Abstract
Recent studies suggest that neutrophils may play a role in antigen presentation. In support of this hypothesis it has been shown that these cells appear to contain cytoplasmic stores of molecules required for this function, i.e. major histocompatibility complex class II (DR) antigen, CD80 and CD86. In this study we have considered a mechanism for the translocation of these preformed molecules onto the cell surface which does not require active synthesis. Cross-linking of the Mac-1 molecule (CD18 + CD11b) was shown to result in rapid cell surface expression of CD80, CD86 and DR antigen on the surface of normal human peripheral blood neutrophils. A distinct subpopulation (approximately 20%) of neutrophils appeared to be enlarged and were found to express significantly elevated levels of these molecules on the cell surface following cross-linking of CD11b when compared with control cells. The level of expression of CD80, CD86 and DR antigen on these large cells was comparable to, and in some cases greater than, the levels found expressed on the surface of monocytes obtained from the same donors. In addition, these cytoplasmic molecules were shown by confocal laser microscopy and by immunoelectron microscopy to be located within secretory vesicles. Following rapid translocation onto the cell surface, CD80 and CD86 appeared to be colocalized within large clusters reminiscent of the supramolecular antigen clusters previously found on conventional antigen-presenting cells. These findings therefore lend further support for the hypothesis that neutrophils may have a role to play in antigen presentation and/or T-cell activation.
Keywords: Neutrophils, CD11b, cross-linking, cytoplasmic stores, antigen presentation
Introduction
Although phagocytic neutrophils are known to play a major role in innate immunity it is now apparent that these cells also regulate specific immune responses and may even function as antigen-presenting cells (APCs).1,2 This seems an unlikely function for these cells since they do not constitutively express the cell surface molecules considered necessary for antigen presentation and subsequent T-cell activation, i.e. major histocompatibility complex (MHC) class II (DR) antigen, CD80 (B7-1) and CD86 (B7-2). Several studies have however, shown that following in vivo and/or in vitro activation by cytokines, neutrophils do appear to express these molecules on the cell surface.3–5 Furthermore, functional studies have now shown that appropriately activated neutrophils can present antigens to helper T cells and can initiate a full-blown T-cell response.6,7
In support of this hypothesis we have previously demonstrated, at the protein and mRNA level, that normal resting peripheral blood neutrophils appear to have significant cytoplasmic stores of these molecules.8 This observation suggests that activated neutrophils have the capacity to actively synthesize the molecules required for antigen presentation. However this mechanism is slow requiring up to 48 hr for full expression of the molecules on the neutrophil surface.3 Because neutrophils are required for rapid responses it seems likely that an additional mechanism(s) may be required for the translocation of these molecules from pre-existing cytoplasmic stores onto the cell surface. In this study we have considered a translocation mechanism initially proposed by Takano et al.9 Using highly purified human peripheral blood neutrophils these workers showed that CD64 (FcγRI), a molecule known to be expressed on the cell surface following activation by interferon-γ, could be translocated from pre-existing cytoplasmic stores onto the cell surface following in vitro cross-linking (X-L) of the Mac-1 heterodimer (CD11b + CD18). Monoclonal antibodies specific for either component mediated this effect but antibodies specific for CD11a or CD11c did not. In this study we have used a modified version of this translocation method using whole blood in place of isolated neutrophils. Using this method we have shown that in vitro cross-linking of CD11b results in rapid (within 2·5 min) neutrophil surface expression of CD18, CD66, CD64, CD80, CD86 and to a lesser extent MHC class II (DR) antigen. The level of expression of CD80, CD86 and MHC class II antigen molecules found on the surface of neutrophils was found to be comparable to, and in some cases higher than, the levels found on the surface of monocytes, i.e. cells known to act as APCs.
Neutrophils are known to contain many preformed molecules required for rapid responses to infection, which are released from different types of granule in a definite hierarchy.10,11 Using confocal microscopy and immunoelectron microscopy we provide evidence in this study that CD80, CD86 and MHC class II antigen are colocalized within neutrophil secretory vesicles.
Materials and methods
Translocation of cytoplasmic antigens onto the surface of normal human peripheral blood leucocytes
Whole blood assay.
Ten microlitres (1 µg) of mouse monoclonal antibody CD11b (clone 2LPM19c, Dako Cytomation A/S, Denmark) was added to 100 µl of whole EDTA anticoagulated blood and incubated at 4° for 30 min. Cells were then washed immediately at room temperature using 2 ml precooled (4°) membrane filtered (0·2 µm Millipore filter) Iscoves culture medium (Gibco BRL, Invitrogen, Paisley, UK) supplemented with 20% heat-inactivated (56° for 30 min) fetal calf serum (I + S). Cells were centrifuged at 200 g for 5 min and following removal of the supernatant, cross-linking antibody, i.e. 5 µl goat F(ab′)2 anti-mouse immunoglobulin G (IgG; Caltag Medsystems Ltd, Oxford, UK) was added to the cell pellet. The optimum concentration of the primary and secondary cross-linking reagents was established by pretitration. Cells were maintained in cross-linking antibody at 37° in a water bath for various time intervals ranging from 2·5 min to 2 hr. Cells were then washed in 2 ml I + S at 200 g for 5 min and incubated for a further 30 min at 4° in 10 µl unconjugated mouse IgG1 myeloma protein (MOPC 21, Sigma-Aldrich Co. Ltd, Poole, UK) at 200 µg/ml in phosphate-buffered saline (PBS) in order to block any free binding sites for mouse IgG and to prevent any non-specific binding to neutrophil Fc gamma receptors. Following centrifugation at 200 g for 5 min the supernatant was removed and cells suspended in 10 µl various fluorochrome (R-phycoerythrin (PE) or fluoroscein isothiocyanate (FITC))-conjugated mouse monoclonal antibodies (see below) and incubated at 4° for 30min. Fluorescence-activated cell sorting (FACS) Lysing (2 ml) solution (Becton Dickinson Biosciences, Pharmingen, Oxford, UK) was then added to each tube to remove contaminating erythrocytes and following a further wash at 200 g for 5 min the resulting leucocyte pellet was finally suspended in 200 µl membrane-filtered PBS for acquisition using a Becton Dickinson flow cytometer. Cell viability was confirmed, by exclusion of propidium iodide, to ensure that cell surface CD antigens were being detected by this method. In all experiments, background binding was assessed using a FITC-conjugated mouse IgG isotype negative control (clone MOPC 21, Becton Dickinson). In addition, several unrelated mouse monoclonal antibodies specific for T-cell antigens were also used to control for non-specific background binding including FITC-conjugated anti-CD4 (clone MT310, Becton Dickinson) and R-PE-conjugated anti-CD8 (clone DK25, Becton Dickinson). All monoclonal antibodies (clone indicated in brackets) used in this study were of the IgG1 isotype with the exception of the one indicated as IgG2b*; from Caltag-Medsystems Ltd (Towcester, UK): CD64–FITC (10·1), CD80–FITC (MEM233), CD86–FITC (BU63), MHC class II-DR antigen–FITC* (To36); from Serotec (Kidlington, Oxford, UK): CD32 (AT10) and CD35 (E11); from DakoCytomation Ltd (Ely, UK): CD3–FITC (UCHT-1), CD16–FITC (DJ130c), CD18–FITC (MHM23), CD66abce–FITC (Kat4C) and CD19–FITC (HO37).
Translocation assay using purified neutrophils.
Neutrophils were isolated from whole blood using a combination of Ficoll-Hypaque density centrifugation, dextran sedimentation and hypotonic lysis as described previously.8 Cells were then adjusted to a final concentration of 1 × 106 cells per 100 µl Iscove's medium supplemented with 20% fetal calf serum. The assay was then conducted as described above for the whole blood method using 100 µl aliquots of cells in place of whole blood.
Image analysis.
Cells prepared as described above for flow cytometry were fixed in 200 µl paraformadehyde (1% in PBS) and 120 µl aliquots cytocentrifuged onto microscope slides coated with 3-aminopropyltriethoxysilane (APES) at 150 g for 5 min. Cells were allowed to air dry for 5 min, stained with haematoxylin and eosin, taken through alcohols, cleared with xylene and mounted in histomount. Cells were then viewed on an Olympus BX51 microscope, photographed at a magnification of ×400 and cell areas measured using Olympus image analysis software.
Detection of cell surface and cytoplasmic CD antigens by flow cytometry, confocal microscopy and immunoelectron microscopy
Cell surface CD antigens.
Ethylenediaminetetraacetic acid (EDTA) anticoagulated whole blood (50 µl) was incubated with fluorochrome-conjugated primary monoclonal antibody (5 µl) at 4° for 30 min. Cells were then washed, by centrifugation at 200 g for 5 min, in 2 ml FACS Lysing solution (Becton Dickinson) to remove excess antibody and to lyse any contaminating erythrocytes. Following aspiration of the supernatant, cells were suspended in 200 µl membrane (Millex-GS 0·22 µm, Millipore S.A., Molsheim, France) filtered PBS for acquisition using a Becton Dickinson flow cytometer. Background staining was established using isotype-matched FITC-conjugated mouse IgG (Becton Dickinson). Results were analysed using Becton Dickinson ‘Cell Quest’ software on an Apple Mac G3 computer. The optimum concentration for cell surface staining was determined for each antibody by pretitration.
Cytoplasmic CD antigens.
This method has previously been described in detail.8 Briefly, EDTA anticoagulated whole blood (50 µl) was incubated for 10min in 2 ml FACS Lysing solution (Becton Dickinson) which contains a mixture formaldehyde and diethylene glycol, thus simultaneously fixing and permeabilizing leucocytes with minimum disruption of cell morphology.8 Following one wash at 200 g for 5 min in PBS, cells were suspended in 50 µl PBS and incubated with 5 µl fluorochrome conjugated primary monoclonal antibody at 4° for 30 min. Cells were then washed at 200 g and suspended in 200 µl PBS for acquisition on the flow cytometer. Cell permeability was confirmed by the uptake of propidium iodide or by binding of monoclonal antibodies specific for intermediate filaments or myeloperoxidase. Background levels were established using an appropriate FITC- or R-PE-conjugated mouse IgG isotype control (Becton Dickinson). In all experiments, cell surface and cytoplasmic staining was measured in parallel for comparison. Antibodies specific for the various types of human neutrophil granules were as follows: for primary azurophilic granules – R-PE conjugated anti-myeloperoxidase (clone H-43–5, Caltag); for secondary neutrophil specific granules – R-PE conjugated anti-lactoferrin (clone 3C5, Caltag); for tertiary gelatinize granules – unconjugated anti-matrix metalloproteinase 9 (MMP-9, clone GE213, Serotec); and for secretory vesicles – FITC-conjugated polyclonal rabbit anti-human serum albumin (Dako). For dual staining studies the following additional antibodies were used: R-PE-conjugated anti-CD80 (clone MEM 233, Caltag) and R-PE-conjugated anti-CD86 (clone BU63, Caltag). In all dual-staining experiments, optimum concentrations of antibody were determined by pretitration and equal volumes of FITC and R-PE conjugated antibodies were added simultaneously. In the case of MMP-9 (gelatinase B) where unconjugated mouse IgG antibody was used, permeabilized cells were preincubated with primary antibody alone at 4° for 30 min, washed and incubated for a further 30 min with R-PE-conjugated rabbit F(ab′)2 anti-mouse IgG (Dako). Any residual binding sites for mouse IgG were blocked by the addition of 10 µl mouse IgG1 myeloma protein (clone MOPC 21, Sigma) at a concentration of 200 µg/ml. Following a further wash in PBS cells were then incubated with FITC conjugated anti-CD80 or CD86, incubated at 4° for 30min, washed in PBS and resuspended in a final volume of 200 µl paraformaldehyde (diluted 1% in PBS) prior to acquisition in the flow-cytometer as described above.
Confocal microscopy.
120 µl of cells prepared for flow cytometry as described above, were cytocentrifuged onto APES-coated microscope slides at 150 g for 5 min, air dried, mounted in Vectashield mounting medium (Vector Laboratories, Peterborough, UK) supplemented with 0·2 mg/ml 4′,6-diamidino-2-phenylindole (DAPI) and the coverslips sealed with nail varnish. Cells were viewed at a magnification of ×630 using a Leica SP2 confocal laser microscope. Lasers were optimized (excitation wavelength) for the three fluorochromes used. Each cell selected was viewed at different Z-levels in order to optimize the image obtained. Using LCS Lite confocal software, individual and merged images were obtained. Background noise was reduced and brightness and contrast optimized using Adobe Photoshop version 7.
Immunoelectron microscopy (pre-embedding method).
Forty ml of EDTA anticoagulated blood was diluted in an equal volume of PBS, layered onto lymphoprep (Nycomed, Pharma AS, Oslo, Norway) gradients and centrifuged at 400 g for 30 min. Neutrophils were recovered from the bottom of these gradients and resuspended in autologous plasma. Contaminating erythrocytes were removed by allowing cells to sediment by 50% of the original volume at 4° by addition of 2 ml 6% w/v dextran in PBS. Cells were then washed in PBS/bovine serum albumin (BSA), divided into two equal aliquots and centrifuged into a tight pellet at 200 g for 5 min in 20 ml sterilin bottles and the supernatant discarded. Following addition of 25 ml FACS Lysing solution for 10 min at room temperature, cells were suspended in blocking buffer (10 mg/ml BSA in PBS) for 30 min at 4°, centrifuged at 200 g for 5 min and the supernatant discarded. To one pellet was added 150 µl of mouse monoclonal anti-CD80 and to the other (negative control) was added 150 µl of PBS. Following incubation at 4° for 2 hr, cells were washed twice in blocking buffer (BSA/PBS) and 150 µl of 10 nm gold particles (British Biocell International Ltd, Cardiff, UK) conjugated with F(ab′)2 goat anti-mouse IgG (diluted 1 : 15 in blocking buffer) was added to each pellet and incubated at 4° overnight. Both pellets were then washed 10 times using 20 ml BSA/PBS blocking buffer in order to minimize non-specific binding of gold particles. Cell pellets were finally placed in 2 ml microcap tubes and allowed to fix overnight in 1 ml 2% glutaraldehyde. Cells were then embedded in gelatine and processed for transmission electron microscopy using a standard protocol. Sections were viewed using a Philips CM10 transmission electron microscope and cells identified as neutrophils by their characteristic morphology were photographed at various magnifications. Negatives were scanned and converted to TIFF images which were viewed using Adobe photoshop and the number of gold particles found to be associated with the cell cytoplasm counted.
Statistical analysis
Image analysis.
Differences in cell area were analysed using a paired t-test.
Kinetic studies.
Differences between background values and the various CD antigens were analysed at each time interval using the Wilcoxon Two Sample Test. 95% confidence intervals were established and significance indicated by a P-value. P-values <0·05 were considered significant.
Results
Translocation of neutrophil cytoplasmic antigens following cross-linking of CD11b
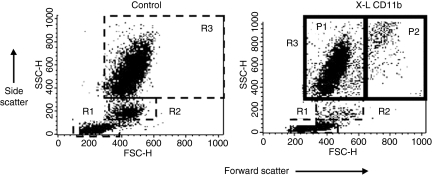
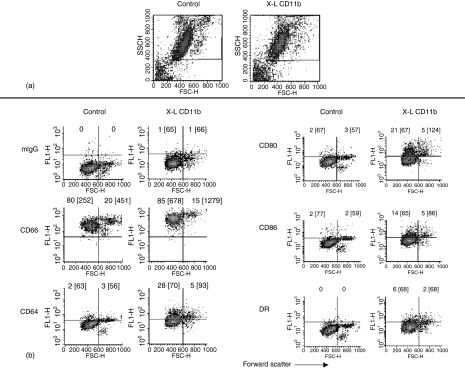
Figure 1 shows a typical example of the effect of cross-linking CD11b on the light scatter properties of normal human peripheral blood leucocytes. Density-plot analysis of forward scatter (FSC = cell size) versus side scatter (SSC = granularity) showed no major change in light scatter properties of lymphocytes or monocytes -gated as regions 1 and 2, respectively. A significant (P < 0·05: Wilcoxon Two Sample Test) reduction in the total number of monocytes was however, evident over time in all experiments (see Table 1). The reason for this reduction is not known but may reflect cell loss because of adherence of these cells to the plastic tubes. The vast majority of neutrophils (gated as region 3) did not show a significant change in light scatter but it was evident that a distinct subpopulation (approximately 20%) of large granular cells appeared following cross-linking of CD11b. All cells appearing in the P2 region were shown to express the granulocyte specific marker CD66 and were also shown to express other markers known to be expressed by but not exclusive for neutrophils, i.e. CD32, CD35, CD16 and CD18. Furthermore, cells in the P2 region did not bind monoclonal antibodies specific for T cells (CD3, CD4, CD8), B cells (CD19) or monocytes (CD14). A small number of CD16 negative eosinophils (generally less than 400 out of 10 000 cells acquired) were also observed in whole blood but these cells were not present in the P2 region – all CD16 positive.
Figure 1.
Effect of cross-linking CD11b on light scatter properties of normal human peripheral blood leucocytes. Dot-plots of forward scatter (cell size) versus side scatter (granularity) showing three main populations of leucocytes before (Control) and following in vitro cross-linking of CD11b (X-L CD11b). Lymphocytes were gated as region 1 (R1), monocytes gated as Region 2 (R2) and neutrophils as region 3 (R3). For the purposes of analysis, neutrophils were further subdivided into two main populations, i.e. P1, corresponding to size and granularity of control neutrophils and larger P2 cells which appear only following cross-linking of CD11b. The P2 gate was set according to the upper limit for forward scatter of control cells to ensure that all cells appearing in the P2 region were a direct result of cross-linking. In this example, cells were incubated at 37° for 30 min in cross-linking reagent: Goat F(ab′)2 anti-mouse IgG.
Table 1. Effect of cross-linking CD11b on peripheral blood mononuclear cell surface expression of CD antigens.
| Incubation period (min) at 37° with cross-linking antibody | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cell type | 0 | 2.5 | 5 | 30 | 60 | 90 | 120 | |
| All lymphocytes | Number of cells in region | 2907 ± 216 | 3429 ± 437 | 3677 ± 300 | 3476 ± 299 | 3256 ± 231 | 3609 ± 332 | 3683 ± 438 |
| mIgG1 – MFI | 1·6 ± 0·2 | 1·8 ± 0·2 | 2·1 ± 02 | 1·9 ± 0·1 | 2·0 ± 0·2 | 1·9 ± 0·1 | 1·9 ± 0·1 | |
| CD64 – MFI | 2·2 ± 0·2 | 2·8 ± 0·4 | 2·9 ± 0·3 | 3·2 ± 0·3 | 3·3 ± 0·6 | 3·2 ± 0·3 | 3·2 ± 0·5 | |
| CD80 – MFI | 3·0 ± 0·4 | 3·9 ± 0·5 | 3·6 ± 0·5 | 4·2 ± 0·5 | 3·8 ± 0·3 | 4·0 ± 0·4 | 3·8 ± 0·5 | |
| CD86 – MFI | 2·2 ± 0·2 | 2·2 ± 0·2 | 2·3 ± 0·2 | 2·3 ± 0·2 | 2·2 ± 0·2 | 2·2 ± 0·2 | 2·0 ± 0·3 | |
| B cells only | MHC Class II –% | 11·7 ± 1·2 | 10·3 ± 0·9 | 11·5 ± 1·2 | 11·5 ± 1·0 | 12·4 ± 1·2 | 11·9 ± 1·4 | 11·2 ± 1·1 |
| MHC Class II – MFI | 76 ± 10·3 | 68·8 ± 8·7 | 65·5 ± 9·4 | 71·5 ± 11·5 | 77·0 ± 16·3 | 71·3 ± 12·2 | 81·3 ± 14·4 | |
| Monocytes | Number of cells in region | 989 ± 144 | 488 ± 162* | 393 ± 102* | 337 ± 48* | 317 ± 22* | 294 ± 36* | 278 ± 40* |
| mIgG1 – MFI | 2·3 ± 0·4 | 5·8 ± 0·5 | 6·7 ± 1·0 | 8·1 ± 1·1 | 8·3 ± 1·2 | 8·3 ± 1·4 | 9·8 ± 2·0 | |
| CD64 – MFI | 27·7 ± 3·6 | 42·3 ± 11·2 | 34·2 ± 4·0 | 33·5 ± 5·8 | 35·3 ± 6·2 | 29·8 ± 3·4 | 30·8 ± 5·3 | |
| CD80 – MFI | 5·5 ± 0·8 | 18·8 ± 3·4* | 17·3 ± 3·7* | 24·2 ± 5·4* | 37·2 ± 8·6* | 24·3 ± 4·9* | 24·2 ± 4·6* | |
| CD86 – MFI | 13·8 ± 2·0 | 23·3 ± 2·7* | 24·2 ± 3·9* | 22·8 ± 2·5* | 23·6 ± 2·7* | 27·2 ± 1·4* | 28·5 ± 1·8* | |
| MHC Class II – MFI | 40·5 ± 8·4 | 46·7 ± 9·4 | 59·3 ± 13·3 | 63·2 ± 14·8 | 73·7 ± 16·9 | 77·0 ± 19·5 | 92·2 ± 22·0 | |
Mean ± SEM of 6 experiments. MFI, mean fluorescence intensity for all cells in region gated. mIgG1, background mouse isotype control.
Significantly (P < 0·05) different from constitutive level expressed at time zero and relative to mouse IgG background values as assessed by the Wilcoxon-Two Sample Test.
Large P2 neutrophils appeared within 2·5 min of addition of cross-linking reagents at 37° with the relative percentage of these cells remaining fairly constant throughout the time course of these kinetic experiments (see Table 2). For the purposes of analysis, ‘normal’ sized neutrophils were gated as population 1(P1) and the larger cells gated as population 2 (P2), see Fig. 1. These large cells were gated according to the upper limit of control neutrophils, i.e. cells were not found in this region in control preparations where no cross-linking reagents had been added. The size of normal control neutrophils as estimated by forward light scatter (FSC) was found to be 463 ± 5 (N = 6). Following cross-linking of CD11b at 37° for 2·5 min the FSC value for cells gated in the P2 region was 795 ± 3·3. This increase was statistically significant (P = 0·002; Wilcoxon Two Sample Test) with the mean percentage increase in size being 71·7 ± 1·5.
Table 2.
Effect of cross-linking CD11b on peripheral blood neutrophil surface expression of CD antigens
| Incubation period (minutes) at 37° with cross-linking antibody | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cell type | 0 | 2·5 | 5 | 30 | 60 | 90 | 120 | |
| All neutrophils | Number of cells in region | 5310 ± 281 | 4812 ± 401 | 4805 ± 407 | 4907 ± 512 | 4848 ± 581 | 4764 ± 641 | 4656 ± 741 |
| mIgG1 – MFI | 2·8 ± 0·4 | 4·5 ± 0·2 | 5·0 ± 0·7 | 7·7 ± 1·1 | 9·1 ± 1·3 | 9·5 ± 1·7 | 9·8 ± 2·5 | |
| CD64 – MFI | 7·1 ± 0·7 | 12·2 ± 0·7* | 13·5 ± 1·1* | 19·7 ± 4·3* | 21·0 ± 4* | 22·8 ± 4·1* | 23·8 ± 6·7* | |
| CD80 – MFI | 7·8 ± 1·3 | 16·2 ± 3·3* | 17·5 ± 3·6* | 25·5 ± 5·5* | 23·0 ± 4·7 | 27·3 ± 5·1* | 28·5 ± 5·4* | |
| CD86 – MFI | 8·7 ± 1·5 | 15·8 ± 6·0* | 14·7 ± 5·5* | 18·2 ± 4·7* | 16·3 ± 4·0 | 12·8 ± 3·1 | 11·7 ± 3·5 | |
| MHC Class II – MFI | 4·2 ± 0·4 | 9·8 ± 1·8* | 9·8 ± 1·5* | 16·8 ± 2·6* | 19·0 ± 4·2* | 21·2 ± 4·4* | 24·2 ± 7·0 | |
| Neutrophils P1 sub-population | Number of cells in region | 5110 ± 262 | 3677 ± 316 | 3932 ± 420 | 3811 ± 615 | 3805 ± 646 | 3768 ± 763 | 3742 ± 777 |
| mIgG1 – MFI | 2·6 ± 0·3 | 3·4 ± 0·3 | 4·3 ± 0·8 | 6·0 ± 0·8 | 6·8 ± 0·7 | 7·2 ± 1·0 | 7·2 ± 1·1 | |
| CD64 – MFI | 6·5 ± 0·6 | 8·8 ± 0·9* | 9·3 ± 0·7* | 14·2 ± 3·8 | 14·8 ± 2·6* | 15·9 ± 3·5* | 17·2 ± 4·9 | |
| CD80 – MFI | 7·0 ± 1·0 | 11·8 ± 2·0 | 13·9 ± 2·9 | 20·3 ± 4·4 | 18·2 ± 3·7 | 23·2 ± 4·5 | 23·8 ± 4·4 | |
| CD86 – MFI | 8·7 ± 1·5 | 13·0 ± 2·9 | 13·0 ± 5·5 | 14·3 ± 4·3 | 13·8 ± 3·9 | 10·2 ± 2·4 | 8·2 ± 2·2 | |
| MHC Class II – MFI | 6·5 ± 0·6 | 8·8 ± 0·9* | 9·3 ± 0·7 | 14·2 ± 3·8 | 14·8 ± 2·6 | 15·9 ± 3·5 | 17·2 ± 4·9 | |
| Neutrophils P2 sub- population | Number of cells in region | 0 | 1092 ± 220 | 739 ± 138 | 811 ± 110 | 801 ± 112 | 770 ± 192 | 763 ± 137 |
| mIgG1 – MFI | 0 | 13·4 ± 1·8 | 17·0 ± 3·9 | 23·5 ± 4·5 | 23·0 ± 2·9 | 22·5 ± 3·9 | 27·2 ± 7·9 | |
| CD64 – MFI | 0 | 38·5 ± 8·6* | 43·3 ± 9·1* | 58·5 ± 15·4* | 63·3 ± 13·9* | 65·8 ± 13·5* | 74·0 ± 13·5* | |
| CD80 – MFI | 0 | 50·8 ± 9·9* | 56·0 ± 14·6* | 80·7 ± 17·1* | 85·0 ± 18·0* | 107·3 ± 30·5* | 105·2 ± 28·2* | |
| CD86 – MFI | 0 | 41·0 ± 11·2* | 38·5 ± 10·9* | 46·3 ± 8·9* | 43·2 ± 6·5* | 37·8 ± 4·2* | 37·7 ± 5·1 | |
| MHC Class II – MFI | 0 | 39·8 ± 6·6* | 48·0 ± 8·7* | 57·8 ± 8·6* | 59·7 ± 10·4* | 57·8 ± 9·8* | 61·3 ± 11·6* | |
Mean ± SEM of six experiments. MFI, mean fluorescence intensity for all cells in region gated. mIgG1, background mouse isotype control.
Significantly (P < 0·05) different from mouse. IgG background as assessed by the Wilcoxon-Two Sample Test.
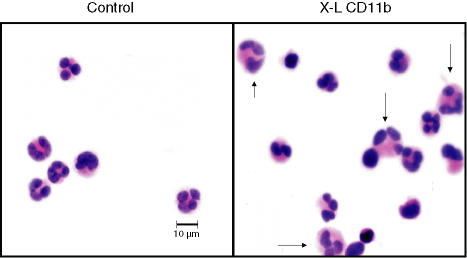
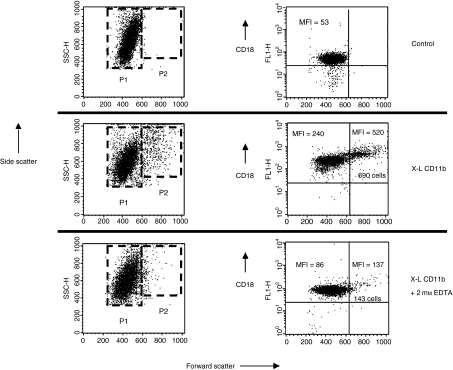
Cytospin preparations of control and cross-linked leucocytes were stained with haematoxylin and eosin (Fig. 2). Neutrophils were identified by their characteristic nuclear shape. It was evident that many individual neutrophils appeared to be much larger than normal with a more voluminous cytoplasm (see arrows). Some small homotypic neutrophil aggregates were also observed in these preparations. Photomicrographs were taken at a magnification of ×630 and the area of individual cell images was measured on the computer screen using image analysis software (Olympus). The mean area of control cells was then compared with cells pretreated with cross-linking reagents at 37° for 2·5 min. Using this measurement of cell area (mm2) a significant (P < 0·0001, paired t-test) increase was observed when control (mean ± SEM for 100 cells = 45 532 ± 572) and cross-linked cells (mean ± SEM for 100 cells = 57 946 ± 917) were compared but the average increase in cell area was only 27%. However, it was noted that 22% of neutrophils exceeded the upper limit for normal control cell area; a value which corresponds well with the proportion of P2 neutrophils (see Fig. 1). When this population was analysed separately it was found that the average increase in area resulting from cross-linking of CD11b was 57% (45 532 ± 572 increased to 71 410 ± 1758). Most of the cells appearing in the P2 region can therefore be accounted for by large individual ‘activated’ neutrophils. We cannot however, rule out the possibility that a few small homotypic neutrophil aggregates contribute to the P2 population. Aggregates may form as a direct result of the cross-linking antibodies or may reflect increased cell surface expression of adhesion molecules translocated onto the cell surface from pre-existing cytoplasmic stores. In support of this hypothesis we have shown that cross-linking of CD11b produced a fivefold increase in surface expression of the adhesion molecule CD18 on the surface of P1 neutrophils and a 10-fold increase on P2 cells. It was also evident that formation of P2 cells and up-regulation of CD18 was almost completely inhibited in the presence of 2 mm EDTA (see Fig. 3). These findings are therefore in good agreement with the findings of Takano et al.9 who showed that translocation of CD antigens onto the surface of neutrophils is mediated by a calcium-dependent protein tyrosine kinase pathway. Similarly, a threefold increase in CD66, a molecule thought to be involved in homotypic adhesion,12 was also observed on all neutrophils following cross-linking of CD11b (see Figs 4 and 5).
Figure 2.
Morphological appearance of leucocytes following in vitro cross-linking of CD11b. Cytospin preparations stained with haematoxylin and eosin of leucocytes before (control) and after cross-linking of CD11b (X-L CD11b) at 37° for 30 min. Some individual neutrophils appear larger than normal with a voluminous cytoplasm as indicated by arrows.
Figure 3.
Effect of cross-linking CD11b on surface expression of CD18. Dot-plots of forward scatter (cell size) versus side scatter (granularity) showing the two main populations of neutrophils before (Control) and following in vitro cross-linking of CD11b (X-L CD11b) conducted in the absence or presence of 2 mm EDTA. Large P2 neutrophils appear only following cross linking of CD11b. The total number of cells appearing in the P2 region is diminished in the presence of EDTA. Dot-plots of forward scatter versus log fluorescence (CD18) show that virtually all neutrophils constitutively express CD18 on the cell surface. The mean fluorescence intensity of binding of FITC-conjugated anti-CD18 increased approximately fivefold on the surface of P1 neutrophils and by 10-fold on P2 cells following cross linking of CD11b at 37° for 30 min. When cross-linking was conducted in the presence of 2 mm EDTA then the MFI value for CD18 expression was essentially normal.
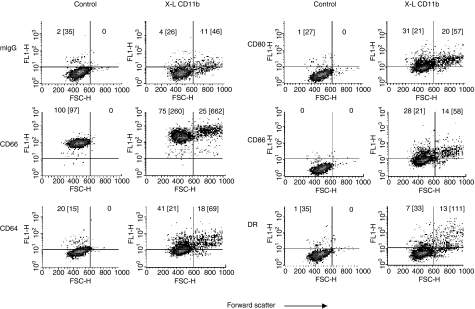
Figure 4.
Effect of cross-linking CD11b on neutrophil surface expression of CD66, CD64, CD80, CD86 and MHC class II (DR) antigen: Whole blood method. Dot-plots of forward scatter versus log fluorescence for all neutrophils gated as R3 (see Fig. 1) are shown for control samples (no cross-linking) and following in vitro cross-linking of CD11b (X-L CD11b) at 37° for 30 min. This figure shows the results of a typical experiment performed using whole blood obtained from one individual donor. Surface expression of CD antigens was measured relative to an appropriate isotype matched mouse IgG isotype control (mIgG1). Quadrant gates were set according to mouse IgG negative control values in each experiment. The horizontal line indicates the upper limit for binding of mouse IgG1 and the vertical line indicates the forward scatter gate placed at the upper limit for normal control neutrophils. The percentage of cells appearing in the upper quadrants (positive cells) is shown for comparison and the mean fluorescence intensity is also indicated in brackets. Cells appearing in the upper left quadrant are therefore P1 neutrophils and the upper right quadrant are P2 cells.
Figure 5.
Effect of cross-linking CD11b on neutrophil surface expression of CD66, CD64, CD80, CD86 and MHC class II (DR) antigen: Purified neutrophils. In order to provide a valid comparison, this assay was performed using the same donor, on the same day, in parallel with the whole blood method as shown above in Fig. 4. Neutrophils were purified using Ficoll-hypaque, dextran sedimentation and hypotonic shock. (a) For analysis, purified neutrophils were gated as indicated by the region shown using dot-plots of forward versus side scatter. (b) Dot-plots of forward scatter versus log fluorescence for all neutrophils are shown for control samples (no cross-linking) and following in vitro cross-linking of CD11b (X-L CD11b) at 37° for 30 min. Surface expression of CD antigens was measured relative to an appropriate isotype-matched mouse IgG isotype control (mIgG1). Quadrant gates were set according to the mouse IgG negative control for the cross-linked preparations. The horizontal line indicates the upper limit for binding of mouse IgG1 and the vertical line indicates the point at which large P2 cells appear. The percentage of cells appearing in the upper quadrants (positive cells) is shown for comparison and the mean fluorescence intensity is also indicated in brackets. Cells appearing in the upper left quadrant are therefore P1 neutrophils and the upper right quadrant are P2 cells.
A typical density plot of forward scatter versus log fluorescence for all neutrophils (Region 3) before (Control) and after cross-linking of CD11b at 37° for 30 min (XL-CD11b) is shown in Fig. 4. In all experiments, background binding was assessed using an isotype control (FITC-conjugated mouse IgG1, Becton Dickinson). It was evident that normal human peripheral blood neutrophils do not constitutively express CD64, MHC class II antigen, CD80 or CD86 on the cell surface. Following cross-linking of CD11b, cell surface expression of these antigens was observed on all neutrophils with higher levels of expression evident on the larger P2 cells which appear in the upper right hand quadrants of these plots. Mean values for CD antigen expression obtained from all six experiments are shown for lymphocytes, monocytes and neutrophils (P1and P2) in Tables 1 and 2.
Lymphocytes (region 1) showed no surface expression of CD64, CD80 or CD86 either before or after cross-linking of CD11b. MHC class II antigen was constitutively expressed on approximately 12% of cells (B cells). No significant change in MHC class II antigen expression was observed over the time course of these kinetic experiments. This applied equally to measurement of the percentage of lymphocytes expressing cell surface MHC class II antigen and to values obtained for mean fluorescence intensity (see Table 1).
Monocytes constitutively express cell surface CD64, MHC class II antigen and to a lesser extent CD86. No surface expression of CD80 was noted. No significant change in CD64 or MHC Class II antigen expression was observed following cross-linking of CD11b. A significant increase in cell surface expression of CD80 and CD86 was however, evident at all time points over the course of these kinetic experiments relative to baseline values at time zero and relative to background binding of mouse IgG1 (see Table 1). No significant differences between CD80 and CD86 levels were observed on monocytes at any time point.
For the purposes of analysis, all neutrophils were gated as region 3 (see Fig. 1) and subdivided into P1 and P2 cells according to their light scatter properties as described above. It was evident that the larger P2 neutrophils expressed significantly higher levels of all three antigens when compared with P1 cells (see Table 2). This increased expression of CD80 and CD86 on P2 cells appears to be specific because these values were significantly elevated in comparison with background mouse IgG1 binding – the only exception being CD86 at 120 min. This increase was not so pronounced with the smaller P1 neutrophils with no significant increase in staining for CD80 or CD86 evident at any time point and MHC class II antigen being significantly increased only at 2·5 min (see Table 2). Significantly elevated levels of CD64 were, however, evident even on P1 neutrophils.
In a separate analysis, the levels of CD80 and CD86 were compared. It was noted that in the early stages (up to and including 1 hr) of the kinetics experiment no significant difference in the level of these molecules was apparent. However, a significantly (P = 0·02; Wilcoxon Two Sample Test) lower value for CD86 was observed at 120 min on P1 cells and between 90 and 120 min on P2 cells. This difference resulted from a fall in the level of CD86 relative to CD80 which remained elevated over the entire 2-hr period of the experiment.
It is also worth noting that the levels of surface expression of MHC class II antigen on P2 neutrophils were comparable (no significant difference observed) to the levels of this antigen expressed on the surface of monocytes. Moreover, the level of CD80 expression on P2 cells was significantly higher, at all time points, than the level observed in parallel on monocytes from the same donors. Similarly, CD86 on P2 neutrophils was also significantly higher than the level found on monocytes but this was apparent only between 30 and 90 min when CD86 expression was highest.
The formation of the larger P2 cells and increased cell surface expression of MHC class II antigen and costimulatory molecules was also found to be inhibited in the presence of 2 mm EDTA similar to the results shown for CD18 in Fig. 3.
Comparison of the whole blood assay with purified neutrophils
As shown in Fig. 5, increased expression of CD64, CD66, CD80, CD86 and to a lesser extent MHC class II antigen was observed on the surface of neutrophils (and in particular on P2 neutrophils) following cross-linking of CD11b when purified neutrophils were used in place of whole blood. This increase was relative to the negative mouse IgG isotype control and to the level of these antigens expressed on the surface of control neutrophils. It was noted however, that control neutrophils purified by Ficoll-Hypaque, dextran sedimentation and hypotonic shock appeared to be spontaneously activated as assessed by the appearance of large P2 cells and by weak expression of surface antigens relative to the negative mouse isotype control. This spontaneous activation was not observed with the whole blood method as shown in Fig. 4. Similarly, detection of MHC class II antigen on P2 neutrophils was also more pronounced with the whole blood method.
Intracellular localization of neutrophil cytoplasmic CD antigens
Confocal microscopy.
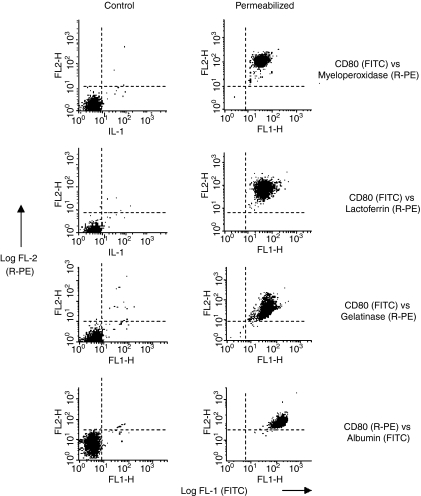
Cytoplasmic CD antigens and neutrophil granule antigens were demonstrated by flow-cytometry within fixed and permeabilized neutrophils (gated as region 3). Dual-staining studies, using various combinations of FITC- and R-PE conjugated antibodies were used to optimize antibody concentrations and to confirm that all neutrophils express cytoplasmic CD80/86 together with neutrophil granule markers, i.e. myeloperoxidase (primary granules), lactoferrin (specific granules), matrix metalloproteinase 9 (gelatinase granules) and human serum albumin (secretory vesicles). These antibodies did not bind to the surface of viable neutrophils (Fig. 6) nor did they show significant binding to control or permeabilized lymphocytes or monocytes. Cytospin preparations of these dual stained preparations were made and the cell nucleus counterstained with DAPI prior to viewing by confocal microscopy.
Figure 6.
Detection of cytoplasmic CD80 by flow-cytometry following fixation and permeabilization of normal human peripheral blood neutrophils: Dual-staining using granule markers. Dot-plots of log fluorescence for FITC (X-axis) versus R-PE (Y-axis) before (Control) and following fixation and permeabilization (Permeabilized) of neutrophils, i.e. cells gated as region 3 – see Fig. 1. Dot-plots of control cells show that monoclonal antibodies specific for CD80 and those specific for the four different types of neutrophil granule, i.e. myeloperoxidase (azurophilic granules), lactoferrin (specific granules), gelatinase (tertiary granules) and human serum albumin (secretory vesicles) do not bind to the surface of neutrophils. In contrast, following fixation and permeabilization, all neutrophils show dual staining.
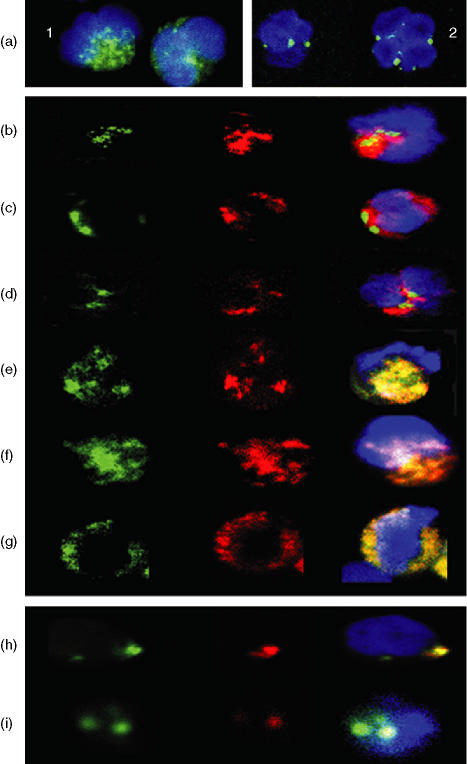
As shown in Fig. 7(a), neutrophil cytoplasmic CD80 and CD86 was confined to the cell cytoplasm with a staining pattern clearly consistent with a granular location. Dual-staining showed that cytoplasmic CD80 (and CD86) was not colocalized within primary, secondary or tertiary (gelatinase) granules (see Fig. 7b) but colocalization was observed within secretory vesicles (see Fig. 7e). Similarly, MHC class II antigen was also found to colocalize with CD80/86 (see Fig. 7f) and CD80 and CD86 were shown to colocalize (Fig. 7g).
Figure 7.
(a-g): Detection of cytoplasmic CD80 by confocal microscopy following fixation and permeabilization of normal human peripheral blood neutrophils: Dual-staining using various granule markers.(a) Confocal image of twopermeabilized neutrophils showing that binding of FITC-conjugated (green) anti-CD80(1) and CD86(2) appears to be confined to granules within the cell cytoplasm. Cell nucleus counterstained with 0.2 mg/ml 40′,6-diamidino-2-phenylindole (DAPI). Each individual cell shown in this figure was approximately 10 ;Cm in diameter. (b) Confocal image of a single permeabilized neutrophil showing green (FITC) alone (CD80), red (R-PE) alone (myeloperoxidase) and the merged image + DAPI. Red and green areas are distinct indicating that CD80 is not located within primary (azurophilic) granules. (c) Confocal image of a single permeabilized neutrophil showing green (FITC) alone (CD80), red (R-PE) alone (lactoferrin) and the merged image + DAPI. Red and green areas are distinct indicating that CD80 is not located within secondary (specific) granules. (d) Confocal image of a single permeabilized neutrophil showing green (FITC) alone (CD80), red (R-PE) alone (MMP-9) and the merged image + DAPI. Red and green areas are distinct indicating that CD80 is not located within tertiary (gelatinase) granules. (e) Confocal image of a single permeabilized neutrophil showing green (FITC) alone (human serum albumin), red (R-PE) alone (CD80) and the merged image + DAPI. Red and green areas are identical indicating that CD80 is located within secretory vesicles. (f) Confocal image of a single permeabilized neutrophil showing green (FITC) alone (CD80), red (R-PE) alone (MHC class II [DR] antigen) and the merged image + DAPI. Red and green areas are identical indicating that these molecules are colocalized within secretory vesicles. (g) Confocal image of a single permeabilized neutrophil showing green (FITC) alone (CD80), red (R-PE) alone (CD86) and the merged image + DAPI. Red and green areas are identical indicating that these molecules are colocalized within secretory vesicles. (h-i) Detection of cell surface CD80 and CD86 following cross-linking of CD11bh. Confocal image of a single neutrophil following cross-linking of CD11b at 37° for 2·5 min showing green (FITC) alone (CD80), red (R-PE) alone (CD86) and the merged image + DAPI. Red and green areas are identical indicating that these molecules are colocalized on the cell surface within a single large cluster. Different z-levels taken through this particular cell confirmed that this was the only area on the surface where these molecules were found to be colocalized. (i) Confocal image of a single neutrophil following cross-linking of CD11b at 37° for 90 min showing green (FITC) alone (CD80), red (R-PE) alone (CD86) and the merged image + DAPI. Two distinct clusters were observed on the surface of this cell. In one cluster only CD80 (FITC) was observed while the other cluster clearly contains both molecules as indicated by the yellow colour in the merged image. Note: In this study many hundreds of confocal images were obtained. For practical reasons the images shown in this figure were therefore selected to illustrate the authors conclusions based on detailed inspection of multiple images.
Following translocation of CD80 and CD86 onto the neutrophil surface, by cross-linking of CD11b, these molecules were observed by confocal microscopy to be colocalized in large polarized clusters (see Fig. 7h). Typically, each cell had a single cluster containing both molecules but cells with multiple clusters were also observed. Cluster formation was evident within a few minutes of addition of cross-linking reagents. Some cells, particularly in the later stages of the kinetic experiment, showed focal areas of segregation where CD86 was apparently lost leaving clusters containing CD80 alone (see Fig. 7i).
Immunoelectron microscopy.
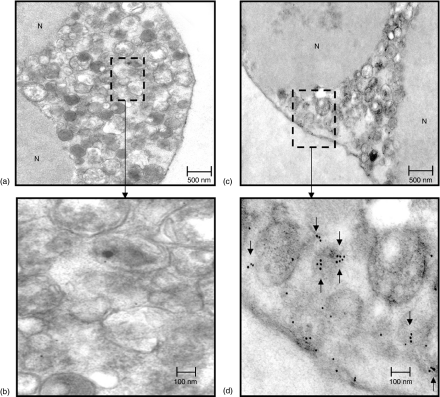
In control neutrophil preparations, where the primary antibody was omitted, a few isolated gold particles were found (mean ± SEM =15 ± 4; N = 8) in the cytoplasm which were not associated with any particular type of granule (see Fig. 8a,b). In neutrophil preparations where anti-CD80 primary antibody was included an increased number of gold particles was observed (126 ± 21; N = 8) within the cell cytoplasm, i.e. no nuclear (N) staining was observed (see Figs 8c,d) with many of these being found in small clusters of three or more. These clusters were not associated with large granules but rather appeared to be free in the cytoplasm or associated with small vesicular structures (see arrows in Fig. 8d).
Figure 8.
Detection of cytoplasmic CD80 by immunoelectron microscopy using 10 nm gold particles conjugated with F(ab′)2 goat anti-mouse IgG. Pre-embedding method. (a, b) Low and high power transmission electron micrographs showing part of a control neutrophil, were primary antibody (mouse anti CD80) was omitted showing a few individual gold particles within the cytoplasm. Clusters of particles were never observed in these preparations. (c, d) Low and high power transmission electron micrographs showing part of a neutrophil, were primary antibody (mouse anti-CD80) was included showing definite clustering (see arrows) of particles within the cell cytoplasm possibly associated with small vesicle like structures within the cell cytoplasm. Immunogold particles did not bind to the cell nucleus nor were they ever found to be associated with large (primary and/or secondary) granules.
Discussion
Following fixation and permeabilization, normal human peripheral blood neutrophils were previously shown, at the protein and mRNA level, to express a variety of antigens normally associated with B-lymphocytes and antigen presenting cells.8 These observations suggest that neutrophils have the capacity to actively synthesize these molecules, thus supporting the hypothesis that activated neutrophils have the potential to function as APCs.2 Because neutrophils contain many preformed molecules, required for rapid immune responses, within cytoplasmic stores13–15 it seems likely that the release and/or surface expression of these molecules may be biphasic with cytoplasmic stores being required for an immediate response and active synthesis for sustained responses.16 The precise mechanism involved in immediate release is not known.
Neutrophils utilize cell surface molecules like the Mac-1 complex (CD11b + CD18) in order to adhere to vascular endothelial cells expressing intracellular adhesion molecule-1; an interaction which in turn leads to up-regulation and increased surface expression of the Mac-1 complex itself.17 Cross-linking of the Mac-1 complex therefore provides a good model for the study of early neutrophil activation. As stated in the Introduction, Takano et al.9 have recently shown that in vitro cross-linking of Mac-1 results in rapid translocation of CD64 (FcγRI), from preformed cytoplasmic stores, onto the neutrophil surface. The original aim of this study was therefore to determine whether this early neutrophil activation signal could also result in translocation of cytoplasmic CD80, CD86 and MHC class II antigen onto the cell surface.
Using a modified (whole blood) version of the original method described by Takano et al.9 we confirmed that rapid translocation of CD64 does indeed occur within a matter of minutes with optimum expression evident by 30 min (see Table 2). We also showed, using six normal donors, that cross-linking of CD11b resulting in rapid, calcium dependent, surface expression of CD80, CD86 and to a lesser and more variable extent MHC class II (DR) antigen.
One unexpected feature of the whole blood version of this assay was the appearance of a distinct subpopulation (approximately 20%) of large cells (forward scatter generally >600 – gated as population 2 – P2) which appeared in a matter of minutes following addition of cross-linking reagents. These cells were identified as neutrophils by their cell surface phenotype and in particular by their expression of the granulocyte-specific marker CD6612 (see Fig. 4). Although flow cytometry is known to count individual cells in single file we did consider the possibility that the P2 cells observed in dot-plots of forward versus side scatter, were simply homotypic aggregates of neutrophils. Some aggregation of neutrophils was noted in cytospin preparations of cross-linked cells (see Fig. 2). This is perhaps not surprising because cross-linking of CD11b was found to increase cell surface expression of adhesion molecules like CD18 and CD66 on the cell surface. As far as we are aware this has not previously been reported, but up-regulation of CD18 and CD66 has been shown to be mediated by neutrophil activators such as N-formylmethionylleucylphenylalanine (FMLP) and phorbol myristate acetate.18,19 Measurement of cell area by image analysis, under direct visual control, before and after cross-linking of CD11b, were compared directly with similar measurements of cell size as assessed by light scatter. From these studies it was concluded that the vast majority of cells in the P2 region could be accounted for by individual enlarged neutrophils, with a distinctive appearance, as shown in Fig. 2, and not by aggregates, which although present, are probably excluded during acquisition by the flow cytometer. This increase in individual cell size was surprisingly rapid (occurring within 2·5 min), but is in good agreement with the findings of others who have demonstrated an increase in neutrophil cell volume of between 35 and 60% following in vitro activation with FMLP.20 Similarly, HL-60 myeloid cells were shown to increase in volume by approximately 125% within 2–3 min when placed in 50% tonicity medium.21
It therefore seems likely that most of the cells appearing in the P2 region are large ‘activated’ neutrophils. The high level of expression of the neutrophil activation marker, CD64 (FcγRI),9 on the surface of P2 neutrophils is consistent with this hypothesis. Similarly, Iking-Konert et al.5 have also demonstrated a distinct subpopulation (17%) of large granular in vivo‘activated’ neutrophils, expressing high levels of CD80, CD86 and DR antigen, in the circulation of patients with active Wegener's granulomatosis.
The generation of large activated neutrophils and up-regulation of CD80, CD86 and MHC class II antigen reported here were all observed following cross-linking of CD11b using whole blood as described in Methods. P2 cell formation and antigen translocation was also observed with purified neutrophils as shown in Fig. 5. It was, however, evident that some spontaneous activation had occurred during the isolation procedure. Others have also noted that neutrophils can be non-specifically activated by Ficoll and/or dextran as assessed by increased myeloperoxidase release, increased expression of FMLP-binding sites and generation of reactive O2 species.22,23 It was concluded by these workers that the use of purified neutrophils ‘may lead to misinterpretation of results’23 and/or ‘may lead to important differences of in vivo polymorphonuclear function being obscured’.24 The whole blood method described in this paper proved to be simple, rapid, reliable, inexpensive and requires only a small volume of blood per test. Activation of cells, prior to cross-linking, was not observed in whole blood control preparations and the differences between control and cross-linked cells was much clearer. The whole blood method also appears to be more sensitive, as evidenced by the detection of weakly expressed antigens like DR which was not detected using purified neutrophils obtained from this particular donor. It also allows for simultaneous analysis of lymphocytes and monocytes from the same donor. The whole blood method, therefore, provides a more efficient in vitro assay for the study of neutrophil activators by flow cytometry.
In this study we were particularly interested in the kinetics of expression of molecules known to play a role in antigen presentation. Kinetic studies showed that these antigens were expressed rapidly over the first 30 min with high levels of surface expression being maintained even after 2 hr of incubation at 37° in the presence of cross-linking reagents. The exception to this appeared to be CD86 where levels fell to background in the later stages of the kinetic experiments. Co-stimulatory molecules (CD80 and CD86) are normally found on the surface of APCs but subtle differences in their kinetics of expression and function have been noted. For example, CD86 is thought to initiate early T-cell activation signals whereas CD80 plays an important role in sustaining the T-cell response.25,26 If neutrophils do indeed function as APCs then differences in the neutrophil CD80 and CD86 kinetics observed here would be consistent with this function.
The whole blood assay also allowed us to study the effects of CD11b cross-linking on lymphocytes and monocytes from these six donors in parallel. No change in cell surface expression of any of the CD antigens studied was observed with lymphocytes (see Table 1). Similarly, no significant change in cell surface expression of MHC class II antigen or CD64 was observed with monocytes but a significant increase in CD80 and CD86 was evident. The overall level of CD80 and CD86 expression found on monocytes was comparable to the level found on P1-neutrophils following cross-linking of CD11b. It is however, of considerable interest to note that the levels of CD80 and CD86 expression induced by cross-linking CD11b on P2-neutrophils was equivalent to or significantly greater than the corresponding monocyte level. P2-neutrophils therefore appear to express MHC class II antigen, CD80 and CD86 at comparable or greater levels than monocytes and for this reason are potential candidates for antigen presenting and/or costimulatory cells within sites of inflammation.
The rapidity with which these molecules appear on the cell surface following cross-linking of CD11b suggests that these molecules are preformed within neutrophil granules. Using various fluorochrome-conjugated monoclonal antibodies known to identify the four main types of human neutrophil granule10,11,27 we showed by confocal laser microscopy that CD80, CD86 and MHC class II antigen were colocalized within secretory vesicles. Preliminary studies using immunoelectron microscopy28 also appeared to confirm this observation with small clusters of immunogold particles associated with small structures within the cell cytoplasm resembling secretory vesicles. This is perhaps not surprising since, several other well characterized receptor molecules have also been found to be contained within secretory vesicles, e.g. complement receptor 1 (CD35) and FMLP-R among others.10 It is also worth noting that cross-linking of CD11b also results in the rapid translocation of CD66 onto the cell surface (see Figs 4 and 5). This molecule has been shown to be present mainly within neutrophil-specific (secondary) granules and tertiary (gelatinized) granules, but has also been found to a lesser extent also within neutrophil secretory vesicles.19 Specific translocation of CD66 from secretory vesicles was observed to occur after short-term activation with fMLP using freshly isolated neutrophils. Such mild treatment does not result in mobilization of CD66 from secondary or tertiary granules but secretory vesicles are readily mobilized under these conditions.19 These observations are therefore consistent with rapid translocation of CD66 from secretory vesicles following cross-linking of CD11b.
Neutrophil granule contents are known to be released in a strict hierarchy with molecules translocating most rapidly from secretory vesicles.10,11 This observation is, therefore, consistent with the kinetic studies reported here, which show that the molecules required for antigen presentation and costimulation are detected on the surface of neutrophils within 2·5 min with optimal expression occurring by 30 min. Exocytosis of molecules from secretory vesicles has been shown to occur within milliseconds29 and transport of molecules from the trans-Golgi network to the plasma membrane via secretory vesicles, visualized in real time using GFP-tagged proteins, was found to be optimal at 20–30 min30 Similarly, translocation of Mac-1 from ‘internal latent pools’ onto the surface fMLP-activated monocytes was shown to occur with 2 min31 and up-regulation of very late antigen-6 on neutrophils in response to fMLP has also been shown to peak at 6 min32 Bezzerides et al.33 have also demonstrated rapid (within 2 min) growth factor mediated translocation of transient receptor potential ion-channel proteins from vesicles held in reserve just under the plasma membrane of HEK-293 cells. These vesicles are docked or tethered by formation of complexes between vesicular soluble N-ethylmaleimide-sensitive fusion protein attachment proteins (SNAP) receptors (v-SNARES) and plasma membrane target molecules (t-SNARES). Vesicles may then fuse completely with the plasma membrane or release their contents into the extracellular space. An unexpected finding in this study was that, following cross-linking of CD11b, both costimulatory molecules appear on the cell surface colocalized within large clusters. Most cells were found to have these molecules concentrated into a single cluster but some cells exhibited more than one. In the early stages, following cross-linking of CD11b, the costimulatory molecules were contained within the same cluster (see Fig. 7h), but as time progressed, segregation occurred with CD86 being lost (see Fig. 7i). The precise mechanism involved in clustering of costimulatory molecules on the surface of cross-linked neutrophils is not known. Brumell et al.34 have, however, shown that neutrophil secretory vesicles have high concentrations of the v-SNARE molecule vesicle-associated membrane protein (VAMP)-2, relative to other granules and that this molecule may bind to the t-SNARE protein syntaxin-4 on the plasma membrane. Syntaxin 4 has also been shown to polarize and accumulate within the lamellipodium as neutrophils respond to chemotactic stimuli.34 It would therefore be of interest to determine whether the clustering of costimulatory molecules observed in this study is linked to ‘hot-spots’ of syntaxin 4 on the plasma membrane of ‘activated’ neutrophils. Co-localization of costimulatory molecules together with various other molecules required to form an immunological synapse with helper T cells35–37 has been previously shown to occur on conventional APCs. Clustering occurs in order to compensate for the relatively low number of synapse-forming molecules found on the surface of APCs. The clusters found on the surface of cross-linked ‘activated’ neutrophils reported here are, therefore, consistent with these supramolecular antigen clusters and further support the hypothesis that activated neutrophils can indeed function as APCs. In this study we have focused exclusively on the effect of cross-linking CD11b as a possible translocation mechanism. It would however, seem likely that other neutrophil activation signals, e.g. fMLP or phorbol esters may also result in rapid translocation of these molecules from cytoplasmic stores onto the plasma membrane. This possibility is currently being investigated in our laboratory.
References
- 1.Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–6. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 2.Ashtekar AR, Saha B. Polys plea. Membership of the club of APCs. Trends Immunol. 2003;24:485–90. doi: 10.1016/s1471-4906(03)00235-7. 10.1016/S1471-4906(03)00235-7. [DOI] [PubMed] [Google Scholar]
- 3.Gosselin EJ, Wardwell K, Rigby WFC, Guyre P. Induction of MHC class II on human polymorphonucler neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-γ, and IL-3. J Immunol. 1993;151:1482–90. [PubMed] [Google Scholar]
- 4.Cross A, Bucknall RC, Cassatella MA, Edwards SW, Moots RJ. Synovial fluid neutrophils transcribe and express class II major histocompatibility complex molecules in rheumatoid arthritis. Arthritis Rheum. 2003;48(10):2796–806. doi: 10.1002/art.11253. [DOI] [PubMed] [Google Scholar]
- 5.Iking-Konert C, Vogt S, Radsak M, Wagner C, Hansch GM, Andrassy K. Polymorphonuclear neutrophils in Wegener's granulomatosis acquire characteristics of antigen presenting cells. Kidney Int. 2001;60:2247–62. doi: 10.1046/j.1523-1755.2001.00068.x. 10.1046/j.1523-1755.2001.00068.x. [DOI] [PubMed] [Google Scholar]
- 6.Fanger NA, Liu C, Guyre PM, et al. Activation of human T cells by major histocompatibility complex class II expressing neutrophils. Proliferation in the presence of superantigens, but not tetanus toxoid. Blood. 1997;89:4128–35. [PubMed] [Google Scholar]
- 7.Radsak M, Iking-Konert C, Stegmaier S, Andrassy K, Hansch GM. Polymorphonuclear neutrophils as accessory cells for T-cell activation. major histocompatibility complex class II restricted antigen-dependent induction of T-cell proliferation. Immunology. 2000;101:521–30. doi: 10.1046/j.1365-2567.2000.00140.x. 10.1046/j.1365-2567.2000.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandilands GP, Hauffe B, Loudon E, Marsh AG, Gondowidjojo A, Campbell C, Ferrier RK, Rodie M. Detection of cytoplasmic CD antigens within normal human peripheral blood leucocytes. Immunology. 2003;108:329–37. doi: 10.1046/j.1365-2567.2003.01591.x. 10.1046/j.1365-2567.2003.01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takano K, Kaganoi J, Yamamoto K, Takahashi A, Kido T, Sasada M. Rapid and prominent up-regulation of high-affinity receptor for immunologlobulin G (FcγRI) by cross-linking of β2 integrins on polymorphonuclear leukocytes. Int J Hematol. 2000;72:48–54. [PubMed] [Google Scholar]
- 10.Borregaard N, Cowland JB. Granules of the human neutrophilic polmorphonuclear leukocyte. Blood. 1997;89:3503–21. [PubMed] [Google Scholar]
- 11.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infection. 2003;5:1317–27. doi: 10.1016/j.micinf.2003.09.008. 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Skubitz KM, Campbell KD, Skubitz APN. Synthetic peptides of CD66a stimulate neutrophil adhesion to endothelial cells. J Immunol. 2000;164:4257–64. doi: 10.4049/jimmunol.164.8.4257. [DOI] [PubMed] [Google Scholar]
- 13.Sengelov H, Boulay F, Kjeldsen L, Borregaard N. Subcellular localization and translocation of the receptor for N-formylmethionyl-leucyl-phenylalanine in human neutrophils. Biochem J. 1994;99(2):473–9. doi: 10.1042/bj2990473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodeberg DA, Morris RE, Babcock GF. Azurophilic granules of human neutrophils contain CD14. Infect Immunity. 1997;65:4747–53. doi: 10.1128/iai.65.11.4747-4753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elbim C, Reglier H, Fay M, Delarche C, Andrieu V, Benna JEI, Gougerot-Pocidalo M-A. Intracellular pool of IL-10 receptors in specific granules of human neutrophils: differential mobilisation by proinflammatory mediators. J Immunol. 2001;166:5201–2507. doi: 10.4049/jimmunol.166.8.5201. [DOI] [PubMed] [Google Scholar]
- 16.Grenier A, Dehoux M, Boutten M, Arce-Vicioso M, Durand G, Gougerot-Pociadalo M-A, Chollet-Martin S. Oncostatin M production and regulation by human polymorphonuclear neutrophils. Blood. 1999;93:1413–21. [PubMed] [Google Scholar]
- 17.Sengelov H, Per Follin Kjeldsen L, Lollike K, Dahlgren C, Borregaard N. Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils. J Immunol. 1995;154:4157–65. [PubMed] [Google Scholar]
- 18.Fernandez-Segura E, Garcia JM, Campos A. Topographic distribution of CD18 integrin on human neutrophils as related to shape changes and movement induced by chemotactic peptide and phorbol esters. Cell Immunol. 1996;171(1):120–5. doi: 10.1006/cimm.1996.0181. 10.1006/cimm.1996.0181. [DOI] [PubMed] [Google Scholar]
- 19.Feuk-Lagerstedt E, Jordan ET, Leffler H, Dahlgren C, Karlsson A. Identification of CD66a and CD66b as the major galectin-3 receptor candidates in human neutrophils. J Immunol. 1999;163:5592–8. [PubMed] [Google Scholar]
- 20.Rosengren S, Henson PM, Worthen GS. Migration-associated Volume changes in neutrophils facilitate the migratory process in vitro. Am J Physiol. 1994;267:1623–32. doi: 10.1152/ajpcell.1994.267.6.C1623. [DOI] [PubMed] [Google Scholar]
- 21.Hallows KR, Packman CH, Knauf PA. Acute cell Volume changes in anisotonic media effect F-actin content of HL-60 cells. Am J Physiol. 1991;261:1154–61. doi: 10.1152/ajpcell.1991.261.6.C1154. [DOI] [PubMed] [Google Scholar]
- 22.Berkow RL, Weisman SJ, Tzeng D, Haak RA, Kleinhans FW, Barefoot S, Baehner RL. Comparative response of human polymorphonuclear leukocytes obtained by counterflow centrifugal elutriation and ficoll-hypaque density centrifugation. II. Membrane potential changes, membrane receptor analysis, membrane fluidity, and analysis of the effects of the preparative techniques. J Lab Clin Med. 1984;104(5):698–710. [PubMed] [Google Scholar]
- 23.Rebecchi IM, Novo F, Julian Y, Campa A. Oxidative metabolism and release of myeloperoxidase from polymorphonuclear leukocytes obtained from blood sedimentation in a ficoll-hypaque gradient. Cell Biochem Funct. 2000;18(2):127–32. doi: 10.1002/(SICI)1099-0844(200006)18:2<127::AID-CBF865>3.0.CO;2-V. 10.1002/(SICI)1099-0844(200006)18:2<127::AID-CBF865>3.3.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 24.Pallister I, Topley N. Chemiluminescence: comparison of whole blood with isolated polymorphonuclear leukocytes after major trauma. J Trauma. 2004;57(2):347–51. doi: 10.1097/01.ta.0000133572.44369.f8. [DOI] [PubMed] [Google Scholar]
- 25.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 26.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 co-stimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–52. doi: 10.1146/annurev.immunol.19.1.225. 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 27.Perretti M, Christian H, Wheller SK, Aiello I, Mugridge KG, Morris JF, Flower RJ, Goulding NJ. Annexin 1 is stored within gelatinase granules of human neutrophil and mobilised on the cell surface upon adhesion but not phagocytosis. Cell Biol Int. 2000;24:163–74. doi: 10.1006/cbir.1999.0468. 10.1006/cbir.1999.0468. [DOI] [PubMed] [Google Scholar]
- 28.Bainton DF. Distinct granule populations in human neutrophils and lysosomal organelles identified by immuno-electron microscopy. J Immunol Methods. 1999;232:153–68. doi: 10.1016/s0022-1759(99)00173-8. [DOI] [PubMed] [Google Scholar]
- 29.Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev. 2003;83:581–606. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- 30.Wacker I, Kaether C, Kromer A, Migala A, Almers W, Gerdes H-H. Microtubule-dependent transport of secretory vesicles visualised in real time with a GFP-tagged secretory protein. J Cell Sci. 1997;110:1453–63. doi: 10.1242/jcs.110.13.1453. [DOI] [PubMed] [Google Scholar]
- 31.Miller LJ, Bainton DF, Borregaard N, Springer TA. Stimulated mobilisation of monocytes Mac-1 and p150,90 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest. 1987;80:535–44. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roussel E, Gingras MC. Transendothelial migration induces rapid expression on neutrophils of granule-release VLA6 used for tissue infiltration. J Leukocyte Biol. 1997;62:356–62. doi: 10.1002/jlb.62.3.356. [DOI] [PubMed] [Google Scholar]
- 33.Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6(8):709–20. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- 34.Brunell JH, Volchuk A, Sengelov H, Borregaard N, Cieutat A-M, Bainton DF, Grinstein S, Klip A. Subcellular distribution of docking/fusion proteins in neutrophils, secretory cells with multiple exocytic compartments. J Immunol. 1995;155:5750–9. [PubMed] [Google Scholar]
- 35.Bromley SK, Burak WR, Johnson KG, et al. The immunological synapse. Annu Rev Immunol. 2001;19:375–96. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 36.Friedl P, Gunzer M. Interaction of T cells with APCs: the serial encounter model. Trends Immunol. 2001;22:187–91. doi: 10.1016/s1471-4906(01)01869-5. 10.1016/S1471-4906(01)01869-5. [DOI] [PubMed] [Google Scholar]
- 37.Faroudi M, Zaru R, Paulet P, Muller S, Valitutti S. Cutting edge. T lymphocyte activation by repeated immunological synapse formation and intermittent signalling. J Immunol. 2003;171:1128–32. doi: 10.4049/jimmunol.171.3.1128. [DOI] [PubMed] [Google Scholar]