Abstract
Major histocompatibility complex (MHC) class II molecules expressed on monocytes may play a role in the control of differentiation of antigen-presenting cells. A soluble LAG-3 (CD223) molecule (sLAG-3) is a natural, high-affinity ligand for MHC class II. It is known to induce maturation of monocyte-derived dendritic cells in vitro and is used as a vaccine adjuvant to induce CD4 T helper type 1 responses and CD8 T-cell responses in vivo. Here, we demonstrate that sLAG-3 (but not an MHC class II-specific monoclonal antibody) reduces the differentiation of monocytes into macrophages in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) as well as their differentiation into dendritic cells in the presence of GM-CSF and interleukin-4, as shown by a decrease in CD14 and CD1a expression, respectively. Dendritic cells derived from monocytes in the presence of sLAG-3 showed impaired antigen-presentation function, as assessed by the reduced capability to induce proliferation of T cells. Our results suggest that activated LAG-3+ lymphocytes present at sites of inflammation may reduce the differentiation of monocytes into macrophages or fully competent antigen-presenting dendritic cells, thus limiting the magnitude of the ongoing T-cell immune responses.
Keywords: cell differentiation, dendritic cell, MHC Class II, monocytes
Introduction
Regulatory mechanisms controlling macrophage or dendritic cell (DC) generation in vivo are poorly understood. Blood monocytes cross the endothelium and enter tissues where they can differentiate to scavenging macrophages or antigen-presenting DC, according to distinct environmental factors that influence their differentiation. For instance, fibroblasts secreting interleukin-6 (IL-6) promote monocyte differentiation to macrophages.1 In vitro, monocytes can either differentiate into macrophages, in response to macrophage or granulocyte–macrophage colony-stimulating factor (M-CSF or GM-CSF), or into DC in response to GM-CSF and IL-4.2,3 In addition to epithelial and fibroblast cells that represent two prominent stromal cell types, monocytes may also encounter activated T or natural killer cells under local inflammatory conditions. Because monocyte-to-DC transition, as well as their migration to the lymph node, are greatly enhanced by these inflammatory conditions characterized by enhanced monocyte phagocytosis and interferon-γ production, we surmised that these activated T (or natural killer) cells may interfere with the control of monocyte switching to either macrophages or DC.
The lymphocyte activation gene-3 protein (LAG-3 or CD223) is expressed on activated T and natural killer cells4–6 and binds to major histocompatibility complex (MHC) class II molecules with a much higher avidity than CD4.7 On T cells, LAG-3 associates with the T-cell receptor : CD3 complex and, like cytotoxic T-lymphocyte antigen-4, negatively regulates signal transduction.8–11 A soluble LAG-3 (sLAG-3) molecule induces DC to mature and migrate to secondary lymphoid organs12–14 where they can prime naive CD4-helper and CD8-cytotoxic T cells.15–17 Monocytes can be activated by T cells and this positive signal is suppressed by LAG-3-specific monoclonal antibodies (mAb).12 Along the same lines, sLAG-3 markedly enhances IL-12 production by monocytes stimulated with infra-optimal concentrations of sCD40 ligand.12 However, the activation of monocytes via engagement of MHC class II molecules by a natural ligand, LAG-3, does not preclude their differentiation outcome in defined cytokine culture conditions. Here, we show that MHC class II molecules play another role in the control of antigen-presenting cell (APC) differentiation by inhibiting monocyte differentiation into either macrophages or into DC following engagement by LAG-3.
Materials and methods
Reagents
Recombinant soluble human LAG-3 immunoglobulin molecules (also termed sLAG-3) were generated by fusing the extracellular domain of human LAG-3 to a human immunoglobulin G1 (hIgG1) Fc portion.18 The resulting recombinant protein was produced in Chinese hamster ovary cells and purified as described previously.18 Preparations contained less than 1 EU/mg endotoxin as determined by the Limulus amoebocyte lysate assay (Biowhittaker, Walkersville, MD).
Purification of human monocytes and culture conditions
Human peripheral blood mononuclear cells were isolated from the venous blood of healthy voluntary donors and purified as previously described.13,14 Monocytes were seeded at a density of 1·5 × 106 cells/well in 12-well plates in 1 ml of complete culture medium (RPMI-1640 supplemented with 10% foetal bovine serum, 2 mm glutamine, and 1 mm pyruvate) with GM-CSF (100 ng/ml, Novartis, Rueil-Malmaison, France) and IL-4 (50 ng/ml, R & D, Minneapolis, MN). In parallel, monocytes were cultured with GM-CSF alone. Human IgG1, MHC class II-specific mAb (I3) or sLAG-3 were added at day 0. Every 2 days the culture medium was renewed with freshly added cytokine.
For lipopolysaccharide (LPS) stimulation, cells were harvested after 4 days of culture with medium, hIgG1, MHC class II-specific mAb, or sLAG-3 and resuspended at a density of 1 × 106 cells/ml in complete culture medium with cytokines and seeded in six- or 12-well plates. LPS (Sigma-Aldrich, St. Louis, MI; 10 ng/ml) was added and cells were incubated for another 24 hr at 37° in 5% CO2.
For hIgG1 cross-linking, monocytes were preincubated with 1 μg/ml hIgG1 for 15 min at 37° and then with 10 μg/ml goat anti-human immunoglobulin. For Fc receptor saturation experiments, monocytes were preincubated with culture medium containing 10% human serum for 30 min at 37°, washed with RPMI-1640 and 1 μg/ml of sLAG-3 was added before culture.
Cytofluorometric analysis
For double staining, cells were processed for 30 min at 4° using fluorescein isothiocyanate (FITC)-CD1a, phycoerythrin (PE)-CD14 (all Becton Dickinson, San Jose, CA), PE-CD86, PE-CD83 (all Coulter, Fullerton, CA) and PE-CD80 (Immunotech, Marseille, France) mAbs. FITC-IgG1 and PE-IgG1 mAbs (all Coulter) were used as negative controls. Stained cells were analysed by fluorescence-activated cell sorting (FACS) using an Epics Elite cytometer (Coulter).
To assess the binding of sLAG-3, monocytes were incubated with phosphate-buffered saline (PBS) containing 10% human serum and 10 mm sodium azide for 30 min at 4° to saturate the Fc receptors. Cells were washed with PBS and processed for double staining (30 min at 4°) using Alexa 488-hsLAG-3 at 10 μg/ml and PE-CD14 (Becton Dickinson) in PBS containing 1% bovine serum albumin and 10 mm sodium azide. Alexa 488-hIgG1 (10 μg/ml) was used as a negative control.
Mixed leucocyte reaction
Cells cultured for 4 days with medium, hIgG1 (1 μg/ml), anti-class II (1 μg/ml), or sLAG-3 (1 μg/ml) were added to 1 × 105 allogeneic peripheral blood lymphocytes (PBL) in round-bottomed 96-well microtitre plates. After 5 days of coculture, cells were pulsed with 1 μCi [3H]thymidine for 18 hr.
Statistics
Data were analysed by the non-parametric Mann–Whitney U rank test and differences with P < 0·05 were considered statistically significant.
Results
APC differentiated in the presence of sLAG-3 have weaker immunostimulatory capacities
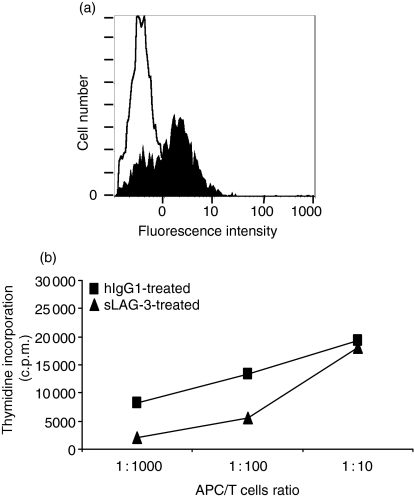
We first assessed the binding of sLAG-3 on freshly purified monocytes. The binding of an Alexa Fluor-coupled sLAG-3 molecule was clearly detectable at 10 μg/ml by FACS analysis, compared to the isotype-matched IgG1 control (Fig. 1a).
Figure 1.
LAG-3 binds MHC class II molecules expressed by monocytes and prevents the immunostimulatory properties of monocyte-derived DC. (a)sLAG-3 binding on monocytes. Freshly purified monocytes were saturated with 10% human serum in 1× PBS/10 mm NaN3 and stained with PE-CD14 mAb (10 μg/ml). The CD14+ cells were analysed for staining with 10 μg/ml hIgG1-(empty histogram) or sLAG-3-coupled Alexa Fluor 488 (filled histogram).(b)Proliferation of a 5-day coculture where allogeneic PBL are stimulated with DC derived from 1 μg/ml hIgG1-treated or LAG-3-treated monocytes. Results are representative of four experiments performed on different PBL samples.
Monocytes were cultured for 4 days in GM-CSF and IL-4 in the presence or absence of 1 μg/ml sLAG-3. APC were then cocultured with naive allogeneic PBL for 5 more days and proliferation was assessed by thymidine incorporation. The sLAG-3-differentiated cells were less immunostimulatory at ratios of 1 : 100 and 1 : 1000 APC : PBL than cells cultured with control hIgG1 (see a representative experiment in Fig. 1b), with a 57 ± 23% and 75 ± 17% mean inhibition for these two ratios, respectively (for four experiments). Immunostimulatory capacities of cells cultured with either medium or 1 μg/ml anti-MHC class II mAb were similar to that of the hIgG1 control condition (not shown).
It was unlikely that the inhibition of alloresponses by sLAG-3 could simply be the result of the masking of stimulatory MHC class II determinants on APC19 because: a non-blocking concentration of sLAG-3 was used (i.e. 1 μg/ml instead of 10 μg/ml), sLAG-3 was added 4 days before initiation of the MLR, and no effect of the addition of anti-MHC class II antibodies at the start of the culture was ever observed in these conditions.
LAG-3 prevents the generation of either CD1a+ DC or CD14+ macrophage from blood monocytes
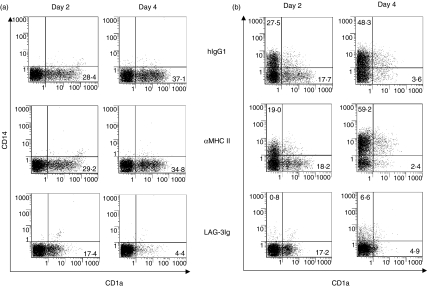
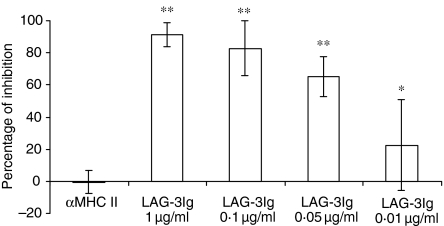
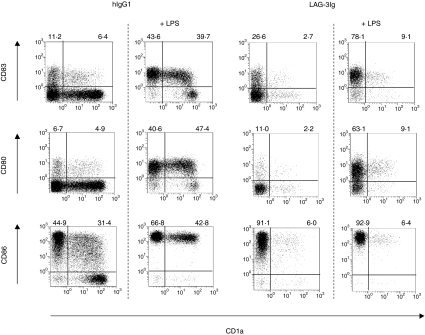
Monocytes were cultured for 6 days in GM-CSF and IL-4 (Fig. 2a) or in GM-CSF alone (Fig. 2b) in the presence or absence of various doses of sLAG-3. For the GM-CSF plus IL-4 conditions, cultures with sLAG-3 did not contain cells with cytoplasmic protrusions and membrane ruffling, as observed in controls: most cells were small, round-shaped and remained positive for myeloperoxidase and α-naphthyl butyrate esterase staining, which are conventional markers for monocytes (data not shown). There was no change between the conditions in terms of cell viability or cell yields (not shown). As shown in Fig. 2(a), cells cultured in the presence of 1 μg/ml sLAG-3 expressed very low levels of CD1a, compared to the control hIgG1 condition. In contrast, cells cultured in medium (not shown), the control hIgG1, or the MHC class II-specific mAb expressed increasing CD1a levels at day 4 (Fig. 2a) and day 6 (not shown) compared to day 2. At day 4 the inhibition of CD1a expression by sLAG-3 was strong, dose-dependent and still measurable at a very low dose (0·01 μg/ml) of sLAG-3 for some but not all monocyte samples (Fig. 3). Addition of sLAG-3 on day 2 of the culture did not inhibit the appearance of CD1a (not shown). Only CD1a expression was affected by the addition of sLAG-3 as expression of CD80, CD83 and CD86 did not change between culture conditions (Fig. 4). Induction of DC maturation by LPS did not rescue CD1a expression in sLAG-3 cultures although it increased CD83, CD80 and CD86 expression on these cells (Fig. 4). There were also no differences in IL-12 or IL-10 concentrations in the supernatant of cells cultured in the different conditions, either before or after LPS stimulation (data not shown). Finally, there was no skewing of the differentiation of monocytes to macrophages, as observed with IL-1020 or IL-61 because there was no difference in CD14 expression until it disappeared on day 2.
Figure 2.
LAG-3 inhibits CD1a or CD14 surface expression during monocyte to DC or to macrophage differentiation. Monocytes were cultured with GM-CSF and IL-4 (a)or GM-CSF(b)and with 1 μg/ml of either hIgG1, MHC class II-specific mAb or sLAG-3. At the indicated times cells were removed and the surface expression of CD1a (a) or CD14 (b) was assessed by FACS analysis. Dot blots show the phenotype of cells of a representative experiment at day 2 and day 4. Percentage of CD1a-positive (a,b) or CD14-positive (b) cells is indicated.
Figure 3.
LAG-3-induced CD1a inhibition during the monocyte-to-DC pathway is dose-dependent. Monocytes were cultured with GM-CSF and IL-4 alone or with 1 μg/ml of either hIgG1, MHC class II-specific mAb, or sLAG-3. At day 4 cells were removed and the surface expression of CD1a was assessed by FACS analysis. Mean fluorescence values were used to calculate the percentage of CD1a expression inhibition by sLAG-3 compared to hIgG1. Data are given as mean of percentage of inhibition ± SD of five independent experiments (*P ≤ 0·05; **P ≤ 0·01).
Figure 4.
LAG-3-differentiated cells show normal expression of costimulatory (CD80/B7.1, CD86/B7.2) and CD83 molecules. Dot plots of double-stained DC derived from hIgG1- or sLAG-3-treated monocytes stimulated or not with LPS (10 ng/ml) are shown. The percentage of positive cells for each marker is indicated. One representative result of five independent experiments is shown.
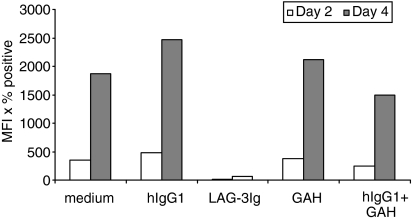
As we used a dimeric LAG-3 immunoglobulin recombinant protein and not the smaller physiological sLAG-3 protein, which is an alternative splicing product found in human serum,17 it was important to show that the dramatic phenotypic changes observed were not the result of FcR signalling. This was made unlikely by four factors: first, the use of a 30-min preincubation period with either 10% human serum (Fig. 5) or 10 μg/ml human IgG1 mAb (not shown) to saturate FcR; second, the use of non-activated monocytes expressing low levels CD64 (high-affinity FcR); third, the use of low sLAG-3 concentrations (down to 0·01 μg/ml) (Fig. 3); and fourth, the fact that direct and strong FcR cross-linking with hIgG1 plus goat anti-human immunoglobulin did not change CD1a expression (Fig. 5).
Figure 5.
Neither direct FcR cross-linking nor FcR saturation with human serum before sLAG-3 addition has any significant effect on CD1a expression. Monocytes were preincubated for 15 min at 37° with 1 μg/ml hIgG1 before the addition of goat anti-human immunoglobulin (10 μg/ml) to directly cross-link FcR (right) or for 30 min at 37° with 10% human serum to saturate FcR before sLAG-3 (1 μg/ml) addition (left). Cells were then cultured with GM-CSF and IL-4 for 6 days. At the indicated times, cells were removed and CD1a expression was assessed by FACS analysis. Data are given as the mean of mean fluorescence intensity (MFI) × percentage positive cells for three independent experiments.
In addition, because there was no obvious skewing from DC differentiation to macrophage generation in the presence of GM-CSF plus IL-4, we tested whether LAG-3 could have an effect on cell surface CD14 expression in conditions where macrophages are generated by culturing monocytes for 6 days in GM-CSF (without IL-4, which down-regulates CD14 expression) in the presence or absence of 1 μg/ml sLAG-3. Cells cultured with medium (not shown), hIgG1, or anti-MHC class II mAb showed increased CD14 expression from day 2 to day 4, while monocytic cells cultured with sLAG-3 expressed very low levels of CD14 together with a reduction in the number of CD1a+ cells (Fig. 2b). There was 67 ± 41% (for six experiments) and 71 ± 19% (for three experiments) inhibition of CD14 expression by sLAG-3 at days 4 and 6, respectively.
Discussion
This study demonstrates that MHC class II signalling induced specifically by a natural ligand, sLAG-3, severely impairs the differentiation of DC from human monocytes. Similarly, monocyte to macrophage ‘in vitro’ differentiation was also significantly hampered and the implication of these findings will be discussed in light of other situations where both the phenotypic and functional properties of these APC have been shown to be altered in vitro or in vivo.
For instance, such an impaired antigen-presentation function of monocyte-derived DC, as assessed by the reduced capability to induce T-cell proliferation, has been described before for Mycobacterium tuberculosis-infected monocytes.21 Inhibition of the differentiation of monocytes infected by M. tuberculosis to fully competent CD1a+ DC is used by the intracellular pathogen as an escape mechanism for evading elimination by the host immune system.21 Similarly, a strong correlation between CD1a expression and effective host immunity in the two forms of leprosy has been reported, with high expression of CD1a in patients with the tuberculoid form (showing strong cell-mediated immunity) and low expression in patients with the lepromatous form (showing weak cell-mediated immunity to the pathogen).22
Many of the substances capable of modifying CD1a expression in vitro are produced in vivo by tumours. These include IL-10, gangliosides, or prostanoids (reviewed in ref. 23). The density of CD1a+ DC has been directly associated with clinical outcome in a variety of human tumour types.23 CD1a may well represent an important mechanism for presentation of non-peptide (e.g. glycolipids) tumour antigens to the immune system and in situ immuno-incompetence could derive from the down-regulation of CD1a expression on APC by tumour cells. As a large fraction of tumor infiltrating lymphocytes (TILs) expressed LAG-3 in human tumours, as detected by immunohistochemistry of tissue sections or FACS analysis of freshly dissociated tumours,24 one cannot exclude the possibility that LAG-3 participates in the observed CD1a down-regulation in DC infiltrating the tumour. As observed with DC,25 MHC class II signalling following sLAG-3 binding on monocytes could lead to activation/phosphorylation of PLCγ2, p72syk, or Akt molecules that may be implicated into CD1a down-regulation.
We conclude that inhibition of both DC and macrophage differentiation are dependent in part upon the specific engagement of MHC class II molecules by sLAG-3. What are the implications of these findings in terms of T-cell response development? On the one hand, sLAG-3 conditioning of DC first incites the professional APC to carry the antigenic information from sites of inflammation to distant lymph nodes within a few hours, leading to increased T-cell responses.13–15 On the other hand, membrane-expressed LAG-3 later (after 1–2 days) inhibits any overshooting of the immune response by down-regulating T-cell receptor signalling into effector T cells, either directly8–11 or indirectly through the suppressor activity of LAG-3+ regulatory T-cell subsets.26 In addition, it may be that increased sLAG-3 production (i.e. the physiological sLAG-3 protein derived from an alternative splicing event17), as a result of the ongoing T-cell activation, blocks further recruitment of APC by reducing the differentiation of monocytes into scavenging macrophages or antigen-presenting DC, as shown in the present study.
Acknowledgments
We wish to thank Dr C. Demeure (Institut Pasteur, Paris) for helpful discussions.
References
- 1.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nature Immunol. 2000;1:510–14. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 2.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2388–592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevée C, Viegas-Pequignot E, Hercend T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171:1393–405. doi: 10.1084/jem.171.5.1393. 10.1084/jem.171.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huard B, Gaulard P, Faure F, Hercend T, Triebel F. Cellular expression and tissue distribution of the human LAG-3-encoded protein, a MHC class II ligand. Immunogenetics. 1994;39:213–17. doi: 10.1007/BF00241263. [DOI] [PubMed] [Google Scholar]
- 6.Workman CJ, Rice DS, Dugger K. Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3) Eur J Immunol. 2002;32:2255–63. doi: 10.1002/1521-4141(200208)32:8<2255::AID-IMMU2255>3.0.CO;2-A. 10.1002/1521-4141(200208)32:8<2255::AID-IMMU2255>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 7.Huard B, Prigent P, Tournier M, Bruniquel D, Triebel F. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3) -Ig fusion proteins. Eur J Immunol. 1995;25:2718–21. doi: 10.1002/eji.1830250949. [DOI] [PubMed] [Google Scholar]
- 8.Huard B, Tournier M, Hercend T, Triebel F, Faure F. Lymphocyte-activation gene 3/major histocompatibility complex class II interaction modulates the antigenic response of CD4+ T lymphocytes. Eur J Immunol. 1994;24:3216–21. doi: 10.1002/eji.1830241246. [DOI] [PubMed] [Google Scholar]
- 9.Hannier S, Tournier M, Bismuth G, Triebel F. CD3/TCR complex-associated LAG-3 molecules inhibit CD3/TCR signaling. J Immunol. 1998;161:4058–65. [PubMed] [Google Scholar]
- 10.Workman CJ, Vignali DA. The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur J Immunol. 2003;33:970–9. doi: 10.1002/eji.200323382. 10.1002/eji.200323382. [DOI] [PubMed] [Google Scholar]
- 11.Workman CJ, Dugger KJ, Vignali DAA. Molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J Immunol. 2002;169:5392–5. doi: 10.4049/jimmunol.169.10.5392. [DOI] [PubMed] [Google Scholar]
- 12.Avice MN, Sarfati M, Triebel F, Delespesse G, Demeure CE. Lymphocyte activation gene-3, a MHC class II ligand expressed on activated T cells, stimulates TNF-alpha and IL-12 production by monocytes and dendritic cells. J Immunol. 1999;162:2748–53. [PubMed] [Google Scholar]
- 13.Andreae S, Piras F, Burdin N, Triebel F. Maturation and activation of dendritic cells induced by lymphocyte activation gene-3 (CD223) J Immunol. 2002;168:3874–80. doi: 10.4049/jimmunol.168.8.3874. [DOI] [PubMed] [Google Scholar]
- 14.Buisson S, Triebel F. MHC class II engagement by its ligand LAG-3 (CD223) leads to a distinct pattern of chemokine receptor expression by human dendritic cells. Vaccine. 2003;21:862–8. doi: 10.1016/s0264-410x(02)00533-9. [DOI] [PubMed] [Google Scholar]
- 15.El mir S, Triebel F. A soluble LAG-3 molecule used as a vaccine adjuvant elicits greater humoral and cellular immune responses to both particulate and soluble antigens. J Immunol. 2000;164:5583–9. doi: 10.4049/jimmunol.164.11.5583. [DOI] [PubMed] [Google Scholar]
- 16.Cappello P, Triebel F, Iezzi M, et al. LAG-3 enables DNA vaccination to persistently prevent mammary carcinogenesis in HER-2/neu transgenic BALB/c mice. Cancer Res. 2003;63:2518–25. [PubMed] [Google Scholar]
- 17.Triebel F. LAG-3. a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24:619–22. doi: 10.1016/j.it.2003.10.001. 10.1016/j.it.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Huard B, Prigent P, Pages F, Bruniquel D, Triebel F. T cell MHC class II molecules downregulate CD4+ T cell clone response following LAG-3 binding. Eur J Immunol. 1996;26:1180–6. doi: 10.1002/eji.1830260533. [DOI] [PubMed] [Google Scholar]
- 19.Subramanyam M, Wands G, Nabioullin R, Tepper MA. Soluble human lymphocyte activation gene-3 modulates allospecific T cell responses. Int Immunol. 1998;10:679–89. doi: 10.1093/intimm/10.5.679. 10.1093/intimm/10.5.679. [DOI] [PubMed] [Google Scholar]
- 20.Allavena P, Piemonti L, Longoni D, Bernasconi S, Stoppacciaro A, Ruco L, Mantovani A. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur J Immunol. 1998;28:359–69. doi: 10.1002/(SICI)1521-4141(199801)28:01<359::AID-IMMU359>3.0.CO;2-4. 10.1002/(SICI)1521-4141(199801)28:01<359::AID-IMMU359>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Mariotti S, Teloni R, Iona E, et al. Mycobacterium tuberculosis subverts the differentiation of human monocytes into dendritic cells. Eur J Immunol. 2002;32:3050–8. doi: 10.1002/1521-4141(200211)32:11<3050::AID-IMMU3050>3.0.CO;2-K. 10.1002/1521-4141(200211)32:11<3050::AID-IMMU3050>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 22.Sieling PA, Jullien D, Dahlem M, Tedder TF, Rea TH, Modlin RL, Porcelli SA. CD1 expression by dendritic cells in human leprosy lesions: correlation with effective host immunity. J Immunol. 1999;162:1851–8. [PubMed] [Google Scholar]
- 23.Coventry B, Heinzel S. CD1a in human cancers: a new role for an old molecule. Trends Immunol. 2004;25:242–8. doi: 10.1016/j.it.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Demeure C, Wolfers J, Martin-Garcia N, Gaulard P, Triebel F. T Lymphocytes infiltrating various tumour types express the MHC class II ligand lymphocyte activation gene-3 (LAG-3): role of LAG-3/MHC class II interactions in cell-cell contacts. Eur J Cancer. 2001;73:1709–18. doi: 10.1016/s0959-8049(01)00184-8. 10.1016/S0959-8049(01)00184-8. [DOI] [PubMed] [Google Scholar]
- 25.Andreae S, Buisson S, Triebel F. MHC class II signal transduction in human dendritic cells induced by a natural ligand, the LAG-3 protein (CD223) Blood. 2003;102:2130–7. doi: 10.1182/blood-2003-01-0273. [DOI] [PubMed] [Google Scholar]
- 26.Huang CT, Workman CJ, Flies D, et al. Role LAG-3 regulatory T cells. Immunity. 2004;21:503–13. doi: 10.1016/j.immuni.2004.08.010. 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]