Abstract
Eosinophil recruitment to the airways, including involvement of haemopoietic eosinophil–basophil progenitors (Eo/B-CFU), is primarily regulated by interleukin-5 (IL-5) and eotaxin. In this study, we investigated the haemopoietic mechanisms in upper and lower airway eosinophilic inflammation. Ovalbumin (OVA) sensitized and challenged BALB/c mice were used to establish isolated upper (UAC), isolated lower (LAC), or combined upper and lower airway (ULAC) inflammation. Airway, blood and bone marrow responses were evaluated in each model. Numbers of airway eosinophils and CD4+ cells were increased significantly in the nasal mucosa in UAC and ULAC mice, and in the lung tissue in LAC and ULAC groups. Levels of IL-5 and eotaxin were increased significantly in the nasal lavage fluid (NL) in UAC and ULAC mice, and in the bronchoalveolar lavage fluid (BAL) in LAC and ULAC groups. The proportion of IL-5-responsive bone marrow Eo/B-CFU was significantly higher than the control in all treatment groups, but peaked much earlier in the ULAC group. Kinetic studies revealed that IL-5 and eotaxin in NL, BAL and serum peaked between 2 and 12 hr after OVA challenge in ULAC mice, and at 24 hr in UAC mice, related to the timing of maximal progenitor responses. These data support the concept that the systemic mechanisms linking rhinitis to asthma depend on the location and extent of airway allergen exposure.
Keywords: allergy, asthma, eosinophils, murine, rhinitis
Introduction
Allergic rhinitis and asthma are both recognized as inflammatory disorders of the airway mucosa, but differ in the location of the inflammatory reaction and clinical manifestations of the disease. There is increasing evidence for close links between the upper and the lower respiratory tracts, including common mechanisms and clinical interactions between rhinitis and asthma.1
Studies performed on respiratory secretions and biopsies of the airways in patients with allergic rhinitis and asthma suggest that eosinophils play a central role in the pathogenesis and clinical expression of the disease.2 The recruitment and activation of these cells appear to be controlled by the release of cytokines such as interleukin-5 (IL-5), specifically involved in eosinophil growth and maturation, 3 and chemotactic agents such as eotaxin, a CC chemokine derived from antigen-stimulated T lymphocytes and airway epithelium, which stimulates the migration of eosinophils from bone marrow into the tissue.4 Eosinophils and their progenitors are released from the bone marrow under the influence of both IL-5 and eotaxin, resulting in an increase of circulating and tissue eosinophils.5, 6
We have previously provided evidence that an important aspect of allergic inflammatory responses is the induction of inflammatory cell progenitor lineage and commitment and maturation, which contribute to disease through the continued production of inflammatory effector cells.7–10 Higher numbers of both circulating eosinophil/basophil colony-forming units (Eo/B-CFU) and CD34+ haemopoietic progenitor cells are demonstrable in the blood of atopic subjects when compared with normal subjects.7, 8 Furthermore, there is an increased responsiveness of these cells to IL-5 in subjects who develop a dual asthmatic response compared with those with only an isolated early response after allergen inhalation.10 A significant increase in the number of bone marrow-derived CD34+ cells expressing the α-subunit of the IL-5 receptor (IL-5Rα)9 as well as CCR311 is found 24 hr after allergen challenge in dual responders, but not in isolated early responders. These results suggest that, in the bone marrow of those subjects who develop a greater eosinophilic airway response after allergen inhalation, a distinct phenotypic switch and migratory response of progenitor cells occurs in response to IL-5 and eotaxin, contributing to the development of blood and tissue eosinophilia.
Most experimental studies of the pathogenesis of allergic rhinitis and asthma employ models of airway allergic inflammation in mice. We have developed murine models with pure upper airway inflammation in the nasal mucosa12 and lower airway inflammation in lung tissue.5 In these models, it has been shown that tissue, blood and bone marrow events initiated in response to allergen provocation are required for the pathogenesis of airway inflammation. However, murine models of allergic rhinitis or asthma13, 14 in mice have focused on one part of the airway in isolation, without studying rhinitis in the context of asthma, leaving the precise haemopoietic mechanisms underlying linkages between upper and lower airway inflammation to be fully clarified. In the present study, we established parallel murine experimental allergic models with isolated upper, isolated lower and both upper and lower airway inflammation to investigate both local and systemic responses in relation to the degree and location of airway involvement.
Materials and methods
Animals
Female BALB/c mice (8–10 week of age) were purchased from Charles River Therion (Troy, NY), and housed under specific pathogen-free conditions for 1 week prior to experimental use. All procedures were performed in accordance with the ethical guidelines of the Canadian Council on Animal Care, and reviewed and approved by the Animal Research Ethics Board at McMaster University.
Allergen sensitization and challenge protocol
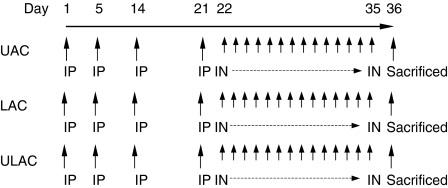
Intraperitoneal ovalbumin (OVA) (Sigma, St. Louis, MO) injections involved precipitating 40 mg/kg of aluminium potassium sulphate with 40 μg/kg OVA, adjusting to pH 6·5, centrifugation then resuspension of the pellet, followed by a 500 μl (1 μg OVA) intraperitoneal injection. Intranasal OVA involved dissolving 25 mg OVA in 1 ml sterile normal saline, followed by intranasal instillation into mice using a sterile pipette. Control mice were sensitized and challenged with diluent. The OVA sensitization and challenge protocol was similar to that described previously.12 It is shown in Fig. 1 and described as follows.
Figure 1.
Protocol for ovalbumin (OVA) intraperitoneal (i.p.) sensitization and subsequent OVA intranasal (i.n.) challenge. UAC, upper airway challenge group; LAC, lower airway challenge group; ULAC, upper and lower airway challenge group.
Mice were studied in four groups (n = 10 for each group).
Group 1: Pure Upper Airway Challenge (UAC).
Five hundred microlitres of 0.005% OVA was injected intraperitoneally (i.p.) on days 1, 5, 14 and 21 and OVA (500 μg/20 μl) was given intranasally (i.n.) daily from day 22 to day 35.
Group 2: Pure Lower Airway Challenge (LAC).
Five hundred microlitres of 0.005% OVA was injected i.p. on days 1, 5, 14 and 21 and OVA (250 μg/10 μl) was given i.n. with anaesthesia seven times between day 22 and day 35. Noses were rinsed with 10 μl normal saline each time after the application of OVA.
Group 3: Upper and Lower Airway Challenge (ULAC).
Five hundred microlitres of 0.005% OVA was injected i.p. on days 1, 5, 14 and 21 and OVA (500 μg/20 μl) was given i.n. without anaesthesia and OVA (250 μg/10 μl) was given i.n. with anaesthesia on alternate days from day 22 to day 35.
Group 4: Control.
Mice were sensitized and challenged with normal saline, using the same volume of solution as used in the OVA-treated mice, applied both i.p. and i.n., respectively.
Kinetic study
The UAC and ULAC mice were also used for kinetic studies; after the daily i.n. OVA challenge from days 22 to 35, the mice were allowed a 5-day recovery interval, then i.n. OVA provocation was performed on day 43. The mice were killed for outcome measurements prechallenge and 2, 12, 24 and 48 hr postchallenge (n = 5 at each time-point).
Outcome measurements
Mice were killed at the times indicated post-i.n. provocation with OVA or normal saline, by cardiac puncture after induction of deep anaesthesia, using a solution that contained ketamine hydrochloride (Ketalean®; Bimeda-MTC, Animal Health Inc., Cambridge, ON, Canada) and xylazine (Rompun®; Bayer Inc. Agriculture Division, Animal Health, Toronto, ON, Canada), diluted in sterile normal saline. Nasal and bronchoalveolar lavages were performed, peripheral blood was drawn, and nose and lung tissue and femoral bone marrow (BM) were removed and processed immediately. Outcome measurements included IL-5 and eotaxin levels in nasal lavage (NL) fluid, bronchoalveolar lavage (BAL) fluid, peripheral blood and BM. Eosinophils and CD4+ cells were counted in the nasal mucosa and lung tissue, as were Eo/B-CFU and CD34+ cells in BM, all at the indicated times after final intranasal OVA challenge.
Tissue preparation
Nose and lung tissues were removed and placed in prelabelled base moulds with frozen tissue matrix (OCT®) (Sakura Finetek USA, Inc. Torrance, CA). Base moulds with tissue in the frozen tissue matrix were plunged into 2-methylbutane (Sigma, St Louis, MO) prechilled in a Dewar flask of liquid nitrogen until the block had almost solidified. The tissue block was removed and placed on dry ice. The embedded tissues were cut into 4-μm sections using a microtome for ultrathin sections (Ultra Cut; Leica, Microsystems, Wetzlar, Germany).
Nasal and bronchoalveolar lavage
Under deep anaesthesia, the neck soft tissue was removed and the trachea was exposed and cannulated using two blunted 18-gauge needles (the first needle pointing toward the head for NL fluid, the second one toward the chest for BAL fluid). One injection of 500 μl phosphate-buffered saline was performed and the fluid was collected with a tube under both nares of the nose for NL fluid; two injections of 250 μl phosphate-buffered saline were injected and withdrawn through the needle for BAL fluid. The NL and BAL fluid were centrifuged for 10 min at 150 g at 4°. The supernatants were stored at −70° for IL-5 and eotaxin assays.
Bone marrow preparation and methylcellulose cultures
After mice were killed by terminal exsanguination via cardiac puncture, one femur was removed from each mouse and freed of soft tissue. BM cells were flushed using 3 ml McCoy's 3+ buffer injected through a 25-gauge needle. To break up cell clumps, the suspension was passed through needles of 18, 20 and 22 gauge. The mixture was then centrifuged for 10 min at 150 g at 4°, and the supernatants were stored at − 70° for IL-5 and eotaxin assays. Suspended cells were separated by density-gradient centrifugation over LymphoPrep (NYCOMED Pharma, Oslo, Norway) for 25 min at 850 g, and the cells at the interface were removed. The mononuclear cells were washed once with McCoy's 3+ buffer, then incubated for 2 hr in plastic flasks at 37° and 5% CO2 to remove adherent cells (which interfere with the growth of progenitor cells in semisolid culture). The non-adherent mononuclear cells (NAMNC) were diluted to a concentration of 5 × 105/ml in McCoy's 3+ buffer and cultured in 35 × 10-mm tissue culture dishes (Falcon Plastics, Oxnard, CA) in a culture medium that comprised 0·9% methylcellulose (The Dow Chemical Company, Midland, MI), 20% fetal calf serum (Gibco BRL), Iscove's Dulbecco's medium (with 1% penicillin–streptomycin, 0·5 μg of 2-mercaptoethanol and 0·1% bovine serum albumin) and the following recombinant mouse (rm) cytokines (R & D Systems Inc., Minneapolis, MN): rmIL-5 (5 ng/ml) in the presence of 1 × 105 NAMNC, rmIL-3 (5 ng/ml) in the presence of 5 × 104 NAMNC, or rm granulocyte–macrophage colony-stimulating factor (GM-CSF; 5 ng/ml) in the presence of 2·5 × 104 NAMNC. Following 6 days of culture, colonies of more than 40 cells were counted using an inverted microscope; Eo/B-CFU were classified using morphological and histological criteria (tight, compact, round refractile cell aggregates). To confirm the identification of colonies as Eo/B-CFU, samples were selected at random from cultures, placed on slides and stained with Diff-Quick (Baxter, McGaw Park, IL), as previously described in detail for both human and murine Eo/B-CFU.5, 14
The NAMNC at a concentration of 5 × 105/ml in McCoy's 3+ buffer were also used to prepare cytocentrifuge slides (Cytospin 3; Shandon Scientific, Sewickly, PA) on 3-aminopropyltriethoxysilane (APTEX)-coated glass slides and stained with Diff-Quick. Eosinophil counts were performed based on morphological and histological criteria (400 cells counted).
Immunostaining
To identify eosinophils in the nasal mucosa, bronchial and lung tissue, slides were fixed in acetone–methanol at room temperature for 10 min, then stained in Diff-Quick; slides were then embedded in Permount (Fisher Scientific, Fair Lawn, NJ). To identify CD4+ lymphocytes in airway tissues and CD34+ cells in BM cytocentrifuge preparations, a streptavidin–biotin complex immunostaining system method was employed, using specific anti-murine monoclonal antibodies (mAbs); anti-murine CD4 mAb [L3T4, rat immunoglobulin G2a (IgG2a), κ; BD Bioscience Canada, Mississauga, ON, Canada); and anti-murine CD34 mAb (rat IgG2a, k; BD Bioscience Canada), as previously described but with some modification.12 Briefly, OCT®-embedded tissue slides and cytocentrifuge slides were fixed at −70° in cold acetone for 15 min before pretreatment (to inhibit endogenous peroxidase activity) with a solution of 0·1% sodium azide and 0·3% hydrogen peroxide for 30 min. Slides were then washed with Tris-buffered saline (TBS), treated for 30 min with TBS containing 1% skimmed milk (Carnation; Nestlé, Don Mills, ON, Canada) and 10% fetal bovine serum (Gibco BRL), followed by incubation for 20 min with sterile water saturated with bovine serum albumin (Sigma) and 10% normal goat serum in TBS to block non-specific reactivity. Each mAb was then added and the mixture was incubated overnight at room temperature. Bound antibodies were then labelled with biotinylated second-stage antibodies appropriate to the isotype of each primary antibody (BD Bioscience Canada) for 2 hr, and detected using a streptavidin–biotin peroxidase detection system (StreptABComplex/HRP, DAKO Canada). Aminoethylcarbazole (AEC; Sigma) was then applied as a chromogen and the sections were counterstained with Mayer's haematoxylin (Sigma) to contrast with the red positive staining of AEC. Negative control sections were similarly treated with the same isotype immunoglobulin at the same concentrations of primary antibodies. As a positive control, murine spleen tissue was stained by the same procedure.
Evaluation and quantification of staining
In the lamina propria of nasal mucosa, all cells expressing positive immunoreactivity for cellular surface marker were counted. The area of the nasal tissue was measured, excluding glands, using an eyepiece with a grid, and the cell count results were expressed as the number of cells/mm2 of lamina propria; the same method of evaluation was employed for lung tissue. For BM evaluation, differential cell counts were performed on cytospin preparations of BM cells after application of Diff-Quick stain; eosinophilic cells on each slide were enumerated by light microscopy and results were expressed as the number of cells per total cells counted or as a percentage of total femoral BM cells. CD34+ cells on BM cytospins were also counted by light microscopy: 1000 mononuclear BM cells were counted and the result was expressed as the absolute number of positive cells per total mononuclear cells of the femoral BM.
Blood smears and serum analyses
Blood samples were obtained by cardiac puncture and blood smears were prepared in duplicate. Total white blood cells were counted in heparinized blood using a haemocytometer. Serum was obtained by centrifugation for 10 min at 150 g and 4° and stored at −70° for IL-5, and eotaxin assays. Differential cell counts were performed on the blood smears using Diff-Quick stain and cells were classified as neutrophils, lymphocytes, monocytes, eosinophils and basophilic cells, based on morphological and histological criteria.
IL-5 assay
IL-5 levels in NL fluid, BAL fluid, serum and BM fluid were assayed by enzyme-linked immunosorbent assay (ELISA) using a mouse IL-5 kit (R & D Systems, Minneapolis, MN). The sensitivity of detection of the ELISA was 5 pg/ml.
Eotaxin assay
Eotaxin levels in NL fluid, BAL fluid, serum and BM fluid were assayed by ELISA using a mouse eotaxin kit (R & D Systems). The sensitivity of detection of the ELISA kit was 5 pg/ml.
Statistics
For all cell counts, stained slides were read randomly and in a blinded manner. All statistical analyses were performed using spss software (Version 10, SPSS Inc., Chicago, IL). All summary statistics were expressed as the mean ± SD. Group mean comparisons were performed on the absolute numbers using repeated measures analysis of variance (anova). After significant group effects, Newman–Keuls post-hoc analysis was used for specific comparisons. All comparisons were two tailed and P < 0·05 was considered statistically significant for all analyses.
Results
BM Eo/B-CFU
Using 5 ng/ml of rmIL-3 in vitro and per 5 × 104 NAMNC plated, all the treatment groups showed significantly higher numbers of Eo/B-CFU than the control group (22·2 ± 3·2, 168·0 ± 27·1, 178·7 ± 14·2 and 101·9 ± 13·4 for control, UAC, LAC and ULAC groups, respectively, P < 0·05) at 24 hr postchallenge, while the number in the ULAC group was significantly lower (P < 0·05) than in the UAC and LAC groups. Using 5 ng/ml of rmIL-5 and rmGM-CSF in vitro, and 1 × 105 and 2·5 × 105 per NAMNC plated, respectively, again Eo/B-CFU counts in all three treatment groups were significantly greater than in the control group at 24 hr (rmIL-5: 23·0 ± 4·0, 87·2 ± 9·6, 70·9 ± 5·4 and 56·5 ± 7·6; rmGM-CSF: 31·3 ± 4·7, 78·5 ± 11·4, 66·3 ± 4·3 and 48·8 ± 6·2 for control, UAC, LAC and ULAC groups, respectively, P < 0·05) while the counts in the ULAC group were significantly lower than in the UAC group at 24 hr (P < 0·05) (Fig. 2).
Figure 2.
Eo/B-CFU in 6-day methylcellulose cultures of murine bone marrow (mean ± SD). Eo/B-CFU in the presence of 5 ng/ml each in rmIL-3, rmIL-5, and rmGM-CSF. Control, control group; UAC, upper airway challenge group; LAC, lower airway challenge group; ULAC, upper and lower airway challenge group.
BM analysis
In comparisons among the four groups, a significant increase in BM eosinophil counts was observed after OVA challenge in all three treatment groups. The number of CD34+ cells also increased significantly in all three treatment groups compared to control. This number in the ULAC mice was significantly lower than in the UAC group at 24 hr postchallenge (P < 0·05) (Table 1).
Table 1.
Pathological changes in nose, lung, peripheral blood and bone marrow 24 hr after final intranasal challenge
| Control | UAC | LAC | ULAC | |
|---|---|---|---|---|
| Nasal mucosa and NL fluid | ||||
| Eosinophils1 | 12·96 ± 19·73 | 459·04 ± 167·07** | 6·88 ± 8·36 | 499·22 ± 183·84** |
| CD4 + cells1 | 167·82 ± 44·54 | 417·80 ± 69·71** | 184·03 ± 24·15 | 379·44 ± 39·26** |
| Eotaxin2 | 1·54 ± 1·89 | 50·85 ± 37·80** | 2·23 ± 1·94 | 44·46 ± 32·73** |
| Lung tissue and BAL fluid | ||||
| Eosinophils1 | 4·33 ± 3·19 | 6·26 ± 5·08 | 1965·30 ± 327·13** | 1584·86 ± 422·52** |
| CD4+ cells1 | 286·11 ± 50·57 | 308·42 ± 79·06 | 624·64 ± 198·44** | 725·57 ± 270·08** |
| IL-53 | 0·57 ± 0·63 | 0·75 ± 0·91 | 11·73 ± 10·05** | 9·34 ± 6·42** |
| Eotaxin3 | 0·13 ± 0·25 | 0·29 ± 0·32 | 102·09 ± 42·14** | 73·94 ± 31·27** |
| Peripheral blood | ||||
| Eosinophils4 | 4·20 ± 1·30 | 11·20 ± 2·30** | 12·80 ± 2·90** | 15·40 ± 3·00** |
| IL-55 | 2·05 ± 1·37 | 19·45 ± 12·49* | 21·30 ± 20·38* | 20·42 ± 17·83* |
| Eotaxin5 | 412·36 ± 55·25 | 443·00 ± 58·41 | 401·62 ± 45·78 | 481·96 ± 102·63 |
| Bone marrow | ||||
| Eosinophils6 | 4·07 ± 2·08 | 7·94 ± 2·40** | 7·51 ± 2·69** | 7·44 ± 2·01** |
| CD34+ cells6 | 16·10 ± 1·66 | 40·10 ± 3·80** | 38·9 ± 5·21** | 26·30 ± 2·36*† |
Number of cells (mean ± SD)/mm
of tissue;
(mean ± SD)ng/ml of NL;
(mean ± SD)ng/ml of BAL;
number of cells (mean ± SD) × 104/ml of blood;
(mean ± SD)ng/ml of blood;
number of cells (mean ± SD) × 106/femur;
P < 0·05,
P < 0·01 compared with the control group,
P < 0·05 compared with UAC group.
Control, control group; UAC, upper airway challenge group; LAC, lower airway challenge group; ULAC, upper and lower airway challenge group.
Pathological changes in airway tissues
The number of eosinophils was increased significantly in the nasal mucosa in both UAC and ULAC mice at 24 hr (P < 0·001), while there was no significant difference in LAC mice when compared with the control group. There was a remarkable increase in the number of eosinophils in lung tissue in LAC and ULAC groups (P < 0·001), but not in UAC mice as compared with controls at 24 hr (Table 1).
A significant increase in the number of CD4+ cells in nasal mucosa occurred 24 hr after i.n. daily challenge with OVA in UAC and ULAC mice (P < 0·00001) but not in LAC mice, compared with the control group. The number of CD4+ cells in lung tissue was significantly elevated in LAC and ULAC mice (P < 0·0001) but not in UAC mice when compared with the control group (Table 1).
Eosinophils in peripheral blood circulation
In peripheral blood, the absolute number of eosinophils was significantly increased in all treatment groups (P < 0·01) when compared with the controls at 24 hr (Table 1).
IL-5 levels in NL fluid, BAL fluid, serum and BM fluid
IL-5 was not detected above background level in NL and BM fluids in any mouse. In BAL fluid, both LAC and ULAC (P < 0·003), but not UAC, mice, had a significantly higher level of IL-5 than controls. IL-5 in serum was elevated significantly in all treatment groups compared to control mice at 24 hr (Table 1).
Eotaxin in NL fluid, BAL fluid, serum and BM fluid
Eotaxin level was elevated significantly in NL fluid in UAC and ULAC (P < 0·0007) mice but not in the LAC group as compared with control mice. In BAL fluid, LAC and ULAC, but not UAC, mice had significantly higher levels of eotaxin than controls (P < 0·00007). Eotaxin levels in serum were not significantly different among the four groups and could not be detected above the measurable limit in BM fluid in all groups (Table 1).
Kinetics of inflammatory changes in BM and tissue
Because BM Eo/B-CFU counts and CD34+ cells in ULAC mice were significantly lower than the other two treatment groups in response to three haemopoietic cytokines 24 hr after the last OVA challenge, a second study was performed to observe the kinetics of inflammatory responses of BM and tissue in this group, using UAC mice as controls.
Bone marrow Eo/B-CFU
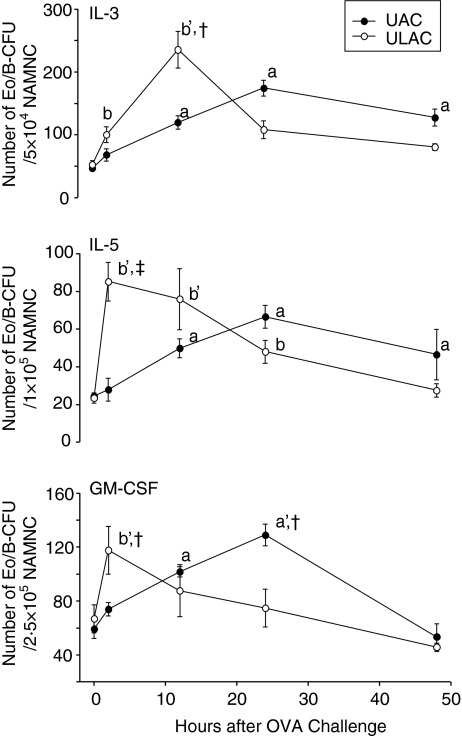
The number of BM Eo/B-CFU in the presence of IL-3, IL-5 and GM-CSF in ULAC mice peaked at 2 or 12 hr after OVA challenge while in the UAC group the peak was 24 hr after OVA challenge (Fig. 3).
Figure 3.
The time–course of Eo/B-CFU in 6-day methylcellulose cultures of murine bone marrow (mean ± SD). Eo/B-CFU in the presence of 5 ng/ml each in rmIL-3, rmIL-5, and rmGM-CSF. UAC, upper airway challenge group; ULAC, upper and lower airway challenge group. aP < 0·05, a'P < 0·01 compared with prechallenge (time 0) in UAC group; bP < 0·05, b'P < 0·01 compared with prechallenge (time 0) in ULAC group; †P < 0·05, ‡P < 0·01 UAC compared with ULAC at the same time-point.
Bone marrow CD34 cells
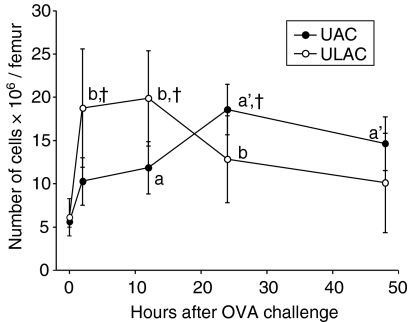
Similarly, BM CD34+ cells increased significantly at 2 hr and peaked at 12 hr in ULAC mice whereas the peak in the UAC group occurred at 24 hr after OVA challenge (Fig. 4). BM eosinophils decreased significantly (P < 0·05) at 2 hr in ULAC mice and 12 hr in the UAC group, followed by a remarkable increase (P < 0·01) at 12 hr in ULAC mice and 24 hr in the UAC group. The eosinophil counts decreased gradually from 12 to 48 hr postchallenge in ULAC mice while they remained at high levels from 24 to 48 hr postchallenge in the UAC group (Fig. 5).
Figure 4.
The time–course of CD34+ cells in murine bone marrow (mean ± SD). UAC, upper airway challenge group; ULAC, upper and lower airway challenge group. aP < 0·05, a'P < 0·01 compared with prechallenge (time 0) in UAC group; bP < 0·05 compared with prechallenge (time 0) in ULAC group; †P < 0·05 UAC compared with ULAC at the same time-point.
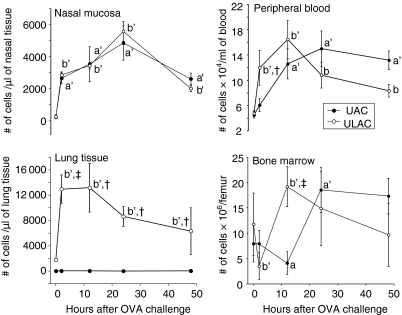
Figure 5.
The time–course of eosinophils in murine nasal mucosa, lung tissue, peripheral blood and bone marrow (mean ± SD). UAC, upper airway challenge group; ULAC, upper and lower airway challenge group. aP < 0·05, a'P < 0·01 compared with prechallenge (time 0) in UAC group; bP < 0·05, b'P < 0·01 compared with prechallenge (time 0) in ULAC group; †P < 0·05, ‡P < 0·01 UAC compared with ULAC at the same time-point.
Eosinophils in blood and tissues
In peripheral blood, eosinophil numbers in UAC mice increased significantly at 12 hr, peaked at 24 hr and remained at significantly high levels at 48 hr after OVA challenge while the absolute number of eosinophils was significantly increased at 2 hr, peaked at 12 hr and also remained at high levels at 24 hr and 48 hr postchallenge in ULAC mice (Fig. 5).
Eosinophils in nasal mucosa in both UAC and ULAC groups were elevated significantly at 2 hr and peaked at 24 hr postchallenge. In lung tissue, the number of eosinophils showed no significant changes in UAC mice during the 48 hr postchallenge period, while numbers increased significantly at 2 hr, peaked at 12 hr, and remained at high levels from 24 to 48 hr after challenge in the ULAC group (Fig. 5).
Cytokine and chemokine responses
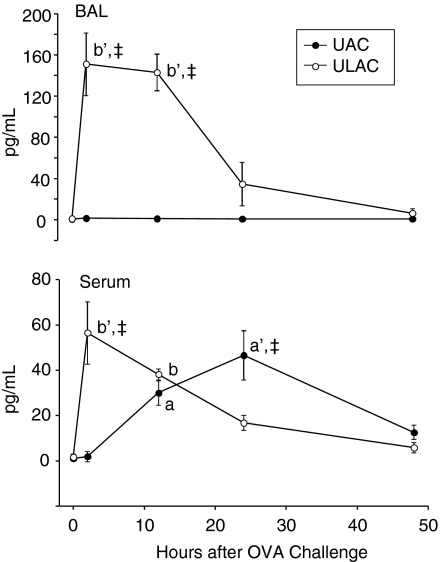
IL-5 levels did not significantly change in BAL fluid in UAC mice, while increases were observed in ULAC mice, peaking at 2 hr, remaining high at 12 hr postchallenge (P < 0·01), and decreasing after 24 hr in ULAC mice (Fig. 6). In serum, IL-5 level was elevated significantly at 12 hr and peaked at 24 hr postchallenge (P < 0·01) in UAC mice, whereas the peak of serum IL-5 occurred 2 hr postchallenge in the ULAC group (P < 0·01) (Fig. 6).
Figure 6.
The time–course of IL-5 levels in murine bronchoalveolar lavage fluid (BAL) and serum (mean ± SD). UAC: upper airway challenge group; ULAC, upper and lower airway challenge group. aP < 0·05, a'P < 0·01 compared with prechallenge (time 0) in UAC group; bP < 0·05, b'P < 0·01 compared with prechallenge (time 0) in ULAC group; ‡P < 0·01 UAC compared with ULAC at the same time-point.
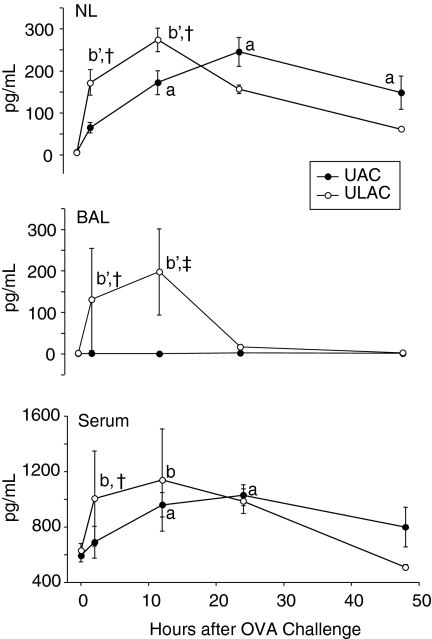
Eotaxin levels did not significantly change in BAL fluid in UAC mice compared to increases at 12 hr, peaking at 24 hr both in NL fluid and serum in these animals (P < 0·05) (Fig. 7). In ULAC mice, eotaxin levels in NL fluid, BAL fluid and serum were elevated significantly at 2 hr, and peaked at 12 hr after challenge (P < 0·05), at which time they started to decrease (Fig. 7).
Figure 7.
The time–course of eotaxin levels in murine nasal lavage fluid (NL), bronchoalveolar lavage fluid (BAL) and serum (mean ± SD). UAC, upper airway challenge group; ULAC, upper and lower airway challenge group. aP < 0·05 compared with prechallenge (time 0) in UAC group; bP < 0·05, b'P < 0·01 compared with prechallenge (time 0) in ULAC group; †P < 0·05, ‡P < 0·01 UAC compared with ULAC at the same time-point.
Discussion
Both local and systemic aspects of allergic (eosinophilic) inflammation at given airway mucosal sites involve, at least in part, haemopoietic (eosinophilopoietic) mechanisms. In the current study, we were able to establish either pure upper and/or lower airways eosinophilic inflammation, utilizing parallel models of sensitization and challenge. The present study has focused, in particular, on key markers of systemic responses, including haemopoietic cytokine- and chemokine-induced BM progenitor cell recruitment during the induction of allergic inflammation in the upper or lower airways.
Sensitization and challenge by the methods described have generated models of airway compartmentalization of inflammatory cellular and molecular responses. In several previous studies of murine experimental allergic rhinitis, the lower airway was not formally examined in parallel;13–15 several previous studies in mice have focused on the lower airway only.14, 16 Although the experimental protocol in this study does not completely reproduce airway allergen exposure in humans, it allows for a study of local and systemic inflammatory/immune responses using aerosolized allergen to challenge the upper and lower airways of the animals simultaneously.
Anatomical factors involved in the localization of inflammatory changes to a specific airway site might include: the amount of antigen presented at any site; alertness of the animals during challenge (involving intact or anaesthetized pharyngeal reflexes); posture of the animals (head-down position for upper airway challenge and upright position for lower airway challenge); and whether or not nasal cavity lavage (after OVA application) is performed. We were able to control exposure of either the upper or the lower airway to the antigen, without contamination of the reciprocal compartment, as evidenced by negative trypan blue staining, respectively, in the tracheal region (lower airway in isolated upper airway challenge), or the nasal cavity (isolated lower airway challenge) (data not shown).
It is known that administration of allergen into the nasal cavities of individuals with allergic rhinitis results in acute nasal reactions that involve the typical symptoms of rhinitis, but not of asthma, accompanied by the release of inflammatory mediators and the development of acute inflammatory changes in the upper airway only.17 As long as the allergen is administered only to the nose and not inhaled into the lower airways, some investigators report no evidence of acute effects on lower airways, 18 compatible with the ‘anatomical control’ of allergen exposure in our models. However, there is evidence that neural stimulation of the nasal mucosa, for example with cold air or histamine, may produce acute bronchoconstriction in asthmatic patients19, 20 and it is not rare to observe lower airway airflow limitation in patients with asthma several hours after nasal-allergen provocation;18–20 nasal-allergen challenge can lead almost invariably to increased lower airway responsiveness when repetitive nasal provocations are administered to induce priming.18, 20 Braunstahl et al. have shown that lower airways eosinophilia develops after nasal-allergen provocation in subjects with allergic rhinitis.21 Given our current findings, and the above evidence in humans, one can hypothesize that isolated allergic rhinitis may eventually cause lower airway responses if the dose of allergen is increased or the duration of allergen exposure is prolonged enough to activate the ‘cross-links’ between upper and lower airways through local and/or systemic pathways. Thus, it is possible that anatomical factors are necessary in determining the degree and localization of airway inflammation, but not sufficient to explain the impact of rhinitis on asthma.1
Exposure to common aeroallergens leads to activation of CD4+ T lymphocytes and the release of cytokines.22 Indeed, we have measured a significant increase in the number of CD4+ cells in nasal mucosa in UAC and ULAC mice, and in lung tissue in LAC and ULAC mice, after daily allergen challenge. In a clinical study, Kelly et al.23 showed an increase in activated CD4+ cells in BAL fluid, accompanied by increases in IL-5 protein following allergen challenge in both rhinitis and asthma patients. Saito et al.12 showed that nasal symptoms and hyper-responsiveness appear, accompanied by significant nasal mucosal changes in CD4+, IL-4+ and IL-5+ cells, in a murine model of isolated allergic rhinitis. Moreover, airway allergen challenge in sensitized subjects elicits eosinophilic inflammation in the airways in both humans2, 24 and mice 5, 12, 25 and is related to enhanced eosinophilopoiesis in the BM. 12, 14, 25
We observed that both immature and mature eosinophils in the BM and peripheral blood increased significantly during OVA challenge in the three different airway inflammatory models, indicating BM participation in each case, in agreement with our previous studies of Eo/B-CFU in blood and BM in patients with a variety of atopic disorders.7, 8, 26–28 The current study formally demonstrates, for the first time, the activation of BM inflammatory cells and their progenitors in situations in which isolated upper airway, isolated lower airway, or both upper and lower airway responses are generated, which has been difficult to demonstrate in human studies.7, 8, 26, 27 This supports the notion that eosinophils develop and mature in the BM from haemopoietic progenitor cells in response to antigen challenge at any one site.
The time point of 24 hr initially used to measure tissue and BM changes was chosen based on our previous protocols.5, 12 Since BM CD34+ cells and Eo/B-CFU counts in ULAC mice were significantly lower than the other two treatment groups at this time-point, we embarked upon a kinetic study. Results indicated that BM eosinophils decreased as early as 2 hr in ULAC mice, accompanied by increases in circulating eosinophils and the development of tissue eosinophilia, suggesting that there was a brief period during which eosinophil recruitment from the BM exceeded production rates. Bone marrow eosinophils peaked at 12 hr, matching the time of increases found in BAL fluid eosinophils, circulating IL-5 levels and BM CD34+ cells; systemic IL-5 may thus be responsible for stimulating more rapid eosinophilopoiesis in this model. While UAC mice had similar profiles in the BM and blood, this occurred almost 10 hr later than ULAC mice, matched by later IL-5 and CD34+ responses.
Other studies have also investigated the time course of BM eosinophils in response to allergen exposure. A study by Ohkawara and co-workers25 using OVA-sensitized mice demonstrated that there was a decrease in BM eosinophil numbers in the first 3–6 hr after airway allergen challenge, followed by airway, blood and marrow eosinophilia that peaked later and persisted for 10–15 days. These data, and results from our study, suggest that progenitor cell expansion in the BM is necessary to restore and increase eosinophil numbers following allergen exposure, with the increase in eosinophil production lasting for the duration of the local airway inflammatory responses.29 These findings are mirrored by changes in CD34+ progenitor cells in the BM during airway allergen challenge: these cells are maintained at high levels during the daily antigen challenge protocol in all three different airway inflammatory models, with a rapid response as early as 2 hr and maximally by 12 hr in ULAC mice. This is in accordance with our previous findings of higher than normal levels of CD34+ cells in studies of blood and marrow in atopic (asthmatic/allergic and rhinitic) individuals8, 10, 30 and in the murine model of isolated rhinitis.12 Indeed, Eo/B-CFU also increased significantly in all three airway inflammatory models, indicating that the BM appears to be also activated functionally. The demonstration of a four-fold increase in the absolute numbers of IL-5-sensitive, Eo/B-CFU at 2 hr in ULAC mice after allergen challenge is in disagreement with another murine model of asthma14 in which the IL-5-sensitive progenitors were maximally responsive at 24 hr. Our recent kinetic studies in human asthmatic subjects31 are compatible with progenitor responses (CD34+ cells expressing IL-5Rα and other cytokine and chemokine receptors) in primed subjects occurring earlier than 24 hr after allergen challenge.
Several studies have stressed the importance of systemic or local effects of IL-5 and eotaxin produced in the airways.5, 13, 32 Based on our observations, we propose that signalling between the airways and BM in response to allergen exposure involves systemic elaboration of haemopoietic/chemotactic mediators. However, both Minshall et al. and Wood et al. have observed that following exposure of airways in mice or human subjects to allergen, there is an increase in CD3+ cells within the BM; many of these cells contain both IL-5 mRNA and protein.30, 33 These results suggest a mechanism by which exposure to allergen at the airway is linked to haemopoietic cytokine production within the BM, a process which also may be operative in our murine models. Circulating and/or locally produced IL-5 can stimulate BM Eo/B progenitors; because IL-5 is detected in serum after allergen challenge in mice25 and because plasma taken from mice after allergen challenge is capable of increasing BM mature eosinophils when injected into naïve recipient mice5, 14 it is likely that both (and not either34, 35) local airway and circulating production of IL-5 critically determine eosinophil production in the BM; eotaxin reciprocally determines eosinophil trafficking to tissues. With regard to eotaxin, its undetectability in BM makes it likely that only locally active eotaxin is involved in the trafficking of eosinophils and their progenitors to the airways6 supported by our kinetic observations of significant increases in eotaxin levels in NL and BAL fluids, which coincide with the eosinophil efflux from BM. Increased IL-5 and eotaxin levels in serum have been shown after allergen challenge and during exacerbation of disease, 36, 37 although levels of these factors do not change in patients with stable asthma and rhinitis38 in whom higher numbers of blood eosinophils and skin test reactivity can be found.
In summary, we have studied local and systemic responses in upper and lower eosinophilic airway inflammation. Our experimental murine models provide useful tools with which to study the factors involved in the development or regulation of airway inflammation after upper or lower airway antigen exposure.
Acknowledgments
The authors would like to thank Drs Roma Sehmi and Gail M. Gauvreau for their valuable input, and Lisa Hayes, Russ Ellis and Jennifer Wattie for their help. This work was supported by the Merck Medical School Grants programme.
Abbreviations
- BAL
bronchoalveolar lavage fluid
- BM
bone marrow
- Eo/B-CFU
eosinophil/basophil colony-forming units
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- IL-5
interleukin 5
- IL-5Ralpha
the alpha sub-unit of the IL-5 receptor
- LAC
pure lower airway challenge
- mAb
monoclonal antibody
- rm
recombinant mouse
- NAMNC
non-adherent mononuclear cells
- NL
nasal lavage fluid
- OVA
ovalbumin
- UAC
pure upper airway challenge
- ULAC
upper and lower airway challenge
References
- 1.Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108:S147–S334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- 2.Kroegel C, Liu MC, Hubbard WC, Lichtenstein LM, Bochner BS. Blood and bronchoalveolar eosinophils in allergic subjects after segmental antigen challenge. Surface phenotype, density heterogeneity, and prostanoid production. J Allergy Clin Immunol. 1994;93:725–34. doi: 10.1016/0091-6749(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 3.Sanderson CJ. The biological role of interleukin 5. Int J Cell Cloning. 1990;8(Suppl. 1):147–53. doi: 10.1002/stem.5530080713. [DOI] [PubMed] [Google Scholar]
- 4.MacLean JA, Ownbey R, Luster AD. T cell-dependent regulation of eotaxin in antigen-induced pulmonary eosinophilia. J Exp Med. 1996;184:1461–9. doi: 10.1084/jem.184.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inman MD, Ellis R, Wattie J, Denburg JA, O'Byrne PM. Allergen-induced increase in airway responsiveness, airway eosinophilia, and bone-marrow eosinophil progenitors in mice. Am J Respir Cell Mol Biol. 1999;21:473–9. doi: 10.1165/ajrcmb.21.4.3622. [DOI] [PubMed] [Google Scholar]
- 6.Palframan RT, Collins PD, Williams TJ, Rankin SM. Eotaxin induces a rapid release of eosinophils and their progenitors from the bone marrow. Blood. 1998;91:2240–8. [PubMed] [Google Scholar]
- 7.Denburg JA, Telizyn S, Belda A, Dolovich J, Bienenstock J. Increased numbers of circulating basophil progenitors in atopic patients. J Allergy Clin Immunol. 1985;76:466–72. doi: 10.1016/0091-6749(85)90728-6. [DOI] [PubMed] [Google Scholar]
- 8.Sehmi R, Howie K, Sutherland DR, Schragge W, O'Byrne PM, Denburg JA. Increased levels of CD34+ hemopoietic progenitor cells in atopic subjects. Am J Respir Cell Mol Biol. 1996;15:645–55. doi: 10.1165/ajrcmb.15.5.8918371. [DOI] [PubMed] [Google Scholar]
- 9.Sehmi R, Wood LJ, Watson R, Foley R, Hamid Q, O'Byrne PM, Denburg JA. Allergen-induced increases in IL-5 receptor alpha-subunit expression on bone marrow-derived CD34+ cells from asthmatic subjects. A novel marker of progenitor cell commitment towards eosinophilic differentiation. J Clin Invest. 1997;100:2466–75. doi: 10.1172/JCI119789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood LJ, Inman MD, Watson RM, Foley R, Denburg JA, O'Byrne PM. Changes in bone marrow inflammatory cell progenitors after inhaled allergen in asthmatic subjects. Am J Respir Crit Care Med. 1998;157:99–105. doi: 10.1164/ajrccm.157.1.9704125. [DOI] [PubMed] [Google Scholar]
- 11.Sehmi R, Dorman S, Baatjes A, et al. Allergen-induced fluctuation in CC chemokine receptor 3 expression on bone marrow CD34+ cells from asthmatic subjects: significance for mobilization of haemopoietic progenitor cells in allergic inflammation. Immunology. 2003;109:536–46. doi: 10.1046/j.1365-2567.2003.01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito H, Howie K, Wattie J, Denburg A, Ellis R, Inman MD, Denburg JA. Allergen-induced murine upper airway inflammation: local and systemic changes in murine experimental allergic rhinitis. Immunology. 2001;104:226–34. doi: 10.1046/j.0019-2805.2001.01253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van de Rijn M, Mehlhop PD, Judkins A, Rothenberg ME, Luster AD, Oettgen HC. A murine model of allergic rhinitis: studies on the role of IgE in pathogenesis and analysis of the eosinophil influx elicited by allergen and eotaxin. J Allergy Clin Immunol. 1998;102:65–74. doi: 10.1016/s0091-6749(98)70056-9. [DOI] [PubMed] [Google Scholar]
- 14.Gaspar Elsas MI, Joseph D, Elsas PX, Vargaftig BB. Rapid increase in bone-marrow eosinophil production and responses to eosinopoietic interleukins triggered by intranasal allergen challenge. Am J Respir Cell Mol Biol. 1997;17:404–13. doi: 10.1165/ajrcmb.17.4.2691. [DOI] [PubMed] [Google Scholar]
- 15.Hellings PW, Hessel EM, Van Den Oord JJ, Kasran A, Van Hecke P, Ceuppens JL. Eosinophilic rhinitis accompanies the development of lower airway inflammation and hyper-reactivity in sensitized mice exposed to aerosolized allergen. Clin Exp Allergy. 2001;31:782–90. doi: 10.1046/j.1365-2222.2001.01081.x. [DOI] [PubMed] [Google Scholar]
- 16.Eum SY, Haile S, Lefort J, Huerre M, Vargaftig BB. Eosinophil recruitment into the respiratory epithelium following antigenic challenge in hyper-IgE mice is accompanied by interleukin 5-dependent bronchial hyperresponsiveness. Proc Natl Acad Sci USA. 1995;92:12290–4. doi: 10.1073/pnas.92.26.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Togias A, Naclerio RM, Proud D, et al. Studies on the allergic and nonallergic nasal inflammation. J Allergy Clin Immunol. 1988;81:782–90. doi: 10.1016/0091-6749(88)90932-3. [DOI] [PubMed] [Google Scholar]
- 18.Corren J, Adinoff AD, Irvin CG. Changes in bronchial responsiveness following nasal provocation with allergen. J Allergy Clin Immunol. 1992;89:611–18. doi: 10.1016/0091-6749(92)90329-z. [DOI] [PubMed] [Google Scholar]
- 19.Fontanari P, Burnet H, Zattara-Hartmann MC, Jammes Y. Changes in airway resistance induced by nasal inhalation of cold dry, dry, or moist air in normal individuals. J Appl Physiol. 1996;81:1739–43. doi: 10.1152/jappl.1996.81.4.1739. [DOI] [PubMed] [Google Scholar]
- 20.Sanico AM, Atsuta S, Proud D, Togias A. Dose-dependent effects of capsaicin nasal challenge: in vivo evidence of human airway neurogenic inflammation. J Allergy Clin Immunol. 1997;100:632–41. doi: 10.1016/s0091-6749(97)70167-2. [DOI] [PubMed] [Google Scholar]
- 21.Braunstahl GJ, Overbeek SE, Kleinjan A, Prins JB, Hoogsteden HC, Fokkens WJ. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol. 2001;107:469–76. doi: 10.1067/mai.2001.113046. [DOI] [PubMed] [Google Scholar]
- 22.Corrigan CJ, Kay AB. Asthma. Role of T-lymphocytes and lymphokines. Br Med Bull. 1992;48:72–84. doi: 10.1093/oxfordjournals.bmb.a072543. [DOI] [PubMed] [Google Scholar]
- 23.Becky Kelly EA, Busse WW, Jarjour NN. A comparison of the airway response to segmental antigen bronchoprovocation in atopic asthma and allergic rhinitis. J Allergy Clin Immunol. 2003;111:79–86. doi: 10.1067/mai.2003.28. [DOI] [PubMed] [Google Scholar]
- 24.Beasley R, Roche WR, Roberts JA, Holgate ST. Cellular events in the bronchi in mild asthma and after bronchial provocation. Am Rev Respir Dis. 1989;139:806–17. doi: 10.1164/ajrccm/139.3.806. [DOI] [PubMed] [Google Scholar]
- 25.Ohkawara Y, Lei XF, Stampfli MR, Marshall JS, Xing Z, Jordana M. Cytokine and eosinophil responses in the lung, peripheral blood, and bone marrow compartments in a murine model of allergen-induced airways inflammation. Am J Respir Cell Mol Biol. 1997;16:510–20. doi: 10.1165/ajrcmb.16.5.9160833. [DOI] [PubMed] [Google Scholar]
- 26.Gibson PG, Manning PJ, O'Byrne PM, Girgis-Gabardo A, Dolovich J, Denburg JA, Hargreave FE. Allergen-induced asthmatic responses. Relationship between increases in airway responsiveness and increases in circulating eosinophils, basophils, and their progenitors. Am Rev Respir Dis. 1991;143:331–5. doi: 10.1164/ajrccm/143.2.331. [DOI] [PubMed] [Google Scholar]
- 27.Otsuka H, Dolovich J, Befus AD, Telizyn S, Bienenstock J, Denburg JA. Basophilic cell progenitors, nasal metachromatic cells, and peripheral blood basophils in ragweed-allergic patients. J Allergy Clin Immunol. 1986;78:365–71. doi: 10.1016/s0091-6749(86)80091-4. [DOI] [PubMed] [Google Scholar]
- 28.Denburg JA, Woolley M, Leber B, Linden M, O'Byrne P. Basophil and eosinophil differentiation in allergic reactions. J Allergy Clin Immunol. 1994;94:1135–41. doi: 10.1016/0091-6749(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 29.Inman MD. Bone marrow events in animal models of allergic inflammation and hyperresponsiveness. J Allergy Clin Immunol. 2000;106:S235–S241. doi: 10.1067/mai.2000.110155. [DOI] [PubMed] [Google Scholar]
- 30.Wood LJ, Sehmi R, Dorman S, et al. Allergen-induced increases in bone marrow T lymphocytes and interleukin-5 expression in subjects with asthma. Am J Respir Crit Care Med. 2002;166:883–9. doi: 10.1164/rccm.2108015. [DOI] [PubMed] [Google Scholar]
- 31.Dorman SC, Sehmi R, Gauvreau GM, et al. The kinetics of bone marrow eosinophilopoiesis and associated cytokines after allergen inhalation. Am J Respir Crit Care Med. 2004;169:565–72. doi: 10.1164/rccm.200307-1024OC. [DOI] [PubMed] [Google Scholar]
- 32.Matthews AN, Friend DS, Zimmermann N, Sarafi MN, Luster AD, Pearlman E, Wert SE, Rothenberg ME. Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci USA. 1998;95:6273–8. doi: 10.1073/pnas.95.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minshall EM, Schleimer R, Cameron L, Minnicozzi M, Egan RW, Gutierrez-Ramos JC, Eidelman DH, Hamid Q. Interleukin-5 expression in the bone marrow of sensitized Balb/c mice after allergen challenge. Am J Respir Crit Care Med. 1998;158:951–7. doi: 10.1164/ajrccm.158.3.9709114. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Palmer K, Lotvall J, et al. Circulating, but not local lung, IL-5 is required for the development of antigen-induced airways eosinophilia. J Clin Invest. 1998;102:1132–41. doi: 10.1172/JCI2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen H, O'Byrne PM, Ellis R, Wattie J, Tang C, Inman MD. The effects of intranasal budesonide on allergen-induced production of interleukin-5 and eotaxin, airways, blood, and bone marrow eosinophilia, and eosinophil progenitor expansion in sensitized mice. Am J Respir Crit Care Med. 2002;166:146–53. doi: 10.1164/rccm.2008161. [DOI] [PubMed] [Google Scholar]
- 36.Braunstahl GJ, Overbeek SE, Fokkens WJ, Kleinjan A, McEuen AR, Walls AF, Hoogsteden HC, Prins JB. Segmental bronchoprovocation in allergic rhinitis patients affects mast cell and basophil numbers in nasal and bronchial mucosa. Am J Respir Crit Care Med. 2001;164:858–65. doi: 10.1164/ajrccm.164.5.2006082. [DOI] [PubMed] [Google Scholar]
- 37.Lilly CM, Woodruff PG, Camargo CA, Jr, Nakamura H, Drazen JM, Nadel ES, Hanrahan JP. Elevated plasma eotaxin levels in patients with acute asthma. J Allergy Clin Immunol. 1999;104:786–90. doi: 10.1016/s0091-6749(99)70288-5. [DOI] [PubMed] [Google Scholar]
- 38.Braunstahl GJ, Fokkens WJ, Overbeek SE, Kleinjan A, Hoogsteden HC, Prins JB. Mucosal and systemic inflammatory changes in allergic rhinitis and asthma: a comparison between upper and lower airways. Clin Exp Allergy. 2003;33:579–87. doi: 10.1046/j.1365-2222.2003.01652.x. [DOI] [PubMed] [Google Scholar]