Abstract
Interleukin-7 (IL-7) is produced by bone marrow and lymphoid stromal cells and is involved in the synthesis, survival and homeostasis of T cells. These attributes are the basis for current strategies to utilize IL-7 as an immune modulator for several clinical conditions to replenish depleted T-cell numbers. Because we had previously determined that IL-7 can induce potent human immunodeficiency virus replication in the otherwise non-permissive CD4+ naïve T-cell compartment, we evaluated here the impact of IL-7 on the phenotype and functional potential of naïve CD4+ T cells in an attempt to understand the mechanism of this induction. We demonstrate that IL-7 mediated the up-regulation of CD25, CD95 and human leucocyte antigen-DR, while it did not alter the expression of CD45RO, CD69, CD40, or CD154. Examination of the cytokine profile of IL-7-treated naïve T cells using a Type1/Type2 Proteome Array indicated a remarkable IL-7-mediated induction of interferon-γ production, while the other cytokines evaluated (IL-2, IL-12, tumour necrosis factor-α, IL-4, IL-5, IL-10 and IL-13) were not affected. Intracellular staining of IL-7-treated naïve T cells for interferon-γ verified the Proteome data. IL-7 did not induce cell cycle proliferation of naïve CD4+ T cells, as evaluated by 7-AAD/pyronin immunostaining and carboxyfluorescein diacetate succinimidyl ester dye tracking. IL-7 treatment of naïve CD4+ T cells induced their ability to prime monocytes, as was indicated by induction of CD80 and CD86 expression on monocytes cocultured with IL-7-treated naïve CD4+ T cells. Collectively, these data indicate that IL-7 signalling is sufficient to phenotypically and functionally prime human CD4+ naïve T cells independent of antigen stimulation.
Keywords: cell surface molecules, cytokines, humans, T cells
Introduction
Immune-based therapeutic intervention is currently a viable option in the treatment of a number of diseases spanning a wide variety of clinical settings, including autoimmune disease, acquired immunodeficiency syndrome (AIDS), transplantation, chemotherapy and age-related immune senescence. One of the potential cytokines for immune-based therapeutic intervention for these conditions is interleukin-7 (IL-7).1–4
IL-7 is produced by bone marrow and lymphoid stromal cells. It plays an important role in both T-cell and B-cell development and in the maintenance of T-cell homeostasis.2,5 To maintain T-cell homeostasis, four major processes may be at play: (1) de novo T-cell synthesis, (2) T-cell survival, (3) T-cell proliferation, and (4) cell death. IL-7 appears to be at the interface of three of these homeostatic mechanisms. IL-7 may enhance de novo T-cell synthesis, as demonstrated by an IL-7-mediated enhancement of a molecular marker of recent thymic emigrants known as T-cell receptor excision circles in thymic explant models.6 However, whether this IL-7-mediated mechanism of enhancing thymic output is functional in humans has not been clearly delineated. IL-7 enhances the survival of early thymocyte progenitors7–9 as well as that of mature T cells by up-regulating Bcl-2 (an anti-apoptotic protein).10 Finally, IL-7 can dramatically expand the existing T-cell pool under lymphopenic conditions.11–14 These collective contributions of IL-7 to the various homeostatic mechanisms establish IL-7 as a possible ‘immuno-regenerative cytokine’.1
Despite the theoretical beneficial functions of IL-7 in the setting of immune-based therapy, the use of IL-7 therapeutically in T-cell-depleted hosts should be approached with caution because of several adverse findings. (1) IL-7 enhances the preferential expansion of autoreactive T cells from the memory T-cell pool.15 (2) IL-7 enhances the expansion of T cells in response to low-affinity antigens, possibly creating a favourable microenvironment for autoimmunity, especially without the appropriate T-cell receptor (TCR) signal.2,16 (3) IL-7 administration in an allogeneic bone marrow transplant mouse model may worsen graft versus host disease, as was reported in one study17 but not in another.12 (4) IL-7 ex vivo induced latent human immunodeficiency virus (HIV) infection.18
We have previously demonstrated that IL-7 can induce the potent replication of HIV IIIB, Bal and primary isolates (302056, 302073, 302142, 302144) in naïve T cells.19 These findings were in contrast to the idea that resting T cells do not support active HIV replication unless they are stimulated to pass beyond the G1b phase of the cell cycle.20–22 Others have demonstrated that resting naïve T cells in lymphoid tissue can support HIV replication without passing through the G1b phase of the cell cycle, suggesting that the environmental milieu may have an impact on HIV infection.23 We believe that IL-7 is a key factor that may regulate HIV infection of naïve T cells. Given the impact of IL-7 on mediating HIV infection of naïve T cells, which are classically non-permissive to HIV infection, and given the current efforts to utilize IL-7, either to purge the latent reservoir of HIV24 or as an ‘immuno-regenerative agent’,1 we evaluated the impact of IL-7 treatment on the phenotype of naïve T cells and its functional consequence. It is critical, however, to establish our definition of naïve CD4+ T cells specifically, based on the growing current knowledge found in the literature.
Naïve T cells are defined as cells that have not yet encountered antigen(s). Traditionally, differential expression of the isoforms of the common leucocyte antigen (CD45) distinguished between naïve and memory T cells, where by CD45RA+ CD45RO− cells are defined as naïve and CD45RA− CD45RO+ are defined as memory T cells.25 Accumulating data indicate that there are at least three populations of CD4+ and CD8+ memory T cells, termed central memory (CM), effector memory (EM) and terminally differentiated effector memory (TDEM) T cells.26,27 The maturation pathway of memory T cells is still unclear but at least for CD8+ T cells, they appear to be generated as part of a linear continuum, where naïve cells give rise to CM, which give rise to EM cells, and ultimately to TDEM T cells.26,28–30 However, others have proposed that EM cells give rise to CM cells.27,31 Functionally, while all memory populations can mount an effector response, CM cells are believed to be more potent in mediating protective immunity because they persist for longer in vivo and have a higher proliferative capacity than EM cells.27,32
The distinction between naïve, CM and EM cells can still be made on the basis of CD45RA expression, where naïve CD4+ T cells are CD45RA+ while CM and EM are CD45RA−.26 However, CD45RA is inadequate to distinguish between naïve and TDEM T cells because like naïve T cells, TDEM T cells are CD45RA+. Additional phenotypic markers (CD62L, CD57, CD27, CD28 and CCR7) can distinguish between these two populations, as summarized in Table 1. Most notably, CD62L, CD28 and CD27 are not expressed on TDEM T cells while they are expressed on naïve T cells and CD57 is expressed on TDEM T cells but not on naïve CD4+ T cells. Functionally, naïve T cells are in a resting state and do not actively secrete cytokines such as IL-2, IL-4, IL-10, or interferon-γ (IFN-γ). Because we have shown that IL-7 mediates the productive infection of HIV in CD4+ CD45RA+ T cells19 we evaluated the impact of IL-7 on the phenotype and functional potential of CD4+ CD45RA+ T cells.
Table 1. Differentiation between T-cell subsets based on cell surface marker expression.
| Marker | Naïve | EM | CM | TDEM |
|---|---|---|---|---|
| CD45RA | + | − | − | + |
| CCR7 | + | − | + | − |
| CD57 | − | − | − | + |
| CD62L | + | − | + | − |
| CD28 | + | + − | + | − |
| CD27 | + | + − | + | − |
| CD45RO | − | + − | + | − |
Table 1 is generated based on published data25,26 and discussions with Drs Mario Roederer, Daniel Douek, and Michael Betts (Vaccine Research Center, NIAID, NIH). Note that the order of Central Memory (CM) and Effector Memory (EM) T cells is not based on lineage hierarchy, given that data exists in favor of both CM giving rise to EM25,27,28 and EM giving rise to CM.26,30 EM, effector memory cells; CM, central memory cells; TDEM, terminally differentiated effector memory cells.
Materials and methods
Cell purification
All cells were purified from healthy laboratory workers between the ages of 20 and 30 years. Nine individuals donated blood for these studies, two males and seven females. Blood samples from these donors were collected using ethylenediaminetetraacetic acid-containing Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ). Peripheral blood mononuclear cells (PBMCs) were then isolated using Ficoll–Hypaque density gradient centrifugation. Subsequently, CD4+ CD45RA+ cells were isolated by negative immunoselection for CD45RA. Briefly, PBMCs were incubated with antibodies (PharMingen, San Diego, CA) to CD45RO (clone UCHL-1), CD14 (clone M5E2), CD8 (clone HIT8a) and CD19 (clone HIB19) for 15 min at room temperature. In some experiments, where indicated, natural killer (NK) cells were depleted by removing CD16+ cells (clone 3GB) and CD56+ cells (clone B159). The cells were then washed with phosphate-buffered saline supplemented with 2% fetal bovine serum and incubated with goat anti-mouse antibody conjugated to Miltenyi microbeads (Miltenyi Biotec, Bergisch Gladback, Germany) for 15 min at 4°. After washing, CD45RA cells were negatively selected using the AutoMacs, as described by the manufacturer (Miltenyi Biotec). The resulting fraction was > 99% viable and contained < 0·02% CD45RO+ and CD19+ cells, < 2% CD14+ cells and < 0·9% CD16+ CD56+ cells in cultures that were depleted for the NK-cell population, and no CD8+ T cells. Human research was conducted in accordance with Institutional Review Board (IRB) approval.
Cell culture
CD45RA+ CD45RO− T cells were cultured in RPMI-1640 medium (Biowhittaker, Walkersville, MD) supplemented with 10% heat-inactivated fetal bovine serum (Sigma, St Louis, MO), 1% penicillin/streptomycin (Gibco-BRL, Grand Island, NY), and 2 mm l-glutamine (Gibco-BRL). The cells were cultured at 1 × 106/ml and were treated with 1000 U/ml IL-7 (PharMingen, San Diego, CA). This dose of IL-7 was determined to be optimal in inducing HIV replication19 and was therefore used to explore further the impact of this dose of IL-7 on the biological properties of CD4+ CD45RA+ T cells. Control cultures were either left untreated or were treated with IL-2 at 100 U/ml (AIDS Research Reagent and Reference Program, Rockville, MD) for the same duration as IL-7-treated cultures. Fresh CD45RA+ T cells were isolated and used immediately as an additional control. All cultures were propagated in a humidified incubator containing 5% CO2.
Cell surface immunofluorescence staining and flow cytometric analysis
Surface staining of enriched CD45RA+ T cells was performed by adding to the cells the appropriate antibodies conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein (PerCP), or allophycocyanin (APC) for 20 min at room temperature in the dark. The enrichment level of the immunoselected T cells was evaluated by immunostaining for CD45RA-FITC (clone L48), CD4-PE (clone SK3), CD8-PerCP (clone SK1), CD45RO-APC (clone UCHL-1), CD14-PE (clone macrophage p9) and CD19-PerCP (clone 4G7). Additional antibodies used included CD45RO-FITC, CCR7 conjugated to anti-rat immunoglobuin G2ak (IgG2ak) -FITC, CD62L-APC, CD57-FITC, CD27-FITC and CD28-APC. Phenotypic analysis of CD45RA+ T cells utilized the following panel: (1) CD4-FITC: CD45RO-PE: CD8-PerCP: CD69-APC (Clone L78); (2) CD4-FITC: CD38-PE (clone HB7): CD8-PerCP: CD45RO-APC; (3) CD95-FITC (clone DX2): CD45RO-PE: CD8-PerCP: CD4-APC; (4) CD4-FITC: CD45RO-PE: CD8-PerCP: CD154-APC (clone TRAP1); (5) CD25-FITC (clone 2A3): CD45RO-PE: CD8-PerCP: CD4-APC; (6) CD4-FITC: HLA-DR-PE (clone L243): CD8-PerCP: CD45RO-APC; (7) CD28-FITC (clone LD28.2): CD45RO-PE: CD8-PerCP: CD4-APC; (8) CD40-FITC (clone 5C3): CD45RO-PE: CD8-PerCP: CD4-APC; and (9) IgG isotypes conjugated to FITC, PE, PerCP, or APC (mouse IgG1 clone X40; mouse Ig2a clone X39; rat IgG2a clone R35-95). A total of 5000 gated events were collected for each panel and the analysis was performed on a FACSCalibur flow cytometer using cellquest software (Becton Dickinson, San Jose, CA). All monoclonal antibodies were purchased from Becton Dickinson or PharMingen (San Diego, CA).
Intracellular immunofluorescence staining and flow cytometric analysis for cytokines and cell cycle
Type 1 (IL-2, IFN-γ) and type 2 (IL-4, IL-10) cytokine expression was evaluated by intracellular immunostaining. Briefly, CD45RA+ T cells were treated with brefeldin A for 4 hr then surface stained with CD45RA-FITC, CD4-PerCP and CD45RO-APC. The cells were then permeabilized with FacsPerm, following the manufacturer's instructions (Becton Dickinson, San Jose, CA), and incubated with either anti-IL-2 (clone MQ1-17H12), anti-IL-10 (clone JES3-19F1), anti-IFN-γ (clone 4S.B3) or anti-IL-4 (clone BD4-8) antibody conjugated to PE for 20 min at room temperature in the dark. The cells were then washed and fixed with 2% formaldehyde. Cell proliferation was analysed by dual staining for DNA/RNA, using 7-AAD/pyronin, and by carboxyfluorescein diacetate succinimidyl ester (CFSE) dye tracking, according to standard protocols.
Proteome array analysis of type 1/type 2 cytokines
Type 1 [IL-2, IL-12p70, IL-8, IFN-γ, tumour necrosis factor-α (TNF-α)] and type 2 (IL-4, IL-5, IL-10, IL-13) cytokines were measured using the SearchLight Human Type 1/Type 2 Multiplex Sandwich enzyme-linked immunosorbent assay (ELISA; Pierce Biotechnology, Rockford, IL), according to the manufacturer's instructions.
Co-culture of monocytes and CD45RA+ T cells
Monocytes were isolated from PBMCs by direct positive immunoselection for CD14 (Miltenyi Biotec). CD45RA+ T cells were isolated as described above. Freshly isolated monocytes were cultured in the presence of extensively washed autologous CD4+ CD45RA+ T cells under various experimental conditions, as indicated. Forty-eight hours post-coculture, the expression of CD80, CD86, CD40, lymphocyte function-associated antigen-1 (LFA-1), intracellular adhesion molecule-1 (ICAM-1), and human leucocyte antigen-DR (HLA-DR) on monocytes (CD14+ cells) was evaluated by flow cytometry.
Statistical analysis
Random effects models were used for analysis.33 The random effects in these models were the subjects. The covariates of interest were day and treatment. The cytokine models included three subjects, two days (day 3 and day 6), and three treatments (untreated, IL-2-treated, and IL-7-treated). Cytokine results below the detection limits were imputed to be half the detection limit. IL-4 results were not analysed because most IL-4 results were below the detection limit. Cytokine results were analysed on the log10 scale. The cytokine models presented do not include a day by treatment interaction (day 3 versus day 6) because these interactions were found to be statistically insignificant (results not shown). Statistical analysis of the impact of IL-7 on CD4+ CD45RA+ T-cell phenotype consisted of models that included four subjects, three days (day 0, day 3, and day 6), and two treatments (IL-2-treated and IL-7-treated). Day 0 results were used as a pretreatment control; hence the treatment effect was nested within days 3 and 6. Models were analysed using the percentage as determined by flow cytometry. Models using alternative scales (i.e. arc sine and logit transformations) were considered but found not to yield substantively different results (results not shown). CD154 results were not analysed, because most CD154 results were 0%. Programming was performed in sas (SAS Institute Inc., sas onlinedoc®, Version 8, Cary, NC: SAS Institute Inc., 2000). Paired t-test was used to compare IFN-γ expression of treated CD4+ CD45RA+ T cells versus untreated cells.
Results
Impact of IL-7 on functional and activation markers of CD4+ naïve T cells
To examine the impact of IL-7 on CD4+ CD45RA+ T cells, we enriched for CD4+ CD45RA+ CD45RO− T cells using negative immunoselection to avoid aberrant activation of the cells by depleting for CD45RO (memory), CD8 (cytotoxic T cells and NK cells that express CD8 dimly), CD19 (B cells) and CD14 (monocytes). In some experiments, where indicated, NK cells were also depleted by removing CD16+ CD56+ cells. By gating for CD4+ T cells alone on the enriched CD45RA+ population, we concordantly enriched for naïve T cells, as this population was > 98% positive for CCR7 (Fig. 1a), CD62L (Fig. 1b), and > 99% negative for CD57 (Fig. 1c), which collectively define a naïve population as summarized in Table 1. In subsequent analyses, we gated on CD4+ CD45RA+ T cells to eliminate any minor populations of CM and EM T cells which both lack CD45RA expression (Table 1). TDEM T cells are also CD45RA+ but represent a minor population, ranging in frequency in our CD45RA+ cultures from 1·35% to 0·16%, depending on which marker is used to identify these cells. Specifically, 1·35% of CD4+ CD45RA+ T cells are CD45RO− CCR7− (Fig. 1a), 0·72% are CD45RO− CD62L− (Fig. 1b), and 0·16% are CD45RO− CD57+ (Fig. 1c). We will refer to this enriched and gated population of CD4+ CD45RA+ cells as CD4+ naïve T cells.
Figure 1.
Enriching for CD4+ CD45RA+ CD45RO− T cells and determining the level of TDEM for subsequent functional studies: PBMCs from venous blood of healthy donors were isolated by Ficoll–Hypaque density gradient centrifugation. The cells were depleted of CD45RO, CD8, CD19, CD14, CD16/CD56, where indicated using magnetic bead immunoselection. A gate was set on CD4+ T cells from the enriched population and the expression of CCR7 (a), CD62L (b), and CD57 (c) was determined. Definition of naïve and TDEM cells is based on a number of phenotypic markers as summarized in Table 1.
The enriched cells were then left untreated or treated with IL-2 or IL-7 for 3 or 6 days prior to analysing the expression of cell surface activation (CD69, CD45RO, CD25, CD95, CD38, HLA-DR) and functional (CD28, CD40, and CD40 ligand) markers on CD4+ CD45RA+ T cells. IL-2 treatment of CD45RA+ T cells was included as a positive control, considering that IL-2 is a survival factor for T cells and generally IL-2-treated CD45RA+ T cells survived better than untreated cells in culture for the duration of these experiments. Additional controls for staining on days 3 and 6 consisted of isolating fresh CD45RA+ T cells at those time points and performing parallel immunostains and flow analysis. These cells denote ‘fresh’ samples that were used to account for any day-to-day variability in the flow cytometer, but consistently generated values that were almost identical to the day 0 stains. Day 3 and day 6 data trends were similar and therefore only day 6 data are presented here. A representative histogram of the impact of IL-7 and IL-2 on the expression of each of the activation and functional molecules examined is shown in Fig. 2 and cumulative data from multiple healthy donors are shown in Table 2. IL-7-treatment of CD4+ CD45RA+ T cells up-regulated CD95 and CD25 expression (Figs 2 and Table 2). Specifically, median percent expression of CD95 on IL-7-treated cells was increased by 11- and 2·5-fold in comparison to fresh or IL-2-treated cells, respectively (Fig. 2a; Table 2). Per cent CD25 expression was up-regulated by approximately nine-fold in comparison to either fresh or IL-2-treated cells (Fig. 2b; Table 2). Per cent CD28 expression on fresh CD4+ CD45RA+ T cells was high (97·4%) and it was not altered by IL-7 or IL-2 treatments (Table 2). CD69 expression was not up-regulated on IL-7-treated cells at either day 3 (data not shown) or day 6 post-treatment and was low (0·78%) on IL-2-treated cells (Table 2). Given that CD69 is an early activation marker, we also examined its expression in response to a 2-hour treatment with IL-7 or IL-2 and it was still not up-regulated (data not shown). The per cent of CD38+ cells was not augmented by IL-7 treatment, but the mean fluorescent intensity (MFI) of its expression was elevated in response to IL-7. IL-2, on the other hand, reduced CD38 expression by approximately 1·2-fold in comparison to either fresh or IL-7-treated CD4+ CD45RA+ T cells (Fig. 2c; Table 2). Per cent HLA-DR expression was up-regulated from a median expression of approximately 2% on fresh or IL-2-treated cells to 4·5% on IL-7-treated cells, which was statistically significant (Fig. 2d; Table 2). Modulation of CD40 expression on IL-7-treated cells was not statistically significant (Table 2). CD40 ligand (CD154) was not detected on CD4+ CD45RA+ T cells and its expression was not altered in response to either IL-7 or IL-2 treatment (Table 2). Finally, CD45RO expression on IL-7-treated CD4+ CD45RA+ T cells was not augmented (Table 2). Collectively, these data indicate that IL-7 induced the up-regulation of the activation molecules CD25, CD95, HLA-DR and CD38 but had no effect on the early activation marker CD69, the late activation marker CD45RO, or the functional markers, CD28, CD40 and CD40 ligand on CD4+ CD45RA+ T cells.
Figure 2.
Impact of IL-7 on activation and functional markers on CD4+ CD45RA+ T cells. Histograms of the expression of CD95 (a), CD25 (b), CD38 (c), and HLA-DR (d)from fresh or 6-day IL-7- or IL-2-treated CD45RA+ CD45RO− T cells are shown from one representative donor. Similar data were observed after 3-day treatments. Grey line corresponds to IL-7 treatment (also denoted by an arrow), black line corresponds to IL-2 treatment, dotted line corresponds to day 0 (freshly isolated) cells, and the thin grey line corresponds to the isotype control.
Table 2. Per cent expressionofphenotypic markers: median (25th, 75th percentile).
| Fresh | IL-7 | IL-2 | |
|---|---|---|---|
| CD95 | 6·86 (6·73, 7·26) | 75·02 (53·13, 81·07) | 31·80 (21·70, 38·29) |
| CD25 | 3·21 (2·77,4·12) | 27·7 (23·61,53·40) | 2·62 (2·36, 2·89) |
| CD28 | 97·44 (95·62, 98·00) | 94·1 (90·87, 95·25) | 91·56 (89·18, 91·72) |
| CD69 | 0 (0, 0) | 0 (0, 0·11) | 0·78 (0·44, 1·07) |
| CD38 | 96·91 (93·69, 97·85) | 94·22 (86·77, 97·01) | 72·9 (71·13, 78·59) |
| HLA-DR | 1·91 (1·53, 2·70) | 4·45 (4·14, 5·37) | 1·80 (1·56, 1·91) |
| CD40 | 2·37 (1·91, 3·23) | 6·83 (5·39,8·47) | 3·59 (3·33, 3·85) |
| CD154 | 0 (0, 0) | 0·06 (0, 0·07) | 0 (0, 0·02) |
| CD45RO | 3·44 (3·05, 3·85) | 3·46 (2·48, 3·82) | 2·22 (1·89, 2·60) |
Impact of IL-7 on type 1/type 2 cytokine expression of CD4+ CD45RA+ T cells
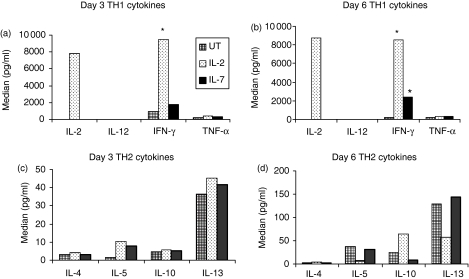
Given that IL-7 altered the phenotype of CD4+ CD45RA+ T cells, we evaluated the impact of IL-7 on the functional potential of CD4+ CD45RA+ T cells by examining the effect of IL-7 on CD4+ CD45RA+ T-cell cytokine profile. CD4+ CD45RA+ T cells were isolated, as previously described. The cells were treated with either IL-7, IL-2, or left untreated for 3 and 6 days. Cytokine production of IL-2, IL-12p70 chain, IFN-γ, TNF-α, IL-4, IL-5, IL-10, IL-13 and IL-8 was evaluated by a type 1/type 2 proteome array. Levels of IL-12p70 chain were below the detection limit of the assay, regardless of cytokine treatment (Fig. 3a,b). IL-8 levels did not change with cytokine treatments (data not shown). By day 6, in comparison to untreated CD45RA+ T cells, IL-7 increased IFN-γ production by approximately 12-fold and 42-fold in response to IL-2 treatment (Fig. 3a,b). However, it did not affect IL-2 levels (Fig. 3a,b). The high value (> 7000 pg/ml) of IL-2 in the IL-2-treated culture is the result of the IL-2 added to the culture (100 U/ml or approximately 7·7 ng/ml). IL-12 remained < 10 pg/ml regardless of IL-2 or IL-7 treatment. TNF-α was secreted in similar amounts by both the IL-7-treated and the IL-2-treated cultures (Fig. 3a,b). IL-7 treatment did not significantly augment type 2 cytokine (IL-4, IL-5, IL-10, IL-13) expression. Interestingly, untreated cultures after incubation for 6 days expressed detectable amount of IL-5, IL-10 and IL-13 but still much less than IFN-γ levels (Fig. 3).
Figure 3.
Impact of IL-7 on cytokine expression of CD45RA+ T cells. CD45RA+ T cells were isolated as described and left untreated or treated with IL-2 or IL-7 for either three (a,c) or 6 days (b,d). Supernatant from the cells was analysed for type 1 (IL-2, IL-12, IFN-γ, TNF-α) (a,b) or type 2 (IL-4, IL-5, IL-10, IL-13) (c,d) cytokine expression using the SearchLight Type 1/Type 2 Proteomic Quantitative Assay (Pierce Biotech, Rockford, IL). Asterisks denote significant values based on random effects models (P < 0·1). Data are representative of the mean concentration (pg/ml) of three different healthy donors. Type 2 cytokine data were not significant between the various treatment conditions and over time.
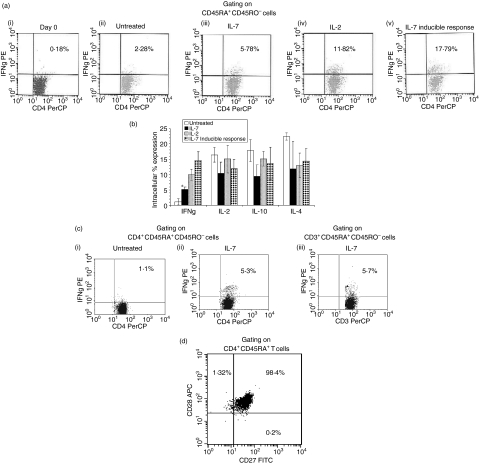
Given that the cytokine proteome assay evaluated bulk cytokine production and that minor contamination of NK cells could account for the elevation in IFN-γ production, we verified the IL-7-mediated IFN-γ response by either including (Figs 4a,b) or depleting (Fig. 4c) NK cells in our immunoselection approach, and re-measuring intracellular IFN-γ by gating on CD4+ CD45RA+ CD45RO− (Figs 4a,b) or on CD3+ CD45RA+ CD45RO− cells [Fig. 4c(iii)], which excludes NK cells from the intracellular cytokine analysis. Both analyses confirmed that IL-7 induces IFN-γ production in naïve T cells, independent of NK cells. Specifically, purified naïve T cells, without NK depletion, were treated with either IL-7, IL-2, or were left untreated then immunostained for intracellular IL-2, IL-4, IL-10, or IFN-γ and surface stained for CD4, CD45RO, and CD45RA on days 0 and 6. These analyses were performed by strictly gating on the CD45RA+ CD45RO− population (Figs 4a and 4b). On day 0, 0·18% of the CD45RO− cells expressed IFN-γ[Fig. 4a(i)] while 6 days of culture (untreated cultures) led to 2·3% of the cells expressing IFN-γ[Fig. 4a(ii)]. Per cent expression of IFN-γ from IL-7-treated cultures was 5·8% by day 6 [Fig. 4a(iii)], which was significant but less than the amount of IFN-γ induced by IL-2 treatment [Fig. 4a(iv)]. To examine the inducible cytokine potential of IL-7-treated cells, 5 days post-treatment with IL-7, the cells were costimulated for 24 hr with anti-CD3/CD28, which resulted in a greater level of IFN-γ expression [18%; Fig. 4a(v)]. IL-7 had no effect on the other cytokines evaluated, as shown in Fig. 4(b). Because IL-7 can augment NK effector function34 we repeated those experiments with starting cultures of naïve T cells that were depleted of NK cells. Despite NK depletion, still 5% of IL-7-treated CD4+ CD45RA+ CD45RO− T cells expressed IFN-γ[Fig. 4c(ii)]. Similar values were obtained by gating on CD3+ CD45RA+ CD45RO− T cells [Fig. 4c(iii)]. Although the predominant cells in the CD4+ CD45RA+ gate are naïve, a small percentage is representative of TDEM cells. This population, depending on which marker one uses to identify them, is in the range of 1·35 to 0·16% (Fig. 1). CD27 and CD28 are two additional markers whose lack of expression, along with CD45RA expression, has also been used to identify TDEM cells (Table 1). We show that our population is negative for CD45RA+ CD27− CD28− cells (Fig. 4b). Approximately 1·32% of the cells were CD45RA+ CD28+ CD27− cells, which are probably naïve cells that have been primed. Collectively, these intracellular data confirm the ELISA data in verifying that IL-7-treatment of CD4+ naïve T cells induces them to express IFN-γ.
Figure 4.
Impact of IL-7 on intracellular cytokine expression of CD4+ CD45RA+ T cells. (a)CD45RA+ T cells were isolated as described in the Materials and methods section but without NK depletion. The cells were stained at day 0 (i), left untreated for 6 days (ii), treated with IL-7 for 6 days (iii), or treated with IL-2 for 6 days (iv). Expression of IFN-γ was evaluated by intracellular immunostaining, where the gate was set on CD4+ CD45RA+ CD45RO− cells for flow cytometric analysis. IL-7-mediated inducible cytokine expression was evaluated by treating the cells with IL-7 for 5 days then stimulating with anti-CD3/CD28 for 24 hr prior to staining for IFN-γ (v). Representative dot plots from one donor are shown in (a)i–v and in (b)the cumulative per cent expression of IFN-γ, IL-2, IL-10 and IL-4 are shown from untreated, IL-7, IL-2, and IL-7-inducible response from at least three donors. Data in (b) represent mean intracellular cytokine per cent expression ± SD. Asterisk denotes significance in comparison to untreated cultures as evaluated by the paired t-test (P > 0·05). (c)CD45RA+ T cells were isolated with NK depletion and intracellular IFN-γ production from IL-7-treated or untreated cultures were determined 6 days post-treatment. Dot blots are representative of intracellular IFN-γ expression from two donors where (i) and (ii) represent untreated and IL-7-treated cultures, respectively, as evaluated by gating on CD4+ CD45RA+ CD45RO− cells, and (iii) represents IL-7-treated cultures by gating on CD3+ CD45RA+ CD45RO− cells. Similar data were obtained with six different donors, where the naïve T cells were isolated without NK depletion. (d) CD45RA+ T cells were immunostained for CD4, CD45RA, CD27 and CD28. A gate was set on the CD4+ CD45RA+ T cells to determine per cent expression of CD27 and CD28 on these cells at day zero.
Impact of IL-7 on cell cycle progression of CD4+ CD45RA+ T cells
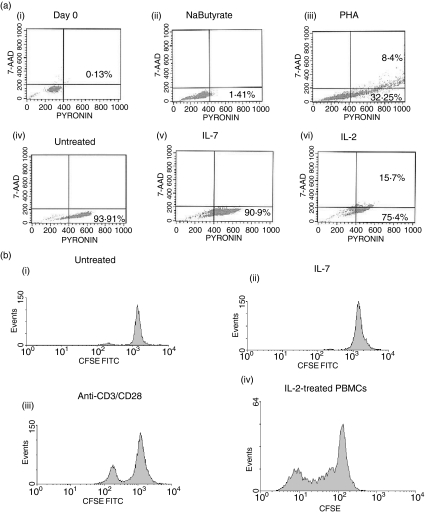
To evaluate the impact of IL-7 on cell cycle progression of CD4+ CD45RA+ T cells, CD45RA+ T cells were purified and left without treatment or treated with IL-7 or IL-2 for 6 days prior to staining with 7-AAD and pyronin to examine the DNA and RNA content, respectively. A positive control for cell cycle progression [phytohaemagglutinin (PHA)-treated CD45RA+ T cells] and a negative control [sodium butyrate (2·5 mm)-treated CD45RA+ T cells], were included in the analysis. PHA induced cell cycle progression, with approximately 8% of the cells entering the S/G2M phase of the cell cycle after 24 hr [Fig. 5a(iii)] while sodium butyrate, an inhibitor of cell cycle progression beyond G1a, successfully blocked progression to G1b [Fig. 5a(ii)]. At day 0, no cell cycling was detected in fresh CD4+ CD45RA+ T cells [Fig. 5a(i)]. Culturing the CD45RA+ T cells without any treatment for 6 days induced 93% of CD4+ CD45RA+ T cells to cycle to G1b [Fig. 5a(iv)]. IL-7 treatment was similar to untreated cultures; both allowed cell cycle progression to the G1b phase of the cell cycle but not beyond this checkpoint [Fig. 5a(iv,v)]. IL-2 treatment for the same duration of time induced approximately 16% of the population to progress to S/G2/M phase of the cell cycle; this subpopulation may be reflective of the IL-2-responsive population within the CD4+ CD45RA+ T cells [Fig. 5a(vi)]. The inability of IL-7 to mediate the proliferation of CD4+ naïve T cells was confirmed by CFSE tracking. Naïve CD4+ T cells were enriched, labeled with CFSE, and treated with either IL-7 or anti-CD3/CD28 or left untreated. A culture of IL-2-treated PBMCs was used as a positive control for the CFSE assay. Six days post-treatment, dilution of CFSE was determined by flow cytometry. IL-7 did not induce cell proliferation while anti-CD3/CD28 induced modest cell division, most probably because these cells are enriched naïve T cells that may require another stimulatory signal for proliferation [Fig. 5b(ii,iii)]. The CFSE-positive control clearly indicated that the assay was working as demonstrated by detectable multiple rounds of cell division in the IL-2-treated PBMC cultures [Fig. 5b(iv)]. These data indicate that IL-7 does not induce active cell proliferation of CD4+ CD45RA+ T cells.
Figure 5.
Impact of IL-7 on CD4+ CD45RA+ T-cell cycle progression. (a)Freshly isolated CD45RA+ T cells (i), sodium butyrate-treated cells (ii), PHA-treated cells (iii), untreated cells (iv), IL-7-treated cells (v), or IL-2-treated cells (vi) were evaluated for cell cycle progression using intracellular staining for 7-ADD and pyronin to evaluate DNA : RNA content. All treatments were for 6 days, except for PHA which was based on 24-hr stimulation. Dot plots shown are representative of three donors. (b)CD45RA+ T cells were stained with CFSE, washed extensively then left untreated (i) or treated with IL-7 (ii) or anti-CD3/CD28 (iii). IL-2-treated PBMCs were CFSE stained as a positive control for the assay (iv). At day 6, CFSE dilution was evaluated by flow cytometry.
Impact of IL-7 on the functional potential of CD4+ CD45RA+ T cells
To examine the functional relevance of IL-7-mediated priming of CD4+ CD45RA+ T cells, we evaluated the ability of IL-7-treated CD4+ CD45RA+ T cells to activate monocytes by augmenting the expression of CD80, CD86, CD40, LFA-1, ICAM-1 and HLA-DR on monocytes. Monocytes were isolated by positive immunoselection using CD14 mAbs conjugated to magnetic microbeads and cultured with CD45RA+ CD45RO− that have been depleted of NK cells. Evaluation of the various markers on CD14+ cells was conducted 48 hr post-coculture. The cultures consisted of (1) monocytes and freshly isolated naïve T cells (denoted as M + F RA+), (2) monocytes and 6-day IL-7-treated naïve T cells that have been extensively washed to remove IL-7 prior to coculturing with the monocytes (denoted as M + IL-7 RA+), (3) monocytes and 6-day untreated naïve cells that contain minimal level of IL-2 (20 U/ml) to maintain the survival of the T cells (denoted as M + UT RA+), and (4) monocytes with media alone (denoted as M + media). IL-7 treatment of naïve T cells mediated the up-regulation of both CD80 and CD86 expression on monocytes (Fig. 6a, b). Specifically, the mean MFI of CD80 and CD86 were elevated by 4·6- and 2-fold, respectively, over CD14+ cells in media alone. Representative histograms for these two markers on monocytes are depicted in Fig. 6(b). CD40 and ICAM-1 expression were not altered under any of the conditions examined while LFA-1 and HLA-DR were up-regulated on monocytes cocultured with untreated or IL-7-treated naïve T cells, indicating that their up-regulation was not IL-7-specific (Fig. 6c). This analysis was performed on six additional donors without the removal of NK cells, yielding similar trends for all markers evaluated (data not shown). These data indicate that IL-7 induces the priming of CD4+ naïve T cells, which induces their ability to augment the function of monocytes.
Figure 6.
Impact of IL-7 treatment of CD4+ CD45RA+ T cells on monocytes. (a)Monocytes were isolated by direct positive immunoselection using CD14 antibodies. Isolated monocytes were cocultured with NK-depleted CD45RA+ T cells under different experimental conditions: fresh CD45RA+ cells (denoted as M + F RA+), CD45RA+ T cells that were treated with IL-7 for 6 days, washed extensively to remove IL-7 (denoted as M + IL-7 RA+), untreated CD45RA+ T cells that were also cultured for 6 days with minimal IL-2 at 20 U/ml (denoted as M + UT RA+), or in media alone (denoted as M + media). Expression of CD80, CD86, CD40, LFA-1, ICAM-1 and HLA-DR on monocytes was evaluated 48 hr after coculturing with the various CD45RA+ T cells. Data presented are mean fold increase of the respective markers on CD14+ cells over monocytes cultured with media alone from two donors (a,c). Six additional donors, where NK cells were not depleted, generated similar data. Representative histograms of CD80 and CD86 on monocytes are shown in (b), with the arrows pointing to monocytes cocultured with day-6 IL-7-treated naïve T cells. The data was reproducible between all individuals but was not statistically significant.
Discussion
Prior to the use of IL-7 in the clinical setting for immune reconstitution, whether to enhance the survival, the proliferation, or de novo synthesis of T cells, the impact of IL-7 on the various T-cell compartments needs to be fully understood. In studies evaluating the susceptibility of naïve CD4+ T cells to productive HIV infection, we demonstrated that IL-7 treatment induced potent replication in this otherwise non-permissive CD4+ naïve T-cell compartment.19 Towards understanding the mechanism of this induction, we evaluated the impact of IL-7 on the biology of these CD4+ naïve T cells.
While a number of studies have evaluated the effect of IL-7 on resting adult and cord blood naïve T cells,35–40 on thymocytes in the whole-lobe human fetal thymic organ model,41 or in conjunction with either suboptimal TCR activation or mitogenic stimulation,42,43 these studies have had either discordant findings or interpreted any IL-7-mediated effects on the phenotype of resting cells as minor. In this study, we specifically addressed the impact of IL-7 on adult naïve CD4+ T cells, without any form of TCR or mitogenic stimulation, not only in relation to changes in the phenotype profile of these cells but more importantly in relation to the functional potential/consequences of IL-7-treatment.
At the core of these studies is the proper classification of cell surface markers that identify naïve T cells. A growing body of evidence indicates that at least four separate populations exist for CD4+ and CD8+ T cells, defined as naïve, effector memory, central memory, and terminally differentiated effector memory T cells (TDEM). Most of these studies are based on observations for CD8+ T cells. Based on the work of Sallusto et al.26 it appears that CD4+ T cells also undergo these differentiation stages. They, however, may be functionally distinct or their differentiation pathway may also be distinct. TDEM cells that are CD45RA+ are more prevalent within the CD8+ T-cell compartment than the CD4+ T-cell compartment.26 Nonetheless, less than 1% of CD4+ CD45RA+ T cells re-express CD45RA on their surface. Because some may have interpreted our naïve cells as being TDEM cells, even if this is largely based on data from the CD8+ T-cell compartment, it was critical for us to prove that those cells are indeed naïve and not TDEM T cells, because both of these populations express CD45RA.
In assessing the impact of IL-7 on naïve T cells, we always depleted for CD8+ T cells, B cells and monocyte/macrophages, and CD45RO. In some experiments, where indicated, NK cells were also depleted. Most importantly, all subsequent analysis involved gating on CD4+ or CD3+ T cells. These CD4+ CD45RA+ T cells are predominantly naïve but may have contained a minor population indicative of TDEM cells. The exact per cent of TDEM cells within the CD4+ CD45RA+ population is difficult to decipher, given that different markers have been used in the literature to discriminate between these CD45RA+ naïve and TDEM populations and none, thus far, have evaluated the collective expression of these various markers on one cell and in relation to the functional definition of TDEM cells. We believe that the observations we have reported here are reflective of the naïve T-cell population and not the TDEM cells for a number of reasons. (1) CD57 expression is believed to more accurately identify TDEM T cells, as evaluated by shorter telomere lengths indicating replicative senescence;44,45 its expression is ≤ 1% of CD4+ T cells and was even lower (0·16%) after enriching for CD45RA+ T cells by depleting for CD45RO. (2) The IFN-γ-producing CD45RA+ T cells express both CD27 and CD28, reflective of a naïve population. (3) IL-7 did not induce this CD45RA+ population to proliferate, which is consistent with previous reports indicating that while IL-7 can induce EM and to a lesser extent CM cells to proliferate, it has no effect on the proliferation of naïve T cells.30 Collectively, these points argue in favour of the population studied being naïve T cells.
The impact of IL-7 on the phenotype of naïve T cells is controversial. Some studies have reported that IL-7 does not alter the phenotype of naïve lymphocytes and has no effect on activation markers.39,46–48 Others have observed that IL-7 induces the up-regulation of certain activation markers (CD25/CD95) but because these changes were not accompanied by conversion of the cells from CD45RA+ to CD45RO+ phenotype, these changes were classified as minimal.40,41 In our study, we included three separate controls (untreated, IL-2-treated, and fresh) with the realization that each has its own limitations. Leaving the cells without treatment for the same duration as IL-7 is not ideal because those cells are not healthy without a growth factor, even though we consistently gated out dead cells. Treating the cells with IL-2 is also not the best control because only a small proportion of naïve T cells are CD25+ and therefore responsive to IL-2. Finally, some may view using fresh cells as not ideal because they have not undergone the same side-by-side comparison of treatment/culturing conditions. Because of all these caveats, we included all three controls but prefer to compare the IL-7 arm to fresh or untreated and view IL-2 effects as providing a notion of how these two cytokines may have differential effects but we do not consider IL-2 treatment as a ‘measuring stick’ of ‘control’ responses. Our data indicate that IL-7 does induce the activation of naïve T cells. This IL-7-mediated priming of CD4+ naïve T cells is sufficient to lead to the induction of cytokine expression, which is a hallmark of naïve T-cell activation, and leads to sufficient interaction with monocytes. These functional consequences mediated by IL-7 were independent of antigen/TCR stimulation. Specifically, IL-7-mediated the priming of CD4+ naïve T cells by up-regulating a number of molecules associated with T-cell activation, such as CD25 (IL-2 receptor α chain), CD95 (Fas receptor), HLA-DR (MHC-II), and CD38 (activation marker). Up-regulation of CD95 (Fas receptor), in particular, suggests that not only the cells are primed but that a mechanism to regulate the immune response may have been initiated. Interaction of CD95 with its ligand ultimately leads to activation-induced cell death. Up-regulation of CD95 alone, nonetheless, is not an adequate predictor of how sensitive a cell will be to Fas-mediated apoptosis. Memory T cells are resistant to Fas-mediated apoptosis even after Fas engagement but naïve T cells are more sensitive to activation-induced cell death.49–52 IL-7 in association with IL-2 primes naïve T cells to Fas-mediated apoptosis.37 This finding is interesting given that IL-7 induced the up-regulation of CD25 on these naïve T cells, suggesting that they would be more responsive to IL-2 signalling. Taken together with the CD95 up-regulation in response to IL-7 treatment this suggests that IL-7 may establish a pathway by regulating the immune response in the absence of TCR engagement, making these cells more responsive to activation-induced cell death.
IL-7-mediated phenotypic alteration of naïve CD4+ T cells are associated with functional consequences, as demonstrated by the induction of IFN-γ production and the ability of IL-7-treated CD4+ CD45RA+ T cells to activate monocytes in a coculture system. Specifically, IL-7 treatment of CD4+ CD45RA+ T cells induced the secretion of considerable levels of IFN-γ, as was demonstrated by ELISA and confirmed by intracellular staining. Given that CD57 may be the best single marker reflective of TDEM cells, it is unlikely that the 5·8% of the IFN-γ-producing CD45RA+ population is from the TDEM cells, because these cells are present at a frequency of 0·16% at the initiation of the culture and they would have to proliferate at least 50-fold during the 6-day treatment to account for the measured level of IFN-γ. Additionally, extensive proliferation in response to IL-7 did not occur as measured by DNA/RNA and CFSE staining. These two points make it unlikely that the source of IFN-γ production from the CD4+ CD45RA+ T cells is the CD45RA+ TDΕΜ cells but rather that it is from the CD45RA+ naïve T cells. Given that NK cells have been shown to be responsive to IL-734 and that they may indirectly contribute to IFN-γ production by naïve T cells, we removed NK cells from the cultures and were still able to detect IFN-γ production in naïve T cells in response to IL-7 treatment. These data suggest that this effect is independent of NK involvement. CD86 activation on monocytes is reported to be mediated by IFN-γ production,53 which is consistent with IFN-γ production by IL-7 treatment of CD4+ CD45RA+ T cells. To our knowledge this is the first report demonstrating the ability of IL-7 to induce naïve T cells to spontaneously express IFN-γ independent of IL-7 plus phorbol 12-myristate 13-acetate/ionomycin stimulation.38 Production of IFN-γ from IL-7-treated naïve CD4+ T cells, without up-regulation of CD45RO, also suggests that the function of these naïve CD4+ T cells has been modified by IL-7, independent of antigen stimulation. A precedent for this finding has been reported for CD8+ T cells. Murali-Krishna and Ahmed demonstrated that homeostatic proliferation of naïve CD8+ T cells under lymphopenic conditions resulted in the priming of CD8+ naïve T cells, as was defined by up-regulation of activation markers and IFN-γ production, without changing their CD45RA/RO phenotype and independent of antigen stimulation.54 This response was eloquently described as CD8+ naïve T cells ‘masquerading’ as memory T cells.54 Using this definition, IL-7-treated naïve CD4+ T cells, in our system, are primed T cells that are masquerading as naïve T cells defined by CD45RA/RO expression.
Reports in support of the ability of IL-7 to induce naïve T-cell proliferation are based on the murine model and are associated with lymphopenic conditions.12,14,55 Under non-lymphopenic conditions, IL-7 does not induce the proliferation of mouse naïve T cells.56In vitro, human IL-7 does not induce adult naïve T cells to proliferate35,36 but is a potent inducer of cord blood naïve T-cell proliferation.36 Given that cord blood T cells are predominantly antigen-inexperienced/recent thymic emigrants whereas adult T cells are mostly memory/primed T cells, this suggests that IL-7 has different effects on T cells at different stages of CD4+ T-cell maturation.36 We previously reported that 4% of CD4+ naïve T cells entered the cell cycle, as measured by Ki67 staining. Ki67, however, does not discriminate between the various phases of the cell cycle and is only a marker of cells entering the cell cycle but not necessarily dividing. Using a more sensitive approach in these studies, 7AAD/pyronin and CFSE immunostaining, confirms the findings that IL-7 does not induce human adult CD4+ naïve T-cell proliferation.35,36 However, culturing CD4+ naïve T cells, independent of cytokine treatment, induced the cells to enter the G1b phase of the cell cycle. This was not apparent in a previous study, which used propidium iodide staining, which does not discriminate between G1b and S/G2/M. It is important to note that the phenotypic and functional data reported here are based on a single dose of IL-7 that was optimum in inducing HIV replication of naïve T cells. Various IL-7 doses may have differential effects on the phenotype and functional potential of naïve T cells.
Our collective findings that the effect of IL-7 on CD4+ CD45RA+ T cells resulted in the up-regulation of some activation markers, production of IFN-γ, and modulation of monocytes may explain several findings regarding the impact of IL-7 on the biology of T cells in a number of models. IL-7 ‘sensitizes’ CD4+ T cells, allowing them to respond to less antigen than that required to stimulate unprimed/naïve cells.57 IL-7 pretreatment induces the potent productive replication of HIV in CD4+ CD45RA+ T cells which are classically non-permissive to productive HIV replication19 and IL-7 pretreatment of CD4+ CD45RA+ T cells significantly enhances their transduction efficiency.38 These effects maybe attributed to the ability of IL-7 to prime CD4+ CD45RA+ T cells without antigenic stimulation. If IL-7-treated cells encounter a second signal, from TCR stimulation or from another lymphokine, these cells may respond more quickly to the stimulation. IL-7 therefore may have a potential use as an adjuvant in vaccine preparations. Conversely, IL-7 has been reported to expand autoreactive T cells, which can lead to autoimmunity.15 The significance of IL-7-mediated antigen/TCR-independent priming of CD4+ CD45RA+ T cells, whether advantageous or disadvantageous, remains to be elucidated.
Unlike previous reports suggesting that such IL-7-mediated phenotypic changes are minor because they do not lead to CD45RO up-regulation,39–41,52 we provide evidence to indicate that these changes are significant and can have functional consequences. IL-7-mediated alteration in naïve T cells can effect their helper-like interaction with other cell types. On the other hand, IL-7-primed naïve T cells, without proper TCR stimulation, may lower the threshold for an immune response with negative consequences, such as the break down in tolerance and induction of autoimmune disease. For these reasons, it is critical that the impact of IL-7 on the biology of T cells be fully understood prior to in vivo IL-7 treatments in the clinical setting.
Acknowledgments
We thank Drs Wright (Westat, Rockville, MD) and Wenbo (USC, Los Angeles, CA) for statistical analyses and Drs Claire Chougnet (Children's Hospital Medical Center, Molecular Immunology, Cincinnati, OH), Mario Roederer, Daniel Douek, and Michael Betts (Vaccine Research Center, NIAID, NIH) for helpful discussions. This work is in partial fulfillment for the Ph.D. degree for EZM from Rush University Medical Center. The work is supported by the Campbell Foundation, the University Committee on Research (UCR), and POI AI055356-01.
References
- 1.Melchionda F, Fry TJ, Mackall CL. Harnessing the immune modulatory effects of IL7 for immunotherapy. Clin Appl Immunol Rev. 2003;4:71–89. 10.1016/S1529-1049(03)00046-1. [Google Scholar]
- 2.Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood. 2002;99:3892–904. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
- 3.Fry TJ, Mackall CL. Interleukin-7 and immunorestoration in HIV. Beyond the thymus. J Hematother Stem Cell Res. 2002;11:803–07. doi: 10.1089/152581602760404603. 10.1089/152581602760404603. [DOI] [PubMed] [Google Scholar]
- 4.Napolitano LA. Approaches to immune reconstitution in HIV infection. Top HIV Med. 2003;11:160–63. [PubMed] [Google Scholar]
- 5.Freitas AA, Rocha BB. Lymphocyte lifespans: homeostasis, selection and competition. Immunol Today. 1993;14:25–29. doi: 10.1016/0167-5699(93)90320-K. 10.1016/0167-5699(93)90320-K. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto Y, Douek DC, McFarland RD, Koup RA. Effects of exogenous interleukin-7 on human thymus function. Blood. 2002;99:2851–58. doi: 10.1182/blood.v99.8.2851. [DOI] [PubMed] [Google Scholar]
- 7.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman IL. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 1997;89:1033–41. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 8.Di Santo JP, Aifantis I, Rosmaraki E, et al. The common cytokine receptor gamma chain and the pre-T cell receptor provide independent but critically overlapping signals in early alpha/beta T cell development. J Exp Med. 1999;189:563–74. doi: 10.1084/jem.189.3.563. 10.1084/jem.189.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pallard C, Stegmann AP, van Kleffens T, Smart F, Venkitaraman A, Spits H. Distinct roles of the phosphatidylinositol 3-kinase and STAT5 pathways in IL-7-mediated development of human thymocyte precursors. Immunity. 1999;10:525–35. doi: 10.1016/s1074-7613(00)80052-7. 10.1016/S1074-7613(00)80052-7. [DOI] [PubMed] [Google Scholar]
- 10.Maraskovsky E, O'Reilly LA, Teepe M, Corcoran LM, Peschon JJ, Strasser A. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1–/– mice. Cell. 1997;89:1011–19. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- 11.Napolitano LA, Grant RM, Deeks SG, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:73–79. doi: 10.1038/83381. 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 12.Alpdogan O, Schmaltz C, Muriglan SJ, et al. Administration of interleukin-7 after allogeneic bone marrow transplantation improves immune reconstitution without aggravating graft-versus-host disease. Blood. 2001;98:2256–65. doi: 10.1182/blood.v98.7.2256. [DOI] [PubMed] [Google Scholar]
- 13.Fry TJ, Connick E, Falloon J, et al. A potential role for interleukin-7 in T-cell homeostasis. Blood. 2001;97:2983–90. doi: 10.1182/blood.v97.10.2983. [DOI] [PubMed] [Google Scholar]
- 14.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 15.Bielekova B, Muraro PA, Golestaneh L, Pascal J, McFarland HF, Martin R. Preferential expansion of autoreactive T lymphocytes from the memory T-cell pool by IL-7. J Neuroimmunol. 1999;100:115–23. doi: 10.1016/s0165-5728(99)00200-3. [DOI] [PubMed] [Google Scholar]
- 16.Bhandoola A, Tai X, Eckhaus M, et al. Peripheral expression of self-MHC-II influences the reactivity and self-tolerance of mature CD4 (+) T cells: evidence from a lymphopenic T cell model. Immunity. 2002;17:425–36. doi: 10.1016/s1074-7613(02)00417-x. [DOI] [PubMed] [Google Scholar]
- 17.Sinha ML, Fry TJ, Fowler DH, Miller G, Mackall CL. Interleukin 7 worsens graft-versus-host disease. Blood. 2002;100:2642–49. doi: 10.1182/blood-2002-04-1082. [DOI] [PubMed] [Google Scholar]
- 18.Smithgall MD, Wong JG, Critchett KE, Haffar OK. IL-7 up-regulates HIV-1 replication in naturally infected peripheral blood mononuclear cells. J Immunol. 1996;156:2324–30. [PubMed] [Google Scholar]
- 19.Steffens CM, Managlia EZ, Landay A, Al-Harthi L. Interleukin-7-treated naive T cells can be productively infected by T-cell-adapted and primary isolates of human immunodeficiency virus 1. Blood. 2002;99:3310–18. doi: 10.1182/blood.v99.9.3310. [DOI] [PubMed] [Google Scholar]
- 20.Korin YD, Zack JA. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J Virol. 1998;72:3161–68. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaleco S, Kinet S, Hassan J, Dardalhon V, Swainson L, Reen D, Taylor N. IL-7 and CD4+ T-cell proliferation. Blood. 2002;100:4676–78. doi: 10.1182/blood-2002-06-1760. [DOI] [PubMed] [Google Scholar]
- 22.Al-Harthi L. IL-7 modulation of HIV-1 infection of naive T cells. Blood. 2002;100:4677–78. [Google Scholar]
- 23.Eckstein DA, Penn ML, Korin YD, et al. HIV-1 actively replicates in naive CD4(+) T cells residing within human lymphoid tissues. Immunity. 2001;15:671–82. doi: 10.1016/s1074-7613(01)00217-5. 10.1016/S1074-7613(01)00217-5. [DOI] [PubMed] [Google Scholar]
- 24.Brooks DG, Hamer DH, Arlen PA, Gao L, Bristol G, Kitchen CM, Berger EA, Zack JA. Molecular characterization, reactivation, and depletion of latent HIV. Immunity. 2003;19:413–23. doi: 10.1016/s1074-7613(03)00236-x. [DOI] [PubMed] [Google Scholar]
- 25.Michie CA, McLean A, Alcock C, Beverley PC. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360:264–65. doi: 10.1038/360264a0. 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 26.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 27.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–34. doi: 10.1038/ni889. 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 28.Appay V, Dunbar PR, Callan M, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–85. doi: 10.1038/nm0402-379. 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 29.Champagne P, Ogg GS, King AS, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–11. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 30.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194:1711–19. doi: 10.1084/jem.194.12.1711. 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–45. doi: 10.1128/JVI.78.11.5535-5545.2004. 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maus MV, Kovacs B, Kwok WW, et al. Extensive replicative capacity of human central memory T cells. J Immunol. 2004;172:6675–83. doi: 10.4049/jimmunol.172.11.6675. [DOI] [PubMed] [Google Scholar]
- 33.Crowder M. Analysis of Repeated Measures. New York: Chapman & Hall; 1990. [Google Scholar]
- 34.Lum JJ, Schnepple DJ, Nie Z, et al. Differential effects of interleukin-7 and interleukin-15 on NK cell anti-human immunodeficiency virus activity. J Virol. 2004;78:6033–42. doi: 10.1128/JVI.78.11.6033-6042.2004. 10.1128/JVI.78.11.6033-6042.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassan J, Reen DJ. Human recent thymic emigrants – identification, expansion, and survival characteristics. J Immunol. 2001;167:1970–76. doi: 10.4049/jimmunol.167.4.1970. [DOI] [PubMed] [Google Scholar]
- 36.Dardalhon V, Jaleco S, Kinet S, et al. IL-7 differentially regulates cell cycle progression and HIV-1-based vector infection in neonatal and adult CD4+ T cells. Proc Natl Acad Sci USA. 2001;98:9277–82. doi: 10.1073/pnas.161272698. 10.1073/pnas.161272698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaleco S, Swainson L, Dardalhon V, Burjanadze M, Kinet S, Taylor N. Homeostasis of naive and memory CD4+ T cells. IL-2 and IL-7 differentially regulate the balance between proliferation and Fas-mediated apoptosis. J Immunol. 2003;171:61–68. doi: 10.4049/jimmunol.171.1.61. [DOI] [PubMed] [Google Scholar]
- 38.Verhoeyen E, Dardalhon V, Ducrey-Rundquist O, Trono D, Taylor N, Cosset FL. IL-7 surface-engineered lentiviral vectors promote survival and efficient gene transfer in resting primary T lymphocytes. Blood. 2003;101:2167–74. doi: 10.1182/blood-2002-07-2224. [DOI] [PubMed] [Google Scholar]
- 39.Soares MV, Borthwick NJ, Maini MK, Janossy G, Salmon M, Akbar AN. IL-7-dependent extrathymic expansion of CD45RA+ T cells enables preservation of a naive repertoire. J Immunol. 1998;161:5909–17. [PubMed] [Google Scholar]
- 40.Cavalieri S, Cazzaniga S, Geuna M, Magnani Z, Bordignon C, Naldini L, Bonini C. Human T lymphocytes transduced by lentiviral vectors in the absence of TCR activation maintain an intact immune competence. Blood. 2003;102:497–505. doi: 10.1182/blood-2003-01-0297. [DOI] [PubMed] [Google Scholar]
- 41.Scripture-Adams DD, Brooks DG, Korin YD, Zack JA. Interleukin-7 induces expression of latent human immunodeficiency virus type 1 with minimal effects on T-cell phenotype. J Virol. 2002;76:13077–82. doi: 10.1128/JVI.76.24.13077-13082.2002. 10.1128/JVI.76.24.13077-13082.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armitage RJ, Namen AE, Sassenfeld HM, Grabstein KH. Regulation of human T cell proliferation by IL-7. J Immunol. 1990;144:938–41. [PubMed] [Google Scholar]
- 43.Morrissey PJ, Goodwin RG, Nordan RP, et al. Recombinant interleukin 7, pre-B cell growth factor, has costimulatory activity on purified mature T cells. J Exp Med. 1989;169:707–16. doi: 10.1084/jem.169.3.707. 10.1084/jem.169.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28− CD8+ T cells imply a replicative history that is distinct from their CD28+ CD8+ counterparts. J Immunol. 1996;156:3587–90. [PubMed] [Google Scholar]
- 45.Batliwalla F, Monteiro J, Serrano D, Gregersen PK, Ostrer H. Oligoclonality of CD8+ T cells in health and disease: aging, infection, or immune regulation? Hum Immunol. 1996;48:68–76. doi: 10.1016/0198-8859(96)00077-8. [DOI] [PubMed] [Google Scholar]
- 46.Dardalhon V, Jaleco S, Rebouissou C, et al. Highly efficient gene transfer in naive human T cells with a murine leukemia virus-based vector. Blood. 2000;96:885–93. [PubMed] [Google Scholar]
- 47.Hassan J, Reen DJ. IL-7 promotes the survival and maturation but not differentiation of human post-thymic CD4+ T cells. Eur J Immunol. 1998;28:3057–65. doi: 10.1002/(SICI)1521-4141(199810)28:10<3057::AID-IMMU3057>3.0.CO;2-Z. 10.1002/(SICI)1521-4141(199810)28:10<3057::AID-IMMU3057>3.3.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 48.Unutmaz D, Kewa I, Ramani VN, Marmon S, Littman DR. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J Exp Med. 1999;189:1735–46. doi: 10.1084/jem.189.11.1735. 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Desbarats J, Wade T, Wade WF, Newell MK. Dichotomy between naive and memory CD4(+) T cell responses to Fas engagement. Proc Natl Acad Sci USA. 1999;96:8104–09. doi: 10.1073/pnas.96.14.8104. 10.1073/pnas.96.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inaba M, Kurasawa K, Mamura M, Kumano K, Saito Y, Iwamoto I. Primed T cells are more resistant to Fas-mediated activation-induced cell death than naive T cells. J Immunol. 1999;163:1315–20. [PubMed] [Google Scholar]
- 51.Kishimoto H, Sprent J. Strong TCR ligation without costimulation causes rapid onset of Fas-dependent apoptosis of naive murine CD4+ T cells. J Immunol. 1999;163:1817–26. [PubMed] [Google Scholar]
- 52.Webb LM, Foxwell BM, Feldmann M. Putative role for interleukin-7 in the maintenance of the recirculating naive CD4+ T-cell pool. Immunology. 1999;98:400–05. doi: 10.1046/j.1365-2567.1999.00906.x. 10.1046/j.1365-2567.1999.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Creery WD, Diaz-Mitoma F, Filion L, Kumar A. Differential modulation of B7-1 and B7-2 isoform expression on human monocytes by cytokines which influence the development of T helper cell phenotype. Eur J Immunol. 1996;26:1273–77. doi: 10.1002/eji.1830260614. [DOI] [PubMed] [Google Scholar]
- 54.Murali-Krishna K, Ahmed R. Cutting edge: naive T cells masquerading as memory cells. J Immunol. 2000;165:1733–37. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 55.Fry TJ, Christensen BL, Komschlies KL, Gress RE, Mackall CL. Interleukin-7 restores immunity in athymic T-cell-depleted hosts. Blood. 2001;97:1525–33. doi: 10.1182/blood.v97.6.1525. [DOI] [PubMed] [Google Scholar]
- 56.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–76. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- 57.Vakkila J, Aysto S, Saarinen-Pihkala UM, Sariola H. Naive CD4+ T cells can be sensitized with IL-7. Scand J Immunol. 2001;54:501–05. doi: 10.1046/j.1365-3083.2001.01001.x. 10.1046/j.1365-3083.2001.01001.x. [DOI] [PubMed] [Google Scholar]