhibits LPS + IFN-γ-induced phosphorylation and degradation of IκBα, thus affecting the translocation of p65 NF-κB and c-Rel to the nucleus while not affecting the total cell level of these proteins.
Figure 3.
rIL-10 decreases nuclear p65 and c-rel levels in a dose dependent manner by reducing phosphorylation of IκBα while not affecting the total induction of these proteins. RAW 264.7 macrophages were cultured with LPS + IFN-γ in the absence or presence of increasing concentrations (0·5 ng/ml, 5 ng/ml and 10 ng/ml) of rIL-10. The cytosolic extracts (CE) and the nuclear extracts (NE) were prepared from nonidet P-40-lysed cells. The levels of c-rel and p65 NF-κB were detected in the NE (a) and CE (b) by Western blotting using antibodies specific to c-rel and p65 NF-κB. The presence of cytosolic total and phosphorylated IκBα was detected by Western blotting using antibodies specific for total IκBα or phosphorylated IκBα (c). The data are representative of three independent experiments.
Involvement of c-rel in IL-12 suppression by IL-10
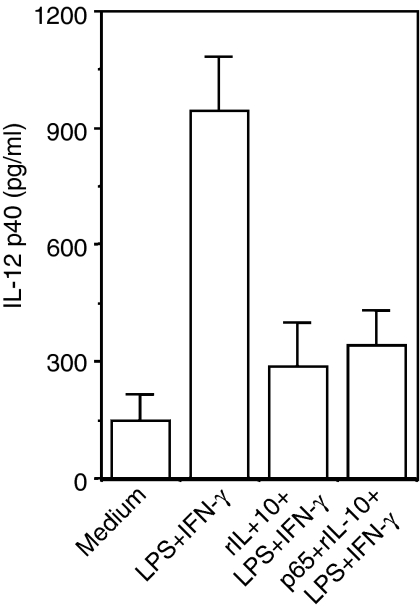
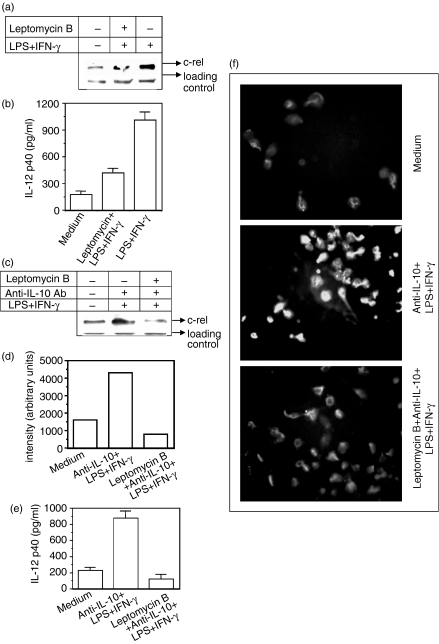
The experiments described above clearly show that the nuclear levels of c-rel were reduced by rIL-10 (Fig. 2a) and that inhibition of IL-10 production by the neutralizing antibody increased the nuclear level of c-rel (Fig. 1c), indicating that IL-10 may negatively target c-rel to exert its effect on IL-12. However, it has also been demonstrated that IL-10 inhibits p65 NF-κB levels in the nucleus and thereby suppresses IL-12 transactivation.17 Thus, it was argued that in vitro transient expression of p65 NF-κB should rescue the inhibitory effect of IL-10 on IL-12 induction. Therefore, in the next experiment, the RAW 264.7 macrophages were transfected with the p65 NF-κB plasmid construct or with mock plasmid (pUC18) and further stimulated with LPS + IFN-γ in the absence or presence of 5 ng/ml of rIL-10 for 48 hr. Over-expression of the p65 NF-κB in the RAW 264.7 macrophages (data not shown) was unable to improve the IL-12 induction status during rIL-10 treatment (Fig. 4) indicating that p65 NF-κB was not the only responsible factor involved in IL-12 suppression by IL-10. Since the c-rel level was increased by anti-IL-10 Ab (Fig. 1c), it was considered necessary to define the role of c-rel in IL-10-mediated IL-12 suppression. To prove a role for c-rel in such regulation, we next blocked the c-rel level within the nucleus by using leptomycin B. The leptomycin B stably retains the c-rel–IκBα complex in the cytoplasm and is a specific inhibitor of Crm1-mediated nuclear export.21, 22 The leptomycin B at a concentration of 10 nm can selectively block nuclear c-rel21, 22 but not other proteins.23, 24 When stimulated with LPS + IFN-γ in the presence of 10 nm leptomycin B, macrophages show a reduction in the nuclear levels of c-rel (Fig. 5a) with a concomitant decline in IL-12 p40 levels (Fig. 5b). This indicated that leptomycin B reduced IL-12 p40 production through a reduction in the nuclear c-rel level but not the NF-κB proteins (data not shown). Having shown in the previous experiment that the up-regulated production of IL-12 by anti-IL-10 Ab was associated with increased levels of nuclear c-rel (Fig. 1), we further investigated whether IL-12 p40 up-regulation by anti-IL-10 Ab could be intercepted by reducing the nuclear c-rel levels by leptomycin B. Therefore, in the next experiment, the macrophages were pretreated with 10 nm of leptomycin B before exposure to anti-IL-10 Ab and LPS + IFN-γ and both the nuclear c-rel level and IL-12 induction were measured. It could be seen that the reduction in c-rel level by the leptomycin B (Fig. 5c,d) inhibited IL-12 p40 induction in macrophages even after treatment with anti-IL-10 Ab, as evident from EIA (Fig. 5e; P < 0·001) or immunofluorescence assay (Fig. 5f). These results once again demonstrate that c-rel is important for IL-10-mediated up-regulation of IL-12 in macrophages.
Figure 4.
In vitro transient expression of p65 NF-κB does not rescue the inhibitory effect of IL-10 on IL-12. The plasmid constructs were transfected into RAW 264.7 macrophage cells using the cationic lipid suspension lipofectin and incubation for 18 hr. The control group received the mock plasmid (pUC18). All the groups were further stimulated with LPS + IFN-γ in the absence or presence of 5 ng/ml of rIL-10 for 48 hr. The amount of IL-12 secreted in the culture supernatants was measured by EIA (mean ± SD). Data are representative of three different experiments.
Figure 5.
Inhibition of c-rel import by leptomycin B prevents IL-12 up-regulation by anti-IL-10 Ab. RAW macrophages were pretreated with 10 nm of lepotmycin B for 1 hr and further stimulated with LPS + IFN-γ either for 1 hr for assaying nuclear accumulation of c-rel in identical amounts of proteins by ECL immunoblot (a) or for 48 hr for quantifying IL-12 levels (mean ± SD) secreted in these cultures (b). In another experiment, RAW 264.7 macrophages were cultured with anti-IL-10 Ab and LPS + IFN-γ in the absence or presence of 10 nm of leptomycin B. The nuclear accumulation of c-rel was detected by ECL immunoblot assay (c) and the gel intensity was quantified by densitometric analysis (d). The IL-12 p40 induction was examined either by EIA (e) or by immunofluorescence microscopy (f) as described in the Materials and methods section. Data are representative of three different experiments.
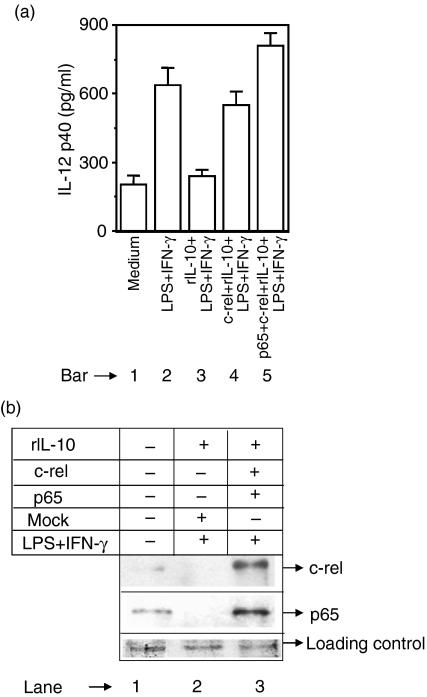
Next we examined whether over-expression of c-rel is able to restore IL-12 during IL-10 treatment. The RAW 264.7 macrophages were transfected with either c-rel alone or with both c-rel and p65 NF-κB. These macrophages were then simulated with LPS + IFN-γ in the presence of 5 ng/ml of rIL-10 and IL-12 p40 production was measured after 48 hr. The IL-12 p40 production was inhibited by rIL-10 in the mock-group (Fig. 6a, compare bar 3 with bar 2) as expected. However, in contrast to p65 (Fig. 4), transfection with c-rel alone could significantly restore IL-12 production in macrophages treated with rIL-10 (Fig. 6a, compare bar 4 with bar 3) demonstrating an important role of c-rel in IL-12 regulation by IL-10. As expected, 11, 18 over-expression of both c-rel and p65 NF-κB in rIL-10-treated macrophages resulted in up-regulation of IL-12 p40 production (Fig. 6a, compare bar 5 with bar 2). In the co-transfected macrophages, over-expression of c-rel and p65 was confirmed by Western blotting (Fig. 6b).
Figure 6.
Over-expression of c-rel restores IL-12 p40 induction in IL-10-treated macrophages. The RAW 264.7 macrophages were transfected with either the c-rel plasmid construct or with both c-rel and p65 NF-κB plasmids using lipofectin. Cells were incubated for 18–20 hr, washed and further stimulated with LPS + IFN-γ in the presence of 5 ng/ml rIL-10. After 48 hr, the amounts of IL-12 p40 (mean ± SD) secreted in the culture supernatants were estimated following EIA (a). The c-rel and the p65 NF-κB levels in the nuclear extracts were detected by Western blotting after 1 hr of stimulation (b). Data are representative of three different experiments.
Discussion
In the present study, we have shown that in addition to the p65 NF-κB, IL-10 also blocks the nuclear c-rel levels. Our results show that the c-rel is also inhibited by IL-10 in addition to p65 NF-κB and it may be responsible for IL-10-mediated suppression of IL-12 induction in RAW 264.7 macrophages. Since transient expression of p65 NF-κB did not improve IL-12 induction status, it is possible that a reduction in c-rel by the IL-10 is mainly responsible for the negative effect of IL-10 on IL-12. This conclusion is supported by the observation that inhibition of nuclear c-rel by leptomycin B prevented the enhancing effect of anti-IL-10 on the production of IL-12. Furthermore, in vitro transient expression of c-rel could restore IL-12 levels in IL-10-treated cells, confirming a role of c-rel in IL-12 regulation by IL-10. The co-immunoprecipitation assay indicated that the c-rel–p50 dimer was not responsible for the enhancing effect of anti-IL-10 Ab on IL-12. It is also possible that the complex interaction between the p65 NF-κB and c-rel is actually responsible for IL-10-mediated suppression of IL-12. Our study provides evidence for a role of c-rel in IL-10-mediated IL-12 suppression, while not ignoring a role of p65 NF-κB in such down-regulation.
The Rel/NF-κB family member proteins are sequestered in the cell cytoplasm, complexed to a family of inhibitory IκB proteins.19 Phosphorylation of IκB by IKKs triggers rapid dissociation of IκB20, 25 and consequently, the Rel/NF-κB proteins are translocated into the nucleus to activate transcription of target genes. A unidirectional negative regulation of IL-12 by IL-10 has been described26 but the molecular mechanisms are controversial. Evidence has revealed that the IL-12 levels were reduced to almost undetectable levels in c-rel–/– macrophages but were not significantly affected in p65–/– macrophages, 12 thereby pointing to the important role of c-rel transcription factor in IL-12 transactivation. The c-rel requirement for IL-12 gene transcription may also be important because of an essential interaction between a c-rel-containing complex and the rel site within the promoter.11, 12 Therefore, inhibition of the c-rel signal is likely to prevent IL-12 induction by reducing transactivation of either p40 or p35 or both. The IL-10 was shown to reduce the phosphorylation of IκBα, which could be responsible for poorer accumulation of c-rel in the nucleus. The inhibition of IKK activity by IL-108 may be one of the mechanisms by which IL-10 prevents degradation of IκBα and the subsequent translocation of c-rel into the nucleus. In addition to IL-12 p40, c-rel also regulates the IL-12 p35 gene27 and therefore, the IL-10-mediated IL-12 p35 regulation may also occur through the c-rel. It is pertinent to note here while c-rel is required for LPS-induced expression of IL-12, c-rel-independent mechanisms of IL-12 expression do exist, as has been shown during Toxoplasma gondii infection of c-rel–/– mice.28 Detailed characterization of the signal pathways involved in such interactions would be of great interest in the generation of pharmacological modulators of immune responses.
Acknowledgments
We thank Ms Ghoussunnisa for technical assistance and Dr Sudip Ghosh for discussions. This work was supported by research fellowships to S.S.R. and N.R. from the Council of Scientific and Industrial Research (CSIR), India. This work was supported by a grant from the Indian Council of Medical Research (48/13/2001-BMS), India, the Third World Academy of Science (02–371 RG/BIO/AS), Italy and a core grant to CDFD from the Department of Biotechnology, Government of India.
Abbreviations
- IL-10
interleukin-10
- IL-12
interleukin-12
- NF-κB
nuclear factor-κB
- IκBα
inhibitory kappa B alpha
- EIA
enzyme immunoassay
References
- 1.D'Andrea A, Rengarayu M, Valginte NM, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–98. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macatonia SE, Hosken NA, Litton M, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–9. [PubMed] [Google Scholar]
- 3.Stobie L, Gurunathan S, Prussin C, Sacks DL, Glaichenhaus N, Wu CY, Seder RA. The role of antigen and IL-12 in sustaining Th1 memory cells in vivo: IL-12 is required to maintain memory/effector Th1 cells sufficient to mediate protection to an infectious parasite challenge. Proc Natl Acad Sci USA. 2000;97:8427–32. doi: 10.1073/pnas.160197797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gubler U, Chua A, Schoenhaut D, et al. Co-expression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci USA. 1991;88:4143–7. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes. an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aste-Amezaga M, Ma X, Sartori A, Trinchieri G. Molecular mechanisms of the induction of IL-12 and its inhibition. J Immunol. 1998;160:5936–44. [PubMed] [Google Scholar]
- 7.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 8.Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS., Jr Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274:31868–74. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- 9.Mukhopadhyay S, Mohanty M, Mangla A, George A, Bal V, Rath S, Ravindran B. Macrophage effector functions controlled by Bruton's tyrosine kinase are more crucial than the cytokine balance of T cell responses for microfilarial clearance. J Immunol. 2002;168:2914–21. doi: 10.4049/jimmunol.168.6.2914. [DOI] [PubMed] [Google Scholar]
- 10.Mukhopadhyay S, Srivastava VML, Murthy PK, Hasnain SE. Poorer NF-κB signaling by microfilariae in macrophages from BALB/c mice affects their ability to produce cytotoxic levels of nitric oxide to kill microfilariae. FEBS Lett. 2004;567:275–80. doi: 10.1016/j.febslet.2004.04.081. [DOI] [PubMed] [Google Scholar]
- 11.Plevy SE, Gemberling JH, Hsu S, Dorner AJ, Smale ST. Multiple control elements mediate activation of the murine and human interleukin 12 p40 promoters. Evidence of functional synergy between C/EBP and Rel proteins. Mol Cell Biol. 1997;17:4572–88. doi: 10.1128/mcb.17.8.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanjabi S, Hoffmann A, Liou HC, Baltimore D, Smale ST. Selective requirement for c-Rel during IL-12 P40 gene induction in macrophages. Proc Natl Acad Sci USA. 2000;97:12705–10. doi: 10.1073/pnas.230436397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukhopadhyay S, Sahoo PK, George A, Bal V, Rath S, Ravindran B. Delayed clearance of filarial infection and enhanced Th1 immunity due to modulation of macrophage APC functions in xid mice. J Immunol. 1999;163:875–83. [PubMed] [Google Scholar]
- 14.Mukhopadhyay S, George A, Bal V, Ravindran B, Rath S. Bruton's tyrosine kinase deficiency in macrophages inhibits nitric oxide generation leading to enhancement of IL-12 induction. J Immunol. 1999;163:1786–92. [PubMed] [Google Scholar]
- 15.Dignam JD, Martin PL, Shastry BS, Roeder RG. Eukaryotic gene transcription with purified components. Meth Enzymol. 1983;101:582–98. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- 16.Ruckdeschel K, Mannel O, Richter K, Jacobi CA, Trulzsch K, Rouot B, Heesemann J. Yersinia outer protein P of Yersinia enterocolitica simultaneously blocks the nuclear factor-kappa B pathway and exploits lipopolysaccharide signaling to trigger apoptosis in macrophages. J Immunol. 2001;166:1823–31. doi: 10.4049/jimmunol.166.3.1823. [DOI] [PubMed] [Google Scholar]
- 17.Driessler F, Venstrom K, Sabat R, Asadullah K, Schottelius AJ. Molecular mechanisms of interleukin-10-mediated inhibition of NF-kappaB activity: a role for p50. Clin Exp Immunol. 2004;135:64–73. doi: 10.1111/j.1365-2249.2004.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–6. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 19.May MJ, Ghosh S. Rel/NF-kappa B and I kappa B proteins: an overview. Semin Cancer Biol. 1997;8:63–73. doi: 10.1006/scbi.1997.0057. [DOI] [PubMed] [Google Scholar]
- 20.Di Donato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappab. Nature. 1997;388:548–54. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 21.Jang BC, Munoz-Najar U, Paik JH, Claffey K, Yoshida M, Hla T. Leptomycin B, an inhibitor of the nuclear export receptor CRM1, inhibits COX-2 expression. J Biol Chem. 2003;278:2773–6. doi: 10.1074/jbc.C200620200. [DOI] [PubMed] [Google Scholar]
- 22.Sachdev S, Hannink M. Loss of IkappaB alpha-mediated control over nuclear import and DNA binding enables oncogenic activation of c-Rel. Mol Cell Biol. 1998;18:5445–56. doi: 10.1128/mcb.18.9.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang TT, Kudo N, Yoshida M, Miyamoto S. A nuclear export signal in the N-terminal regulatory domain of IkappaBalpha controls cytoplasmic localization of inactive NF-kappaB/IkappaBalpha complexes. Proc Natl Acad Sci USA. 2000;97:1014–19. doi: 10.1073/pnas.97.3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harhaj EW, Sun SC. Regulation of RelA subcellular localization by a putative nuclear export signal and p50. Mol Cell Biol. 1999;19:7088–95. doi: 10.1128/mcb.19.10.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mercurio F, Zhu H, Murray BW, et al. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–6. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 26.Xia CQ, Kao KJ. Suppression of interleukin-12 production through endogenously secreted interleukin-10 in activated dendritic cells: involvement of activation of extracellular signal-regulated protein kinase. Scand J Immunol. 2003;58:23–32. doi: 10.1046/j.1365-3083.2003.01268.x. [DOI] [PubMed] [Google Scholar]
- 27.Grumont R, Hochrein H, O'Keeffe M, Gugasyan R, White C, Caminschi I, Cook W, Gerondakis S. c-Rel regulates interleukin 12 p70 expression in CD8(+) dendritic cells by specifically inducing p35 gene transcription. J Exp Med. 2001;194:1021–32. doi: 10.1084/jem.194.8.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason N, Aliberti J, Caamano JC, Liou HC, Hunter CA. Identification of c-Rel-dependent and -independent pathways of IL-12 production during infectious and inflammatory stimuli. J Immunol. 2002;168:2590–4. doi: 10.4049/jimmunol.168.6.2590. [DOI] [PubMed] [Google Scholar]