Abstract
Schistosomiasis mansoni, a tropical helminthic disease, is caused by disseminated worm eggs that induce CD4+ T-cell mediated granulomatous inflammation and fibrosis. T suppressor cell activity has been proposed as one of the mechanisms active in the down-modulation of the murine disease during the chronic stage (16–20 weeks of the infection). In recent years a new category of the CD4+ CD25+ T regulatory (Treg) lymphocyte has been identified that maintains immune tolerance to self, and also functions in the regulation of parasite-induced immunopathology. The Foxp3 gene which encodes the transcription factor Scurfin was found to be expressed by and required for the generation of CD4+ CD25+ T reg. At 8 weeks of the infection Foxp3 gene expression of splenocytes was similar to that of naïve mice, but increased fourfold by 16 weeks. In contrast, granulomatous livers at 8 and 16 weeks showed 10- and 30-fold increases, respectively, in gene expression compared with normal liver. The percentage of granuloma CD4+ CD25+ T cells rose from 12% at 8 weeks to 88% at 16 weeks of the infection. Foxp3 expression was 3·5-fold higher in the CD4+ CD25+ versus the CD4+ CD25− T cells in the 8 week infection granulomas. As a novel observation neuropilin-1 membrane expression, a recently identified marker for Treg, was correlated with Foxp3 expression in the granuloma CD4+ CD25+ but not the CD25− cells. Co-incubation with polyclonal stimulation of CD4+ CD25+ splenic cells with CD4+ CD25− cells suppressed proliferation of the latter. Retroviral transfer of the Foxp3 gene at the onset of granuloma formation enhanced fourfold Foxp3 expression in the granuloma CD4+ CD25+ T cells and strongly suppressed full granuloma development. Gene transfer also significantly enhanced transforming growth factor-β, interferon-γ and interleukin-4 but not interleukin-10 expression. It is concluded, that CD4+ CD25+, Foxp3+ Treg cells also regulate schistosome egg-induced immunopathology.
Keywords: Schistosome granuloma, Foxp3 gene expression
Introduction
Schistosomiasis mansoni, a chronic disease of the tropics is caused by disseminated worm eggs that induce CD4+ T-cell mediated granulomatous inflammation, fibrosis and occasional death.1 In the murine model of the disease the acute stage (8–10 weeks) of the vigorous granulomatous response dominated by type 2 cytokine production2 is spontaneously downmodulated at the chronic stage (16–20 weeks) with diminished granuloma development.3,4 Over the years several mechanisms have been proposed to be active during downmodulation. These included suppressor T cells5,6 and their factors,7 idiotypic antibodies8 and T-cell anergy.9 Diminished granuloma development is protective against hepatosplenic disease.10 Infected hosts incapable of regulating high tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) production develop severe hepatosplenic schistosomiasis.11,12
In recent years a new category the CD4+ CD25+ T regulatory (Treg) lymphocytes have been identified that maintain immune tolerance to self and prevent auto-immune diseases.13,14 As yet no specific membrane marker exists that differentiates between activated and regulatory CD25+ T cells. More recently the Foxp3 gene which encodes a forkhead-winged helix transcription factor Scurfin15 was found to be expressed by and required for the generation of CD4+ CD25+ Treg.16,17 Moreover, retroviral gene transfer of Foxp3 converted naïve CD4+ T cells to CD4+ CD25+ Treg and Foxp3-transduced adoptively transferred T cells prevented autoimmune inflammatory bowel disease.18 Several publications indicated that the CD4+ CD25+ Treg subset, which spontaneously arises in the thymus,19 can also be peripherally induced by antigen20 and functions in the regulation of parasite-induced immunopathology.21–25 We localized CD4+ CD25+ T cells within the liver granulomas of infected mice; therefore, it was of interest to characterize their function and association with Foxp3 gene expression in the regulation of egg-induced immunopathology.
Materials and methods
Mice, parasites, infection and antigen preparation
Female CBA/Jk mice 6–8 weeks old-from Jackson Laboratories (Bar Harbor, ME), were used in this study. Mice were housed under specific-pathogen-free conditions at the Wayne State University School of Medicine animal facility. The animals were kept according to the criteria set by the American Association for the Accredition of Laboratory Animal Care. Biomphalaria glabrata snails infected with the Puerto Rican strain of Schistosoma mansoni worms were obtained from Biomedical Research Institute (Rockville, MD). Infective stage cercariae were isolated from the infected snails. Mice were infected subcutaneously with 30 cercariae. Soluble egg antigens (SEA) were prepared from homogenized eggs as previously described.26
Granuloma isolation and cell separation
Hepatic granulomas were isolated and enzymatically dispersed following the previously described method.27 Granuloma cells were used for the CD4+ T-cell separation. Some of the granuloma samples were mixed with Trizol (Invitrogen, Carlsbad, CA) and immediately frozen in liquid nitrogen for RNA preparation.
Purification of CD4+, CD25+ and CD25− cells
CD4+ cells were isolated from dispersed granuloma cells by positive selection using a mini magnetic cell sorting system (MACS) according to the protocols provided by the manufacturer (Miltenyi Biotec, Auburn, CA). Briefly, viable granuloma cells were suspended in phosphate-buffered saline supplemented with 2 mm ethylenediaminetetraacetic acid and 0·5% bovine serum albumin at a density of 107 cells in 90 µl of buffer and 10 µl of MACS CD4+ microbeads. The mixture was incubated for 15 min at 4–6°. After the incubation cells were washed and applied to a mini MACS separator. The negative cells were washed through the column. CD4+ cells retained on the column were eluted by removing the column from the magnetic field and flushing out the cells with 1 ml of elution buffer. The purity of the CD4+ cells was determined by staining the eluted cells with fluoroscein isothiocyanate (FITC)-conjugated anti-CD4 antibody by flow cytometry using FACSCalibur Sorter (BD Biosciences, Sunnyvale, CA). The isolated CD4+ cells were usually >95% positive for CD4+. The cells were then stained with phycoerythrin-conjugated anti-CD25 antibody (BD PharMingen, San Diego, CA). Anti-neuropilin (NP)-1 (H286, rabbit polyclonal immunoglobulin G, IgG) and corresponding isotype control antibody (normal rabbit IgG, control IgG) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). FITC-labelled goat anti-rabbit-IgG from Caltag Laboratories (Burlingame, CA) were used as secondary reagents.28 CD4+ CD25+ cells were sorted into two populations: CD25+ NP− and CD25+ NP+. Subpopulations of CD4+ cells: (CD4+ CD25− and CD4+ CD25+ cells) and CD4+ CD25+ cells (CD25+ NP− and CD25+ NP+) were separated by two-colour sorting on a FACSVantage Sorter (BD Biosciences). All populations were >98% pure on reanalysis.
Proliferation assay
Proliferation assays were performed with CD4+, CD4+ CD25+ and CD4+ CD25− cells isolated from spleen cells of naïve, 8 and 16 week infected mice. Additional assays were also performed by coincubation of CD4+ CD25+ and CD4+ CD25− cells at a 3:1 ratio. Cells were cultured at a cell density of 1 × 105/well in 200 µl of complete RPMI-1640 in a 96-well plate with plate-bound anti-CD3 (5 µg/ml) and soluble anti-CD28 (2 µg/ml) in culture medium for 72 hr. Naïve spleen cells were irradiated at 3000 rad and 3 × 105 cells were added into each well which served as antigen-presenting cells. Wells were pulsed with 0·5 µCi/well of [3H]thymidine (ICN Pharmaceutical, Inc., Costa Mesa, CA) for the last 8–12 hr of culture and then harvested with a Harvester 96, Match IIIM (TOMTEC, Orange, CT) and thymidine incorporation was counted in a 1450 Microbeta Plus, Liquid Scintillation Counter (PerkinElmer Life Sciences, Downers Grove, IL). Proliferation data are presented as mean ± SE of c.p.m. values. Experiments were repeated twice.
Liver histology and granuloma measurement
Liver histology and granuloma measurements were done on formalin-fixed 5 µm thick sections cut at 250 µm depth interval from one another. Slides were stained for haematoxylin and eosin or Masson's stain. Liver granuloma areas were measured in vector alone and Foxp3 vector gene recipient mice by computerized morphometry using a Scion software program (Scion Corp., Frederick, MD). Granuloma areas were expressed as square micrometers.
Retroviral construct and gene transfer
The Foxp3 expression vector used in these studies was kindly provided by Drs Shimon Sakaguchi and Shohei Hori (Kyoto University, Japan).18 The control vector, MIGR1 was received from Dr Warren Pear (University of Pennsylvania, Philadelphia, PA, USA). These vectors were amplified by transforming into DH5α competent bacteria and the DNA was isolated from the transformants using Qiagen plasmid DNA extraction kit.
In vivo gene delivery
Mice were injected iv with the Foxp3 retroviral construct vector or MIGR1 vector alone using TransIT-In Vivo Gene Delivery System (Mirus Corporation, Madison, WI) following the vendor's protocol. Mice were injected with 10 µg DNA of Foxp3 vector or control vector four times in 2 ml of the delivery fluid29 between 6 and 8 weeks of the infection period.
RNA and reverse transcription
RNA was extracted from the frozen liver, spleen, granuloma, or CD4+ CD25− and CD4+ CD25+ cells samples using Trizol (Invitrogen). CD25+ NP− and CD25+ NP+ cells were also processed for RNA preparation. Isolated pure RNA for reverse transcriptase–polymerase chain reaction (RT–PCR) was prepared by treating RNA samples with RNase free DNAse I (Promega Corporation, Madison, WI). Further, samples were extracted with buffered phenol and then chloroform/isoamylalcohol (24 : 1). The recovered RNA was ethanol precipitated and stored in RNAse-free distilled water. The purity of RNA samples was assessed spectrophotometrically. RT experiments were carried out by adding 8 µg of RNA for cDNA synthesis using random primers and murine Moloney leukaemia virus reverse transcriptase (Gibco/BRL) in a volume of 80 µl reaction mixture as reported earlier.30
Real-time quantitative PCR analyses
The presence of relative mRNA species was evaluated by the SYBR Green method using the ABI Prism 7700 Sequence Detector System (Applied Biosystems, Foster City, CA). Primers were designed for PCR by using Gene Runner Software (Hasting Software, Inc., Hasting, NY). The RNA sequences used for primer design were obtained from the Gene Bank (Table 1). All primer sets had a calculated annealing temperature of 60°. RT–PCR was performed in duplicate in a 25-µl reaction volume containing 2× SYBR Green PCR Master Mix (Applied Biosystems), 900 nm of each primer, 2–3 µl of cDNA and the volume was adjusted with pure distilled water. The RT–PCR amplifications were 2 min at 50° followed by 40 cycles of denaturation for 15 s and annealing/extension at 60° for 1 min Real-Time PCR data were analysed using v.1.7 Sequence Detection Software (PE Biosystems, Foster City, CA). Relative expression of the genes was calculated by using the comparative Ct method as described earlier.31 Relative transcript levels were normalized to the 18SrRNA genes. For comparison, transcript levels of naïve, uninfected mice were set at 1 and data from infected mice are reported as fold increase over 1 baseline. Dissociation curves were used to verify the specificity of the PCR products.
Table 1. Sequences of the murine primers used for the real-time PCR.
| Gene | Primer | Sequence |
|---|---|---|
| Foxp3 | Forward | 5′-GGC CCT TCT CCA GGA CAG A-3′ |
| Reverse | 5′-GCT GAT CAT GGC TGG GTT GT-3′ | |
| IFN-γ | Forward | 5′-GGA TGC ATT CAT GAG TAT TGC-3′ |
| Reverse | 5′-CCT TTT CCG CTT CCT GAG G-3′ | |
| IL-4 | Forward | 5′-AGA TCA TCG GCA TTT TGA ACG-3′ |
| Reverse | 5′-TTT GGC ACA TCC ATC TCC G-3′ | |
| IL-10 | Forward | 5′-GGA CAA CAT ACT GCT AAC CGA C-3′ |
| Reverse | 5′-GGG CAT CAC TTC TAC CAG G-3′ | |
| TGF-β | Forward | 5′-CTC CAC CTG CAA GAC CAT-3′ |
| Reverse | 5′-CTT AGT TTG GAC AGG ATC TGG-3′ | |
| 18SrRNA | Forward | 5′-CGG CTA CCA CAT CCA AGG AA-3′ |
| Reverse | 5′-GCT GGA ATT ACC GCG GCT-3′ |
Statistical analyses
Data are presented as mean ± SE. Statistical analyses were performed by using Student's two-tailed t-test. Values were considered significant when P < 0·05.
Results
Foxp3 expression in the spleens and granulomatous livers of infected mice
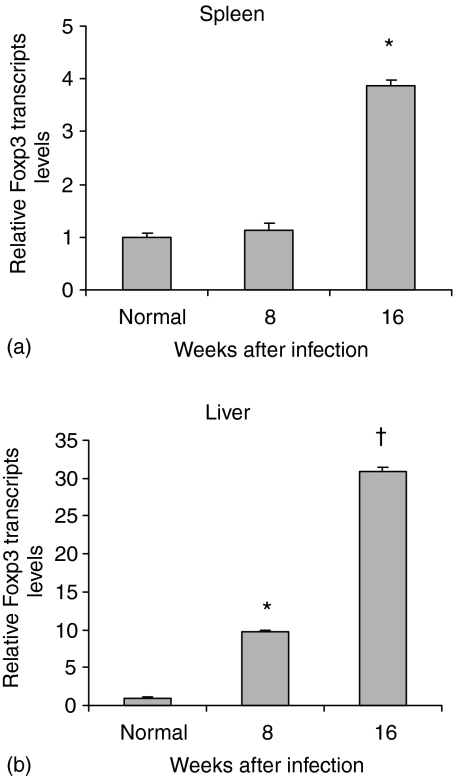
As a first step we examined Foxp3 expression during the acute (8w) and chronic (16w) stages of the infection. As Fig. 1(a) shows, compared with normal spleen of uninfected mice, that the message level at 8 weeks was not elevated but by 16 weeks about a fourfold increase was observed. In contrast, granulomatous livers showed elevated message levels at both 8 and 16 weeks of the infection. The strong increase in gene expression between 8 and 16 weeks is noteworthy (Fig. 1b).
Figure 1.
Relative Foxp3 gene expression in the spleens and granulomatous livers of Schistosoma mansoni infected mice. Real-time RT–PCR assay was performed for the spleens (a) and livers (b) of normal, uninfected mice and those at 8 and 16 weeks after infection. Calculation of relative levels is described in Materials and Methods. Data presented as means ± SE of six to eight mice in each group. Statistically significant differences were observed when compared with control naïve group: *, P < 0·001; †, P < 0·0001.
Association of Foxp3 expression with the CD4+ CD25+ subset
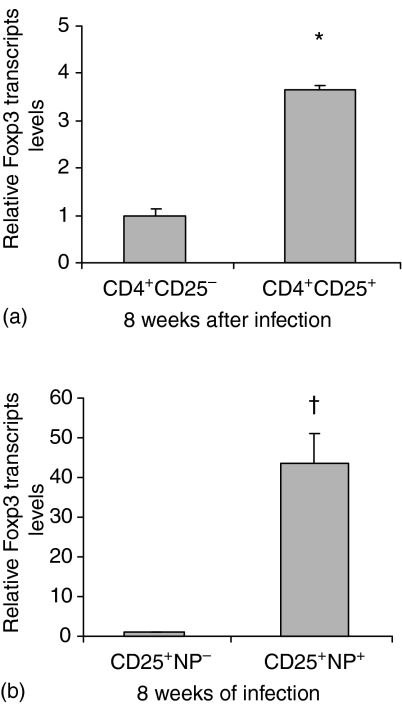
To locate Treg within the CD25 subset the column-purified granuloma CD4+ T cells were sorted into CD25− and CD25+ subsets. Table 2 shows the percentage of the subsets established by flow cytometry and the calculated absolute numbers of cells within the tissues of uninfected and infected mice. Compared with uninfected mice, the percentage and absolute numbers of splenic CD25+ T cells were elevated at 8 and 16 weeks of the infection. Spleens of the downmodulated 16 weeks infected mice had a lower percentage of CD25+ T cells compared with the 8 weeks infected counterpart. In contrast, a major change was seen in the granuloma cell populations where the percentage and numbers of CD25+ Treg within the downmodulated lesions increased about threefold over those of the 8 weeks infection granulomas. In addition to the 17% CD25+ T-cell content Fig. 2(a) indicates that within the 8w infection vigorous granulomas it is the CD4+ CD25+ subset that is predominantly expressing the Foxp3 message.
Table 2. Percentage and absolute numbers of CD4+ T-cell subsets in spleens and granulomas of infected mice.
| (No. of cells × 106)* | |||
|---|---|---|---|
| Tissue | Uninfected | 8 week infected | 16 week infected |
| Spleen | |||
| CD4+ CD25+ | 8 (0·54) | 17 (2·72) | 12 (2·59) |
| CD4+ CD25− | 92 (6·21) | 83 (13·28) | 88 (19·00) |
| Granuloma | |||
| CD4+ CD25+ | − (−) | 25 (1·87) | 88 (5·23) |
| CD4+ CD25− | − (−) | 75 (5·62) | 12 (0·71) |
Percentage of CD4+ T subsets obtained by flow cytometry in three repeats.
Calculations based on mean total spleen cell counts of at least 8 mice. Uninfected: 45 × 106, 15% CD4+ T cells; 8 week infected: 100 × 106, 16% CD4+ T cells; 16 week infected: 120 × 106, 18% CD4+ T cells. Mean total granuloma cell count of 10 mice: 8 week infected: 50 × 106, 15% CD4+ T cells; 16 week infected: 35 × 106, 17% CD4+ T cells.
Figure 2.
Relative Foxp3 gene expression in the subset of CD4+ cells in the liver granulomas from 8 weeks infected mice. Foxp3 transcript levels of CD4+ CD25+ cells were calculated as fold increase over the mean values of the CD4+ CD25−. The mean values of the latter were normalized to 1 and the relative increase was calculated by dividing the CD25+ value by the CD25− value. Real-time RT–PCR assay was performed in the (a) CD4+ CD25− and CD4+ CD25+ and (b) CD25+ NP− and CD25+ NP+ subsets of CD4+ and CD4+ CD25+ cells. Data presented as means ± SE of six to eight mice. Statistically significant differences were observed when CD4+CD25− were compared with CD4+ CD25+ and CD25+ NP− with CD25+ NP+ cells: *, P < 0·05; †, P < 0·01.
We also sought a correlation between the display of NP-1 membrane marker and CD4+ CD25+ Treg that express Foxp3. As Fig. 2(b) shows the CD4+ CD25+ subset was further sorted into NP− and NP+ groups. Examination by RT–PCR Foxp3 transcript levels demonstrated that only the CD25+ NP+ cells expressed Foxp3.
CD4+ CD25+ T cells suppress CD4+ CD25− T-cell proliferation
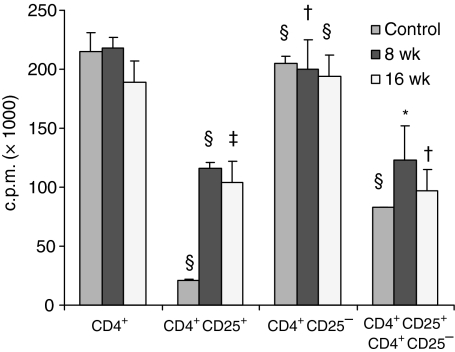
To assign a regulatory function to the CD25+ subset coincubation studies were carried out. Figure 3 illustrates the proliferative capacity of unsorted CD4+ cells and the CD25−, CD25+ sorted subsets from spleens of naïve, 8 and 16 week infected mice. Upon polyclonal T-cell receptor (TCR) stimulation the CD4+ T cells from the three groups showed strong proliferation. The CD25+ subset of naïve mice proliferated only minimally. The 8 and16 week infection-derived CD25+ cells exhibited 50% lower proliferation, whereas the CD25− subset showed strong proliferation, similar to that of the CD4+ cells. Coincubation at a 3 : 1 ratio of the CD25+ subset with the CD25− cells from both infection stages resulted in significant suppression of proliferation compared with the level of the CD25− cells.
Figure 3.
Proliferative response of CD4+, CD4+ CD25+ and CD4+ CD25− cells isolated from spleens of naïve, 8 and 16 weeks infected mice. Proliferation assays were performed by stimulating the cells with plate bound anti-CD3 and anti-CD28 in culture for 72 hr. Data presented as means ± SE of six to eight mice in each group. Experiments were repeated twice. Statistically significant difference observed when compared with their respective group subsets of CD4+ cells: *, P < 0·05; †, P < 0·01; ‡, P < 0·001 and §, P < 0·0001.
Foxp3 and cytokine gene expression in granuloma CD4+ CD25−, CD25+ subsets following retroviral gene transfer of Foxp3
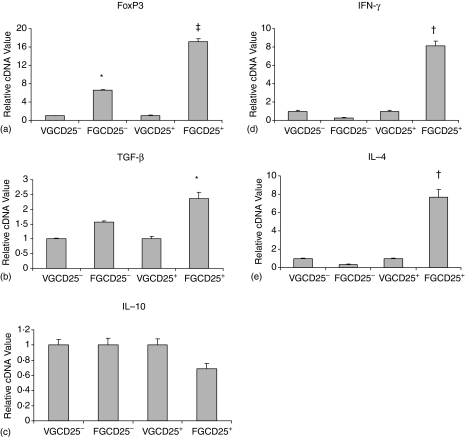
To prove that Foxp3 gene can induce the generation of Treg, Foxp3 gene in a retroviral construct was adoptively transferred at the onset of granuloma development to mice. In Fig. 4 for ease of comparison the cDNA values of CD25− and CD25+ cells derived from control, empty MIGR1 vector (VG) recipients were standardized to 1. Interestingly after retroviral transfer of Foxp3 gene (FG Fig. 4a) Foxp3 expression in both the CD25− and the CD25+ subsets showed enhanced gene expression, however, the increase in the CD25+ subset was significantly higher. A direct comparison between empty vector recipients of the CD25− and CD25+ values showed a 1·4-fold increase in cDNA value of CD25+ cells over the CD25− population. This lower expression (see Fig. 2a) is attributed to the interference by the empty vector.
Figure 4.
Relative Foxp3 (a) and cytokine genes (TGF-β, b, IL-10, c, IFN-γ, d, IL-4, e) expression in granuloma CD4+ CD24− and CD4+ CD25+ cells following retroviral gene transfer of Foxp3 in 8 weeks infected mice. In (a–d) the mean values of VGCD25− cells and VGCD25+ cells (derived from control MIGR1 vector recipient mice) were normalized to 1 and the relative increase shown by FGCD25+ cells (derived from Foxp3 gene recipient mice) was calculated as in Fig. 2. Real time RT–PCR assays were performed in cells from MIGR1 vector or Foxp3 gene transferred mice. Data presented as means ± SE of five mice in each group. Experiment was repeated twice. Statistically significant differences were observed when compared with MIGR1 vector alone group: *, P < 0·05; †, P < 0·001 and ‡, P < 0·0001.
Transforming growth factor-β (TGF-β) expression showed a different pattern. Compared with the baseline values of the two subsets in MIGR1 vector recipients a significant increase in message level was found only in the CD25+ subset of Foxp3 gene recipients (Fig. 4b).
The IFN-γ and interleukin (IL)-4 cytokine messages showed a similar pattern of expression. Post-gene transfer a significant increase was noticed only in the CD25+ subset (Fig. 4d, e).
In an additional assay, IL-10 expression was also examined. As Fig. 4(c) shows IL-10 message in granuloma CD25+ cells of Foxp3 gene recipients was not significantly different compared with the level of the control CD25− cells.
Retroviral Foxp3 gene transfer suppresses liver granuloma development
Having observed elevated Foxp3, TGF-β and Th0 type cytokine gene expression in the CD25+ subsets of Foxp3 gene recipients, development of liver granuloma in MIGR1 vector alone or Foxp3 gene-treated mice was examined. Table 3 shows that repeated injections of the Foxp3 retroviral construct between 6 and 8 weeks of the infection into mice during the incipient stage of granuloma formation severely suppressed the development of liver granulomas (P < 0·05).
Table 3. Effect of retroviral Foxp3 gene transfer on the liver granuloma size of infected mice.
| Group | Mean granuloma area (× 103µm2 ± SEM) |
|---|---|
| Control MIGR1 vector | 142·88 ± 4·97 (100)* |
| Foxp3 MIGR1 vector | 75·79 ± 2·84 (100)** |
Values are mean ± SEM from five mice per group, P < 0·05.
No. of granulomas measured.
Mean liver granuloma area × 103 µm2 of 18–20 weeks infected mice: 71·32 3·3 (13 mice).
Discussion
In the present study an association between a Treg subset and regulation of schistosome egg-induced granulomatous pathology is described. The Treg subset is identified as the CD4+ CD25+ Foxp3-expressing T lymphocytes present in the spleen and granulomas of infected mice. The Foxp3 molecular marker is considered to be specific for Treg cells.16,32 Therefore it is noteworthy that compared with normal liver the Foxp3 message was elevated in the 8 week infection granulomatous tissue and increased ∼30 fold in livers of chronically infected mice that carry the downmodulated granulomas.3 Recently NP-1 receptor was found to be displayed on murine CD4+ CD25+ Treg cells which also expressed high levels of Foxp3. Examination of 8 week infection granuloma CD4+ CD25+ cells confirmed the association of the NP-1 marker and Foxp3. This membrane marker will in the future be very useful to separate CD4+ CD25+ Treg cells from CD4+ CD25+ activated T cells. Whereas the CD25− subset of acute or chronic infection CD4 splenocytes showed vigorous proliferation upon polyclonal TCR stimulation, their CD25+ counterparts exhibited significantly lower proliferative capacity. Moreover, coincubation of the CD25− and CD25+ subsets showed significant suppression of the CD25− cell proliferation. Taken together these data indicate that the CD25+ subset exhibited characteristic Treg function.33 Our data extend recently published results in which liver granuloma CD4+ CD25+ T cells of schistosome-infected mice were shown to suppress the polyclonally stimulated naïve CD4+ T-cell proliferation.24 To directly demonstrate the association of the induced Foxp3 transcription factor with Treg function we carried out retroviral transfer of the Foxp3 gene to infected recipients with developing granulomas. The transfer induced low gene expression in the CD25−, but significantly higher one in the CD25+ granuloma CD4+ lymphocytes. Moreover, the NP-1+, not the NP-1− subset of the CD4+ CD25+ cells showed a strong expression of Foxp3 further underscoring the utility of this marker in the identification of Treg. The most significant finding of this study is the observed strong suppression of granuloma development in the acutely infected gene-transferred recipients. The degree of suppression achieved was similar to that observed in downmodulated granulomas of chronically infected mice. Thus, Foxp3 expression could be induced in extrathymic, predominantly CD25+ T cells that migrated into the lesions and exerted antipathology suppressor function. The mechanism(s) whereby Treg suppress T effector cell functions is still being investigated. In different systems IL-10,34 cytotoxic T lymphocyte antigen-435 or TGF-β36 have been implicated as mediators of suppression. In experimental candidiasis CD4+ CD25+ T cells isolated from mesenteric nodes secreted IL-4, IL-10 and TGF-β,22 whereas this subset in the dermis of Leishmania-infected mice exerted suppression over CD4+ CD25− cells by both IL-10-dependent and -independent mechanisms.21 Previous observations made by us and other laboratories established the antipathologic role of IL-10 chiefly at the acute stage of the periovum granulomatous inflammation.37–39 A recent publication also showed that in the infected C57BL/6 mice the CD4+ CD25+ granuloma T cells were the main producers of IL-10.24 Therefore, it was of interest that the forced expression of Foxp3 gene in infected recipients greatly boosted TGF-β but not IL-10 expression in the CD25+ subset. Moreover, messages for both IFN-γ and IL-4 became elevated indicating that enhanced Foxp3 expression also shifted the immune response to a T helper 0 profile. The sum total of changes in cytokine gene expression led to the suppression of the granulomatous response. Whether suppression mediated by TGF-β alone, or the combined effect of TGF-β and IFN-γ was instrumental in the mediation of suppression remains to be elucidated. Adoptively enhanced TGF-β gene expression prevented autoimmune diabetes in NOD mice but also caused fibrosis in the pancreas.40 Although in the present study the suppressed granulomas did not contain more collagen-staining tissue than the control lesions, this undesirable side-effect of enhanced fibrosis needs further examination. As shown in Fig. 1 at 16 weeks of the infection livers of mice bearing the downmodulated granulomas had greatly elevated Foxp3 transcripts. Moreover, the percentage of CD4+ CD25+ cells greatly increased within the downmodulated granulomas. This, combined with previous and recent observations on elevated TGF-β expression within livers41 and downmodulated liver granulomas42 raises the question of an association between elevated number of CD4+ CD25+ Treg, Foxp3, TGF-β expression and the spontaneous downmodulation of the periovum granulomatous response. When such an association is firmly established, a novel perhaps major mechanism among the previously suggested regulatory pathways5–9 will have been revealed.
In sum, the present study has shown that in agreement with previous publications21–23 CD4+ CD25+ Treg cells provide protection not only in autoimmune13,14 but also in worm parasite-induced immunopathology. Artificially enhanced induction of Treg cell activity may thus be considered as a modality in the suppression of the schistosome egg-induced immunopathology.
Acknowledgments
The work was supported by Public Health Service grants AI12913 (D.L.B.), AI46240 (T.R.R.) from the National Institute of Allergy and Infectious Diseases and AR-42541 (A.P.H.). Schistosome life stages for this work were supplied through NIH NIAID contract No. NO1-AI55270. We are indebted to Drs Sakaguchi and Hori, Japan for providing us with the retroviral construct and Dr Pear for supplying the vector.
References
- 1.Ross AG, Bartley PB, Sleigh AC, Olds GR, Li Y, Williams GM, McManus DP. Schistosomiasis. N Engl J Med. 2002;346:1212–20. doi: 10.1056/NEJMra012396. 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- 2.Boros DL. T helper cell populations, cytokine dynamics and pathology of the schistosome egg granuloma. Microbes Infect. 1999;1:511–6. doi: 10.1016/s1286-4579(99)80090-2. [DOI] [PubMed] [Google Scholar]
- 3.Boros DL, Pelley RP, Warren KS. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J Immunol. 1975;114:1437–41. [PubMed] [Google Scholar]
- 4.Colley DG. Immune responses to a soluble schistosomal egg antigen preparation during chronic primary infection with Schistosoma mansoni. J Immunol. 1975;115:2150–6. [PubMed] [Google Scholar]
- 5.Colley DG. Adoptive suppression of granuloma formation. J Exp Med. 1976;143:2696–700. doi: 10.1084/jem.143.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chensue SW, Wellhausen SR, Boros DL. Modulation of granulomatous hypersensitivity II. Participation of Lyt1+ and Lyt2+ T lymphocytes in the suppression of granuloma formation and lymphokine production in Schistosoma mansoni-infected mice. J Immunol. 1981;127:363–7. [PubMed] [Google Scholar]
- 7.Fidel PL, Jr, Boros DL. Regulation of granulomatous inflammation in murine schistosomiasis. V. Antigen-induced T cell-derived suppressor factors downregulate proliferation and IL-2, but not IL-4, production by CD4+ effector T cells. J Immunol. 1991;146:1941–8. [PubMed] [Google Scholar]
- 8.Parra JC, Gazzinelli G, Goes AM, Moyes RB, Rocha R, Colley DG, Doughty BL. Granulomatous hypersensitivity to Schistosoma mansoni egg antigens in human schistosomiasis. II. In vitro granuloma modulation induced by polyclonal idiotypic antibodies. J Immunol. 1991;147:3949–54. [PubMed] [Google Scholar]
- 9.Stadecker MJ, Flores Villanueva PO. Accessory cell signals regulate Th-cell responses: from basic immunology to a model of helminthic disease. Immunol Today. 1994;15:571–4. doi: 10.1016/0167-5699(94)90219-4. 10.1016/0167-5699(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 10.Gazzinelli G, Colley DG. Human immune responses during schistosomiasis mansoni. Rev Soc Bras Med Trop. 1992;25:125–34. doi: 10.1590/s0037-86821992000200006. [DOI] [PubMed] [Google Scholar]
- 11.Adewusi OI, Nix NA, Lu X, Colley DG, Secor WE. Schistosoma mansoni: relationship of tumor necrosis factor-alpha to morbidity and collagen deposition in chronic experimental infection. Exp Parasitol. 1996;84:115–23. doi: 10.1006/expr.1996.0097. 10.1006/expr.1996.0097. [DOI] [PubMed] [Google Scholar]
- 12.Mwatha JK, Kimani G, Kamau T, et al. High levels of TNF, soluble TNF receptors, soluble ICAM-1 and IFN-γ but low levels of IL-5, are associated with hepatosplenic disease in human schistosomiasis mansoni. J Immunol. 1998;160:1992–9. [PubMed] [Google Scholar]
- 13.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 14.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 15.Brunkow ME, Jeffrey EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 16.Khattri R, Cox T, Yasayako SA, Ramsdell F. An essential role for Scurfin in CD4+ CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 17.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 18.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 19.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity. production of CD25+ CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–26. [PubMed] [Google Scholar]
- 20.Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25+ CD4+ regulatory T cells by oral antigen administration. J Immunol. 2001;167:4245–53. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- 21.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+ CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–7. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 22.Xu D, Liu H, Komai-Koma M, Campbell C, McSharry C, Alexander J, Liew FY. CD4+ CD25+ regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J Immunol. 2003;170:394–9. doi: 10.4049/jimmunol.170.1.394. [DOI] [PubMed] [Google Scholar]
- 23.Montagnoli C, Bacci A, Bozza S, Gaziano R, Mosci P, Sharpe AH, Romani L. B7/CD28-dependent CD4+ CD25+ regulatory T cells are essential components of the memory-protective immunity to Candida albicans. J Immunol. 2002;169:6298–308. doi: 10.4049/jimmunol.169.11.6298. [DOI] [PubMed] [Google Scholar]
- 24.Hori S, Carvalho TL, Demengeot J. CD25+ CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur J Immunol. 2002;32:1282–91. doi: 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Hesse M, Piccirillo CA, Belkaid Y, et al. The pathogenesis of schistosomiasis is controlled by cooperating IL-10 producing innate effector and regulatory T cells. J Immunol. 2004;172:3157–66. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- 26.Boros DL, Warren KS. Delayed hypersensitivity granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970;132:488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ragheb S, Boros DL. Characterization of granuloma T lymphocyte function from Schistosoma mansoni-infected mice. J Immunol. 1989:3239–46. [PubMed] [Google Scholar]
- 28.Bruder D, Probst-Kepper M, Westendorf AM, et al. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol. 2004;34:623–30. doi: 10.1002/eji.200324799. [DOI] [PubMed] [Google Scholar]
- 29.Liu F, Song YK, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–66. doi: 10.1038/sj.gt.3300947. 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 30.Gerard HC, Wang Z, Whittum-Hudson JA, El-Gabalawy H, Goldbach-Mansky R, Bardin T, Schumacher HR, Hudson AP. Cytokine and chemokine mRNA produced in synovial tissue chronically infected with Chlamydia trachomatis and C. pneumoniae. J Rheumatol. 2002;29:1827–35. [PubMed] [Google Scholar]
- 31.Gerard HC, Branigan PJ, Schumacher HR, Jr, Hudson AP. Synovial Chlamydia trachomatis in patients with reactive arthritis/Reiter's syndrome are viable but show aberrant gene expression. J Rheumatol. 1998;25:734–42. [PubMed] [Google Scholar]
- 32.Gavin M, Rudensky A. Control of immune homeostasis by naturally arising regulatory CD4+ T cells. Curr Opin Immunol. 2003;15:690–6. doi: 10.1016/j.coi.2003.09.011. 10.1016/j.coi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 34.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25 (+) CD4 (+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen W, Wahl SM. TGF-beta: The missing link in CD4 (+) CD25 (+) regulatory T cell-mediated immunosuppression. Cytokine Growth Factor Rev. 2003;14:85–9. doi: 10.1016/s1359-6101(03)00003-0. [DOI] [PubMed] [Google Scholar]
- 37.Flores Villanueva PO, Reiser H, Stadecker MJ. Regulation of T helper cell responses in experimental murine schistosomiasis by IL-10. Effect on expression of B7 and B7–2 costimulatory molecules by macrophages. J Immunol. 1994;153:5190–9. [PubMed] [Google Scholar]
- 38.Boros DL, Whitfield JR. Endogenous IL-10 regulates IFN-γ and IL-5 cytokine production and the granulomatous response in schistosomiasis mansoni-infected mice. Immunology. 1998;94:481–7. doi: 10.1046/j.1365-2567.1998.00544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wynn TA, Cheever AW, Williams ME, Hieny S, Caspar P, Kuhn R, Muller W, Sher A. IL-10 regulates liver pathology in acute murine schistosomiasis mansoni but is not required for immune down-modulation of chronic disease. J Immunol. 1998;160:4473–80. [PubMed] [Google Scholar]
- 40.Grewal IS, Grewal KD, Wong FS, Wang H, Picarella DE, Janeway CA, Jr, Flavell RA. Expression of transgene encoded TGF-beta in islets prevent autoimmune diabetes in NOD mice by a local mechanism. J Autoimmun. 2002;19:9–22. doi: 10.1006/jaut.2002.0599. 10.1006/jaut.2002.0599. [DOI] [PubMed] [Google Scholar]
- 41.Kresina TF, He Q, Degli Esposti S, Zern MA. Gene expression of transforming growth factor beta 1 and extracellular matrix proteins in murine Schistosoma mansoni infection. Gastroenterology. 1994;107:773–80. doi: 10.1016/0016-5085(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 42.Singh KP, Gerard HC, Hudson AP, Boros DL. Dynamics of collagen, MMP and TIMP gene expression during the granulomatous, fibrotic process induced by Schistosoma mansoni eggs. Ann Trop Med Parasitol. 2004;98:581–93. doi: 10.1179/000349804225021316. 10.1179/000349804225021316. [DOI] [PubMed] [Google Scholar]