Abstract
Dendritic cells (DCs) are the most potent antigen-presenting cells and populate many tissues where they may participate in inflammatory reactions. The infiltration of polymorphonuclear leucocytes (PMNLs) into tissues is a prominent feature of inflammation. The mechanisms of PMNL recruitment depend on chemotactic factors and adhesion molecules expressed on endothelial cells. The aim of the present study was to determine whether DCs participate in the early recruitment of PMNLs. Dendritic cells derived from peripheral blood monocytes were used for this study. PMNLs incubated with culture supernatant (CS) from untreated or from tumour necrosis factor-α (TNF-α)-treated (1 hr, 100 U/ml, 37°) monocyte-derived DCs (moDCs) had increased surface expression of both CD11b and CD18. Moreover, both untreated and TNF-α-treated moDCs induced PMNL chemotaxis. By blocking CXCL8, CXCL5, CXCL7 and Pan GRO (CXCL1, CXCL2, CXCL3), we observed that CXCL8/interleukin-8 might be the chemokine that induced the PMNL chemotactic activity in the CS of untreated and TNF-α-treated moDC. Furthermore, we investigated the regulation of CXCL8 production in moDCs by adhesion molecule engagement. Our data demonstrated that CD31, CD18, CD29 and CD49d participated in the adhesion of immature moDCs to endothelium. Moreover, engagement of domains 1–3 of CD31, but not of CD29 or CD18, decreased the production of CXCL8 by immature but not mature moDCs (which display lower CD31 levels than immature moDCs). Overall, these results suggest that DCs not only trigger a specific immune response, but also the innate immune response by recruiting PMNLs. Furthermore, our results also suggest that CXCL8 production by immature DCs might be regulated by signalling through CD31 during their migration through the vascular endothelium.
Keywords: CXCL8/iL.8, migration, neutrophils, PECAM-1/CD31
Introduction
Inflammation is characterized by the movement of polymorphonuclear leucocytes (PMNLs) and monocytes from the blood into the tissues. The mechanism of leucocyte infiltration into inflammatory sites has been extensively studied. This process involves chemotactic factors produced in the inflamed tissue and adhesion molecules expressed on endothelial cells.1 The endothelial component comprises activation of the endothelium by cytokines such as interleukin (IL)-1, tumour necrosis factor-α (TNF-α) or bacterial lipopolysaccharide (LPS). These factors increase the surface expression of the membrane glycoproteins E-selectin, intercellular adhesion molecule-1 (ICAM-1)/CD54 and vascular cell adhesion molecule-1 (VCAM-1)/CD106 on endothelial cells.1 Molecules on the endothelium interact with sialyl Lewis X containing molecule, β2 integrins (CD11/CD18) and very-late activation antigen type 4 (VLA-4) (CD49d/CD2) on leucocytes.2 Chemotactic factor-dependent PMNL migration involves binding of the factors to specific membrane receptors on PMNLs and largely depends on CD11/CD18 leucocyte surface molecules. Several chemotactic factors such as C5a, LTB4 and chemokines can induce PMNL migration.3 Among chemokines, those belonging to the CXC family and containing the amino acid sequence Glu-Leu-Arg (ELR) motif attract PMNLs via CXCR1 or CXCR2 on the PMNLs.4
Dendritic cells (DCs) are bone marrow-derived leucocytes with the unique capacity to induce primary immune responses. The cells are released into the bloodstream and seed non-lymphoid as well as lymphoid tissues, playing a role as sentinels.5 The proinflammatory signals that initiate acute inflammation and PMNL recruitment can also activate DCs. Among the proinflammatory signal factors, TNF-α plays a key role.6 Activation of DCs induces a maturation process that involves the loss of antigen capture function, up-regulation of major histocompatibility complex (MHC) class II and costimulatory molecules, as well as changes in chemokine receptor expression required for their movement via afferent lymphatics from the periphery to T-cell areas in the lymph node.7 Based on the chemokine expression of DCs, it has been suggested that these cells contribute to PMNL recruitment at peripheral sites of inflammation, 8–11 although the chemokine produced by the DCs did not always correlate with leucocyte infiltration.12 Moreover, under normal, non-inflamed conditions, no PMNL infiltration occurred despite the presence of DCs or Langerhans' cells in the skin. Therefore, in the present study we investigated whether DCs participate in the induction of PMNL infiltration in response to TNF-α, an inflammatory cytokine. Our results demonstrated that untreated and TNF-α-treated monocyte-derived DCs (moDCs) activated and recruited PMNLs by producing mainly CXCL8. Moreover, our data indicated that signalling through CD31 DC down-regulated CXCL8 production by immature moDCs, but not by TNF-α-treated moDCs.
Materials and methods
Monoclonal antibodies
A number of monoclonal antibodies (mAbs) that recognize antigens present on DCs were used in vitro. These included mAbs anti-CD1b (Wm25, IgG1; The 5th International Workshop on Human Leucocyte Differentiation Antigens), anti-HLA-DR [HB-55, IgG2a; American Tissue Culture Collection (ATCC), Rockville, MD], anti-CD86 (FUN-1; IgG1; PharMingen, San Diego CA) and anti-CD83 (HB15A, IgG2b; Immunotech, Marseille, France). Other mAbs used were anti-CD14 (3C10, IgG2b) and anti-CD18 (TS1/18, IgG1) (both from the ATCC), anti-CD29 (P4G11, IgG1; Santa Cruz Biotechnology, Santa Cruz, CA), anti-CD11b (2LPM19c, IgG1; Dako Corp., Carpinteria, CA), anti-CD31 (mAb 2B3, IgG1; Dr Ramón Villela, Servei d'Immunologia, Hospital Clinic, Barcelona, Spain) and mAb 5H2 (IgG2a generated by fusion of P3U1 myeloma cells with spleen cells from a BALB/c mouse immunized with purified human PMNL).
The neutralizing mouse IgG1 mAb against human CXCL8 (MAB208; R & D Systems, Minneapolis, MN) was used to block CXCL8 activity. Other neutralizing mAbs used were MAB276 (IgG1) against GRO (CXCL1, CXCL2, CXCL3), MAB254 (IgG1) against CXCL5/ENA-78, and MAB393 (IgG1) against CXCL7/NAP-2 (all from R & D Systems).
Reagents
Recombinant human TNF-α (specific activity, 5 × 107 U/mg), IL-4 (500 U/ml), granulocyte–macrophage colony-stimulating factor (GM-CSF) (800 U/ml) and CXCL8 (285 ng/ml) were from Sigma (St Louis, MO). All of these cytokines contained less than 1 ng/mg of LPS. Each of the cytokines was diluted immediately before use in 0·1% (v/v) LPS-free human serum albumin (HSA) (Grifols Institut, Barcelona, Spain).
Culture of moDCs from peripheral blood
Human DCs were generated from peripheral blood mononuclear cells (PBMCs) as previously described.13 Briefly, peripheral blood was obtained from healthy donors. First, the PBMCs were isolated by centrifugation through a Ficoll–Hypaque (Pharmacia LKB Biotech, Piscataway, NJ) gradient. Then, monocytes were isolated through a Percoll gradient, as reported previously.14 The purest monocyte fraction was recovered and cultured for 5–7 days in RPMI-1640 supplemented with 2 mm l-glutamine, 50 µg/ml gentamicin, 50 µm 2-mercaptoethanol, 800 U/ml GM-CSF, 500 U/ml IL-4 (Sigma) and 10% fetal bovine serum (FBS) (Gibco, Rockville, MD). The moDCs were identified by immunofluorescence staining of the cells with mAbs to HLA-DR, CD1b and CD86. The moDC preparations containing < 3% contaminating cells were used for the experiments. Whenever necessary, another Percoll gradient purification was performed to further enrich for moDCs after culture with GM-CSF and IL-4, i.e. 5–7 days.
For evaluation of moDC adherence to human umbilical vein endothelial cells (HUVEC), the moDCs were labelled with [51Cr]sodium chromate (20 µCi/ml) (New England Nuclear Corporation, Boston, MA) by incubation for 30 min at 37°, as described previously.
Human PMNL purification from normal donors
Human PMNLs were purified, as described previously, from acid–citrate–dextrose (ACD)-heparin-anticoagulated venous blood of healthy donors.15 Briefly, leucocyte-rich plasma was collected after red cells were sedimented with 6% dextran-saline (Kabi Pharmacia, Uppsala, Sweden). The PMNLs were then purified by discontinuous Percoll gradient centrifugation, washed, and resuspended to 2·5 × 106 PMNLs/ml in RPMI-1640, 0·5% HSA, 10 mm HEPES, pH 7·4. This method yielded PMNLs of > 95% purity and > 98% cell viability.
MoDC culture supernatants (CS)
To analyse the production of activators or chemotactic factors by moDCs, they were cultured in RPMI-1640 with 10% (v/v) FBS and GM-CSF + IL-4, as described above. The cells were then either unstimulated or stimulated with TNF-α (10–1000 U/ml) for 1–16 hr, depending on the experiment. After stimulation, the cells were washed twice with RPMI-1640 and incubated for 3 hr (37°, 5% CO2) at 2 × 106 moDCs/ml RPMI-1640, 0·5% (v/v) HSA, 10 mm HEPES (pH 7·4). At the end of the incubation, the CS were collected and frozen at −70° until the migration studies and enzyme-linked immunosorbent assays (ELISAs) for CXCL8 and GRO were performed.
PMNL chemotaxis assays
For chemotaxis assays, 0·1 ml of medium containing 2 × 105 PMNLs was added to the upper surface of the polyvinyl propylene (PVP)-free polycarbonate filters (bearing 5-µm pores) in Transwell culture plate inserts (6·5-mm diameter, Transwell 3415; Costar, Cambridge, MA). To the lower compartment, 0·6 ml of RPMI-1640 containing 10 mm HEPES and 0·5% (v/v) HSA was added together with different dilutions of moDC CS or the chemotactic stimulus CXCL8 or fMet-Leu-Phe (fMLP). After incubation (60 min), migration was stopped by removing the upper compartment. The cells that had migrated into the lower compartment were recovered. The contents of the lower chamber (migrated cells) were analysed by using a Cytoron flow cytometer (Ortho Diagnostic Systems, Inc., Raritan, NJ) equipped with the software program absolute. According to the positivity of PMNLs for either CD16 or CD15, a gate on a forward-scatter versus side-scatter dot-plot was defined. Cells located in the gate were considered as migrated PMNLs and counted by flow cytometry. Results were expressed as the percentage of the total PMNLs added above the filter, which transmigrated the filter. All the stimulation conditions were performed in triplicate.
PMNL activation
Activation of PMNLs was assessed by analysing CD11b and CD18 expression and myeloperoxidase (MPO) release by PMNLs. Analysis of CD11b and CD18 expression on PMNLs was performed by flow cytometry. PMNL were incubated for 10 min with different dilutions of moDC CS. Then, the expression of CD11b and CD18 on PMNLs was analysed by immunofluorescence staining.
MPO release was performed by incubating 5 × 106 PMNLs in the presence of cytochalasin B (5 µm) at various concentrations of moDC CS or fMLP for 30 min at 37°. The reaction was terminated by placing the tubes in an ice bath followed by sedimentation of PMNL by centrifugation. The MPO activity in supernatants was assayed by colour reaction with o-phenylenediamine (Sigma), and the absorbance was determined at 492 nm. The PMNLs were washed twice with phosphate-buffered saline (PBS) containing 0·1% HSA and lysed by the addition of 50 µl of H2O and freezing. Samples were thawed and MPO was also assayed. The percentage of MPO in the PMNL supernatant relative to total MPO was determined and the results are expressed as the relative change in released MPO (1 = the release of MPO in untreated PMNLs).
CXCL8 and GROα detection
CXCL8 and CXCL1 concentrations in moDC CS were detected by sandwich ELISA. In brief, BD Falcon™ 96-well Clear ELISA Plates were coated overnight at 4° in coating buffer with MAB208 (1 µg/ml) for CXCL8 or with MAB276 (5 µg/ml) for CXCL1. The next day, the plates were washed and non-specific binding sites were blocked by incubating the wells with PBS containing 2% (v/v) bovine serum albumin (BSA) in coating buffer for 30 min at 37°. Serially diluted chemokine standards and samples were then added and incubated overnight at 4°. The wells were then washed and polyclonal goat anti-CXCL8 or anti-CXCL1 was added. After 90 min of incubation at 37°, the wells were washed and peroxidase anti-goat IgG was added for 60 min at 37°. Then, 50 µl of o-phenylenediamine substrate solution was added and incubation continued for 10–30 min. The absorbance was read at 492 nm.
Reverse transcription–polymerase chain reaction (RT–PCR)
Quantification of mRNA for CXCL8, CXCL5, CXCL6/GCP-2 and CXCL7 was performed by RT–PCR. Total cellular RNA was isolated from 106 purified untreated and TNF-α-treated moDCs by using Trizol LS Reagent (Gibco BRL, Grand Island, NY).16 Synthesis of cDNA was performed according to the manufacturer's recommendations (Superscript II; Gibco BRL) followed by standard polymerase chain reaction (PCR) amplification. These primers had the following sequence: CXCL5: sense 5′-GCGTTTGTTTACAGACCACGCAAG-3′, and antisense 5′-CCACTGTCCAAAATTTTCTGGATGAC-3′, with the predicted product of 184 bp. The CXCL6 sense 5′-GCACTTGTTTACGCGTTACGCTG-3′ and antisense 5′-CCACTGTCCAAAATTTTCTGGATGAC-3′ generated the predicted product of 184 bp. The CXCL7 sense 5′-CCACCAAAGGACAAACTAAGAGAA-3′ and antisense 5′-CAGATTCATCACCTGCCAATTT-3′ generated the predicted product of 273 bp. The CXCL8 sense 5′-CTTGGCAGCCTTCCTGATTT-3′ and antisense 5′-CTCAGCCCTCTTCAAAAACT-3′ generated the predicted product of 433 bp. The cDNA was amplified for 40 cycles in the PCR. Amplified products, together with a DNA ladder as a size standard, were resolved on 2% (w/v) agarose and the products were visualized following staining with ethidium bromide.
Migration blocking experiments
In some experiments, moDC CS were treated for 20 min at room temperature with the neutralizing mAbs indicated, at 20–40 µg/ml. These mAb-treated CS were then tested for inducing PMNL migration. In some experiments, the moDCs were treated with 10 µm 5-lipoxygenase-activating protein inhibitor (MK886; Biomol Research Laboratories, Inc., Plymouth Meeting, PA) for 30 min at 37°. Afterwards, moDC CS were harvested, as described above, and tested for PMNL migration.
Culture of HUVEC
Endothelial cells were isolated from human umbilical vein and cultured in flasks or in 96-well plates as described by Jaffe et al.17 Briefly, endothelial cells were grown in RPMI-1640 containing 2 mm l-glutamine, 2-mercaptoethanol (2-ME), sodium pyruvate, penicillin G/streptomycin and supplemented with 20% (v/v) FBS (Gibco BRL), 25 µg/ml of endothelial cell growth supplement (Collaborative Res., Lexington, MA) and 45 µg/ml of heparin (Sigma). Cells were cultured in gelatin-coated culture flasks. The HUVEC were detached by using 0·025% trypsin/0·01% versene (Sigma) and cultured in 96-well flat-bottom plates until confluent.
MoDC adhesion to HUVEC
Adhesion of moDCs to HUVEC was performed as previously described.13 Briefly, 5 × 105[51Cr]moDC/ml were added over HUVEC monolayers. After incubation (for 45 min), adhesion was stopped by washing to remove non-adherent moDCs. The attached moDCs were lysed by the addition of 0·5% (v/v) Triton-X-100 and then 51Cr incorporation was quantified. The results were expressed as a percentage of the total [51Cr]moDCs added to the HUVEC, recovered in each fraction. For adhesion-blocking experiments, moDCs were preincubated with the indicated mAbs for 20 min before plating. All experiments were performed in triplicate.
Co-ligation assay
Tissue culture plates (96-well flat bottom; Nunc, Rochester, NY) were coated overnight at 4° with 5H2, 7E4 or control mAb (Bi24) at 20 µg/ml in PBS. After two washings with PBS, mAb-coated wells were incubated with HSA (5 mg/ml) at 37° for 2 hr to block uncoated sites. Afterwards, moDCs and LPS-treated moDCs were added to each well and incubated for 24 hr. Finally, the CS were recovered and frozen at −70° until ELISA assays for CXCL8 were performed.
Immunofluorescence and flow cytometric analysis
Suspensions of moDCs or PMNL were incubated successively with pooled AB serum (10%), for 30 min, to block Fc receptors, with primary mAb for 60 min at an optimal concentration (2–5 µg/ml) and with R-phycoerythrin (RPE)-conjugated goat anti-mouse (Dako) immunoglobulin for 30 min. All incubation and washing procedures were carried out at 4° in RPMI-1640 supplemented with 5% FBS and 0·1% NaN3. Cells labelled successively with irrelevant isotype-matched primary mAbs and RPE-conjugated goat anti-mouse immunoglobulin were used as negative controls. Fluorescence intensity was analysed by using a Cytoron flow cytometer. Dead cells were excluded by gating with propidium iodide (PI).
Fluorescence-activated cell sorter (FACS) analysis of the DNA content was performed as described previously.18 In brief, 24 hr after engagement of CD31 or control on moDCs, the cells were washed in ice-cold PBS and resuspended in PBS containing 5% (v/v) glucose and 5% (v/v) glycerol. Cells were permeabilized by the dropwise addition of 95% (v/v) ice-cold ethanol to a final concentration of 80% and incubation at 4° for 15 min. Thereafter, cells were stored at −20° until analysis. Prior to analysis, cells were washed in PBS, resuspended in PBS containing 1·25 µg/ml of PI (Sigma) and 50 µg/ml of RNase A (Sigma) and incubated in the dark for 15 min at 4°. The DNA content of moDCs was measured by analysing fluorescence intensity with a Cytoron flow cytometer.
Bone marrow (BM)-derived DCs
BM-derived DC were generated from BALB/c mice (6–8 weeks old) according to a method described by Inaba et al. with the modifications of Lutz et al.19, 20 In brief, femurs and tibias were removed from mice and mechanically isolated from the surrounding tissues; before removing epiphyses, BM cells were flushed with RPMI-1640. Fresh BM cells were cultured in Iscove's modified Dulbecco's medium (IMDM), supplemented with 10% (v/v) heat-inactivated FCS and 30% (v/v) J558/GM-CSF conditioned medium, at 2 × 106 cells/ml in 100-mm bacteriological Petri dishes. On day 5 of culture, DCs were isolated by transferring the non-adherent and loosely adherent cells to new culture plates (leaving behind the adherent macrophages), incubating the plates at 37° for at least 2 hr, and then repeating the procedure to remove any contaminating macrophages. The DC were further enriched (> 95% purity) by density-gradient centrifugation with a dense BSA solution 1·080 g/ml, 21 resuspended in sterile PBS and injected into the air pouch. For DC maturation, on day 10, non-adherent cells were collected, centrifuged at 300 g for 5 min at room temperature, and resuspended in 10 ml of fresh RPMI-1640 into a fresh 100-mm tissue culture plastic dish containing 100 U/ml recombinant murine (rm)GM-CSF and 1 µg/ml of LPS (Sigma).
In vivo air pouch model
Male BALB/c mice, 6–8 weeks of age, were anesthetized. Dorsal air pouches were raised by injecting sterile air (3 ml) subcutaneously into the mice 3 and 6 days before treatment started, as described previously.22 On day 0, some mice were intrapouch injected with sterile RPMI-1640, TNF-α (10 ng), or untreated or TNF-α-treated (for 1 hr or 24 hr) BM-derived DCs (106/200 µl). Two hours later, while anesthetized, exudates were obtained from mice at various intervals by lavage of the pouches twice with sterile PBS (3 ml). Aspirates were centrifuged (at 500 g for 15 min at room temperature), the cell pellets were resuspended in PBS (500 µl) and PMNLs were enumerated by using a Cytoron (Ortho Diagnostics) flow cytometer equipped with the software program absolute.
Statistical analysis
Analysis of variance (anova) was used for statistical analysis of the data, with individual group means compared by using post hoc Student Newman Kules analysis, the Dunnett multiple comparisons test or the Tukey-Kramer Multiple Comparisons test (a P-value of > 0·05 was considered non-significant).
Results
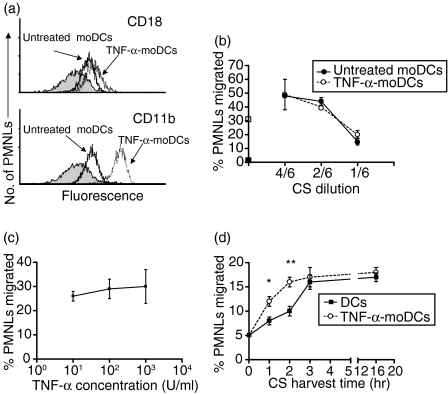
Monocyte-derived DCs were generated in vitro from human monocytes with GM-CSF + IL-4 in the presence of 10% (v/v) FBS, as previously described.13 After 7 days, these moDCs expressed high levels of HLA-DR, CD86, CD1a, b and c, and low levels of CD14, but not of CD83.13 These cells were able to stimulate allogeneic PBMC in a mixed leucocyte culture.13 A purity of > 95% moDCs was achieved by Percoll density-gradient enrichment. We first examined the ability of untreated and of TNF-α (100 U/ml, 1 hr at 37°)-treated moDCs to activate PMNLs. By using CS (harvested at 3 hr) from moDC as a stimulator, we analysed the expression of the integrins CD11b and CD18 on PMNLs. Figure 1(a) shows that CS from moDC (solid line) stimulated the PMNL up-regulation of CD18 (upper panel) and of CD11b (lower panel). However, stimulation with CS from TNF-α-treated moDCs (broken line) induced higher levels of CD18 (upper panel) and of CD11b (lower panel) on PMNLs compared to CS from untreated moDCs (solid line). It should be noted that the cytokines used for moDC differentiation, as well as the TNF-α added to stimulate the moDCs, were not present during generation of the CS. Activation of PMNLs was also examined by measuring the release of MPO. Incubation of PMNLs with CS from TNF-α-treated moDCs increased MPO release by PMNLs up to the levels induced by fMLP treatment (TNF-α-treated moDCs, 45 ± 5%; fMLP, 37 ± 6%; n = 3, P < 0·01). However, in PMNLs treated with CS from untreated moDCs, we detected only a slight increase of MPO release (12 ± 8%; n = 3).
Figure 1.
Polymorphonuclear leucocyte (PMNL) activation (a) and migration (b, c and d). (a) CD18 and CD11b expression on PMNLs. The culture supernatant (CS) from monocyte-derived dendritic cells (moDCs) and from tumour necrosis factor-α (TNF-α) (100 U/ml, 1 hr at 37°)-treated moDCs was harvested after 3 hr, as described in the Materials and methods. Then, the leucocytes were incubated with the CS from moDCs and moDC-TNF-α for 10 min. Finally, PMNLs were stained with monoclonal antibodies (mAbs) to CD18 or to CD11b and analysed by flow cytometry. (b) PMNL migration induced by CS from moDCs and TNF-α (100 U/ml)-treated moDC harvested after 3 hr. Different dilutions of CS were added below the polycarbonate filters and the PMNLs were added above. They were incubated for 60 min, as described in the Materials and methods. The basal (▪) and fMLP (□)-induced PMNL migration is marked on the y axis. (c) PMNL migration induced by CS derived from moDCs treated for 1 hr with different concentrations of TNF-α (10–1000 U/ml). (d) PMNL migration induced by CS (diluted 1: 6) from unstimulated or from TNF-α-treated (100 U/ml) moDCs harvested at different time-points after moDC stimulation. In (a), one representative experiment of three is shown. For (b), (c) and (d), the results are expressed as the percentage of added PMNLs that migrated through the filter, and values represent the mean ± standard error of the mean (SEM) of three or four separate experiments performed in triplicate. **P < 0·01; *P < 0·05 (post hoc Student Newman Kules test).
We next examined the capacity of the CS from untreated and from TNF-α-treated moDCs to induce PMNL migration. Although there was a difference in PMNL activation by CS, the CS from untreated and from TNF-α-treated moDCs induced PMNL migration to the same extent at all dilutions tested (Fig. 1b). To determine the optimal concentration of TNF-α required to stimulate DCs, we performed dose–response experiments. The dose–response curve of Fig. 1(c) shows that similar PMNL migration by moDCs was induced at all the TNF-α concentrations used. All of these experiments were carried out with CS harvested after 3 hr of moDC stimulation. In order to determine the kinetics of the chemotactic factor release by moDCs treated or untreated with TNF-α, we harvested CS from moDC at different time-points. When the CS were harvested at shorter time-points of incubation (at 1 and 2 hr instead of 3 hr), a slightly higher migration of PMNLs was observed with CS from TNF-α-treated moDCs compared with that from untreated moDC (Fig. 1d). In contrast, at longer incubation time-points (16 hr), both CS from TNF-α-treated moDCs and from untreated moDCs induced PMNL migration to the same extent (Fig. 1d).
To identify the chemotactic factors present in the moDC-derived CS responsible for the migration of PMNLs, we next examined whether neutrophil-active chemokines such as CXCL8, CXCL6, CXCL7 and CXCL5 released by moDCs may be involved. As shown in Fig. 2, mRNA from CXCL8 (lane 5), CXCL5 (lane 3) and CXCL7 (lane 1) was detected, but not mRNA from CXCL6 (lane 2) in either TNF-α-treated or untreated moDCs. The presence in the CS of other chemokines (such as CXCL1) active for PMNLs was also analysed. Although it has been described that LPS induces the expression of CXCL1, CXCL2 and CXCL3, 11 we did not detect significant levels of CXCL1 in any of the CS tested (data not shown). In order to determine the PMNL chemotactic factors present in the CS of moDC that might be responsible for PMNL migration, blocking experiments in the presence of neutralizing mAbs to CXCL8, CXCL5, CXCL7 and pan GRO (CXCL1, CXCL2, CXCL3) were performed. Figure 3 shows that only neutralizing anti-CXCL8 significantly inhibited the migration of PMNLs in response to CS from untreated and TNF-α-treated moDCs. However, the percentage of inhibition for untreated moDCs CS was lower (but not statistically significant) than the inhibition for TNF-α-treated moDC CS (54 versus 74%, respectively). Moreover, the CXCL8 mAb concentration used in this assay almost completely blocked the migration of PMNLs induced by rh-CXCL8 (10−8 m) (data not shown). Overall, these results indicate that CXCL8 is responsible for most of the chemotactic activity observed in CS from TNF-α-treated moDCs.
Figure 2.
Reverse transcription–polymerase chain reaction (RT–PCR) analysis for CXCL7 (lane 1), CXCL6 (lane 2), CXCL5 (lane 3), α-actine (lane 4) and CXCL8 (lane 5) mRNA in monocyte-derived dendritic cells (moDCs) and tumour necrosis factor-α (TNF-α)-treated moDCs. RNA was extracted from moDCs that were either treated or untreated with TNF-α.
Figure 3.
Effect of monoclonal antibodies (mAbs) against CXCL8, CXCL5, CXCL7 and pan GRO on culture supernatant (CS)-induced polymorphonuclear leucocyte (PMNL) migration. CS were treated or not treated with the indicated mAb for 30 min at room temperature and then tested for their ability to induce PMNL migration. Data are expressed as the percentage of added PMNLs that migrated and represent the mean ± standard error of the mean (SEM) of four experiments performed in triplicate. **P < 0·01; ***P < 0·001 (post hoc Student Newman Kules test).
The amount of CXCL8 in the CS of TNF-α-treated or untreated moDCs was next determined by using ELISA analysis. As shown in Fig. 4(a), CS from both TNF-α-treated and untreated moDCs contained a significant concentration of CXCL8. However, the concentration of CXCL8 in the CS of TNF-α-treated moDCs was higher than that in the CS of untreated moDC. We also determined CXCL8 production by stimulation of moDCs with LPS, a well-known maturation stimulus of DCs. Figure 4(a) shows that treatment of moDCs with 100 ng/ml of LPS stimulated a higher production of CXCL8 than stimulation with 100 U/ml of TNF-α. The effect of TNF-α on the production of CXCL8 from moDCs was statistically significant at concentrations ranging from 10 to 1000 U/ml of TNF-α (Fig. 4b), reaching a plateau at 100 U/ml.
Figure 4.
CXCL8 concentrations in monocyte-derived dendritic cells (moDCs), tumour necrosis factor-α (TNF-α)-treated moDC and lipopolysaccharide (LPS)-treated moDC culture supernatants (CS). (a) After stimulation with 100 U/ml of TNF-α or 100 ng/ml of LPS, the cells were incubated for 3 hr in RPMI-1640 containing 5 mg/mol human serum albumin (HSA). Then, the supernatants were recovered and diluted, and CXCL8 was assayed by enzyme-linked immunosorbent assay (ELISA). (b) Different dilutions of CS from moDC and CS from TNF-α-treated moDC. Data represent the mean ± standard deviation (SD) of one representative experiment of three. *P < 0·05; **P < 0·01; ***P < 0·001 (post hoc Student Newman Kules test).
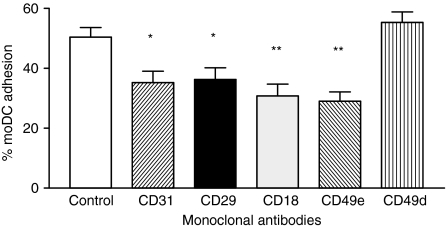
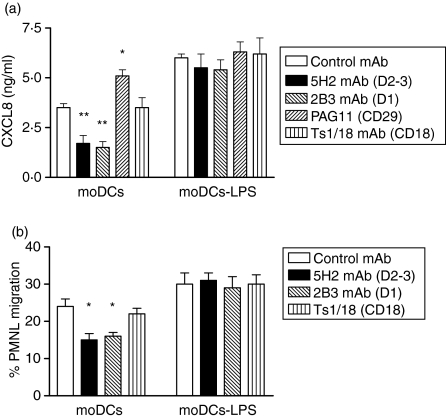
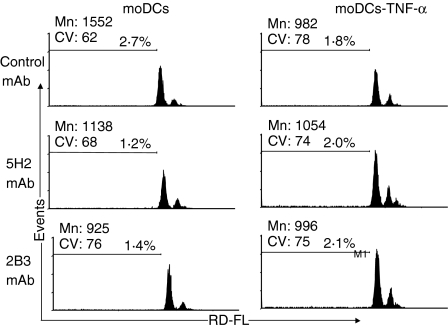
It is known that in the absence of an inflammatory environment, PMNL infiltration does not occur spontaneously, despite the presence of DCs in the tissues. We speculated that CXCL8 production by DCs could be regulated by signals received while precursor ‘immature’ DCs migrate through the endothelium to seed in the non-lymphoid organs. To address this hypothesis, we first studied the putative molecules involved in moDC adhesion to endothelium. Figure 5 shows that the adhesion of moDCs to HUVEC was partially blocked by mAbs against CD31, CD18, CD29 and CD49e, but not by mAb to CD49d. Therefore, we next examined whether these molecules present on moDCs could trigger intracellular signals capable of modulating CXCL8 production. To achieve this, CD31, CD18 and CD29 on untreated moDCs and mature moDCs were co-ligated by immobilized mAbs to CD31 (5H2 for domains 2–3 and 2B3 for domain 1), CD18 (TS1/18), CD29 (PAG11) or an isotype-control mAb, and CXCL8 production was assessed. Figure 6(a) shows that co-ligation of CD31 with immobilized 5H2 or 2B3 on untreated moDCs (but not on LPS-treated moDC), significantly decreased CXCL8 production. In contrast, control or CD18 mAb had no effect on CXCL8 secretion, whereas CD29 mAb slightly increased the levels of CXCL8 by untreated moDCs (Fig. 6a). Similarly to LPS-treated moDC, co-ligation of CD31 with immobilized 5H2 on TNF-α-treated moDCs did not decrease the CXCL8 production (data not shown). Furthermore, CS from CD31 co-ligated DCs (with lower CXCL8 levels) showed a reduced ability to induce PMNL migration (Fig. 6b). To exclude that the lower levels of CXCL8 resulted from cell death induced by CD31 engagement, we measured apoptosis in moDCs. Therefore, flow cytometric analysis of PI-stained moDCs was performed. The fragmented DNA of apoptotic cells incorporates less PI than the intact DNA of normal cells; thus, the DNA content, as determined in single cells by fluoresence-activated cell sorting (FACS), provides a good estimation of the number of apoptotic (hypodiploid) cells in a cell population.18 Fig. 7 shows that after 24 hr of culture, control (isotype mAb) or CD31 engagement on moDCs had no effect on the apoptotic level, either on untreated or on TNF-α-treated moDC.
Figure 5.
Monocyte-derived dendritic cell (moDC) adhesion to human umbilical vein endothelial cells (HUVEC). [51Cr]-Labelled moDCs were preincubated for 20 min at room temperature with the indicated monoclonal antibody (mAb). Thereafter, moDCs were layered over unstimulated HUVEC monolayers for 30 min at 37°. Results are expressed as the percentage of added moDCs that were adherent. Data represent the mean ± standard deviation (SD) of one representative experiment of three. *P < 0·05; **P < 0·01 (post hoc Student Newman Kules test).
Figure 6.
Effect of CD31, CD29 and CD18 ligation on CXCL8 production (a) and polymorphonuclear leucocyte (PMNL) migration (b) by monocyte-derived dedritic cells (moDCs). Untreated or lipopolysaccharide (LPS)-treated moDCs were cultured on immobilized CD31 monoclonal antibody (mAb) (5H2; 2B3), CD29 mAb (PAG11) or CD18 mAb (TS1/18) for 24 hr. Afterwards, the level of CXCL8 (a) and the chemotaxis ability of PMNL (b) in culture supernatant (CS) was determined. Data represent the mean ± standard error of the mean (SEM) of four independent experiments for (a) and of two for (b). **P < 0·01, *P < 0·05 (Dunnett multiple comparisons test).
Figure 7.
Fluorescence-activated cell sorter (FACS) analysis of the DNA content of monocyte-derived DCs (moDCs) or of TNFα-treated moDCs after 24 hr of engagement by CD31 monoclonal antibody (mAb) or isotype-control mAb. moDCs were harvested, permeabilized and stained with propidium iodide. Cells per sample were analysed by FACS and cell counts were plotted against propidium iodide fluorescence. The percentage of cells of the hypodiploid (apoptotic) population is indicated on each graph. CV, coefficient of variation; Mn, mean.
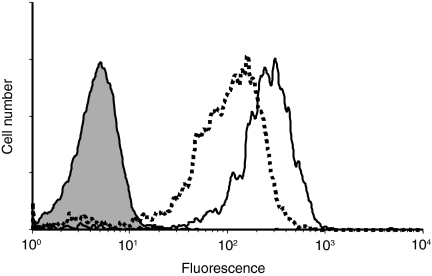
The lack of effect of CD31 co-ligation on LPS or on TNF-α-treated moDCs led us to examine the expression of CD31 on untreated and on TNF-α-treated moDCs. Figure 8 shows that TNF-α decreased the expression of CD31 on moDCs.
Figure 8.
Flow cytometric analysis of CD31 expression on untreated or on tumour necrosis factor-α (TNF-α)-treated monocyte-derived dendritic cells (moDCs). Cells were either untreated (solid line) or treated (dotted line) for 24 hr with TNF-α (100 U/ml) before staining with anti-CD31 monoclonal antibody (mAb) (clone 5H2). Controls consisted of untreated (filled histogram) or of TNF-α-treated moDCs (not shown owing to overlap) incubated with an isotype-matched irrelevant mAb. The data presented are representative of three independent experiments.
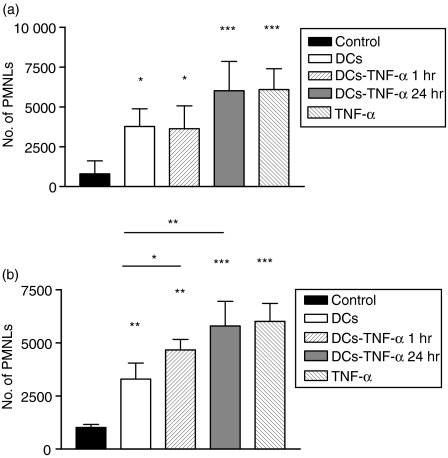
The ability of DCs to recruit PMNLs was also examined in a murine dorsal air pouch model. This model allows cellular analyses of closed-spaced inflammatory responses. As previously described, 22 at time 0 (before the administration of stimulator) or 3 hr after the administration of sterile vehicle (RPMI-1640), a mild PMNL infiltration was observed inside the pouch (Fig. 9a). On the contrary, the instillation of 10 ng of mouse TNF-α (specific activity 1 × 107 U/mg) into the pouch induced a PMNL-rich inflammatory exudate. The same degree of PMNL infiltration was observed after the administration of BM-derived DCs treated with TNF-α for 24 hr. It is important to point out that, in order to avoid the administration of cytokine into the air pouch, TNF-α-treated DCs were washed thoroughly before the administration. Furthermore, a lower, but significant, PMNL infiltration was observed after administration of untreated BM-derived DCs or DCs treated for 1 hr with TNF-α. In this case we did not observe a significant difference between untreated and TNF-α-treated DCs. However, we did observe a difference when CS from BM-derived DCs were used instead of DCs. Figure 9(b) shows that CS from BM-derived DCs treated with TNF-α for 1 or 24 hr induced higher PMNL infiltration than CS from untreated, BM-derived DCs. These data indicate that untreated and TNF-α-treated murine BM-derived DCs or CS derived from DCs are capable of recruiting PMNLs, at least in a closed space.
Figure 9.
In vivo polymorphonuclear leucocyte (PMNL) migration induced by bone marrow-derived dendritic cells (BM-DCs) or by culture supernatant (CS) derived from BM-DCs. Murine air pouches were raised, and RPMI (200 µl), tumour necrosis factor-α (TNF-α) (10 ng/100 µl), untreated BM-DCs (5 × 105/100 µl) or tumour necrosis factor (TNF-α) (10 ng for 1 or 24 hr)-treated BM-DC were injected intrapouch (a). In another set of experiments, CS from untreated or from TNF-α-treated BM-DC were harvested and injected intrapouch (b). Two hours later, the mice were killed, the exudates collected and PMNLs counted by using a Cytoron flow cytometer. Data represent the mean ± standard error of the mean (SEM) of eight independent experiments for the results shown in (a) and of six independent experiments for the results shown in (b). ***P < 0·001, **P < 0·01, *P < 0·05 (Tukey-Kramer Multiple Comparisons test).
Discussion
The margination and emigration of PMNLs is, in part, induced by chemotactic factors produced in the inflamed tissue, mainly by activated endothelial cells and macrophages. Although DCs are known to secrete several chemokines, 8, 23 the role of resident immature and mature DCs in PMNL emigration is not well known. In this study we demonstrate that both immature and mature DCs produce factors that can activate and recruit PMNLs, and the major factor mediating this effect is secreted CXCL8. Furthermore, our results also demonstrate that CXCL8 production by mature DCs is greater than the production by immature DCs. Moreover, CXCL8 production can be modulated by signalling through CD31 only in immature DCs. The results presented herein are consistent with other reports that have measured chemokine mRNA, showing that mature and immature DCs are able to produce CXCL8.24 The activation of PMNLs is clearly demonstrated by the up-regulation of CD11b and CD18, which was higher after culture with CS from mature DC (Fig. 1a). This result could be related to the higher level of CXCL8 found in the CS of TNF-α-treated DCs than in untreated DCs. However, the variation in CXCL8 concentration did not induce a difference in PMNL migration with CS harvested at 3 hr (Fig. 1b). Nevertheless, a greater PMNL migration was induced with TNF-α-treated CS harvested at earlier time-points than the migration induced with CS from untreated moDCs (Fig. 1d), strongly suggesting a difference in the kinetics of CXCL8 production upon DC maturation. It should be mentioned that these studies have focused on early responses, as we treated DCs for only 1 hr and harvested CS for the subsequent 3 hr. It is well known that DC maturation induces a change in the expression of surface molecules5 and chemokines.8 This was reflected, in our studies, by the down-modulation of CXCL7 and CXCL5 transcripts (Fig. 2). However, neither CXCL7 and CXCL5, nor GRO chemokines (CXCL1, CXCL2 and CXL3) were responsible for the PMNL chemotactic activity observed, as chemokine-blocking mAbs did not inhibit the effect. However, a mAb to CXCL8 significantly blocked the migration of PMNLs induced by CS from TNF-α-treated DC. Interestingly, the same mAb was less effective in inhibiting the PMNL migration induced by unstimulated moDC CS, suggesting that this CS contained other chemotactic factors for PMNLs besides CXCL8 (Fig. 3). Related to this observation, it has been shown that DCs produce macrophage inflammatory protein-2γ (MIP-2γ)12 and LTB425 chemokines that are active for PMNLs. However, LTB4 has been ruled out in our studies, as treatment of moDCs with the specific inhibitor of 5-lipoxygenase (MK886) had no effect on the production of PMNL chemotactic activity (data not shown).
Freshly isolated blood DCs have been shown to express very low levels of CXCL8, 9 although this expression may vary under different conditions.24 For example, in vitro it has been shown that DCs cultured in medium containing autologous serum express more CXCL8 than when cultured with FCS. As monocytes produce more CXCL8 than moDCs, and autologous serum-cultured moDCs require a combination of stimuli for full maturation, the authors suggested that autologous serum-cultured moDCs are less differentiated.24 Clearly, the regulation of CXCL8 expression and production by moDCs is not well defined, although soluble factors and adhesive interactions probably both participate. Here we demonstrate that multiple adhesion molecules, including CD31, CD29, CD18 and CD49e, contribute to moDC adhesion to endothelium, as blocking mAbs to these molecules (but not to CD49d) inhibited adhesion (Fig. 5). Furthermore, among these adhesion molecules, only ligation of CD31 on unstimulated moDCs down-modulated CXCL8 production, without any effect on stimulated and matured moDCs (Fig. 6a). These observations suggest that the interaction of DC precursors with CD31, for example on endothelial cells during transendothelial migration, might provide an intermediate maturation signal that results in the dampening down of CXCL8 production. This would presumably be the case when they become sentinel DCs in tissues, which do not recruit PMNLs under physiological conditions. The interaction of blood DCs with CD31 is supported by the findings that different subsets of DCs require CD31 for transendothelial migration.26 Those migrated DCs with low or absent production of CXCL8, will start again to produce CXCL8 upon encountering an inflammatory stimulus. However, our findings indicate that such intermediate DCs, when fully matured, will no longer respond to negative signals through CD31, at least for CXCL8 production. In contrast to immature moDCs, co-ligation of PBMCs with mAb 1B5 to CD31 is reported to induce CXCL8 production.27 The difference between this study by Chen et al. and ours may be attributed not only to the mAb used, as they used 1B5 which binds domain 4–5, 28 but also the type of cells used. As Chen et al. employed PBMC, it is not possible to exclude an indirect effect of other cytokines produced by CD31-expressing cells, e.g. T lymphocytes. The down-regulation of CD31 on mature DCs shown in this study (Fig. 8) and by others29 may partially explain the lack of signalling through CD31 on mature DCs. However, even though we cannot find an effect on CXCL8 production, there is still a substantial level of CD31 expression on mature moDCs, so it is unlikely that this could be the only explanation. This suggests that the lack of an effect of CD31 engagement on mature DCs could be related to the maturation process that might modify the recruitment of signalling molecules to the CD31 intracellular immunoreceptor tyrosine inhibitory motifs.30, 31 However, this needs to be investigated in greater detail.
The pattern of migration and recruitment of DCs and other inflammatory cells constitutes an important and complex matter. Although the migration process depends on chemotaxis factors, it also relies on the interaction with adhesion molecules present on the luminal surface of the vascular endothelium. Thus, the physiology of cells is constantly changing, depending on their interactions with other cells and with the extracellular matrix. Several physiological and pathological factors can modify the adhesion molecules and therefore the signals received by transmigrated cells. Moreover, it is known that differences on adhesion molecules are present within different compartments of the human body. Therefore, a signal such as CD31 that can be important to shut down DCs CXCL8 production might be irrelevant whether DCs have began the maturation process.
The finding related to the participation of DCs in the recruitment of PMNLs is an important issue, not only in inflammatory pathology but also in DC-based immunotherapy. This recruitment mechanism observed in the in vitro experiments has also been observed in a murine dorsal air pouch model in vivo where the administration of immature BM-derived DCs or CS derived from those cells and administered directly into the air pouch, induced PMNL accumulation (Fig. 9).
The difference in PMNL migration observed when we injected CS rather than DCs inside the air pouch could be related to the kinetics of production of chemokine or to other factors released by the host that might influence the physiology of intrapouch DCs. Furthermore, TNF-α stimulation of these DCs, for 24 hr prior to injection, induced a significantly greater PMNL recruitment as did such stimulation of moDCs in vitro. This finding may be important when administering DCs for immunotherapy, as PMNL recruitment and accumulation could alter the immunostimulatory capacity of DCs. This point is currently under investigation at our laboratory.
Acknowledgments
We thank Marcos Barbosa and Florencia Quiroga for their technical assistance, and Dr Verónica Garcia for critical reading of the manuscript. We are also grateful to Sanatorio Municipal ‘Julio Mendez’ for supplying human umbilical vein cord. This work was supported by ‘beca Ramón Carrillo-Arturo Oñativia’ and by a grant from CONICET PIP0905/98, Universidad de Buenos Aires (TM16, UBACYT 2004–07, M019), and AGENCIA (BID 1201/OC-AR PICT 11.702).
References
- 1.Pober JS, Cotran RS. Cytokines and endothelial cell biology. Physiol Rev. 1990;70:427–51. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- 2.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 3.Mollinedo F, Borregaard N, Boxer LA. Novel trends in neutrophil structure, function and development. Immunol Today. 1999;20:535–7. doi: 10.1016/s0167-5699(99)01500-5. [DOI] [PubMed] [Google Scholar]
- 4.Clark-Lewis I, Dewald B, Geiser T, Moser B, Baggiolini M. Platelet factor 4 binds to interleukin 8 receptors and activates neutrophils when its N terminus is modified with Glu-Leu-Arg. Proc Natl Acad Sci USA. 1993;90:3574–7. doi: 10.1073/pnas.90.8.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Sedgwick JD, Riminton DS, Cyster JG, Korner H. Tumor necrosis factor: a master-regulator of leukocyte movement. Immunol Today. 2000;21:110–3. doi: 10.1016/s0167-5699(99)01573-x. [DOI] [PubMed] [Google Scholar]
- 7.Kimber I, Cumberbatch M, Dearman RJ, Bhushan M, Griffiths CE. Cytokines and chemokines in the initiation and regulation of epidermal Langerhans cell mobilization. Br J Dermatol. 2000;142:401–12. doi: 10.1046/j.1365-2133.2000.03349.x. [DOI] [PubMed] [Google Scholar]
- 8.Sallusto F, Palermo B, Lenig D, et al. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol. 1999;29:1617–25. doi: 10.1002/(SICI)1521-4141(199905)29:05<1617::AID-IMMU1617>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Penna G, Vulcano M, Roncari A, Facchetti F, Sozzani S, Adorini L. Cutting edge: differential chemokine production by myeloid and plasmacytoid dendritic cells. J Immunol. 2002;169:6673–6. doi: 10.4049/jimmunol.169.12.6673. [DOI] [PubMed] [Google Scholar]
- 10.Lore K, Sonnerborg A, Spetz AL, Andersson U, Andersson J. Erratum to ‘Immunocytochemical detection of cytokines and chemokines in Langerhans’ cells and in vitro derived dendritic cells'. J Immunol Methods. 1998;218:173–87. doi: 10.1016/s0022-1759(98)00171-9. [DOI] [PubMed] [Google Scholar]
- 11.Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003;170:5165–75. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- 12.Cao X, Zhang W, Wan T, et al. Molecular cloning and characterization of a novel CXC chemokine macrophage inflammatory protein-2 gamma chemoattractant for human neutrophils and dendritic cells. J Immunol. 2000;165:2588–95. doi: 10.4049/jimmunol.165.5.2588. [DOI] [PubMed] [Google Scholar]
- 13.Jancic C, Chuluyan HE, Morelli A, et al. Interactions of dendritic cells with fibronectin and endothelial cells. Immunology. 1998;95:283–90. doi: 10.1046/j.1365-2567.1998.00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuluyan HE, Issekutz AC. VLA-4 integrin can mediate CD11/CD18-independent transendothelial migration of human monocytes. J Clin Invest. 1993;92:2768–77. doi: 10.1172/JCI116895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drake WT, Lopes NN, Fenton JWJ, Issekutz AC. Thrombin enhancement of interleukin-1 and tumor necrosis factor-α induced polymorphonuclear leukocyte migration. Lab Invest. 1992;67:617–27. [PubMed] [Google Scholar]
- 16.Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 17.Jaffe EA, Nachman RL, Becker CR, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphological and immunologic criteria. J Clin Invest. 1973;52:2745–56. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 19.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 21.Shaw J, Grund V, Durling L, Crane D, Caldwell HD. Dendritic cells pulsed with a recombinant chlamydial major outer membrane protein antigen elicit a CD4(+) type 2 rather than type 1 immune response that is not protective. Infect Immun. 2002;70:1097–105. doi: 10.1128/IAI.70.3.1097-1105.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–9. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 23.Vulcano M, Albanesi C, Stoppacciaro A, et al. Dendritic cells as a major source of macrophage-derived chemokine/CCL22 in vitro and in vivo. Eur J Immunol. 2001;31:812–22. doi: 10.1002/1521-4141(200103)31:3<812::aid-immu812>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 24.Vissers JL, Hartgers FC, Lindhout E, Teunissen MB, Figdor CG, Adema GJ. Quantitative analysis of chemokine expression by dendritic cell subsets in vitro and in vivo. J Leukoc Biol. 2001;69:785–93. [PubMed] [Google Scholar]
- 25.Harizi H, Gualde N. Dendritic cells produce eicosanoids, which modulate generation and functions of antigen-presenting cells. Prostaglandins Leukot Essent Fatty Acids. 2002;66:459–66. doi: 10.1054/plef.2002.0383. [DOI] [PubMed] [Google Scholar]
- 26.de la Rosa G, Longo N, Rodriguez-Fernandez JL, Puig-Kroger A, Pineda A, Corbi AL, Sanchez-Mateos P. Migration of human blood dendritic cells across endothelial cell monolayers: adhesion molecules and chemokines involved in subset-specific transmigration. J Leukoc Biol. 2003;73:639–49. doi: 10.1189/jlb.1002516. [DOI] [PubMed] [Google Scholar]
- 27.Chen W, Knapp W, Majdic O, Stockinger H, Bohmig GA, Zlabinger GJ. Co-ligation of CD31 and FcgRII induces cytokine production in human monocytes. J Immunol. 1994;152:3991–7. [PubMed] [Google Scholar]
- 28.Muller WA. CD31 workshop panel report. In: Kishimoto T, von Kikutani H, dem Borne AEGK, et al., editors. Leucocyte Typing VIWhite Cell Differentiation Antigens. New York and London: Garland Publishing, Inc.; 1997. pp. 362–4. [Google Scholar]
- 29.Ebner S, Lenz A, Reider D, Fritsch P, Schuler G, Romani N. Expression of maturation-/migration-related molecules on human dendritic cells from blood and skin. Immunobiology. 1998;198:568–87. doi: 10.1016/S0171-2985(98)80079-X. [DOI] [PubMed] [Google Scholar]
- 30.Jackson DE. The unfolding tale of PECAM-1. FEBS Lett. 2003;540:7–14. doi: 10.1016/s0014-5793(03)00224-2. [DOI] [PubMed] [Google Scholar]
- 31.Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1. New roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol. 2003;23:953–64. doi: 10.1161/01.ATV.0000071347.69358.D9. [DOI] [PubMed] [Google Scholar]