Abstract
We have previously shown that macrophages from interleukin (IL)-12p40 gene knockout (IL-12/IL-23−/−) mice have a bias towards the M2 activation profile, spontaneously secreting large quantities of transforming growth factor-β1 (TGF-β1) and producing low levels of nitric oxide (NO) in response to lipopolysaccharide (LPS) and interferon-γ (IFN-γ). To verify whether the activation profile of dendritic cells (DCs) is also influenced by the absence of IL-12/IL-23, bone marrow-derived DCs from IL-12/IL-23−/− and C57BL/6 mice were evaluated. At first we noticed that ≈ 50% of the C57BL/6 DCs were dead after LPS-induced maturation, whereas the mortality of IL-12/IL-23−/− DCs was < 10%, a protective effect that diminished when recombinant IL-12 (rIL-12) was added during maturation. Similarly to macrophages, mature IL-12/IL-23−/− DCs (mDCs) produced higher levels of TGF-β1 and lower levels of NO than C57BL/6 mDCs. NO release was IFN-γ-dependent, as evidenced by the poor response of IFN-γ−/− and IL-12/IL-23−/−IFN-γ−/− mDCs. Nevertheless, IFN-γ deficiency was not the sole reason for the weak NO response observed in the absence of IL-12/IL-23. The high level of TGF-β1 secretion by IL-12/IL-23−/− mDCs could explain why exogenous IFN-γ partially restored the NO production of IFN-γ−/− mDCs, while IL-12/IL-23−/− IFN-γ−/− mDCs remained unresponsive. We also showed that CD4+ T-cell proliferation was inhibited by C57BL/6 mDCs, but not by IL-12/IL-23−/− mDCs. IFN-γ and NO appear to mediate this antiproliferative effect because this effect was not observed in the presence of mDCs from IFN-γ−/− or IL-12/IL-23−/− IFN-γ−/− mice and it was attenuated by aminoguanidine. We conclude that the presence of IL-12/IL-23 during LPS-induced maturation influences the activation profile of DCs by a mechanism that is, only in part, IFN-γ dependent.
Keywords: dendritic cells, IL-12, IL-23, nitric oxide, TGF-β1
Introduction
Dendritic cells (DCs) are the key link between innate and adaptative immunity. Certain features of DCs, including their presence at sites of antigen entry and their ability to migrate from peripheral sites to secondary lymphoid organs, to stimulate naïve T cells and to drive T helper 1/T helper 2 (Th1/Th2) polarization, illustrate their pivotal role in the generation of adaptative immune responses.1,2 Yet, besides their ability to modulate T-cell responses, DCs may also communicate between themselves, exerting a broad influence in the immune system. Evidence in this direction has been gathered from data showing that cytokines produced by DCs, particularly interleukin (IL)-12 and IL-23, can act in an autocrine manner and modulate the activation pattern of these cells.3,4
IL-12 is an heterodimeric cytokine formed by two chains – p35 and p40 – encoded by genes located on different chromosomes.5 For generation of the bioactive IL-12p70 molecule, both genes must be expressed in the same cell.6 The IL-12p40 chain associates with a p19 chain to originate another heterodimeric cytokine, named IL-23.7 Moreover, the β1 chain is shared by IL-12 and IL-23 receptors, this molecule being associated with IL-12Rβ2 or IL-23R chains to specifically interact with IL-12 or IL-23 cytokines, respectively.7–9 Because of these characteristics, the signalling cascades of IL-12 and IL-23 are found to be similar, but not identical,10 which might explain why the biological effects of these cytokines have similarities and differences.9
Several studies indicate that DCs not only produce IL-12 and IL-23 but also respond to them. The autocrine activity of these cytokines is evidenced by the fact that DCs express IL-12R11 and IL-23R.12 In the last few years, it has been reported that one of the major effects of IL-12 on these cells is the induction of interferon-γ (IFN-γ) production,13 which involves Stat4 and T-bet transcriptional factors.14,15 Because of this effect of IL-12, some of its autocrine activities on DCs seem to be mediated by endogenous IFN-γ.3 The direct effect of IL-23 on IFN-γ production by DCs has not yet been evaluated, but IL-23 induces IL-12 secretion by DCs,12 which might contribute to IFN-γ production by these cells. Moreover, the referred study also shows that IL-23 promotes peptide presentation equally well for both CD8– and CD8+ DC subsets, whereas IL-12 acts selectively on CD8− DCs.
We have previously shown that the absence of IL-12 and IL-23 affects the functional profile of peritoneal macrophages.16 That is, macrophages from IL-12p40-deficient (IL-12/IL-23−/−) mice spontaneously secrete high levels of transforming growth factor-β1 (TGF-β1) and, when activated with IFN-γ and/or lipopolysaccharide (LPS), release low levels of nitric oxide (NO) compared to macrophages from C57BL/6 mice. Thus, according to the M1/M2 functional profiles described by Mills and collaborators,17 IL-12/IL-23−/− macrophages show a phenotype similar to M2 macrophages obtained from Th2-prone mouse strains (BALB/c and DBA/2). These cells differ from M1 macrophages of the Th1-prone mouse strains (C57BL/6 and B10.A) that spontaneously secrete low levels of TGF-β1 and respond to LPS or recombinant IFN-γ (rIFN-γ) with vigorous NO release.
Based on the previous considerations, the present study investigated whether the activation profile of DCs is influenced by the absence of IL-12 and IL-23, as previously reported for peritoneal macrophages.16 This objective was achieved by comparing the production of NO and TGF-β1 by immature DCs (iDCs) and mature DCs (mDCs) derived from the bone marrow of IL-12/IL-23−/− and C57BL/6 mice. In addition, by analysing mDCs obtained from IFN-γ−/− and IL-12/IL-23−/− IFN-γ−/− (double knockout) mice, we were able to determine the role of IFN-γ in NO production and in the modulation of T-cell proliferation. This study complements our previous findings in peritoneal macrophages by demonstrating that IL-12 and/or IL-23 influence the activation profile of bone marrow-derived DCs. The results presented here show that DC polarization, regarding NO and TGF-β1 production, occurs during LPS-induced maturation. In addition, our data indicate that NO release by mDCs is IFN-γ-dependent, while the up-regulation of TGF-β1 secretion, as a result of the absence of IL-12/IL-23, is IFN-γ-independent.
Materials and methods
Mice
Six- to 8-week-old IL-12/IL-23−/− (p40−/−),18 IFN-γ−/− (GKO - IFN-γKO),19 IL-12/IL-23−/−IFN-γ−/− and C57BL/6 male mice were bred in our animal facilities at the University of São Paulo. IL-12/IL-23−/− IFN-γ−/− mice were obtained by crossing IL-12/IL-23−/− and IFN-γ−/− mice.
DC generation
DCs were generated in vitro from bone marrow cells, as described by Inaba et al.20 with some modifications. Briefly, cells removed from the femurs were cultured with 20 ng/ml of recombinant granulocyte–macrophage colony-stimulating factor (rGM-CSF; a gift from Dr Brian Kelsall, Yale University, School of Medicine, CT) in Dulbecco's modified Eagle's minimal essential medium (DMEM) supplemented with 5% (v/v) fetal calf serum (FCS), 10−5 m 2-mercaptoethanol (2-ME), 2 mm l-glutamine, 1 mm sodium pyruvate and 100 µg/ml gentamycin (all reagents were purchased from Gibco BRL, Rockville, NY). The medium with rGM-CSF was renewed on day 4 of culture. Cells were harvested on day 7 of culture with flushes of cold DMEM. DCs were matured with 1 µg/ml of LPS from Escherichia coli (Sigma, St Louis, MO) for 18 hr, as described by Suri et al.21
Cell phenotypic analysis
Cells (106) were incubated for 20 min at 4° with monoclonal antibodies (mAbs) to CD16/CD32 (FcgR II/III, 2·4G2; Fc block) and stained for 30 min at 4° with fluorescein isothiocyanate (FITC) or phycoerythrin (PE)-labelled mAb (1 µg/106 cells) to CD11c (HL3), major histocompatibility complex (MHC) class II (I-Ab, AF6-120·1) and CD86 (B7·2, Gl-1) (PharMingen, San Diego, CA). Cells were analysed by flow cytometry (FACScalibur™; Becton Dickinson, Mountain View, CA) using cellquest™ software (Becton Dickinson).
Detection of NO and TGF-β1 in culture supernatants
Cells were cultured with 2·5 ng/ml of rIFN-γ (PharMingen) and the supernatants were harvested 48 hr later. Culture supernatants were tested for NO by using the Griess reaction. Briefly, 50 µl of the supernatant was incubated with 50 µl of Griess reagent for 5 min at room temperature, and the NO2 concentration was determined by measuring the absorbance at 550 nm in reference to a standard NaNO2 solution. Latent plus bioactive TGF-β1 was quantified by using the TGF-β1  Imunoassay System (Promega, Madison, WI), according to the manufacturer's instructions. All supernatant samples were activated by acid treatment before determination of TGF-β1. TGF-β1 measured in the culture medium supplemented with 3% (v/v) fetal calf serum (FCS) ranged from 450 to 550 pg/ml. The amount of TGF-β1 in the supernatants was determined by subtracting the TGF-β1 value of the culture medium from that obtained in the supernatant sample.
Imunoassay System (Promega, Madison, WI), according to the manufacturer's instructions. All supernatant samples were activated by acid treatment before determination of TGF-β1. TGF-β1 measured in the culture medium supplemented with 3% (v/v) fetal calf serum (FCS) ranged from 450 to 550 pg/ml. The amount of TGF-β1 in the supernatants was determined by subtracting the TGF-β1 value of the culture medium from that obtained in the supernatant sample.
Cell proliferation
Splenic CD4+ T cells sorted by positive selection by using anti-CD4 mAb conjugated with magnetic microbeads (Miltenyi Biotec GMBH, Bergisch Gladbach, Germany) were stained with the vital dye 5,6-carboxy-succinimidyl-fluorescein-ester (CSFE; Molecular Probes, Eugene, OR), as previously described.22 Cells (5 × 105) were then cultured for 72 hr with 5 µg/ml of plate-bound anti-CD3 mAb (PharMingen) in the presence of mDCs at a DC : CD4+ T-cell ratio of 1 : 10. In some experiments, mDC : CD4+ T-cell cultures were treated with rIFN-γ (2·5 ng/ml) and/or aminoguanidine (0·1 mm) (Sigma). CD4+ T-cell proliferation was evaluated in a flow cytometer by measuring the CSFE fluorescence intensity.
Statistical analysis
Statistical analysis was performed by unpaired analysis of variance (anova) non-parametric tests. Differences between groups were considered significant when P < 0·05.
Results
Generation of DCs from the bone marrow of IL-12/IL-23−/− and C57BL/6 mice
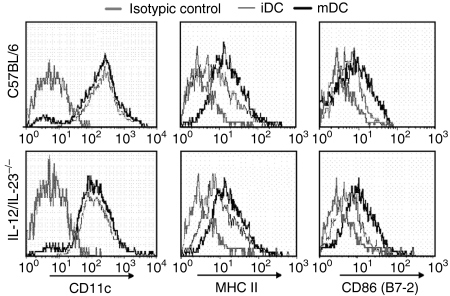
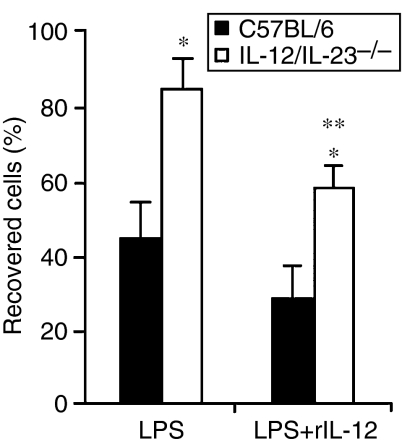
To evaluate the possibility that the absence of IL-12/IL-23 influences the activation profile of DCs, bone marrow cells from IL-12/IL-23−/− and C57BL/6 mice were differentiated with rGM-CSF, matured or not matured with LPS, and phenotyped for surface markers. According to this analysis, immature IL-12/IL-23−/− and C57BL/6 DCs (iDCs) expressed high levels of CD11c and basal levels of MHC class II and CD86 (Fig. 1). After LPS-stimulated maturation, a similar increase of MHC class II and CD86 expression was observed in the DCs from both groups of mice. Interestingly, however, less than 50% of mature C57BL/6 DCs (mDCs) were recovered from LPS-stimulated cell cultures, compared to almost 90% of IL-12/IL-23−/− mDCs under the same culture conditions (Fig. 2). Moreover, following LPS-induced maturation, the percentage of cells staining with Trypan blue was always higher in C57BL/6 DCs, i.e. 50% of C57BL/6 mDCs and 7% of IL-12/IL-23−/− mDCs (data not shown). To verify whether IL-12 is involved in the mortality of C57BL/6 mDCs, experiments were carried out in which DCs were matured with LPS in the presence of rIL-12. Under these conditions, the number of IL-12/IL-23−/− mDCs was reduced by 30%, with only ≈ 60% being recovered (Fig. 2). Taken together, these data indicate that IL-12 contributes to DC mortality after LPS-induced maturation.
Figure 1.
Phenotypic analysis of immature dendritic cells (iDCs) and mature DCs (mDCs) from interleukin (IL)-12/IL-23−/− and C57BL/6 mice. Bone marrow-derived DCs were cultured in the absence (iDCs) or presence (mDCs) of lipopolysaccharide (LPS) (1 µg/ml) for 18 hr. After washing, cells (106) were stained with fluorescence-labelled anti-CD11c, monoclonal anti-major histocompatibility complex (MHC) class II and monoclonal anti-CD86 (B7-2) immunoglobulins, and analysed by flow cytometry.
Figure 2.
Percentages of interleukin (IL)-12/IL-23−/− and C57BL/6 dendritic cells (DCs) recovered after lipopolysaccharide (LPS)-induced maturation in the presence or absence of recombinant IL-12 (rIL-12). Bone marrow-derived DCs (106) were cultured with LPS (1 µg/ml) or with LPS + rIL-12 (2·5 ng/ml) for 18 hr. The experiments were repeated twice, with the same pattern of results obtained on each occasion. Data represent the mean value ± standard deviation (SD) of a representative experiment. Standard deviation values were calculated from triplicate samples. *P < 0·05 versus C57BL/6 mature DCs (mDCs). **P < 0·05 versus treatment with LPS.
Production of NO and TGF-β1 by IL-12/IL-23−/− and C57BL/6 DCs
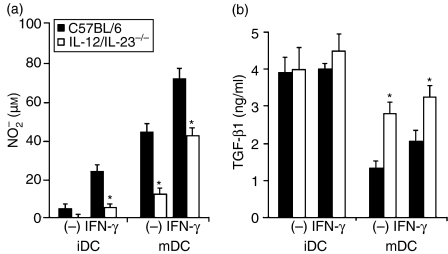
NO and TGF-β1 levels were determined in the culture supernatants of iDCs and mDCs from IL-12/IL-23−/− and C57BL/6 mice, activated or not activated with rIFN-γ. iDCs from both groups of mice produced low levels of NO(Fig. 3a). After stimulation with rIFN-γ, only C57BL/6 iDCs released a considerable quantity of NO. Although LPS-induced maturation had a positive effect on NO production by both types of DC, NO levels in the supernatant of IL-12/IL-23−/− mDCs were significantly lower than NO levels in the supernatant of C57BL/6 mDCs. This could not be attributed to cell death, because > 90% of IL-12/IL-23−/− mDCs were recovered after 48 hr of culture in the presence or absence of rIFN-γ (data not shown). iDCs from both groups of mice produced high levels of TGF-β1 (latent plus bioactive), even in the presence of rIFN-γ(Fig. 3b). LPS-induced maturation down-regulated the secretion of TGF-β1 by both types of DC, but the supernatant of IL-12/IL-23−/− mDCs contained almost twice the concentration of TGF-β1 than C57BL/6 mDCs, and this difference persisted after activation with rIFN-γ. These results indicate that DC polarization, regarding TGF-β1 and NO secretion, occurs during LPS-induced maturation. In addition, TGF-β1 production by DCs correlates inversely with their ability to release NO in response to IFN-γ.
Figure 3.
Nitric oxide (NO) and transforming growth factor-β1 (TGF-β1) production by immature dendritic cells (iDCs) and mature DCs (mDCs) from interleukin (IL)-12/IL-23−/− and C57BL/6 mice. Bone marrow-derived DCs were cultured in the absence (iDCs) or presence (mDCs) of lipopolysaccharide (LPS) (1 µg/ml) for 18 hr. After washing, the cells (106) were incubated for a further 48 hr, with or without recombinant interferon-γ (rIFN-γ) (2·5 ng/ml).(a)NO was quantified in culture supernatants by using the Griess reaction. The experiments were repeated four times, with the same pattern of results obtained on each occasion.(b)TGF-β1 was quantified in culture supernatants by enzyme-linked immunosorbent assay (ELISA). The experiments were repeated twice, with the same pattern of results obtained on each occasion. Data represent the mean values ± standard deviation (SD) of a representative experiment. SD values were calculated from triplicate samples. *P < 0·05 versus C57BL/6 DCs.
Role of IFN-γ in the production of NO and TGF-β1 by mDCs
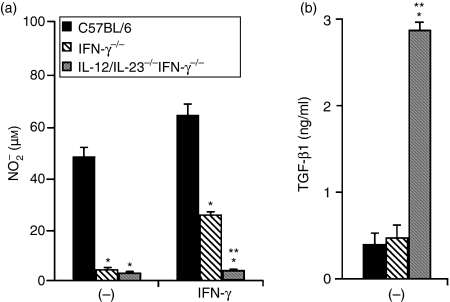
Several reports have described the ability of IL-12 to induce IFN-γ secretion by DCs,13 raising the possibility that some of the autocrine activities attributed to IL-12 might be mediated by endogenous IFN-γ.3 To address this issue, we analysed NO and TGF-β1 production by mDCs derived from the bone marrow of IFN-γ−/− and IL-12/IL-23−/− IFN-γ−/− mice. As shown in Fig. 4(a), IFN-γ−/− and IL-12/IL-23−/− IFN-γ−/− mDCs produce negligible amounts of NO, indicating that this response is IFN-γ dependent. To evaluate whether this deficiency could be restored by exogenous IFN-γ, IFN-γ−/− and IL-12/IL-23−/− IFN-γ−/− mDCs were also analysed after stimulation with rIFN-γ. We observed that the ability of IFN-γ−/− mDCs to release NO was only partially restored by treating these cells with rIFN-γ, suggesting that the response to exogenous IFN-γ is amplified by endogenous IFN-γ. Remarkably, in contrast to IFN-γ−/− mDCs, IL-12/IL-23−/− IFN-γ−/− mDCs remained essentially unresponsive, even in the presence of rIFN-γ. This finding could not be attributed to cell death, as > 90% of IFN-γ−/− and IL-12/IL-23−/− IFN-γ−/− were recovered after 48 hr of culture in the presence or absence of rIFN-γ (data not shown). These data are particularly interesting because they indicate that, in the absence of IL-12 and IL-23, there is an IFN-γ-independent mechanism that exerts a negative control over NO production. In fact, the quantity of TGF-β1 produced by IL-12/IL-23−/− IFN-γ−/− mDCs was almost six times higher than that produced by IFN-γ−/− mDCs (Fig. 4b), suggesting that the ability of IL-12 and/or IL-23 to down-regulate TGF-β1 secretion is IFN-γ independent. Thus, in IL-12/IL-23−/− IFN-γ−/− mDCs, both the secretion of a high level of TGF-β1 and the absence of IFN-γ could result in an impaired NO response.
Figure 4.
Nitric oxide (NO) and transforming growth factor-β1 (TGF-β1) production by mature DCs (mDCs) from interferon-γ−/− (IFN-γ−/−), interleukin (IL)-12/IL-23−/− IFN-γ−/− and C57BL/6 mice. Bone marrow-derived DCs were cultured in the presence of lipopolysaccharide (LPS) (1 µg/ml) for 18 hr. After washing, the cells (106) were further incubated for 48 hr, with or without recombinant IFN-γ (rIFN-γ) (2·5 ng/ml).(a)NO was quantified in culture supernatants by using the Griess reaction. Experiments were repeated four times, with the same pattern of results obtained on each occasion.(b)TGF-β1 was quantified in culture supernatants by enzyme-linked immunosorbent assay (ELISA). Experiments were repeated twice, with the same pattern of results obtained on each occasion. Data represent the mean values ± standard deviation (SD) of a representative experiment. SD values were calculated from triplicate samples. *P < 0·05 versus C57BL/6 mDCs. **P < 0·05 versus IFN-γ−/− mDCs.
Role of IFN-γ in the ability of mDCs to inhibit CD4+ T-cell proliferation
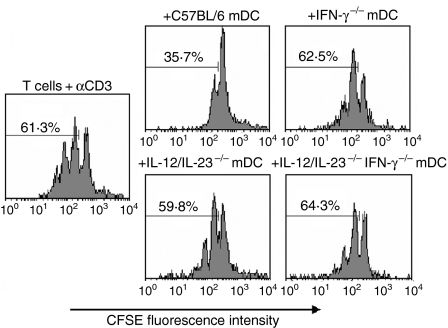
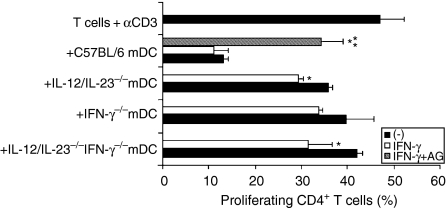
While the major role of DCs in the immune response is antigen presentation to T cells, NO produced by these cells exerts a negative effect on T-cell proliferation.23–26 In an effort to understand the role of IL-12/IL-23 and IFN-γ in the regulatory effect of DCs, we compared the ability of mDCs obtained from C57BL/6, IL-12/IL-23−/−, IFN-γ−/− and IL-12/IL-23−/− IFN-γ−/− mice to inhibit CD4+ T-cell proliferation. In these experiments, CD4+ T cells isolated from the spleen of C57BL/6 mice were labelled with CSFE and cultured with plate-bound anti-CD3 mAb in the presence of mDCs. After 72 hr of culture, ≈ 60% of CD4+ T cells proliferated in the absence of mDCs (Fig. 5). The presence of C57BL/6 mDCs reduced the percentage of proliferating CD4+ T cells to 35·7%, a suppressive effect not observed for IL-12/IL-23−/−, IFN-γ−/− or IL-12/IL-23−/− IFN-γ−/− mDCs. To better characterize the role of IFN-γ on the modulation of CD4+ T-cell proliferation, C57BL/6, IL-12/IL-23−/−, IFN-γ−/− and IL-12/IL-23−/− IFN-γ−/− mDCs were treated with rIFN-γ and/or aminoguanidine. As shown in Fig. 6, treatment with rIFN-γ resulted in a small decrease in the proliferative response of CD4+ T cells co-cultured with IL-12/IL-23−/− and IL-12/IL-23−/− IFN-γ−/− mDCs. On the other hand, treatment of C57BL/6 mDCs with aminoguanidine, an inducible nitric oxide synthase (iNOS) inhibitor, significantly restored CD4+ T-cell proliferation, even in the presence of exogenous IFN-γ. For IL-12/IL-23−/−, IFN-γ−/− and IL-12/IL-23−/− IFN-γ−/− mDCs, the aminoguanidine treatment resulted in a small increase in CD4+ T-cell proliferation (data not shown). These results indicate that NO is required for the antiproliferative effect of mDCs. The data presented in Fig. 4(a), showing that IL-12/IL-23−/−, IFN-γ−/− and IL-12/IL-23−/− IFN-γ−/− mDCs release little NO in response to rIFN-γ (compared to C57BL/6 mDCs), could explain why treatment with IFN-γ had only a minor effect on the proliferation of CD4+ T cells.
Figure 5.
Modulation of T-cell proliferation by mature DCs (mDCs) from interleukin (IL)-12/IL-23−/−, interferon-γ−/− (IFN-γ−/−), IL-12/IL-23−/− IFN-γ−/− and C57BL/6 mice. CD4+ T cells purified from the spleen of C57BL/6 mice with anti-CD4 magnetic beads were stained with 5,6-carboxy-succinimidyl-fluorescein-ester (CSFE). CD4+ T cells (106) were then cultured with plate-bound monoclonal anti-CD3 (5 µg/ml) in the presence or absence (control) of mDCs (105). The proliferation of gated CD4+ T cells was determined by analysing the CSFE fluorescence intensity using a flow cytometer. Experiments were repeated three times, with the same pattern of results obtained on each occasion.
Figure 6.
Effects of interferon-γ (IFN-γ) and aminoguanidine (AG) on the modulation of T-cell proliferation by mature dendritic cells (mDCs) from interleukin (IL)-12/IL-23−/−, IFN-γ−/−, IL-12/IL-23−/− IFN-γ−/− and C57BL/6 mice. CD4+ T cells, purified from the spleen of C57BL/6 mice by using anti-CD4 magnetic beads, were stained with 5,6-carboxy-succinimidyl-fluorescein-ester (CSFE). CD4+ T cells (106) were then cultured with plate-bound monoclonal anti-CD3 (5 µg/ml) in the presence or absence (control) of mDCs (105). The mDC : CD4+ T-cell cultures were treated with recombinant IFN-γ (rIFN-γ) (2·5 ng/ml) and/or AG (0·1 mm). The proliferation of gated CD4+ T cells was determined by analysing the CSFE fluorescence intensity by using a flow cytometer. Experiments were repeated twice, with the same pattern of results obtained on each occasion. Data represent the mean values ± standard deviation (SD) of a representative experiment. SD values were calculated from triplicate samples. *P ≤ 0·05 versus untreated cells. **P ≤ 0·05 versus rIFN-γ-treated cells.
Discussion
We have previously shown that peritoneal macrophages from IL-12/IL-23−/− mice spontaneously secrete high levels of TGF-β1 and, when activated with rIFN-γ and/or LPS, release low levels of NO compared to macrophages from C57BL/6 mice.16 Thus, regarding TGF-β1 and NO production, IL-12/IL-23−/− macrophages behave similarly to BALB/c macrophages, which are representative of the M2 activation profile described by Mills and collaborators.17 In the present work, we extend these findings by showing that the absence of IL-12 and IL-23 also affects the production of TGF-β1 and NO by bone marrow-derived DCs. However, the different profiles exhibited by IL-12/IL-23−/− and C57BL/6 DCs were only evident after LPS-induced maturation. We have also shown that the ability of mDCs to release NO is IFN-γ-dependent, while the up-regulation of TGF-β1 secretion found in IL-12/IL-23−/− mDCs is IFN-γ-independent. Moreover, in contrast to TGF-β1 and NO production, there was no difference between IL-12/IL-23−/− and C57BL/6 DCs regarding the expression of MHC class II and CD86, even in mDCs, which express higher levels of these molecules than iDCs. Our data also show that, in the absence of IL-12 and IL-23, mDCs display an increased survival and a lower capacity to down-modulate CD4+ T-cell proliferation, an effect mediated by NO.
From these results we extrapolated that the polarization of DCs, in terms of TGF-β1 and NO production, does not occur during their generation in vitro. Supporting this notion, our data show that TGF-β1 levels were similarly high in IL-12/IL-23−/− and C57BL/6 iDCs, and NO production in response to rIFN-γ was negligible in both groups. Therefore, we postulate that IL-12 and IL-23 are not secreted during DC generation or, alternatively, that bone marrow precursors are not targets for these cytokines, at least in terms of TGF-β1/NO polarization. The fact that transcription of IL-12p35 mRNAs occurs only late during DC development, at the CD86+ stage,27 supports the first interpretation. Following LPS maturation, however, we observed a clear polarization of DCs that was determined by their capacity to produce IL-12 and IL-23, as previously reported for peritoneal macrophages.16 In agreement with this observation, it has been demonstrated that DC modulation by inflammatory cytokines and microbial components is strictly dependent on the potential of the stimuli to induce IL-12 secretion.28,29
Production of NO by C57BL/6 and IL-12/IL-23−/− mDCs inversely correlated with their ability to secrete TGF-β1. Data showing the potential of IL-12 in suppressing the expression of mRNA for TGF-β1 in monocytes and bone marrow-derived macrophages30 might explain the high level of TGF-β1 secretion by IL-12/IL-23−/− mDCs. In turn, as TGF-β is a powerful inhibitor of NO production,31–33 the low levels of NO released by IL-12/IL-23−/− mDCs are somehow expected. Regarding the role of IL-23 in DC polarization, further studies need to be performed to determine its effects on the production of TGF-β1 and NO. Our data, showing that IFN-γ−/− and IL-12/IL-23−/−IFN-γ−/− mDCs have a profound deficiency in the ability to release NO, indicate that this response is mediated by IFN-γ. Besides that, the observation that NO deficiency in IFN-γ−/− mDCs is only partially restored by treating cells with rIFN-γ suggests that endogenous IFN-γ amplifies the NO response to exogenous IFN-γ. Moreover, the fact that exogenous stimulation with IFN-γ had no effect on NO production by IL-12/IL-23−/− IFN-γ−/− mDCs is compatible with the notion that the high level of TGF-β1 secretion, as a result of the absence of IL-12 and IL-23, may exert a negative control over NO production. Indeed, we found high levels of TGF-β1 in the culture supernatants of IL-12/IL-23−/− IFN-γ−/− mDCs. In addition, our results, showing that TGF-β1 secretion is up-regulated in both IL-12/IL-23−/− and IL-12/IL-23−/− IFN-γ−/− mDCs, but not in IFN-γ−/− mDCs, indicate that this process is regulated by IL-12/IL-23 in an IFN-γ-independent manner, a finding similar to that previously described for peritoneal macrophages.16
NO production by macrophages correlates with the resistance to intracellular infections, whereas in DCs it is associated with cell death and the inhibition of T-cell proliferation.23–26,34,35 In a previous study, we observed that the bias of IL-12/IL-23−/− macrophages towards an M2 activation profile is characterized by a deficiency in the control of intracellular Trypanosoma cruzi replication.16 Here, we described that the activation profile of IL-12/IL-23−/− mDCs correlates with an improved survival during LPS-induced maturation and a decreased ability to down-modulate CD4+ T-cell proliferation. Moreover, the fact that maturation of IL-12/IL-23−/− DCs in the presence of added rIL-12 increases cell mortality is indicative of the involvement of this cytokine in DC death. It is probable that the high level of NO released by C57BL/6 mDCs is responsible for their intense pro-apoptotic and antiproliferative activities. Our observation that adding aminoguanidine to the cell cultures inhibited the antiproliferative effect of C57BL/6 mDCs supports this interpretation. The data showing that IL-12/IL-23−/−, IFN-γ−/− and IL-12/IL-23−/− IFN-γ−/− mDCs release little NO in response to rIFN-γ could explain why the treatment of these cells with IFN-γ had only a minor effect on CD4+ T-cell proliferation.
From our observation that LPS-induced MHC class II and CD86 expression do not require IL-12 and IL-23, we can envisage that mDCs of both TGF-β1 and NO secretion profiles may have a similar potential to present antigens. The above data are particularly interesting because they indicate that, concerning the induction of molecules involved in antigen presentation, LPS-induced maturation overcomes the absence of IL-12 and IL-23. This is somehow intriguing, because IL-23 is known to stimulate DCs to secrete IL-1212 which, in turn, induces the production of IFN-γ (a cytokine known to up-regulate MHC class II and CD86 expression) or the expression of IFN-γ mRNA.13 In fact, in peritoneal macrophages, IL-12 is able to induce the expression of these molecules by a mechanism dependent in endogenous IFN-γ.3
Our findings lead us to conclude that IL-12 and IL-23 have an important role in the modulation of the DC activation profile. These cytokines, acting through IFN-γ-dependent and -independent pathways, may signal DCs, macrophages and lymphocytes in the same direction, culminating in the optimization of mechanisms responsible for eliminating micro-organisms from host tissues.3 Determining the source of IFN-γ during LPS-induced maturation would be an important step in understanding the molecular pathways responsible for NO release by DCs. However, experiments carried out in our laboratory failed to detect (by using intracellular staining) the production of IFN-γ by DCs stimulated with LPS. Nevertheless, it is possible that the amount of IFN-γ produced during LPS-induced maturation is too low to permit its detection by intracellular staining, but sufficient to prime DCs for NO production. The involvement of IL-12 and/or IL-23 in the polarization of DCs towards an NO- or TGF-β1-dominant profile consolidates the autocrine role of these cytokines. Further studies are in progress to determine the relative contribution of IL-12 and IL-23 in the TGF-β1/NO polarization of mDCs.
Acknowledgments
This study was supported by grants from FAPESP and CNPq (Brazil). We thank Dr Pieter Leenen for helpful discussion, Rogério Nascimento for technical assistance, and Silvia Massironi and Thais Marques for IL-12/IL-23−/− IFN−/− mice. We also thank Dr Luiz Vicente Rizzo for providing the founders for the IL-12/IL-23−/− and IFN-γ−/− mouse colony.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 3.Bastos KRB, Marinho CRF, Barboza R, Russo M, Alvarez JM, D'Império Lima MR. What kind of message does IL-12/IL-23 bring to macrophages and dendritic cells? Microbes Infect. 2004;6:630–6. doi: 10.1016/j.micinf.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Puccetti P, Belladonna ML, Grohmann U. Effects of IL-12 and IL-23 on antigen-presenting cells at the interface between innate and adaptive immunity. Crit Rev Immunol. 2002;22:373–90. [PubMed] [Google Scholar]
- 5.Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–45. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gubler U, Chua AO, Schoenhaut DS, et al. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci USA. 1991;88:4143–7. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 8.Desai BB, Quinn PM, Wolitzky AG, Mongini PK, Chizzonite R, Gately MK. IL-12 receptor. II. Distribution and regulation of receptor expression. J Immunol. 1992;148:3125–32. [PubMed] [Google Scholar]
- 9.Wiekowski MT, Leach MW, Evans EW, et al. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J Immunol. 2001;166:7563–70. doi: 10.4049/jimmunol.166.12.7563. [DOI] [PubMed] [Google Scholar]
- 10.Parham C, Chirica M, Timans J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 11.Grohmann U, Belladonna ML, Bianchi R, Orabona C, Ayroldi E, Fioretti MC, Puccetti P. IL-12 acts directly on DC to promote nuclear localization of NF-kappaB and primes DC for IL-12 production. Immunity. 1998;9:315–23. doi: 10.1016/s1074-7613(00)80614-7. [DOI] [PubMed] [Google Scholar]
- 12.Belladonna ML, Renauld JC, Bianchi R, et al. IL-23 and IL-12 have overlapping, but distinct, effects on murine dendritic cells. J Immunol. 2002;168:5448–54. doi: 10.4049/jimmunol.168.11.5448. [DOI] [PubMed] [Google Scholar]
- 13.Frucht DM, Fukao T, Bogdan C, Schindler H, O'Shea JJ, Koyasu S. IFN-gamma production by antigen-presenting cells: mechanisms emerge. Trends Immunol. 2001;22:556–60. doi: 10.1016/s1471-4906(01)02005-1. [DOI] [PubMed] [Google Scholar]
- 14.Fukao T, Frucht DM, Yap G, Gadina M, O'Shea JJ, Koyasu S. Inducible expression of Stat4 in dendritic cells and macrophages and its critical role in innate and adaptive immune responses. J Immunol. 2001;166:4446–55. doi: 10.4049/jimmunol.166.7.4446. [DOI] [PubMed] [Google Scholar]
- 15.Lugo-Villarino G, Maldonado-Lopez R, Possemato R, Penaranda C, Glimcher LH. T-bet is required for optimal production of IFN-gamma and antigen-specific T cell activation by dendritic cells. Proc Natl Acad Sci USA. 2003;100:7749–54. doi: 10.1073/pnas.1332767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastos KR, Alvarez JM, Marinho CR, Rizzo LV, Lima MR. Macrophages from IL-12p40-deficient mice have a bias toward the M2 activation profile. J Leukoc Biol. 2002;71:271–8. [PubMed] [Google Scholar]
- 17.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–73. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 18.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–42. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 20.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suri RM, Austyn JM. Bacterial lipopolysaccharide contamination of commercial collagen preparations may mediate dendritic cell maturation in culture. J Immunol Methods. 1998;214:149–63. doi: 10.1016/s0022-1759(98)00048-9. [DOI] [PubMed] [Google Scholar]
- 22.Lyons AB. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Methods. 2000;243:147–54. doi: 10.1016/s0022-1759(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 23.Bonham CA, Lu L, Li Y, Hoffman RA, Simmons RL, Thomson AW. Nitric oxide production by mouse bone marrow-derived dendritic cells: implications for the regulation of allogeneic T cell responses. Transplantation. 1996;62:1871–7. doi: 10.1097/00007890-199612270-00033. [DOI] [PubMed] [Google Scholar]
- 24.Punjabi CJ, Laskin DL, Heck DE, Laskin JD. Production of nitric oxide by murine bone marrow cells. Inverse correlation with cellular proliferation. J Immunol. 1992;149:2179–84. [PubMed] [Google Scholar]
- 25.Powell TJ, Jenkins CD, Hattori R, MacPherson GG. Rat bone marrow-derived dendritic cells, but not ex vivo dendritic cells, secrete nitric oxide and can inhibit T-cell proliferation. Immunology. 2003;109:197–208. doi: 10.1046/j.1365-2567.2003.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corinti S, Pastore S, Mascia F, Girolomoni G, Powell TJ, Jenkins CD, Hattori R, MacPherson GG. Regulatory role of nitric oxide on monocyte-derived dendritic cell functions. J Interferon Cytokine Res. 2003;23:423–31. doi: 10.1089/107999003322277838. [DOI] [PubMed] [Google Scholar]
- 27.Morelli AE, Zahorchak AF, Larregina AT, Colvin BL, Logar AJ, Takayama T, Falo LD, Thomson AW. Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation. Blood. 2001;98:1512–23. doi: 10.1182/blood.v98.5.1512. [DOI] [PubMed] [Google Scholar]
- 28.Hilkens CM, Kalinski P, de Boer M, Kapsenberg ML. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood. 1997;90:1920–6. [PubMed] [Google Scholar]
- 29.Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000;164:4507–12. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson KA, Aung H, Wu M, Toossi Z. Modulation of transforming growth factor beta-1 gene expression by interleukin-12. Scand J Immunol. 2000;52:271–7. doi: 10.1046/j.1365-3083.2000.00772.x. [DOI] [PubMed] [Google Scholar]
- 31.Bogdan C, Paik J, Vodovotz Y, Nathan C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-beta and interleukin-10. J Biol Chem. 1992;267:23301–8. [PubMed] [Google Scholar]
- 32.Vodovotz Y, Bogdan C, Paik J, Xie QW, Nathan C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J Exp Med. 1993;178:605–13. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinson DM, LeClaire RD, Lorsbach RB, Parmely MJ, Russell SW. Regulation by transforming growth factor-beta 1 of expression and function of the receptor for IFN-gamma on mouse macrophages. J Immunol. 1992;149:2028–34. [PubMed] [Google Scholar]
- 34.Lu L, Bonham CA, Chambers FG, Watkins SC, Hoffman RA, Simmons RL, Thomson AW. Induction of nitric oxide synthase in mouse dendritic cells by IFN-gamma, endotoxin, and interaction with allogeneic T cells: nitric oxide production is associated with dendritic cell apoptosis. J Immunol. 1996;157:3577–86. [PubMed] [Google Scholar]
- 35.Stanford A, Chen Y, Zhang XR, Hoffman R, Zamora R, Ford HR. Nitric oxide mediates dendritic cell apoptosis by downregulating inhibitors of apoptosis proteins and upregulating effector caspase activity. Surgery. 2001;130:326–32. doi: 10.1067/msy.2001.116411. [DOI] [PubMed] [Google Scholar]