Abstract
We report the effects of hemicellulase-treated Agaricus blazei (ABH) on the maturation of bone-marrow-derived dendritic cells (BMDCs). ABH activated immature BMDCs, inducing up-regulation of surface molecules, such as CD40, CD80 and major histocompatibility complex class I antigens, as well as inducing allogeneic T-cell proliferation and T helper type 1 cell development. However, unlike lipopolysaccharide (LPS), ABH did not stimulate the BMDCs to produce proinflammatory cytokines, such as interleukin-12 (IL-12) p40, tumour necrosis factor-α, or IL-1β. In addition, ABH suppressed LPS-induced DC responses. Pretreatment of DCs with ABH markedly reduced the levels of LPS-induced cytokine secretion, while only slightly decreasing up-regulation of the surface molecules involved in maturation. ABH also had a significant impact on peptidoglycan-induced or CpG oligodeoxynucleotide-induced IL-12p40 production in DCs. The inhibition of LPS-induced responses was not associated with a cytotoxic effect of ABH nor with an anti-inflammatory effect of IL-10. However, ABH decreased NF-κB-induced reporter gene expression in LPS-stimulated J774.1 cells. Interestingly, DCs preincubated with ABH and then stimulated with LPS augmented T helper type 1 responses in culture with allogeneic T cells as compared to LPS-stimulated but non-ABH-pretreated DCs. These observations suggest that ABH regulates DC-mediated responses.
Keywords: dendritic cells, cytokines/interleukins, co-stimulation/costimulatory molecules, fungi
Introduction
Agaricus blazei Murill is a mushroom native to Brazil that is consumed as food for its nutritional and medicinal properties.1, 2 There have been a number of studies regarding its antitumour, 3–10 antimutagenic, 11–15 antimicrobial16 and immunomodulatory17–20 activities. It has been suggested that this basidiomycete might affect the function of some immunocompetent cells21–23 but little is known about its effects on dendritic cells (DCs). DCs play a critical role in innate and adaptive immunity. They have the ability to recognize a variety of microbial pathogens through a limited repertoire of surface molecules, such as Toll-like receptors (TLRs), as the first line of defence24 and are also the most potent antigen-presenting cells known to prime naïve T cells.25 Exposure to pathogens, cytokine stimulation [e.g. tumour necrosis factor (TNF-α) or interleukin-1β (IL-1β)], and CD40–CD40 ligand interactions activate DCs.26, 27 The activation of DCs is termed ‘maturation’, in which DCs undergo morphological and functional changes. Mature DCs have high levels of surface expression of costimulatory molecules and major histocompatibility complex (MHC) antigens for efficient T-cell activation.28 In addition, they are able to release large amounts of inflammatory mediators, such as TNF-α or IL-12, which are involved in subsequent immune responses.29, 30 IL-12 is a T helper type 1 (Th1)-promoting cytokine and stimulates cell-mediated immunity;31 TNF-α plays a pivotal role in host defence.32, 33 However, these molecules are also likely to be associated with the pathogenesis of autoimmune diseases.34–37
The innate immune system responds to the constituents of microbial pathogens (cell wall components and nucleic acids, etc.) as ‘danger signals’. Recent studies have demonstrated that it also recognizes fungal components (reviewed in ref. 38). Although opportunistic fungal infection often becomes a serious problem in compromised hosts, few fungi are professional pathogens. This raises the question of whether non-pathogenic fungi, including edible mushrooms, also serve as ‘danger signals’ in healthy individuals and trigger unnecessary responses by the host. Therefore, we investigated the effects of A. blazei on DC maturation. It has been difficult to abstract an essence from A. blazei because of its low aqueous solubility. Therefore, the A. blazei used here was digested with hemicellulase to achieve solubility in water. Hemicellulase-treated A. blazei (ABH) activated DCs, promoting their maturation, but the activation was quite different from bacterially induced DC maturation. ABH did not stimulate DCs to produce proinflammatory cytokines. Furthermore, ABH inhibited mature DC-mediated responses; it strongly suppressed the production of cytokines, particularly IL-12p40, and slightly down-regulated the expression of costimulatory molecules and MHC antigens in lipopolysaccharide (LPS)-treated DCs. However, ABH-pretreated DCs enhanced Th1 responses in cultures with allogeneic T cells. Our results indicate that ABH has both stimulatory and inhibitory effects on DC maturation. It activates DCs but might inhibit unnecessary or excessive responses of DCs to ABH itself.
Materials and methods
Mice
BALB/c and C57BL/6 mice were purchased from Charles River Japan, Inc. (Yokohama, Japan). C3H/HeN and C3H/HeJ mice were purchased from Japan SLC, Inc. (Hamamatsu, Japan). Experiments were carried out in accordance with the Guide for Animal Experimentation, University of Yamanashi.
Hemicellulase-treated Agaricus blazei
Hemicellulase-treated A. blazei (AB fraction H, ABH) is the source material of the commercial product ‘Agaricus blazei practical compound (ABPC®)’ supplied by Japan Applied Microbiology Research Institute Ltd. (Yamanashi, Japan). Mycelia of A. blazei Murill were digested with 0·1% hemicellulase for 1 hr. After heat-inactivation of the enzyme, the filtrate of the degradation product was lyophilized. ABH comprises 63·3% carbohydrates, 30·9% proteins, 0·3% lipids and other minor components. The freeze-dried samples were dissolved in distilled water at a concentration of 10% (w/v), and diluted with culture medium at final concentrations of 0·001–0·1%. The samples were examined for endotoxin by a Limulus amoebocyte lysate assay (Seikagaku Corp., Tokyo, Japan). The endotoxin contents of samples used here were less than 0·02 EU per 1 mg of sample.
Reagents
The following reagents were purchased from Sigma-Aldrich (St Louis, MO): LPS (from Escherichia coli O111:B4), peptidoglycan (PGN, from Staphylococcus aureus), 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT), polymyxin B and α-methyl d-mannoside. Fluorescein-conjugated ovalbumin (OVA) was obtained from Molecular Probes, Inc. (Eugene, OR). Curdlan, a linear (1→3)-β-d glucan, was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). CpG DNA was synthesized by Sigma Genosys (Ishikari, Japan). The DNA sequence was 5′-TGACTGTGAACGTTCGAGATGA-3′.39
Bone marrow-derived dendritic cells
Bone marrow-derived dendritic cells (BMDCs) were generated as described previously.40 Briefly, BM cells were obtained from the tibiae and femurs of mice and seeded into bacteriological Petri dishes (InaOptica Co., Ltd, Osaka, Japan) at 2 × 106 cells/dish in 10 ml RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Logan, UT), 2 mm l-glutamine, 50 μm 2-mercaptoethanol, and 20 ng/ml murine granulocyte–macrophage colony-stimulating factor (GM-CSF; PeproTech EC Ltd, London, UK). On day 3, another 10 ml culture medium containing 20 ng/ml GM-CSF was added to each dish. On day 6, half of the culture was collected and centrifuged. The cell pellet was resuspended in 10 ml fresh culture medium containing 20 ng/ml GM-CSF and returned to the original dishes. On day 9, non-adherent cells were harvested and cultured in the presence or absence of various doses of test substances. After 24 hr, non-adherent cells were used for fluorescence-activated cell sorter (FACS) analyses, T-cell activation, MTT assay and RNA isolation as described below. Supernatants from cultures with 4 × 105 DCs in 24-well plates (BD Labware, Franklin Lakes, NJ) were collected for cytokine and prostaglandin E2 (PGE2) measurements. In one experiment, DCs were preincubated with 100 μg/ml anti-CD11b (Clone M1/70, Chemicon International, Temecula, CA) or 100 mmα-methyl d-mannoside.
FACS analyses
BMDCs (5 × 105) were washed and resuspended in phosphate-buffered saline. After blocking Fc receptors on the cell surface with Fc Block™ (PharMingen, San Diego, CA), the cells were stained for 30 min on ice with saturating amounts of antibodies. As a control, isotype-matched antibodis with irrelevant specificity were used. Cells were washed twice and analysed using FACSCalibur™ and CELLQuest™ software (BD Biosciences, San Jose, CA). The following antibodies were used in this study (all purchased from PharMingen): Fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated anti-CD11c (clone HL3), anti-CD40 (clone 3/23), anti-CD80 (clone 16-10A1), anti-CD86 (clone GL1), anti-H-2Kd (clone SF1-1.1), and anti-I-A/I-E (clone M5/114.15.2). For the uptake of OVA, BMDCs (5 × 105) were pulsed with fluorescein-conjugated OVA (100 μg/ml) for 1 hr at either 4° or 37°. Uptake was stopped by washing the cells with ice-cold PBS, followed by staining with PE-conjugated anti-CD11c on ice. The fluorescent signals of DCs were measured by FACS. The value remaining after deducting the mean fluorescence at 4° from that at 37° was divided by the mean fluorescence at 4°. This calculated value was defined as the uptake index.
Allogeneic T-cell activation
Spleen cells were obtained from C57BL/6 mice and incubated with the following antibodies (all purchased from PharMingen): anti-Ly-6G (clone RB6-8C5), anti-Ly-76 (clone TER-119), anti-B220 (clone RA3-6B2), and anti-I-A/I-E (clone M5/114.15.2). After washing twice, the cells were incubated with bead-coupled secondary antibody (Dynal, Oslo, Norway). CD3+ T cells (H-2b) were enriched from these cells by magnetic negative depletion according to the manufacturer's instructions (> 90% CD3+ T cells). Triplicate cultures of 2 × 105 enriched T cells were cultured in round-bottomed 96-well plates (BD Labware) with titrated numbers of BMDCs (H-2d), which had been cultivated under various conditions for 24 hr, washed and irradiated (15 Gy). After a 3-day incubation, the cultures were pulsed with 1 μCi/well [3H]thymidine (Amersham Biosciences, Piscataway, NJ) for the final 16 hr. Incorporation of radioactivity was determined using a microplate scintillation counter (Packard, Meriden, CT). For measurement of cytokines, 4 × 106 enriched T cells were seeded into 24-well plates with 4 × 105 BMDCs. After 3 days, the supernatants were harvested and used for enzyme-linked immunosorbent assay (ELISA).
Determination of cytokines and PGE2
Culture supernatants were examined by ELISA for IL-12p40, TNF-α, IL-1β (BD Biosciences), interferon-γ (IFN-γ), IL-4, IL-10 (BioSource International, Inc., Camarillo, CA), and PGE2 (R & D Systems Inc., Minneapolis, MN). The limits of detection were 10, 5, 10, 1, 5, 13 and 8 pg/ml, respectively. Assays were performed according to the manufacturers' instructions.
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from DCs with TRIzol® Reagent (Invitrogen Corp., Carlsbad, CA) according to the manufacturer's instructions. Aliquots of 1 μg total RNA were reverse transcribed into cDNA, and the RT-generated DNA was amplified by PCR for IL-12p40 and β-actin using a ThermoScript™ RT-PCR System (Invitrogen Corp.). Reverse transcription-untreated RNA samples were also used to detect samples laced with genomic DNA. The primers used were as follows: IL-12p40 sense, 5′-CCCATTCCTACTTCTCCCTCAA-3′ and antisense, 5′-GAGGAACGCACCTTTCTGGTTA-3′ (designed using Primer Express™ software from Applied Biosystems); β-actin sense, 5′-ACCCACACTGTGCCCATCTA-3′ and antisense, 5′-CGGAACCGCTCATTGCC-3′.41 The expected sizes of PCR products were 100 base pairs (bp) for IL-12p40 and 289 bp for β-actin. Thermal cycling profiles consisted of preincubation at 94° for 2 min followed by 25 cycles (IL-12p40) or 20 cycles (β-actin) of 94° for 1 min, 50° for 1 min, and 72° for 1 min. The PCR products were electrophoresed in 1·5% agarose gels and visualized by ethidium bromide staining.
NF-κB reporter assay
Murine macrophage J774.1 cells were supplied by the Cell Resource Center for Biochemical Research, Institute of Development, Aging and Cancer, Tohoku University (Sendai, Japan). The cells were seeded into 100-mm dishes at 2 × 106 cells/dish 2 days before transfection. The cells were then transfected with a plasmid containing four copies of the NF-κB consensus sequence fused to a TATA-like promoter and secreted alkaline phosphatase (SEAP) gene, pNFκB-SEAP (BD Biosciences) in the presence of PolyFect® transfection reagent (QIAGEN GmbH, Hilden, Germany). After 8 hr, the cells were harvested, washed once, and cultured with or without ABH and LPS in 24-well plates at 5 × 105 cells/well for 12 hr. The culture supernatants were then heat-inactivated and SEAP levels were examined using a chemiluminescent detection kit (Roche Diagnostics GmbH, Mannheim, Germany). The light signals were measured using a Luminescencer JNR (ATTO Corp., Tokyo, Japan).
Cell viability
DCs (2 × 105/ml) or J774.1 cells (5 × 105/ml) were cultured in 96-well plates in a final volume of 200 μl. After overnight culture, 20 μl of MTT (5 mg/ml) was added, followed by a further 4-hr incubation. After removing 100 μl of the supernatant from each well, the formazan formed in the cells was dissolved by addition of 100 μl of acidic isopropanol to each well. The optical density was measured at 570 nm.
Statistical analyses
Statistical analyses of data were performed by analysis of variance (anova) followed by Newman–Keuls test.
Results
ABH induced up-regulation of surface molecules on BMDCs
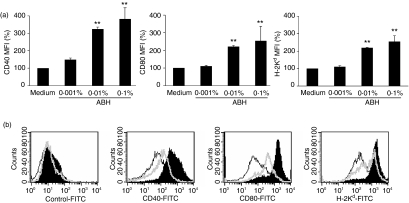
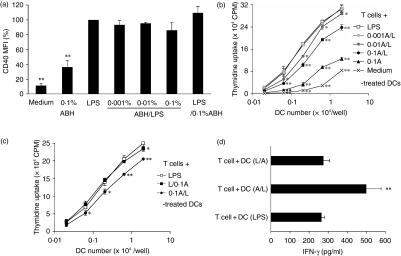
DCs used in this study were generated by culturing bone marrow precursors in the presence of GM-CSF for 10 days. Non-adherent cells were harvested from the cultures and stained with antibodies against the surface molecules involved in the maturation of DCs and with the appropriate isotype controls. FACS analysis showed that > 80% of these cells were CD11c+. LPS or TNF-α induced up-regulation of costimulatory molecules (CD40, CD80 and CD86) and MHC antigens (H-2Kd and I-Ad/I-Ed) on the cells, that is, generated fully mature DCs (data not shown). We used BMDCs generated in vitro to investigate the effects of ABH on DC maturation. The maturation of DCs was characterized by up-regulation of costimulatory molecules and MHC antigens, cytokine production, and loss of the capability to capture antigens. Therefore, instead of LPS and TNF-α, ABH was added to the cultures at final concentrations of 0·001–0·1% for the last 24 hr. The mean fluorescence intensities (MFIs) of CD40, CD80 and H-2Kd staining on CD11c+ DCs were increased in an ABH dose-dependent manner (Fig. 1a). When cultured in the presence of 0·1% ABH (optimal stimulation), they increased by 3·8-, 2·5- and 2·6-fold, respectively, as compared to medium-only controls. However, they were lower than those of DCs with optimal LPS stimulation (1 μg/ml). The MFIs of these molecules on LPS-treated DCs were 17·7-, 13·3- and 6·5-fold, respectively, of medium-only control values (Fig. 1b). Inactivated hemicellulase without A. blazei had little influence on CD40 expression of DCs at concentrations of 0·001–0·1% (0·9–1·3-fold that of medium-only control).
Figure 1.
Effects of ABH on surface molecule expression on DCs. (a) BMDCs were prepared from BALB/c mice and treated with or without 0·001–0·1% ABH for the last 24 hr. FACS analyses determined the expression of CD40, CD80 and H-2Kd on CD11c+ DCs. Data show MFIs of surface molecule staining relative to medium-only controls (set as 100%). Data are means ± SD of five separate experiments. MFI values of CD40, CD80 and H-2Kd on untreated DCs were 65, 249 and 395, respectively. **P < 0·01, compared with Medium (untreated DCs). (b) BMDCs were untreated (open histogram, black line), or were treated with 1 μg/ml LPS (closed histogram), or 0·1% ABH (open histogram, grey line). Data are representative of five separate experiments.
ABH-mediated DC maturation is not the result of endotoxin contamination of ABH preparation
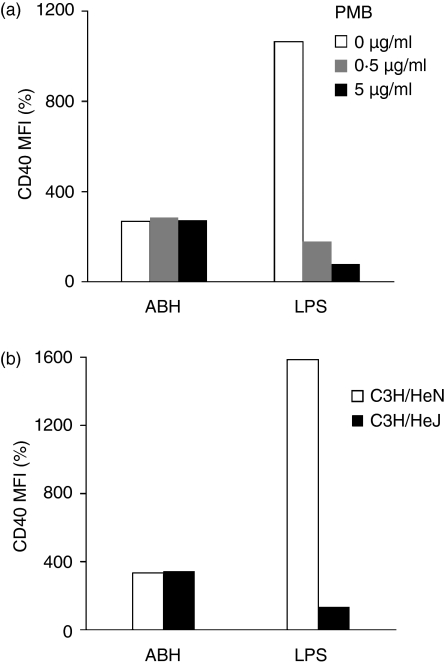
The maximum endotoxin levels of cultures containing ABH were less than 0·02 EU/ml (equivalent to 8 pg/ml LPS) in the present study. To eliminate the possibility that the up-regulation of surface molecules on ABH-treated DCs might have been caused by endotoxin contamination, test substances were treated with polymyxin B, an inhibitor of LPS, 1 hr before addition to DC cultures (Fig. 2a). Pretreatment of LPS (30 ng/ml) with polymyxin B (0·5 μg/ml) significantly decreased LPS-induced up-regulation of CD40, as a representative marker for DC maturation, on DCs (1·8-fold that of medium only control), and LPS (30 ng/ml) treated previously with polymyxin B (5 μg/ml) induced no maturation of DCs. In contrast, polymyxin B did not inhibit 0·1% ABH-mediated up-regulation of CD40 (2·7-, 2·9- and 2·7-fold at polymyxin B concentrations of 0, 0·5 and 5 μg/ml, respectively). Polymyxin B had no effect on DC maturation by itself, because CD40 expression on untreated or TNF-α-treated DCs remained the same regardless of polymyxin B treatment (data not shown). Furthermore, BMDCs generated from C3H/HeN and C3H/HeJ mice, which are sensitive and hyposensitive to LPS, respectively, were cultivated with LPS or ABH for 24 hr, and the expression of CD40 on the DCs was compared (Fig. 2b). DCs from C3H/HeN were fully matured by LPS stimulation (3 ng/ml), whereas those from C3H/HeJ showed reduced responsiveness to the same LPS stimulation. However, there was no difference in the up-regulation of CD40 between ABH-treated DCs generated from C3H/HeN and C3H/HeJ (3·3- and 3·4-fold, respectively, as compared to controls).
Figure 2.
(a) Pretreatment of ABH with polymyxin B. ABH (1%) and LPS (30 ng/ml) were pretreated with or without polymyxin B (0·5 and 5 μg/ml) for 1 hr. They were then added to the DC cultures and diluted 1 : 10. After 24 hr, FACS analyses determined the expression of CD40 on the DCs. Data show MFI of CD40 staining relative to medium-only control (set as 100%). (b) Response of DCs from LPS-hyposensitive mice to ABH. BMDCs were generated from C3H/HeN and C3H/HeJ mice, and were incubated with or without 0·1% ABH or 3 ng/ml LPS for 24 hr. FACS analyses determined the expression of CD40 on these DCs. Data show MFI of CD40 staining relative to medium-only controls (set as 100%). MFI values of CD40 staining on untreated DCs from C3H/HeN and C3H/HeJ were 82 and 77, respectively.
ABH-treated DCs enhanced allogeneic T-cell proliferation and Th1-cell development
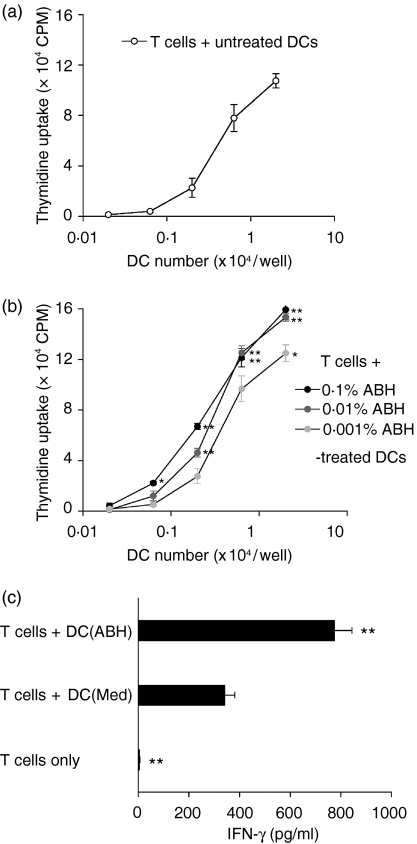
DCs that had been treated with 0·001–0·1% ABH for 24 hr and washed were cultured with allogeneic T cells for a further 3 days, and T-cell proliferation and cytokine levels in the culture supernatants were measured. DCs that had received no treatment were also cultured with allogeneic T cells. T cells showed no activation without DCs. Consistent with the up-regulation of surface molecules on ABH-treated DCs, the DCs activated allogeneic T cells in an ABH dose-dependent manner (Fig. 3a,b). In addition, culture of T cells with 0·1% ABH-treated DCs increased IFN-γ (Fig. 3c) but not IL-4 (data not shown) release as compared with the culture of T cells with untreated DCs. ABH did not stimulate IFN-γ production in DC-only cultures (data not shown).
Figure 3.
Allogeneic T-cell activation by ABH-treated DCs. (a, b) BMDCs were incubated with or without 0·001–0·1% ABH for 24 hr. DCs were harvested, washed and irradiated. Purified allogeneic T cells were cultured with titrated numbers of untreated (a) or ABH-treated (b) DCs for 3 days. The cultures were pulsed with [3H]thymidine for the final 16 hr, and incorporation of radioactivity was determined. Data are the mean c.p.m. ± SD of triplicate cultures.(a) and (b) show the results of an experiment carried out at the same time. Similar results were obtained in three separate experiments. *P < 0·05 and **P < 0·01 compared with T cells + untreated DCs.(c) IFN-γ release of T-cell cultures with DCs. Purified allogeneic T cells were cultured alone (T cells only) or with untreated DCs [T + DC(Med)] or 0·1% ABH-treated DCs [T + DC(ABH)] for 3 days. Culture supernatants were examined for cytokine levels by ELISA. Data are means ± SD of three separate experiments. **P < 0·01, compared with T + DC(Med).
ABH-treated DCs decreased antigen uptake
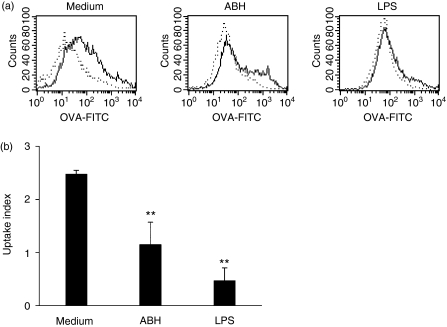
DCs lose the capability to capture antigens with maturity. To further confirm maturation of ABH-treated DCs, they were incubated with FITC-OVA at either 4° or 37° for 1 hr (Fig. 4). DCs that had received no treatment showed uptake of OVA at 37°, while those treated with 1 μg/ml LPS showed little uptake of OVA at 37°. DCs treated with 0·1% ABH also showed considerably decreased uptake of OVA, but a subpopulation still possessed a high capacity to capture antigen.
Figure 4.
Antigen uptake of ABH-treated DCs. (a) BMDCs were incubated with or without 0·1% ABH or 1 μg/ml LPS for 24 hr. These DCs were then incubated with FITC-OVA. FITC-OVA uptake was assayed at 37° (solid line), and background staining at 4° served as a reference (dotted line) Data are representative of three separate experiments. (b) Uptake index was defined as described in the Materials and methods section. Data are means ± SD of three separate experiments. **P < 0·01, compared with Medium (untreated DCs).
ABH did not stimulate DCs to produce proinflammatory cytokines
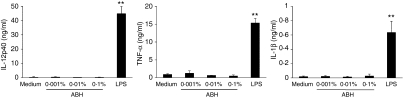
After incubation of DCs with LPS or ABH for 24 hr, the culture supernatants were examined for cytokine levels. Curiously, although LPS stimulation (1 μg/ml) markedly increased the release of IL-12p40, TNF-α and IL-1β, ABH had no effect, even at a concentration of 0·1%(Fig. 5). IL-10, a potent inhibitor of these cytokines, was also undetectable in DC cultures treated with 0·001–0·1% ABH (data not shown).
Figure 5.
Cytokine release of ABH-treated DCs. BMDCs were incubated with or without 0·001–0·1% ABH or 1 μg/ml LPS for 24 hr. Culture supernatants were examined for IL-12p40, TNF-α, or IL-1β levels by ELISA. Data are means ± SD of three to five separate experiments. **P < 0·01, compared with Medium (untreated DCs).
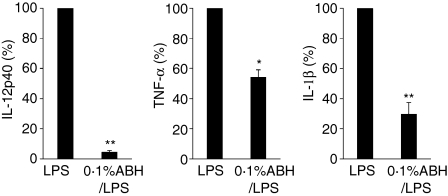
ABH-pretreated DCs decreased LPS-induced cytokine secretion
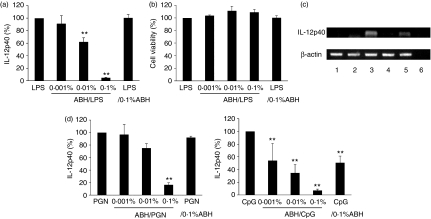
To examine whether ABH has inhibitory effects on DC maturation, DCs were cultured with a combination of LPS and ABH. To clarify the effects of ABH, DCs were stimulated with a low dose of LPS (1 ng/ml). DCs were pretreated with or without 0·1% ABH for 1 hr and then stimulated with 1 ng/ml LPS for a further 24 hr. Culture supernatants were examined for cytokine levels. As the cytokine levels varied from experiment to experiment, cytokine levels in LPS-stimulated DC cultures that had not received ABH pretreatment were set as 100%. As shown in Fig. 6, pretreatment of DCs with 0·1% ABH reduced LPS-induced IL-12p40, TNF-α and IL-1β secretion to 4·7, 54·3 and 29·9%, respectively. In contrast to the case of ABH, curdlan, a linear, (1→3)-β-d-glucan, did not show inhibition of LPS-induced IL-12p40 release (data not shown). As ABH had a much greater impact on LPS-induced IL-12p40 production, further examinations were carried out with regard to IL-12p40 inhibition. DCs were pretreated with 0·001–0·1% ABH for 1 hr, followed by the addition of 1 ng/ml LPS (abbreviated to ABH/LPS). Pretreatment of DCs with ABH inhibited LPS-induced IL-12p40 production in an ABH dose-dependent manner (Fig. 7a). However, this inhibition was not the result of a cytotoxic effect of ABH, as ABH showed no impairment of cell viability under the same conditions (Fig. 7b). IL-10 was undetectable from cultures with ABH/LPS-treated DCs (data not shown). Inactivated hemicellulase without A. blazei also had no effect on LPS-induced IL-12p40 release (data not shown). On the other hand, DCs that had been stimulated with 1 ng/ml LPS for 1 hr followed by the addition of 0·1% ABH (abbreviated to LPS/ABH) released IL-12p40, as observed with control DCs. The effects of ABH/LPS and LPS/ABH on IL-12p40 release were also confirmed at the transcriptional level (Fig. 7c). ABH/LPS but not LPS/ABH inhibited IL-12p40 mRNA expression. This inhibition was not specific for LPS-mediated responses as it was also observed in PGN- or CpG DNA-induced IL-12p40 release (Fig. 7d).
Figure 6.
Inhibitory effects of ABH on LPS-induced cytokine release. BMDCs, which had received 0·1% ABH treatment for 1 hr, were stimulated with 1 ng/ml LPS for 24 hr (0·1% ABH/LPS). Culture supernatants were examined for IL-12p40, TNF-α, or IL-1β levels by ELISA. Cytokine levels in LPS-stimulated DC cultures (LPS) served as controls (set as 100%). Data are means ± SD of five separate experiments. Supernatants of LPS-treated DCs contained 15·0 ± 10·0 ng/ml IL-12p40, 4·4 ± 2·2 ng/ml TNF-α, and 68·0 ±10·4 pg/ml IL-1β (mean ± SD). *P < 0·05, **P < 0·01 compared with LPS (LPS-treated DCs).
Figure 7.
Inhibition of IL-12p40 release by ABH pretreatment of DCs. BMDCs were pretreated with or without 0·001–0·1% ABH for 1 hr, and then stimulated with 1 ng/ml LPS for 24 hr (0·001–0·1% ABH/LPS), or were preincubated with 1 ng/ml LPS for 1 hr, and then treated with 0·1% ABH for 24 hr (LPS/0·1% ABH). (a) Culture supernatants were examined for IL-12p40 levels. IL-12p40 levels in LPS-stimulated DC cultures (LPS) served as controls (set as 100%). Data are means ± SD of five separate experiments. **P < 0·01, compared with LPS (LPS-treated DCs). (b) Cell viability was assayed by MTT assay and is expressed as a percentage of the OD value obtained from LPS-treated DC cultures. Data are means ± SD of three separate experiments. (c) DCs were lysed, and total RNA was prepared for RT-PCR analysis. PCR amplification of the housekeeping gene, β-actin, was performed on each sample. Lane 1, no treatment; lane 2, 0·1% ABH treatment; lane 3, 1 ng/ml LPS treatment; lane 4, ABH/LPS treatment; lane 5, LPS/ABH treatment; lane 6, negative control. (d) Instead of LPS, DCs were stimulated with 0·3 μg/ml PGN or 10 μg/ml CpG DNA. Culture supernatants were examined for IL-12p40 levels. IL-12p40 levels in PGN- or CpG DNA-stimulated DC cultures (PGN or CpG) served as controls (set as 100%). Data are means ± SD of three to five separate experiments. Supernatants of DCs treated with PGN or CpG DNA contained 6·0 ± 2·6 or 4·6 ± 0·8 ng/ml IL-12p40 (mean ± SD), respectively. **P < 0·01, compared with PGN (PGN-treated DCs) or CpG (CpG DNA-treated DCs).
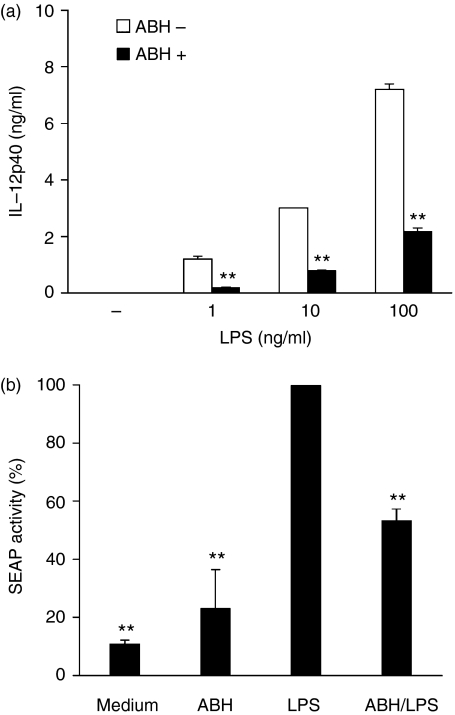
ABH-pretreated macrophages reduced LPS-induced NF-κB activity
Next, we investigated NF-κB activity in ABH-treated J774.1 cells. As DCs were seriously damaged during transfection with a plasmid containing the NF-κB-driven SEAP reporter gene, we used a murine macrophage cell line instead of DCs. As was the case with DCs, LPS induced IL-12p40 production in J774.1 cells. In addition, ABH inhibited LPS-induced IL-12p40 production (Fig. 8a), but did not impair the viability of J774.1 cells (data not shown). Consequently, the cells were treated with or without ABH, LPS, or ABH/LPS after transfection, and SEAP levels in the culture supernatants were determined (Fig. 8b). SEAP activity in LPS-stimulated cell culture was set at 100%. ABH-treated cells expressed SEAP at higher levels than untreated cells (23·2% versus 11·0%, respectively). In contrast, ABH/LPS-treated cells showed a reduction of SEAP expression to 53·4% of that in control cultures.
Figure 8.
Effects of ABH on IL-12p40 secretion and NF-κB activity in a macrophage cell line. (a) J774.1 cells were pretreated with or without 0·1% ABH for 1 hr, and then stimulated with the indicated concentrations of LPS for 24 hr. IL-12p40 levels in culture supernatants were then determined. Data are means ± SD of two separate experiments. **P < 0·01, compared with non-ABH-pretreated cells (ABH-) at each concentration of LPS. (b) J774.1 cells were transfected with a plasmid containing the NF-κB-driven SEAP reporter gene. The cells were pretreated with or without 0·1% ABH for 1 hr, and then stimulated with or without 100 ng/ml LPS for 12 hr. SEAP levels in culture supernatants were then determined. SEAP activity in LPS-stimulated cell culture was set as 100%. Data are means ± SD of three separate experiments. The light signal was 5427 ± 918 relative light units (mean ± SD) in LPS-stimulated cell culture supernatants. **P < 0·01, compared with LPS (LPS-treated J774.1 cells).
ABH-pretreated DCs slightly inhibited LPS-induced up-regulation of surface molecules and also suppressed allogeneic T-cell proliferation but not Th1-cell development
To further examine the inhibitory effects of ABH on DC maturation, the expression of surface molecules was investigated on DCs cultivated with ABH and LPS for 24 hr. As shown in Fig. 9(a), CD40 expression on ABH/LPS-treated DCs was down-regulated at ABH concentrations of 0·001–0·1%. However, ABH had a very slight impact on the expression of surface molecules as compared to IL-12p40 production, as 0·1% ABH/LPS-treated DCs showed decreases in CD40, CD80, CD86, H-2Kd and I-A/I-E expression to only 86, 89, 85, 96 and 96%, respectively, of those of LPS-treated DCs. ABH/LPS-treated DCs expressed these molecules at far higher levels than untreated DCs or those treated with 0·1% ABH. On the other hand, LPS/ABH-treated DCs expressed CD40 similarly to those treated with LPS. ABH/LPS-treated DCs suppressed allogeneic T-cell proliferation, and LPS/ABH showed little impairment of this proliferation (Fig. 9b,c). However, it was interesting to note that culture of T cells with ABH/LPS-treated DCs released larger amounts of IFN-γ than culture with LPS- or LPS/ABH-treated DCs (Fig. 9d). ABH/LPS did not stimulate IFN-γ production in DC-only cultures (data not shown).
Figure 9.
Inhibition of CD40 up-regulation and allogeneic T-cell activation by ABH pretreatment. BMDCs were incubated as described in Fig. 7. (a) FACS analyses determined the expression of CD40 on DCs. Data show MFI of CD40 staining relative to LPS-stimulated DCs (set as 100%). Data are means ± SD of three separate experiments. **P < 0·01, compared with LPS (LPS-treated DCs). (b, c) Purified allogeneic T cells were cultured with titrated numbers of DCs for 3 days. Data are mean c.p.m. ± SD of triplicate cultures. Similar results were obtained in three separate experiments. LPS/0·1% ABH and 0·001–0·1% ABH/LPS treatments were further abbreviated to L/0·1A and 0·001–0·1A/L. *P < 0·05, **P < 0·01 compared with T cells + LPS-treated DCs. (d) IFN-γ release of T-cell cultures with DCs. Purified allogeneic T cells were cultured with DCs treated with LPS [T + DC(LPS)], ABH/LPS [T + DC(A/L)], or LPS/ABH [T + DC(L/A)]. Culture supernatants were examined for cytokine levels by ELISA. Data are means ± SD of three separate experiments. **P < 0·01, compared with T + DC(LPS).
PGE2 and complement receptor 3 (CR3, CD11b/CD18) were not involved in the inhibitory effects of ABH on LPS-induced IL-12p40 production
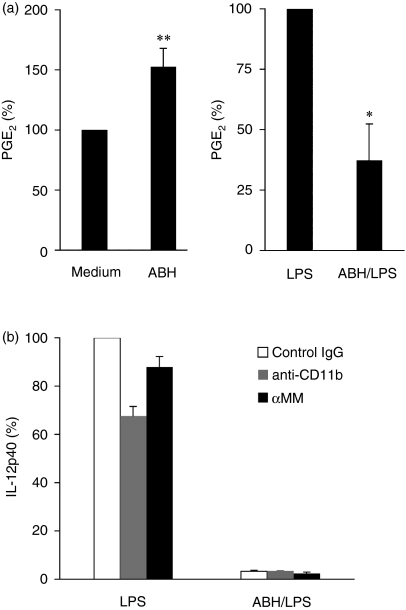
We attempted to find mediators that play important roles in the effects of ABH on LPS-induced IL-12p40 production. First, we focused on PGE2, which has been shown to inhibit IL-12 production in LPS-stimulated DCs.42, 43 The supernatants of untreated cultures and of those treated with 0·1% ABH, 1 ng/ml LPS, or ABH/LPS were examined for endogenous PGE2(Fig. 10a). In addition, culture medium containing 0·1% ABH was also examined for exogenous PGE2. DCs that had received no treatment released small amounts of PGE2 (mean of three separate experiments; 28·6 pg/ml). ABH-treated DCs released slightly larger amounts of PGE2 than untreated controls, while LPS-treated DCs produced large amounts of PGE2 (mean of three separate experiments; 204·6 pg/ml). However, pretreatment of DCs with ABH reduced LPS-induced PGE2 production. PGE2 was not detected in culture medium containing ABH. Next, DCs were preincubated with an antibody specific for the I-domain of CD11b or α-methyl d-mannoside (αMM), which are known to inhibit the binding of soluble zymosan to the lectin domain of macrophage CR3, 44 or control immunoglobulin G, followed by treatment with LPS or ABH/LPS for 24 hr. Culture supernatants were examined for IL-12p40 levels (Fig. 10b). Although anti-CD11b monoclonal antibody suppressed LPS-induced IL-12p40 secretion to some extent, as described previously, 45αMM did not show such suppression. The inhibitory effects of ABH on IL-12p40 secretion remained intact in ABH/LPS-treated DC cultures with anti-CD11b or αMM.
Figure 10.
PGE2 and CR3 showed no relation to inhibition of LPS-induced IL-12p40 production by ABH. (a) Supernatants from untreated cultures and those treated with 0·1% ABH, 1 ng/ml LPS, or ABH/LPS were collected for PGE2 measurement. PGE2 levels in untreated (Medium) or LPS-treated (LPS) DC cultures were set as 100%. Data are means ± SD of three separate experiments. Supernatants of untreated or LPS-treated DCs contained 28·6 ±17·2 pg/ml, or 204·6 ± 91·2 pg/ml PGE2 (mean ± SD), respectively. **P < 0·01, compared with Medium (untreated DCs); *P < 0·05, compared with LPS (LPS-treated DCs). (b) BMDCs were preincubated with anti-CR3 (100 µg/ml), α-methyl d-mannoside (αMM, 100 mm), or control IgG, followed by treatment with 1 ng/ml LPS or 0·1% ABH/LPS for 24 hr. Culture supernatants were examined for IL-12p40 levels. IL-12p40 levels in LPS-stimulated DC cultures with control IgG set as 100%. Data are means ± SD of two separate experiments. Supernatants of control DCs contained 22·8 ± 4·1 ng/ml IL-12p40 (mean ± SD)
Discussion
We investigated the effects of ABH on DCs in the present study. DCs are key players in innate and adaptive immunity. On the other hand, the ABH used here consists mainly of polysaccharides. There have been many studies of fungal polysaccharides that interact with DCs (reviewed in ref. 46). In particular, pathogenic fungal polysaccharides (and their derivatives) are thought to serve as pathogen-associated molecular patterns (PAMPs) for recognition by the host innate immune system. For example, cell wall polysaccharides of Candida albicans are recognized by TLRs and induce host immune responses.4747–50 Kikuchi et al. demonstrated that soluble β-glucan derived from Candida induced maturation of BMDC and IL-12 production.51 We are interested in whether edible mushrooms belonging to the non-pathogenic fungi are also involved in DC maturation. If they also activate DCs as strongly as C. albicans, they may be unsuitable for use in humans because of safety concerns. However, A. blazei has been eaten for many years because of its beneficial medical effects. Based on our observations, we assumed that it has a regulatory effect on the immune system.
In the present study, ABH stimulated immature BMDCs to up-regulate the surface molecules involved in maturation. This activation of ABH-treated DCs resulted in an increase of its capacity to induce proliferation of allogeneic T cells and enhanced IFN-γ responses in culture with T cells. However, the responses to optimal ABH stimulus were far weaker than those to optimal LPS stimulus. In addition, although DCs produced IL-12p40, TNF-α and IL-1β in response to LPS, they showed no increases in release of these proinflammatory cytokines in response to ABH. This indicated that ABH interacted with DCs in a manner different to bacterial constituents, such as LPS. Therefore, we considered two possibilities. First, the mechanism by which ABH induces up-regulation of the surface molecules may not be associated with cytokine production. Second, ABH may have inhibitory as well as stimulatory effects on DC maturation, and the inhibitory effects may interrupt DC activation by the stimulatory effects. If the second hypothesis is correct, ABH may inhibit LPS-mediated responses as well as its own stimulatory effects. To confirm this, DCs were cultured with a combination of ABH and LPS. As expected, DCs pretreated with ABH reduced LPS-induced cytokine secretion and up-regulation of surface molecules on DCs. However, the degrees of inhibition differed among the responses. ABH strongly inhibited LPS-induced cytokine production, in particular, that of IL-12p40, and this inhibition was also observed in PGN- or CpG DNA-induced IL-12p40 production, while ABH showed only slight inhibition of LPS-induced up-regulation of costimulatory molecules and MHC antigens on DCs. The DCs expressed them at far higher levels than untreated DCs. Neither the cytotoxic effect nor IL-10 was associated with these effects. However, ABH was shown to reduce LPS-induced NF-κB activity in a macrophage cell line. Strangely, ABH/LPS-treated DCs augmented the Th1 response in culture with allogeneic T cells. IL-12 is widely recognized as a major inducer of Th1 cell development. Th1 cells characteristically produce IFN-γ.52 Kawakami et al. demonstrated that Cryptococcus neoformans, a fungus that causes opportunistic infection, suppressed macrophage IL-12p40 production, and that IL-10 was not involved in this suppression.53 Several investigators have also reported the suppression of macrophage IL-12 production by other infectious pathogens.54, 55 This suppression of IL-12 production is considered to be a mechanism by which pathogens evade host defences, as the Th1 response plays a critical role in the elimination of these pathogens from the host.56–58 ABH may have a suppressive effect on the Th1 response. However, it may also have other effects that enhance it, as ABH treatment enhanced IFN-γ release in allogeneic mixed lymphocyte reaction. Our observations indicated that ABH induced weak maturation of DCs but also strongly inhibited the DC response in proinflammatory cytokine production. Although the physiological relevance of these observations remains to be determined, ABH resulted in enhancement of Th1-cell development. This suggested that ABH might affect T-cell differentiation without the involvement of proinflammatory mediators. An acute inflammatory reaction must not be caused by ingestion of edible mushrooms, unlike infection by pathogens. The properties of ABH may be advantageous in avoiding the inappropriate effects of proinflammatory cytokines.
We attempted to find mediators involved in inhibition of IL-12p40 production in ABH/LPS-treated DCs. Several studies have shown that PGE2 has some effects on DC maturation. While PGE2 promotes DC maturation in concert with proinflammatory cytokines, such as TNF-α, IL-1β and IL-6, 42, 59 it has been shown to inhibit the production of TNF-α, IL-6 and IL-12 in LPS-stimulated DCs.43, 60, 61 In addition, PGE2 is involved in DC migration and T-cell differentiation by DCs.62, 63 As some of these effects are similar to those of ABH, the culture supernatants were examined to determine whether DCs overproduced PGE2 in response to ABH. However, ABH also decreased LPS-induced PGE2 release similarly to that of proinflammatory cytokines. This result suggested that ABH/LPS-treated DCs did not release the large amounts of PGE2 that inhibit LPS-induced IL-12p40 production. Next, a previous study demonstrated that DCs reduced the levels of mRNA and secretion of proinflammatory cytokines, such as IL-1α, IL-1β, IL-6, IL-12p70 and TNF-α after internalization of circulating apoptotic cells in peripheral tissues.64 This inhibition was at least partially mediated through iC3b–CR3 interaction, and was considered to contribute to induce/maintain tolerance to self-antigens. Furthermore, Marth and Kelsall reported that CR3 signalling regulated IL-12 production.45 CR3 is also one of the receptors for β-glucans, which constitute a large part of ABH-derived polysaccharides. Therefore, we preincubated DCs with an antibody to CR3 or α-methylmannoside, inhibitors of the lectin domain of CR3. However, the inhibitory effects of ABH on IL-12p40 remained intact under these conditions.
As other cases of the regulation of proinflammatory cytokine production by DCs, a recent study demonstrated a novel function of complement C1q.65 C1q suppressed the myeloid differentiation factor 88 (MyD88)-dependent pathway, resulting in reduction of NF-κB activity, which is responsible for TLR-mediated responses including proinflammatory cytokine production. C1q showed strong inhibition of IL-12p40 secretion and weak inhibition of TNF-α secretion in LPS- or CpG DNA-stimulated BMDCs. However, C1q did not suppress LPS-induced up-regulation of costimulatory molecules (CD40, CD86) in Myd88-deficient DCs, indicating that the inhibitory effects of C1q may have little influence on the Myd88-independent pathway in TLR-mediated signals. Although we did not investigate TLR-signalling pathways in ABH-treated DCs, except for NF-κB activity, the results of this previous study and those reported here have some common features. In the present study, we hypothesized that ABH might have both stimulatory and inhibitory effects on DC maturation, and that these effects might interact with each other. However, we only demonstrated inhibitory effects of ABH against bacteria-mediated stimuli on DC maturation, and the two independent effects were not necessarily confirmed directly. Further studies to search for the essential components in ABH and their mechanisms of action are currently in progress.
In conclusion, ABH stimulated immature DCs to up-regulate the expression of costimulatory molecules and MHC antigen, but did not increase the production of inflammation-inducible cytokines. ABH-pretreated DCs inhibited some bacteria-mediated DC responses. However, ABH-mediated DCs enhanced the Th1 response in allogeneic mixed lymphocyte reaction. The antithetical effects of ABH may help to maintain immunological homeostasis.
References
- 1.See D, Mason S, Roshan R. Increased tumor necrosis factor alpha (TNF-alpha) and natural killer cell (NK) function using an integrative approach in late stage cancers. Immunol Invest. 2002;31:137–53. doi: 10.1081/imm-120004804. [DOI] [PubMed] [Google Scholar]
- 2.Ahn WS, Kim DJ, Chae GT, Lee JM, Bae SM, Sin JI, Kim YW, Namkoong SE, Lee IP. Natural killer cell activity and quality of life were improved by consumption of a mushroom extract, Agaricus blazei Murill Kyowa, in gynecological cancer patients undergoing chemotherapy. Int J Gynecol Cancer. 2004;14:589–94. doi: 10.1111/j.1048-891X.2004.14403.x. [DOI] [PubMed] [Google Scholar]
- 3.Kawagishi H, Inagaki R, Kanao T, Mizuno T, Shimura K, Ito H, Hagiwara T, Nakamura T. Fractionation and antitumor activity of the water-insoluble residue of Agaricus blazei fruiting bodies. Carbohydr Res. 1989;186:267–73. doi: 10.1016/0008-6215(89)84040-6. [DOI] [PubMed] [Google Scholar]
- 4.Ito H, Shimura K, Itoh H, Kawade M. Antitumor effects of a new polysaccharide-protein complex (ATOM) prepared from Agaricus blazei (Iwade strain 101) ‘Himematsutake’ and its mechanisms in tumor-bearing mice. Anticancer Res. 1997;17:277–84. [PubMed] [Google Scholar]
- 5.Fujimiya Y, Suzuki Y, Oshiman K, et al. Selective tumoricidal effect of soluble proteoglucan extracted from the basidiomycete, Agaricus blazei Murill, mediated via natural killer cell activation and apoptosis. Cancer Immunol Immunother. 1998;46:147–59. doi: 10.1007/s002620050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebina T, Fujimiya Y. Antitumor effect of a peptide-glucan preparation extracted from Agaricus blazei in a double-grafted tumor system in mice. Biotherapy. 1998;11:259–65. doi: 10.1023/a:1008054111445. [DOI] [PubMed] [Google Scholar]
- 7.Mizuno M, Minato K, Ito H, Kawade M, Terai H, Tsuchida H. Anti-tumor polysaccharide from the mycelium of liquid-cultured Agaricus blazei mill. Biochem Mol Biol Int. 1999;47:707–14. doi: 10.1080/15216549900201773. [DOI] [PubMed] [Google Scholar]
- 8.Takaku T, Kimura Y, Okuda H. Isolation of an antitumor compound from Agaricus blazei Murill and its mechanism of action. J Nutr. 2001;131:1409–13. doi: 10.1093/jn/131.5.1409. [DOI] [PubMed] [Google Scholar]
- 9.Ohno N, Furukawa M, Miura NN, Adachi Y, Motoi M, Yadomae T. Antitumor beta glucan from the cultured fruit body of Agaricus blazei. Biol Pharm Bull. 2001;24:820–8. doi: 10.1248/bpb.24.820. [DOI] [PubMed] [Google Scholar]
- 10.Lee YL, Kim HJ, Lee MS, Kim JM, Han JS, Hong EK, Kwon MS, Lee MJ. Oral administration of Agaricus blazei (H1 strain) inhibited tumor growth in a sarcoma 180 inoculation model. Exp Anim. 2003;52:371–5. doi: 10.1538/expanim.52.371. [DOI] [PubMed] [Google Scholar]
- 11.Menoli RC, Mantovani MS, Ribeiro LR, Speit G, Jordao BQ. Antimutagenic effects of the mushroom Agaricus blazei Murrill extracts on V79 cells. Mutat Res. 2001;496:5–13. doi: 10.1016/s1383-5718(01)00227-3. [DOI] [PubMed] [Google Scholar]
- 12.Delmanto RD, de Lima PL, Sugui MM, da Eira AF, Salvadori DM, Speit G, Ribeiro LR. Antimutagenic effect of Agaricus blazei Murrill mushroom on the genotoxicity induced by cyclophosphamide. Mutat Res. 2001;496:15–21. doi: 10.1016/s1383-5718(01)00228-5. [DOI] [PubMed] [Google Scholar]
- 13.Martins de Oliveira J, Jordao BQ, Ribeiro LR, Ferreira da Eira A, Mantovani MS. Anti-genotoxic effect of aqueous extracts of sun mushroom (Agaricus blazei Murill lineage 99/26) in mammalian cells in vitro. Food Chem Toxicol. 2002;40:1775–80. doi: 10.1016/s0278-6915(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 14.Barbisan LF, Scolastici C, Miyamoto M, Salvadori DM, Ribeiro LR, da Eira AF, de Camargo JL. Effects of crude extracts of Agaricus blazei on DNA damage and on rat liver carcinogenesis induced by diethylnitrosamine. Genet Mol Res. 2003;2:295–308. [PubMed] [Google Scholar]
- 15.Guterrez ZR, Mantovani MS, Eira AF, Ribeiro LR, Jordao BQ. Variation of the antimutagenicity effects of water extracts of Agaricus blazei Murrill in vitro. Toxicol Vitro. 2004;18:301–9. doi: 10.1016/j.tiv.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Sorimachi K, Ikehara Y, Maezato G, Okubo A, Yamazaki S, Akimoto K, Niwa A. Inhibition by Agaricus blazei Murill fractions of cytopathic effect induced by western equine encephalitis (WEE) virus on VERO cells in vitro. Biosci Biotechnol Biochem. 2001;65:1645–7. doi: 10.1271/bbb.65.1645. [DOI] [PubMed] [Google Scholar]
- 17.Talorete TP, Isoda H, Maekawa T. Agaricus blazei (class Basidiomycotina) aqueous extract enhances the expression of c-Jun protein in MCF7 cells. J Agric Food Chem. 2002;50:5162–6. doi: 10.1021/jf011566p. [DOI] [PubMed] [Google Scholar]
- 18.Kuo YC, Huang YL, Chen CC, Lin YS, Chuang KA, Tsai WJ. Cell cycle progression and cytokine gene expression of human peripheral blood mononuclear cells modulated by Agaricus blazei. J Laboratory Clin Med. 2002;140:176–87. doi: 10.1067/mlc.2002.126717. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu S, Kitada H, Yokota H, Yamakawa J, Murayama T, Sugiyama K, Izumi H, Yamaguchi N. Activation of the alternative complement pathway by Agaricus blazei Murill. Phytomedicine. 2002;9:536–45. doi: 10.1078/09447110260573047. [DOI] [PubMed] [Google Scholar]
- 20.Chen L, Shao HJ, Su YB. Coimmunization of Agaricus blazei Murill extract with hepatitis B virus core protein through DNA vaccine enhances cellular and humoral immune responses. Int Immunopharmacol. 2004;4:403–9. doi: 10.1016/j.intimp.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Mizuno M, Morimoto M, Minato K, Tsuchida H. Polysaccharides from Agaricus blazei stimulate lymphocyte T-cell subsets in mice. Biosci Biotechnol Biochem. 1998;62:434–7. doi: 10.1271/bbb.62.434. [DOI] [PubMed] [Google Scholar]
- 22.Sorimachi K, Akimoto K, Ikehara Y, Inafuku K, Okubo A, Yamazaki S. Secretion of TNF-alpha, IL-8 and nitric oxide by macrophages activated with Agaricus blazei Murill fractions in vitro. Cell Struct Funct. 2001;26:103–8. doi: 10.1247/csf.26.103. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima A, Ishida T, Koga M, Takeuchi T, Mazda O, Takeuchi M. Effect of hot water extract from Agaricus blazei Murill on antibody-producing cells in mice. Int Immunopharmacol. 2002;2:1205–11. doi: 10.1016/s1567-5769(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 24.Kopp E, Medzhitov R. Recognition of microbial infection by Toll-like receptors. Curr Opin Immunol. 2003;15:396–401. doi: 10.1016/s0952-7915(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 25.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 26.Rescigno M, Granucci F, Citterio S, Foti M, Ricciardi-Castagnoli P. Coordinated events during bacteria-induced DC maturation. Immunol Today. 1999;20:200–3. doi: 10.1016/s0167-5699(98)01427-3. [DOI] [PubMed] [Google Scholar]
- 27.Reis e Sousa C, Sher A, Kaye P. The role of dendritic cells in the induction and regulation of immunity to microbial infection. Curr Opin Immunol. 1999;11:392–9. doi: 10.1016/S0952-7915(99)80066-1. [DOI] [PubMed] [Google Scholar]
- 28.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 29.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verhasselt V, Buelens C, Willems F, De Groote D, Haeffner-Cavaillon N, Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J Immunol. 1997;158:2919–25. [PubMed] [Google Scholar]
- 31.Trinchieri G. Interleukin-12. a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 32.Beutler B. TNF, immunity and inflammatory disease: lessons of the past decade. J Invest Med. 1995;43:227–35. [PubMed] [Google Scholar]
- 33.Mannel DN, Echtenacher B. TNF in the inflammatory response. Chem Immunol. 2000;74:141–61. [PubMed] [Google Scholar]
- 34.McIntyre KW, Shuster DJ, Gillooly KM, et al. Reduced incidence and severity of collagen-induced arthritis in interleukin-12-deficient mice. Eur J Immunol. 1996;26:2933–8. doi: 10.1002/eji.1830261219. [DOI] [PubMed] [Google Scholar]
- 35.Parks E, Strieter RM, Lukacs NW, Gauldie J, Hitt M, Graham FL, Kunkel SL. Transient gene transfer of IL-12 regulates chemokine expression and disease severity in experimental arthritis. J Immunol. 1998;160:4615–9. [PubMed] [Google Scholar]
- 36.Criscione LG, St Clair EW. Tumor necrosis factor-alpha antagonists for the treatment of rheumatic diseases. Curr Opin Rheumatol. 2002;14:204–11. doi: 10.1097/00002281-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Shanahan JC, St Clair W. Tumor necrosis factor-alpha blockade: a novel therapy for rheumatic disease. Clin Immunol. 2002;103:231–42. doi: 10.1006/clim.2002.5191. [DOI] [PubMed] [Google Scholar]
- 38.Roeder A, Kirschning CJ, Rupec RA, Schaller M, Korting HC. Toll-like receptors and innate antifungal responses. Trends Microbiol. 2004;12:44–9. doi: 10.1016/j.tim.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Cho HJ, Hayashi T, Datta SK, Takabayashi K, Van Uden JH, Horner A, Corr M, Raz E. IFN-alpha beta promote priming of antigen-specific CD8+ and CD4+ T lymphocytes by immunostimulatory DNA-based vaccines. J Immunol. 2002;168:4907–13. doi: 10.4049/jimmunol.168.10.4907. [DOI] [PubMed] [Google Scholar]
- 40.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Meth. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 41.du Breuil RM, Patel JM, Mendelow BV. Quantitation of beta-actin-specific mRNA transcripts using xeno-competitive PCR. PCR Meth Appl. 1993;3:57–9. doi: 10.1101/gr.3.1.57. [DOI] [PubMed] [Google Scholar]
- 42.Rieser C, Bock G, Klocker H, Bartsch G, Thurnher M. Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells. synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603–8. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2-issued prostaglandin E2 enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol. 2002;168:2255–63. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- 44.Xia Y, Vetvicka V, Yan J, Hanikyrova M, Mayadas T, Ross GD. The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J Immunol. 1999;162:2281–90. [PubMed] [Google Scholar]
- 45.Marth T, Kelsall BL. Regulation of interleukin-12 by complement receptor 3 signaling. J Exp Med. 1997;185:1987–95. doi: 10.1084/jem.185.11.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buentke E, Scheynius A. Dendritic cells and fungi. APMIS. 2003;111:789–96. doi: 10.1034/j.1600-0463.2003.11107810.x. [DOI] [PubMed] [Google Scholar]
- 47.Netea MG, Van Der Graaf CA, Vonk AG, Verschueren I, Van Der Meer JW, Kullberg BJ. The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J Infect Dis. 2002;185:1483–9. doi: 10.1086/340511. [DOI] [PubMed] [Google Scholar]
- 48.Kataoka K, Muta T, Yamazaki S, Takeshige K. Activation of macrophages by linear (1→3)-beta-D-glucans. Impliations for the recognition of fungi by innate immunity. J Biol Chem. 2002;277:36825–31. doi: 10.1074/jbc.M206756200. [DOI] [PubMed] [Google Scholar]
- 49.Tada H, Nemoto E, Shimauchi H, et al. Saccharomyces cerevisiae- and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14- and Toll-like receptor 4-dependent manner. Microbiol Immunol. 2002;46:503–12. doi: 10.1111/j.1348-0421.2002.tb02727.x. [DOI] [PubMed] [Google Scholar]
- 50.Jouault T, Ibata-Ombetta S, Takeuchi O, Trinel PA, Sacchetti P, Lefebvre P, Akira S, Poulain D. Candida albicans phospholipomannan is sensed through toll-like receptors. J Infect Dis. 2003;188:165–72. doi: 10.1086/375784. [DOI] [PubMed] [Google Scholar]
- 51.Kikuchi T, Ohno N, Ohno T. Maturation of dendritic cells induced by Candida beta-D-glucan. Int Immunopharmacol. 2002;2:1503–8. doi: 10.1016/s1567-5769(02)00084-x. [DOI] [PubMed] [Google Scholar]
- 52.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 53.Kawakami K, Qureshi MH, Koguchi Y, Nakajima K, Saito A. Differential effect of Cryptococcus neoformans on the production of IL-12p40 and IL-10 by murine macrophages stimulated with lipopolysaccharide and gamma interferon. FEMS Microbiol Lett. 1999;175:87–94. doi: 10.1111/j.1574-6968.1999.tb13605.x. [DOI] [PubMed] [Google Scholar]
- 54.Karp CL, Wysocka M, Wahl LM, Ahearn JM, Cuomo PJ, Sherry B, Trinchieri G, Griffin DE. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–31. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 55.de Diego J, Punzon C, Duarte M, Fresno M. Alteration of macrophage function by a Trypanosoma cruzi membrane mucin. J Immunol. 1997;159:4983–9. [PubMed] [Google Scholar]
- 56.Kawakami K, Tohyama M, Teruya K, Kudeken N, Xie Q, Saito A. Contribution of interferon-gamma in protecting mice during pulmonary and disseminated infection with Cryptococcus neoformans. FEMS Immunol Med Microbiol. 1996;13:123–30. doi: 10.1016/0928-8244(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 57.Finke D, Brinckmann UG, ter Meulen V, Liebert UG. Gamma interferon is a major mediator of antiviral defense in experimental measles virus-induced encephalitis. J Virol. 1995;69:5469–74. doi: 10.1128/jvi.69.9.5469-5474.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Torrico F, Heremans H, Rivera MT, Van Marck E, Billiau A, Carlier Y. Endogenous IFN-gamma is required for resistance to acute Trypanosoma cruzi infection in mice. J Immunol. 1991;146:3626–32. [PubMed] [Google Scholar]
- 59.Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–42. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 60.Hedi H, Norbert G. Inhibition of IL-6, TNF-alpha, and cyclooxygenase-2 protein expression by prostaglandin E2-induced IL-10 in bone marrow-derived dendritic cells. Cell Immunol. 2004;228:99–109. doi: 10.1016/j.cellimm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 61.Vassiliou E, Jing H, Ganea D. Prostaglandin E2 inhibits TNF production in murine bone marrow-derived dendritic cells. Cell Immunol. 2003;223:120–32. doi: 10.1016/s0008-8749(03)00158-8. [DOI] [PubMed] [Google Scholar]
- 62.Scandella E, Men Y, Legler DF, Gillessen S, Prikler L, Ludewig B, Groettrup M. CCL19/CCL21-triggered signal transduction and migration of dendritic cells requires prostaglandin E2. Blood. 2004;103:1595–601. doi: 10.1182/blood-2003-05-1643. [DOI] [PubMed] [Google Scholar]
- 63.Lee JJ, Takei M, Hori S, et al. The role of PGE2 in the differentiation of dendritic cells: how do dendritic cells influence T-cell polarization and chemokine receptor expression? Stem Cells. 2002;20:448–59. doi: 10.1634/stemcells.20-5-448. [DOI] [PubMed] [Google Scholar]
- 64.Morelli AE, Larregina AT, Shufesky WJ, et al. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood. 2003;101:611–20. doi: 10.1182/blood-2002-06-1769. [DOI] [PubMed] [Google Scholar]
- 65.Yamada M, Oritani K, Kaisho T, et al. Complement C1q regulates LPS-induced cytokine production in bone marrow-derived dendritic cells. Eur J Immunol. 2004;34:221–30. doi: 10.1002/eji.200324026. [DOI] [PubMed] [Google Scholar]